Abstract
Background
Infection of open fractures remains a significant cause of morbidity and mortality to patients worldwide. Early administration of prophylactic antibiotics is known to improve outcomes; however, increasing concern regarding antimicrobial resistance makes finding new compounds for use in such cases a pressing area for further research. CSA-90, a synthetic peptidomimetic compound, has previously demonstrated promising antimicrobial action against Staphylococcus aureus in rat open fractures. However, its efficacy against antibiotic-resistant microorganisms, its potential as a therapeutic agent in addition to its prophylactic effects, and its proosteogenic properties all require further investigation.
Questions/purposes
(1) Does prophylactic treatment with CSA-90 reduce infection rates in a rat open fracture model inoculated with S aureus, methicillin-resistant S aureus (MRSA), and methicillin-resistant Staphylococcus epidermidis (MRSE) as measured by survival, radiographic union, and deep tissue swab cultures? (2) Does CSA-90 reduce infection rates when administered later in the management of an open fracture as measured by survival, radiographic union, and deep tissue swab cultures? (3) Does CSA-90 demonstrate a synergistic proosteogenic effect with bone morphogenetic protein 2 (BMP-2) in a noninfected rat ectopic bone formation assay as assessed by micro-CT bone volume measurement? (4) Can CSA-90 elute and retain its antimicrobial efficacy in vitro when delivered using clinically relevant agents measured using a Kirby-Bauer disc diffusion assay?
Methods
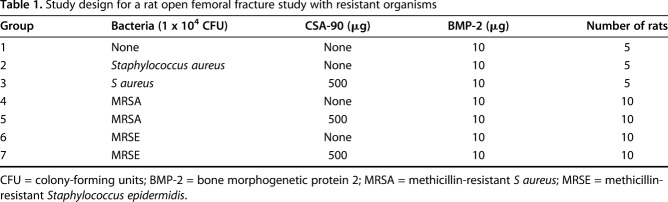
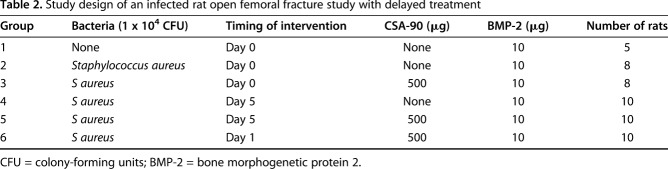
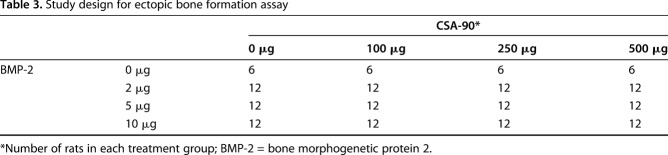
All in vivo studies were approved by the local animal ethics committee. In the open fracture studies, 12-week-old male Wistar rats underwent open midshaft femoral fractures stabilized with a 1.1-mm Kirschner wire and 10 µg BMP-2 ± 500 µg CSA-90 was applied to the fracture site using a collagen sponge along with 1 x 104 colony-forming units of bacteria (S aureus/MRSA/MRSE; n = 10 per group). In the delayed treatment study, débridement and treatment with 500 µg CSA-90 were performed at Day 1 and Day 5 after injury and bacterial insult (S aureus). All animals were reviewed daily for signs of local infection and/or sepsis. An independent, blinded veterinarian reviewed twice-weekly radiographs, and rats showing osteolysis and/or declining overall health were culled at his instruction. The primary outcome of both fracture studies was fracture infection, incorporating survival, radiographic union, and deep tissue swab cultures. For the ectopic bone formation assay, 0 to 10 µg BMP-2 and 0 to 500 µg CSA-90 were delivered on a collagen sponge into bilateral quadriceps muscle pouches of 8-week-old rats (n = 10 per group). Micro-CT quantification of bone volume and descriptive histologic analysis were performed for all in vivo studies. Modified Kirby-Bauer disc diffusion assays were used to quantify antimicrobial activity in vitro using four different delivery methods, including bone cement.
Results
Infection was observed in none of the MRSA inoculated open fractures treated with CSA-90 with 10 of 10 deep tissue swab cultures negative at the time of cull. Median survival was 43 days (range, 11-43 days) in the treated group versus 11 days (range, 8-11 days) in the untreated MRSA inoculated group (p < 0.001). However, delayed débridement and treatment of open fractures with CSA-90 at either Day 1 or Day 5 did not prevent infection, resulting in early culls by Day 21 with positive swab cultures (10 of 10 for each time point). Maximal ectopic bone formation was achieved with 500 μg CSA-90 and 10 μg BMP-2 (mean volume, 9.58 mm3; SD, 7.83), creating larger bone nodules than formed with 250 μg CSA-90 and 10 μg BMP-2 (mean volume, 1.7 mm3; SD, 1.07; p < 0.001). Disc diffusion assays showed that CSA-90 could successfully elute from four potential delivery agents including calcium sulphate (mean zone of inhibition, 11.35 mm; SD, 0.957) and bone cement (mean, 4.67 mm; SD, 0.516).
Conclusions
CSA-90 shows antimicrobial action against antibiotic-resistant Staphylococcal strains in vitro and in an in vivo model of open fracture infection.
Clinical Relevance
The antimicrobial properties of CSA-90 combined with further evidence of its proosteogenic potential make it a promising compound to develop further for orthopaedic applications.
Introduction
Infection of open fractures remains a significant cause of morbidity and mortality to patients worldwide [20, 23]. Even when wounds are not visibly contaminated, it is accepted that open fractures have a substantially increased risk of becoming infected compared with equivalent closed fractures [15]. Although there is some controversy regarding the exact treatment window for exploration, débridement, and fixation, it generally is accepted that for most fractures, this should occur within 6 to 24 hours and systemic antibiotic prophylaxis usually is delivered for 72 hours or until definitive wound closure [9, 10, 13].
Staphylococcus aureus is frequently isolated in open fracture infections along with other coagulase-negative Staphylococci including Staphylococcus epidermidis [8]. Although S epidermidis is considered a less virulent pathogen than S aureus, there is evidence to suggest that S epidermidis strains may be acquiring more invasive properties and are just as effective at forming biofilms, particularly on orthopaedic implants [7]. The incidence of community-acquired antibiotic-resistant strains such as methicillin-resistant S aureus (MRSA) and methicillin-resistant S epidermidis (MRSE) is increasing, particularly in the United States, with rates of up to 25% reported [4, 18]. With increasing resistance to conventional antibiotics, there is a pressing need to identify and develop novel antimicrobial therapies, particularly for orthopaedic applications.
CSA-90 is a small synthetic peptidomimetic compound based on endogenous cationic antibacterial peptides such as LL-37. Its steroid-like structure is able to disrupt cell membranes and therefore confers a broad range of activity against Gram-positive and Gram-negative bacteria, including vancomycin and methicillin-resistant strains [3, 6, 12]. CSA-90 demonstrates reduced cytotoxicity and improved in vivo stability compared with endogenous peptides and has also shown proosteogenic potential, a desirable attribute in the management of open fractures [11, 17]. Furthermore, in vitro evidence suggests LL-37 may be able to prevent biofilm formation [14]. Schindeler et al. [17] recently showed that local treatment with CSA-90 could prevent infection with S aureus and facilitate union in a rat open fracture model.
In view of the challenges facing open fracture management and the orthopaedic potential of CSA-90, we sought to further examine the breadth of CSA-90’s antimicrobial action to include antibiotic-resistant strains. Having already shown promise as a prophylactic agent [17], the natural progression was to hypothesize whether CSA-90 might be effective in the treatment of established open fracture infection. Proosteogenic potential of CSA-90 had already been suggested in vitro in synergy with bone morphogenetic protein 2 (BMP-2) [17] and required further testing in vivo to determine whether this might provide an additional clinical benefit in orthopaedic-specific applications. Lastly, antimicrobial prophylaxis is rarely delivered locally, with few antibiotics being available for local use and limited methods for delivery. We performed preliminary studies to identify possible carriers that would be required to develop CSA-90 further as a therapeutic agent.
Our study aimed to answer four key research questions: (1) Does prophylactic treatment with CSA-90 reduce infection rates in a rat open fracture model inoculated with S aureus, MRSA, and MRSE as measured by survival, radiographic union, and deep tissue swab cultures? (2) Does CSA-90 reduce infection rates when administered later in the management of an open fracture as measured by survival, radiographic union, and deep tissue swab cultures? (3) Does CSA-90 demonstrate a synergistic proosteogenic effect with BMP-2 in a noninfected rat ectopic bone formation assay as assessed by micro-CT bone volume measurement? (4) Can CSA-90 elute and retain its antimicrobial efficacy in vitro when delivered using clinically relevant agents as measured using a Kirby-Bauer disc diffusion assay?
Materials and Methods
Rat Open Femoral Fracture Model
Studies were carried out under the Children’s Hospital at Westmead/Children’s Medical Research Institute Animal Ethics Committee approval K339. Animals were purchased from the Animal Resources Centre (Canning Vale, Western Australia) and housed in pairs with access to food and water ad libitum. Surgical anesthesia was induced with intraperitoneal ketamine (70 mg/kg) and xylazine (10 mg/kg) and maintained with inhaled isoflurane as required. The right distal femora of 12-week-old male Wistar rats were first stabilized using a 1.1-mm Kirschner wire; then a midshaft femoral fracture was created using the Einhorn drop-weight apparatus [1]. The fracture site was opened and the periosteum stripped around the fracture to recreate an open injury. A ¼-inch square absorbable collagen sponge was pretreated in a sterile hood with 30 μL experimental agent (10 μg BMP-2 ± 500 μg CSA-90) and then dosed with 1 x 104 colony-forming units (CFU) of bacteria immediately before implantation (Table 1). The sponge was wrapped circumferentially around the fracture and the wound closed in layers with 4-0 VicrylTM (Ethicon, Somerville, NJ, USA). Animals recovered on a heated pad and were given subcutaneous saline and buprenorphine (0.1 mg/kg) for postoperative analgesia. The rats were reviewed daily by veterinary nurses (KM, LP) throughout the 6-week study period for any developing signs of infection and underwent twice-weekly radiographs under general anesthesia (inhaled isoflurane) using a digital x-ray (Faxitron, Tucson, AZ, USA) at 30 kV with x 2 magnification.
Table 1.
Study design for a rat open femoral fracture study with resistant organisms
For the delayed-treatment study, depending on the experimental group, the fracture site was reopened at Day 1 or 5 and the wound irrigated, débrided, and then treated with 500 μg CSA-90 as previously described (Table 2). This study was only performed with S aureus and not repeated with MRSA and MRSE.
Table 2.
Study design of an infected rat open femoral fracture study with delayed treatment
Reagents
CSA-90 (molecular weight 851 g/mol) was produced in the laboratory of Paul B. Savage PhD, at Brigham Young University (Provo, UT, USA), courtesy of N8 Medical (Columbus, OH, USA). Recombinant human BMP-2 was purchased as part as of the Infuse® kit from Medtronic (Macquarie Park, NSW, Australia).
Bacterial Culture
S aureus (American Type Culture Collection [ATCC]-12600) was used as well as MRSA and MRSE strains obtained from David Isaacs (The Children’s Hospital at Westmead, NSW, Australia). Bacteria were grown on lysogeny broth agar plates and single colonies picked for overnight culture in lysogeny broth before surgical inoculation. Bacteria were quantified using a spectrophotometer (Cary® 300 UV-Vis; Agilent, Santa Clara, CA, USA) at 600 nm with an optical density of 1 valued to represent 1 x 109 CFU/mL.
Survival
Twice-weekly blinded radiographs were reviewed by an independent veterinarian (RM) and those animals showing radiologic evidence of infection (osteolysis, loss of metalwork fixation) or declining overall health (loss of body weight, lethargy, pyrexia, poor coat condition, nonweightbearing, inflammation of the surgical site) were euthanized earlier than the 6-week study endpoint with carbon dioxide at his instruction.
Microbiologic Analysis
Right femora were harvested using an aseptic technique and a deep tissue microbiologic swab taken from the surgical site. Swabs were agitated in 1 mL sterile lysogeny broth and then cultured overnight at 37o C before being reported as either positive (turbid) or negative (clear) as previously published [17]. Negative control swabs were performed with each batch to confirm sterility of the technique.
Radiographic and Micro-CT Analysis
Fracture union was assessed by an orthopaedic surgeon (RM) blinded to treatment group using the modified Radiographic Union Scale for Tibia (RUST) score on 3- (lateral only) and 6-week (AP and lateral) postoperative radiographs [21]. This scoring system has been validated for use in rodent models of fracture healing [19].
Nondestructive ultrastructural analysis was performed with the SkyScan 1174 micro-CT scanner (Bruker MicroCT, Kontich, Belgium), later upgraded to the SkyScan 1272 scanner for the open fracture study with MRSA. The intramedullary Kirschner wire was removed and samples were scanned wrapped in saline-soaked gauze at 50 kV with a 0.5-mm aluminum filter. Ten-micrometer pixel resolution images were reconstructed using NRecon (Bruker MicroCT) and analyzed using CTAn software (Bruker MicroCT). A global threshold to define bone tissue was set at 0.3 g/cm3 calcium hydroxyapatite calibrated using two phantom samples of a known density. Bone volume (BV), tissue volume (TV), and %BV/TV were calculated from a cylindrical volume of interest that included all the woven callous and remodeling bone 100 slices above and below the fracture line.
Histologic Analysis
Specimens were first fixed in 10% formalin for 48 hours and stored in 70% ethanol before being fully decalcified in 0.34 mol/L EDTA (pH 8.0), embedded in paraffin, cut to 5-μm sections on a Leica RM2155 microtome (Leica Biosystems, Wetzlar, Germany), and stained with hematoxylin and eosin or alcian blue and picrosirius red. Stained sections were scanned digitally with the AperioTM CS2 digital pathology slide scanner (Leica Biosystems) and images captured using Leica’s ImageScope software for descriptive analysis. Representative samples of each group were selected from the median bone volume values based on micro-CT data.
Rat Ectopic Bone Formation Assay
CSA-90 was tested in an ectopic bone formation model, in which 4-mm-diameter discs of Helistat® absorbable collagen sponge (Integra Lifesciences, Plainsboro, NJ, USA) containing BMP-2 ± CSA-90 were implanted bilaterally into the quadriceps muscles of 8-week-old male Wistar rats to induce a nodule of bone. To find the therapeutic window of both drugs alone and in combination, 0- to 10-μg doses of BMP-2 were combined with 0- to 500-μg doses of CSA-90 (Table 3). Rats were monitored daily by veterinary nurses (KM, LP) and underwent weekly radiographs until the study endpoint at 4 weeks, when micro-CT quantification of the resultant bone formation was performed. For this analysis, a freehand volume of interest was drawn to include the entire nodule but excluding the adjacent femur.
Table 3.
Study design for ectopic bone formation assay
In Vitro Disc Diffusion Assay
Modified Kirby-Bauer disc diffusion assays were performed using lysogeny broth agar plates. Six millimeter diameter Number 1 Whatman® paper discs (Sigma-Aldrich, St Louis, MO, USA) were prepared in a sterile environment with 10 μg to 500 μg CSA-90 and compared with 10-μg gentamicin discs as a positive control (Oxoid Limited, Hampshire, UK). Plates were spread with 1.5 x 108 CFU (equivalent to 0.5 McFarland turbidity standard), the discs applied, and incubated at 37o C for 24 hours before two independent observers (TLC, RM) measured the maximal zone of bacterial growth inhibition (radius from the center of the disc). Assays were run in triplicate with S aureus, MRSA, and MRSE.
Potential delivery methods for CSA-90 were tested in the same assay (S aureus only) using a 10-μL carrier agent to deliver 500 μg CSA-90 per disc. The delivery agents tested were: saline; 1% poly(vinylpyrrolidone) (PVP); 0.25% dihydrous calcium sulphate; and sucrose acetate isobutyrate mixed 50:50 with absolute ethanol to obtain a suitable viscosity for coating the discs. These reagents were obtained from Sigma-Aldrich. Similar 6-mm diameter x 2.5-mm bone cement discs were prepared in a silicone mold created from a three-dimensional-printed reverse template. Twenty-five milligrams of CSA-90 were added to 10 g CMWTM 1 bone cement mix (DePuy, Blackpool, UK) compared with 80 mg gentamicin prepared in the same volume as a positive control.
Statistical Analysis
Statistical power calculations and analyses were performed using GraphPad Prism® (GraphPad Software, La Jolla, CA, USA) and the cutoff for significance for all tests was set to p < 0.05. Statistical analysis of the disc diffusion assays and the bone formation assays was performed using a one-way analysis of variance and post hoc Dunn’s test. In vivo studies were powered to infection rate and fracture union based on means and variances from a previously published study [17] and were compared using Fisher’s exact test. Survival curves were analyzed using the log-rank (Mantel-Cox) test with correction for multiple comparisons.
Results
Prophylactic Use of CSA-90 Mitigates Open Fracture Infection and Facilitates Union
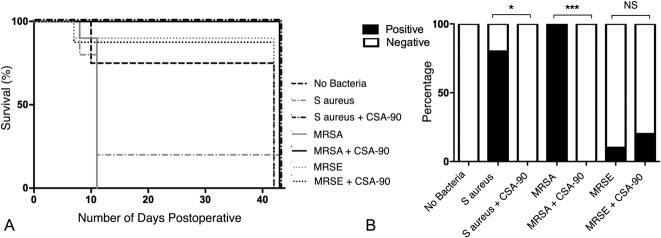
All (10 of 10) untreated rats with open fractures inoculated with MRSA and four of five rats inoculated with S aureus developed severe infection of the fracture site requiring euthanasia by Day 11 postoperatively (Fig. 1A). Deep tissue swab cultures confirmed the presence of infection at the surgical site in these animals (Fig. 1B). The single rat inoculated with S aureus, but surviving to 6 weeks without obvious clinical or radiographic signs of infection, had negative deep tissue swab cultures at the time of cull. However, this fracture failed to unite within 6 weeks, unlike the noninfected controls and MRSE inoculated groups, suggesting possible low-grade infection.
Fig. 1 A-B.
(A) The Kaplan-Meier curves illustrate the effect of local CSA-90 treatment on animal survival after open fractures were inoculated with S aureus, MRSA, and MRSE. (B) The bar graph shows the percentage of positive deep tissue swab cultures in each group at the time of cull. NS = not significant.
All rats treated with 500 μg CSA-90 after inoculation with S aureus (10 of 10) or MRSA (10 of 10) survived to the end of the study without any clinical or radiologic evidence of infection, despite intramedullary metalwork remaining in situ for the study duration. All (20 of 20) deep tissue swab cultures in these CSA-90-treated groups were negative at the time of cull. Log-rank (Mantel-Cox) analysis of the Kaplan-Meier survival curves showed that the CSA-90–treated S aureus group had increased survival compared with untreated S aureus controls (median survival, 43 versus 11 days; p = 0.016). CSA-90-treated MRSA fractures also survived longer than untreated MRSA controls (median survival, 43 days; range, 11-43 days versus 11 days; range, 8-11 days; p < 0.001). No difference was observed between the CSA-90-treated and untreated MRSE groups, because the rats appeared to spontaneously overcome the 1 x 104 CFU MRSE bacterial burden without any clinical signs of infection developing (median survival, 43 versus 43 days; p = 0.871).
All MRSA and S aureus inoculated fractures treated with 500 μg CSA-90 united by the 6-week study endpoint, which is illustrated by lateral radiographs, micro-CT sagittal sections, and histologic sections (Fig. 2). Micro-CT quantification of bone around the fracture region showed an increased bone volume around the fracture site (mean, 189.4 mm3; SD, 25.35; 95% confidence interval [CI], 168.2-210.6 mm3) in the CSA-90–treated MRSA group compared with the untreated MRSA group (mean, 139.7 mm3; SD, 18.49; 95% CI, 124.2-155.1 mm3; p = 0.016) (Fig. 3A).
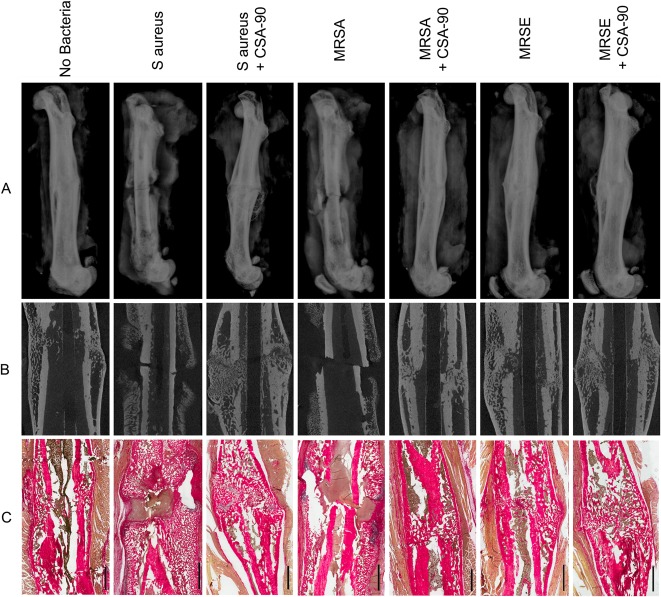
Fig. 2 A-C.
(A) Lateral femur radiographs taken at the time of cull show representative samples from each of the open fracture groups inoculated with S aureus, MRSA, and MRSE. Radiographic union (single-plane) is demonstrated in the CSA-90-treated groups. (B) Micro-CT sagittal reconstructions of the same femora further confirm union and demonstrate the bony remodeling achieved around the fracture site in the treatment groups. (C) Histologic sections, stained with alcian blue and picrosirius red, further illustrate bony remodeling in the treated fractures, whereas inflammatory cell debris and pus are visible around the fracture site in the infected nonunions. Scale bars = 2 mm.
Fig. 3 A-B.
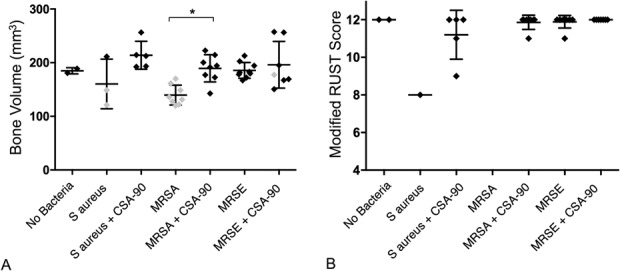
(A) The scatterplot illustrates the bone volume (mm3) (mean + SD) of fracture callus in the infected open fracture groups, calculated from micro-CT analysis of a predefined volume of interest. The gray diamonds represent cases with confirmed positive deep swab cultures. (B) The scatterplot illustrates the modified RUST score (a validated measure of fracture union) of the fractures at 6 weeks, as assessed by a blinded orthopaedic surgeon (DGL) (maximum score 12). *p < 0.05.
Nineteen of 20 of the CSA-90–treated infected fractures achieved a modified RUST score of ≥ 11, consistent with fracture union (Fig. 3B) [19]. None (zero of 10) of the untreated MRSA fractures reached the study endpoint and so were not included in the analysis. No difference was identified between the MRSE treated and untreated groups with all (20 of 20) fractures achieving a modified RUST score of ≥ 11.
CSA-90 Inhibits Growth of MRSA and MRSE In Vitro
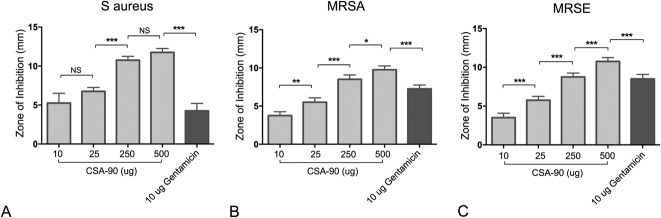
An antimicrobial effect of CSA-90 against MRSE was demonstrated in vitro in a modified Kirby-Bauer disc diffusion assay (mean growth inhibition with 500-μg dose, 10.75 mm; range, 10-11 mm). A clear dose-dependent response was seen across assays with S aureus, MRSA, and MRSE (Fig. 4). Five hundred micrograms of CSA-90 inhibited more bacterial growth than 10 μg gentamicin tested against S aureus (mean difference, 7.5 mm; 95% CI, 6.16-8.83; p < 0.001), MRSA (mean difference, 2.5 mm; 95% CI, 1.17-3.83; p < 0.001), and MRSE (mean difference, 2.25 mm; 95% CI, 0.92-3.58; p < 0.001).
Fig. 4 A-C.
The bar graphs (mean + SD) show the diameter of bacterial growth inhibition around 6-mm-diameter treated discs and a clear dose-response of CSA-90 against (A) S aureus, (B) MRSA, and (C) MRSE. *p < 0.05; **p < 0.01; ***p < 0.001; NS = not significant.
Delayed Treatment With 500 μg CSA-90 Does Not Prevent S aureus Infection Developing in Open Fractures
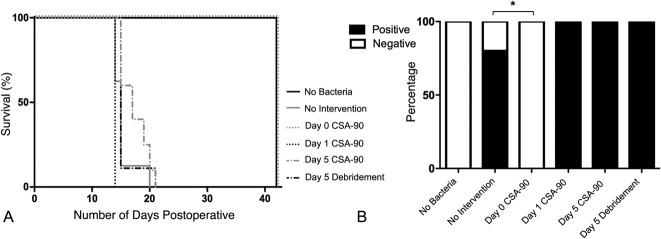
Kaplan-Meier survival curves and deep tissue cultures from the delayed treatment study (Fig. 5A) confirmed previous findings that S aureus fracture infection could be prevented by administering 500 μg CSA-90 at the time of inoculation (Day 0) [17]. However, neither débridement alone nor débridement in conjunction with 500 μg CSA-90 at Day 1 or Day 5 was able to prevent S aureus infection developing in the open fractures. All rats in these groups had both clinical and radiologic signs of infection, requiring premature cull by Day 21. All had positive deep tissue swab cultures from the fracture site (Fig. 5B). Further histologic analysis of the femurs confirmed the presence of infection around the nonunited fracture site (Fig. 6).
Fig. 5 A-B.
(A) The Kaplan-Meier curves illustrate the effect of delayed CSA-90 treatment on animal survival after open fractures were inoculated with S aureus. (B) The bar graph shows the percentage of positive deep wound swab cultures in each group of the delayed treatment open fracture study. SAIB = sucrose acetate isobutyrate; NS = not significant.
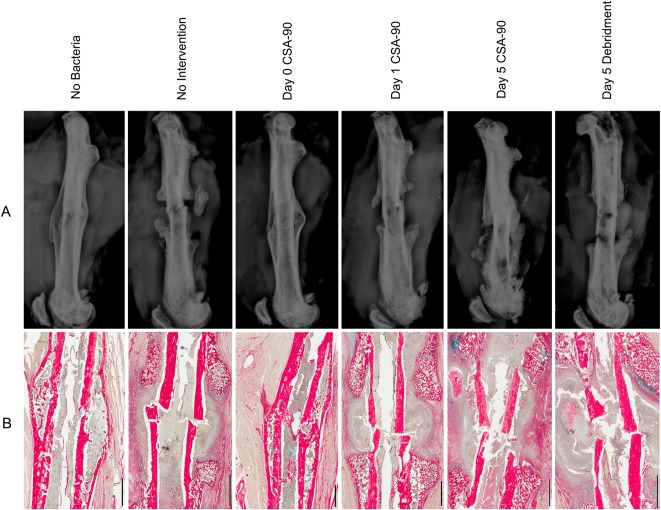
Fig. 6 A-B.
(A) Lateral femur radiographs taken at the time of cull show representative samples from each of the open fracture groups inoculated with S aureus and treated with CSA-90 and/or débridement at Day 0, 1, or 5 after surgery. Noninfected and those treated at Day 0 demonstrate fracture union (single-plane). (B) Corresponding histologic sections, stained with alcian blue and picrosirius red, confirm union in the noninfected and Day 0 treatment groups. Inflammatory cell debris and pus are clearly visible around the nonunited fractures of the nontreated and delayed treatment groups. Scale bars = 2 mm.
CSA-90 Enhances BMP-2–induced Bone Formation
Maximal ectopic bone formation was achieved with 500 μg CSA-90 and 10 μg BMP-2. This therapeutic combination created bone nodules with a mean volume of 9.58 mm3 (SD, 7.83; 95% CI, 4.60-14.56), visibly larger than bone nodules formed with the same 10-μg dose of BMP-2 and 250 μg CSA-90 (mean volume, 1.7 mm3; SD, 1.07; 95% CI, 1.02-2.30; p < 0.001) (Fig. 7A).
Fig. 7 A-B.
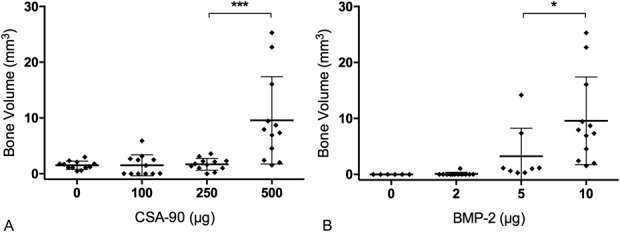
The scatterplots (mean + SD) show micro-CT analysis of the bone volume of ectopic nodules in the muscle pouch assay with (A) 10 µg BMP-2 in conjunction with increasing doses of CSA-90 and (B) 500 µg CSA-90 with increasing doses of BMP-2. *p < 0.05; ***p < 0.001.
Five hundred micrograms of CSA-90 without BMP-2 resulted in no bone formation in this assay. The 500 μg CSA-90 and 10 μg BMP-2 combination also produced greater bone formation than 500 μg CSA-90 with 5 μg BMP-2 (mean difference, 6.32 mm3; 95% CI, 0.16-12.48; p = 0.043) (Fig. 7B). Consequently, 10 μg BMP-2 was used in addition to 500 μg CSA-90 in the open fracture studies.
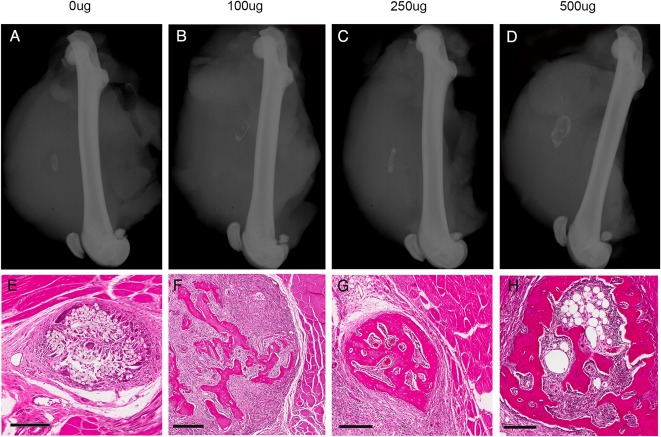
Lateral radiographs demonstrate the relative size of the ectopic bone nodules in relation to the quadriceps and femur with minimal ectopic bone visible at the lower CSA-90 doses (Fig. 8A-B), but discrete nodules seen at 250-μg and 500-μg doses (Fig. 8C-D). Corresponding histologic sections illustrate the evolving bony architecture of the nodules with a cortical shell developing around pseudomarrow space cavities in the larger bone nodules (Fig. 8E-H).
Fig. 8 A-H.
(A-D) Lateral plain radiographs demonstrate the relative size of ectopic bone nodules formed in the quadriceps muscle pouch assay using collagen discs treated with 10 µg BMP-2 and increasing doses of CSA-90. (E-H) Corresponding histologic sections stained with hematoxylin and eosin illustrate the bony architecture of each nodule with a dense cortical shell enclosing pseudomarrow space cavities. Scale bars = 200 µm.
CSA-90 Inhibits Bacterial Growth From Four Potential Delivery Agents, Including Bone Cement
Further disc diffusion assays performed using discs coated with 500 μg CSA-90 in different delivery agents showed that CSA-90 could elute effectively from PVP, a commercially available hydrogel (mean zone of inhibition, 12 mm; SD, 0.816); calcium sulphate, often used for filling bone defects (mean, 11.25 mm; SD, 0.957); and sucrose acetate isobutyrate, an injectable sugar-based delivery system (mean, 11.5 mm; SD, 0.577) (Fig. 9A). It was also shown that CSA-90 could be mixed into bone cement, elute, and still inhibit S aureus growth in the same disc diffusion assay (mean, 4.7 mm; SD, 0.516) (Fig. 9B).
Fig. 9 A-B.
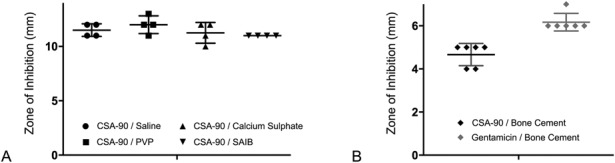
The scatterplots (mean + SD) show (A) the diameter of S aureus growth inhibition around 6-mm-diameter discs treated with 500 µg CSA-90 in different delivery agents and (B) the same assay repeated using 6-mm-diameter x 2.5-mm discs of DePuy CMW 1 bone cement mixed with either CSA-90 or gentamicin. ***p < 0.001.
Discussion
CSA-90 has previously shown promise as a local prophylactic antimicrobial in the management of open fractures inoculated with S aureus [17]. We sought to broaden its potential therapeutic range by testing it in a preclinical model with common antimicrobial resistant strains, where it was demonstrated to mitigate infection in MRSA inoculated open fractures. In the subsequent study testing delayed treatment, 500 µg CSA-90 administered at the time of surgical débridement on Day 1 or Day 5 failed to facilitate normal fracture healing.
Clearly this study represents a rather rudimentary animal model for hypothesizing on novel antimicrobial strategies for the management of such a complex clinical problem as open fracture infection. However, such models do enable control of confounding variables such as comorbidities, mechanism of injury, delay in presentation, microbiologic differences, and variations in management to study a single parameter, which would be impossible in clinical studies. Furthermore, success in a small rodent model can by no means guarantee efficacy in the human population; however, it is an important step in directing further pharmaceutical development in larger animals. Testing local delivery of CSA-90 in combination with routine systemic antibiotic prophylaxis will also be a key step in further identifying the clinical potential of this novel agent.
Despite demonstrating dose-dependent inhibition of MRSE growth by CSA-90 in the disc diffusion assay, 1 x 104 CFU of the same strain was insufficient to cause a clinical infection in the open fracture model, presumed as a result of the lesser virulence of S epidermidis compared with S aureus. Nonetheless, we have included these data not only for transparency, but also to inform future studies, because the biofilm-forming phenotype of S epidermidis [7] will continue to be a pathogen of interest to orthopaedic researchers.
A further limitation of our study is the deep tissue swab technique used to determine the presence of infection at the time of cull. Turbidity is recognized as a surrogate measure to quantify bacteria in a solution. Gram stain would have confirmed the presence of bacteria, but not necessarily have identified contaminants, which would likely also be Gram-positive cocci. Gene sequencing or the addition of a detectable tag to the inoculating bacteria would provide a robust method of identifying the source of infection; however, this was outside the scope of this study.
Lastly, micro-CT analysis was performed in the open fracture studies as a secondary measure of fracture union and infection. Measurements were limited to BV and TV because the callous architecture was found to be so heterogeneous and temporarily sensitive that extensive quantification of trabecular structure was not felt to offer any clinically relevant information in this context.
Prophylactic treatment with 500 µg CSA-90 delivered locally to the fracture site shortly after injury reduced infection rates in both S aureus and MRSA inoculated models. Using the same dose of CSA-90 for treatment of established S aureus infection at Day 1 and Day 5 postinjury was less successful. Previous studies looking at delayed treatment of open femoral fractures in rats likewise failed to fully eradicate S aureus with local administration of tobramycin and vancomycin-loaded polymethylmethacrylate beads at 2, 6, or 24 hours [2]. Only those animals receiving both systemic antibiotic prophylaxis and surgical débridement within 2 hours of inoculation had undetectable bacterial loads 2 weeks later [16]. These findings are aligned with our current focus on CSA-90 as a prophylactic agent; however, it is hypothesized that at higher doses, CSA-90 may exhibit biofilm activity, as suggested by studies of the endogenous cationic peptide LL-37 [14].
The ectopic bone formation study supported the hypothesis that CSA-90 may have proosteogenic potential; however, this was only demonstrated in combination with BMP-2. In the open fracture study, it was not possible to ascertain whether the increased bone volume seen in the CSA-90-treated compared with the untreated MRSA fractures was the result of antimicrobial or proosteogenic action, but we suspect the former given that no difference was identified between CSA-90-treated and untreated noninfected controls in a previous study [17].
In the rodent models described in this article, we used an acellular collagen sponge to deliver CSA-90 to the surgical site. However, there is potential to develop other carrier systems for use in the orthopaedic setting. One alternative is an injectable sugar-based compound, sucrose acetate isobutyrate, which has been successfully used in vivo as a BMP-2 delivery method [5]. Our in vitro work demonstrated that CSA-90 could maintain its antimicrobial properties eluting from calcium sulphate, commonly used for filling bone voids, and polymethylmethacrylate bone cement, both of which could be used for delivery around an open fracture in a bead format [22]. We were particularly surprised that despite being a small peptide compound, CSA-90 did not appear to be denatured after mixing with the highly exothermic polymethylmethacrylate bone cement reaction. Limited quantitative comparison of delivery methods was performed at this preliminary stage.
In conclusion, CSA-90 shows antimicrobial action against antibiotic-resistant Staphylococcal strains in vitro. The antimicrobial properties of CSA-90 combined with further evidence of its proosteogenic potential, when administered in combination with BMP-2, make it a promising compound to develop further for orthopaedic applications.
Acknowledgments
Ross Matthews BVSc, Dip Vet Clin Path, Department of Animal Care, Westmead Hospital, Westmead, New South Wales, Australia, was involved in the blinded review of radiographs and animals displaying signs of infection.
Footnotes
The institution of one or more of the authors has received, during the study period, grant funding and nonfinancial support from N8 Medical (Las Vegas, NV, USA; PBS, DGL, AS); Novartis Pharma (Basel, Switzerland), Celgene (Summit, NJ, USA), and Amgen (Thousand Oaks, CA, USA; DGL, AS); and Hyundai Help for Kids (Macquarie Park, NSW, Australia; TLC) unrelated to this work. One author (CG) is an employee of N8 Medical. One of the authors (PBS) holds patents for CSA-90 licensed to N8 Medical. This study was funded by the National Health and Medical Research Council (DGL; NHMRC Project Grant GNT1106982). N8 Medical supplied CSA-90 for the study under the terms of a material transfer agreement.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Each author certifies that his or her institution approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at The Children’s Hospital at Westmead (Westmead, NSW, Australia) and Brigham Young University (Provo, UT, USA).
References
- 1.Bonnarens F, Einhorn TA. Production of a standard closed fracture in laboratory animal bone. J Orthop Res. 1984;2:97–101. [DOI] [PubMed] [Google Scholar]
- 2.Brown KV, Walker JA, Cortez DS, Murray CK, Wenke JC. Earlier débridement and antibiotic administration decrease infection. J Surg Orthop Adv. 2010;19:18–22. [PubMed] [Google Scholar]
- 3.Bucki R, Niemirowicz K, Wnorowska U, Byfield FJ, Piktel E, Wątek M, Janmey PA, Savage PB. Bactericidal activity of ceragenin CSA-13 in cell culture and in an animal model of peritoneal infection. Antimicrob Agents Chemother. 2015;59:6274–6282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen AF, Schreiber VM, Washington W, Rao N, Evans AR. What is the rate of methicillin-resistant Staphylococcus aureus and Gram-negative infections in open fractures? Clin Orthop Relat Res. 2013;471:3135–3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng TL, Valtchev P, Murphy CM, Cantrill LC, Dehghani F, Little DG, Schindeler A. A sugar-based phase-transitioning delivery system for bone tissue engineering. Eur Cell Mater. 2013;26:208–221. [DOI] [PubMed] [Google Scholar]
- 6.Epand RF, Pollard JE, Wright JO, Savage PB, Epand RM. Depolarization, bacterial membrane composition, and the antimicrobial action of ceragenins. Antimicrob Agents Chemother. 2010;54:3708–3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gill SR, Fouts DE, Archer GL, Mongodin EF, Deboy RT, Ravel J, Paulsen IT, Kolonay JF, Brinkac L, Beanan M, Dodson RJ, Daugherty SC, Madupu R, Angiuoli SV, Durkin AS, Haft DH, Vamathevan J, Khouri H, Utterback T, Lee C, Dimitrov G, Jiang L, Qin H, Weidman J, Tran K, Kang K, Hance IR, Nelson KE, Fraser CM. Insights on evolution of virulence and resistance from the complete genome analysis of an early methicillin-resistant Staphylococcus aureus strain and a biofilm-producing methicillin-resistant Staphylococcus epidermidis strain. J Bacteriol. 2005;187:2426–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gosselin RA, Roberts I, Gillespie WJ. Antibiotics for preventing infection in open limb fractures. Cochrane Database Syst Rev. 2004;1:CD003764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gustilo RB, Merkow RL, Templeman D. The management of open fractures. J Bone Joint Surg Am. 1990;72:299–304. [PubMed] [Google Scholar]
- 10.Halawi MJ, Morwood MP. Acute management of open fractures: an evidence-based review. Orthopedics. 2015;38:1025–1033. [DOI] [PubMed] [Google Scholar]
- 11.Lai XZ, Feng Y, Pollard J, Chin JN, Rybak MJ, Bucki R, Epand RF, Epand RM, Savage PB. Ceragenins: cholic acid-based mimics of antimicrobial peptides. Acc Chem Res. 2008;41:1233–1240. [DOI] [PubMed] [Google Scholar]
- 12.Leszczynska K, Namiot D, Byfield FJ, Cruz K, Zendzian-Piotrowska M, Fein DE, Savage PB, Diamond S, McCulloch CA, Janmey PA, Bucki R. Antibacterial activity of the human host defence peptide LL-37 and selected synthetic cationic lipids against bacteria associated with oral and upper respiratory tract infections. J Antimicrob Chemother. 2013;68:610–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okike K, Bhattacharyya T. Trends in the management of open fractures. A critical analysis. J Bone Joint Surg Am. 2006;88:2739–2748. [DOI] [PubMed] [Google Scholar]
- 14.Overhage J, Campisano A, Bains M, Torfs EC, Rehm BH, Hancock RE. Human host defense peptide LL-37 prevents bacterial biofilm formation. Infect Immun. 2008;76:4176–4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patzakis MJ, Wilkins J. Factors influencing infection rate in open fracture wounds. Clin Orthop Relat Res. 1989;243:36–40. [PubMed] [Google Scholar]
- 16.Penn-Barwell JG, Murray CK, Wenke JC. Early antibiotics and débridement independently reduce infection in an open fracture model. J Bone Joint Surg Br. 2012;94:107–112. [DOI] [PubMed] [Google Scholar]
- 17.Schindeler A, Yu NY, Cheng TL, Sullivan K, Mikulec K, Peacock L, Matthews R, Little DG. Local delivery of the cationic steroid antibiotic CSA-90 enables osseous union in a rat open fracture model of Staphylococcus aureus infection. J Bone Joint Surg Am. 2015;97:302–309. [DOI] [PubMed] [Google Scholar]
- 18.Stevens DL. Community-acquired Staphylococcus aureus infections: increasing virulence and emerging methicillin resistance in the new millennium. Curr Opin Infect Dis. 2003;16:189–191. [DOI] [PubMed] [Google Scholar]
- 19.Tawonsawatruk T, Hamilton DF, Simpson AH. Validation of the use of radiographic fracture-healing scores in a small animal model. J Orthop Res. 2014;32:1117–1119. [DOI] [PubMed] [Google Scholar]
- 20.Tay WH, de Steiger R, Richardson M, Gruen R, Balogh ZJ. Health outcomes of delayed union and nonunion of femoral and tibial shaft fractures. Injury. 2014;45:1653–1658. [DOI] [PubMed] [Google Scholar]
- 21.Whelan DB, Bhandari M, Stephen D, Kreder H, McKee MD, Zdero R, Schemitsch EH. Development of the radiographic union score for tibial fractures for the assessment of tibial fracture healing after intramedullary fixation. J Trauma Acute Care Surg. 2010;68:629–632. [DOI] [PubMed] [Google Scholar]
- 22.Wininger DA, Fass RJ. Antibiotic-impregnated cement and beads for orthopaedic infections. Antimicrob Agents Chemother. 1996;40:2675–2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu N. Economic burden of illness among US patients experiencing fracture non-union. Orthop Res Rev. 2013;5:21–33. [Google Scholar]