Abstract
Background
Survival of cruciate-retaining (CR) TKA is generally good, but there may be important differences in survivorship among devices, and different designs may not all be equally patellar-friendly. Large registry databases are needed to identify small but important differences between devices.
Questions/purposes
The purposes of this study were (1) to assess the long-term survivorship of the most common CR TKA devices with revision for any reason as the endpoint and compare the revision risk of these devices after controlling for the potentially confounding variables of age, sex, hospital volume, and primary diagnosis; and (2) to analyze these same devices with revision for secondary resurfacing of the patella as a separate endpoint.
Methods
Data were collected from the Finnish Arthroplasty Register. Over 95% of all primary TKAs are captured in the Finnish Register. We assessed Kaplan-Meier (KM) survivorship for each of the four most frequently used CR TKA designs used between years 2005 and 2015: Triathlon CR (n = 34,337), Nexgen CR Flex (n = 15,723), PFC Sigma CR (n = 15,541), and Vanguard CR (n = 9461), with revision for any reason as the endpoint. Revision was defined as a reoperation in which at least one of the components was exchanged (including insert exchange). Revisions in which the patella was not resurfaced at the primary operation and was resurfaced in the revision were studied as a separate endpoint. The mean followup times were 4.0 (range, 0-11.0) years for Triathlon CR, 3.8 (range, 0-11.0) years for Nexgen CR Flex, 5.1 (range, 0-11.0 ) years for PFC Sigma CR, and 4.9 (range, 0-10.9) years for Vanguard CR (p < 0.001). The group demographics were clinically comparable. We compared the risk of revision of these devices in the Cox multiple regression model with adjustment for hospital volume, age, sex, and primary diagnosis. There were some differences in the incidence of patellar resurfacing at the time of index arthroplasty (Nexgen CR flex 18.7%, PFC Sigma CR 18.4%, Triathlon CR 11.3%, Vanguard CR 14.4%), which was controlled by the Cox model. Implant survival analyses for Triathlon CR, Nexgen CR Flex, and PFC Sigma CR were also performed at the hospital level for the 25 largest TKA providers in Finland.
Results
The overall 10-year KM survivorships were 96% (95% confidence interval [CI], 95-96) for Nexgen CR Flex, 96% (95% CI, 96-97) for PFC Sigma CR, 94% (95% CI, 93-95) for Triathlon CR, and 94% (95% CI, 93-95) for Vanguard CR. After controlling for potential confounding variables like age, sex, hospital volume, and primary diagnosis, both Triathlon CR (hazard ratio [HR], 1.4; 95% CI, 1.2-1.6; p < 0.01) and Vanguard CR (HR, 1.4; 95% CI, 1.2-1.6; p < 0.01) had an increased risk for revision compared with the Nexgen CR Flex (the reference device). When revision with patellar resurfacing served as the endpoint, after controlling for those same confounding variables, Triathlon CR had a higher risk for revision than Nexgen CR Flex (HR, 1.8; 95% CI, 1.4-2.2; p < 0.01).
Conclusions
Despite slight differences among the studied devices, the overall 10-year survivorship of the current devices studied was good. However, there were differences in implant survival between the study devices, especially when revision for late patellar resurfacing was analyzed. Further studies adjusted for additional hospital and surgeon variables will be needed to examine and confirm our results.
Level of Evidence
Level III, therapeutic study.
Introduction
Long-term survivorship of the implant selected is critical in a common surgical procedure like TKA to avoid unnecessary revision operations. The desire to improve survivorship has led to numerous design innovations in TKA and the selection of available devices is wide [16]. However, during the last decade, improvement in the survival of new devices compared with earlier designs has been rare [2]. Evaluating long-term survivorship of both new devices and those already on the market is important to provide the best evidence-based care for patients [2]. Although overall survivorship of cruciate-retaining (CR) TKA is high, there may be differences between designs, especially in patellofemoral articulation, in which design characteristics are sought that make the TKA more “patella-friendly.” Although there are large studies that show no difference in outcome whether the patella has been resurfaced in the primary operation, there might be a difference, for example, in anterior knee pain between TKA designs [1, 6, 11].
Survival of TKA devices is traditionally assessed by survival analysis with all-cause revision as the endpoint. National joint replacement registries are currently the most important source of high-volume comparative survival data for TKA [4]. In a large study of Australian surgeons’ preferences, low revision rate was only the 10th most important reason cited to choose an implant. However, when only high-volume TKA surgeons were studied, reducing the risk of complications was one of the most important reasons for TKA device selection, suggesting that the most experienced surgeons value register data [14]. Although primary TKA in general has shown survivorship > 90% at longer than 10 years followup, registry data have revealed considerable differences in implant survival among different primary TKA devices [3, 9, 13]. The majority of the most popular TKA devices are used globally, and the implant survival data are available in annual reports from major registries [3, 9, 13]. However, implant survival is dependent on numerous factors other than implant design alone and factors such as sex or hospital volume might also have an effect. The Finnish Arthroplasty Register has gathered these data from primary and revision TKAs in Finland between 2005 and 2017, allowing it to assess these characteristics of primary TKA.
The aims of this study were to (1) assess long-term survivorship of the most common CR TKA devices with revision for any reason as the endpoint and compare the revision risk of these devices after controlling for potentially confounding variables like age, sex, hospital volume, and primary diagnosis; and (2) analyze these same devices with revision for secondary resurfacing of the patella as a separate endpoint.
Patients and Methods
The Finnish Register has collected information on total joint arthroplasties performed in Finland since 1980 [10]. Orthopaedic units are obligated to provide all information essential for maintenance of the register to the Finnish National Institute for Health and Welfare. Dates of death are obtained from the Population Information System maintained by the Population Register Centre. The Finnish Register data capture percentage is high, above 95% in primary TKA, when compared with the Hospital Discharge Register [5]. Data from years 2005 onward are currently based on implant catalog numbers and therefore the precision of device identification has increased, making these data very reliable and thus forming the timeframe for this report. The Finnish healthcare system is publicly funded and every hospital takes care of all patients in their geographic region. More complex operative procedures such as TKA are rarely undertaken in the private sector, where patients must pay for operations themselves. In addition, the patient population in Finland is relatively homogenous with few variations in the patient population between regions.
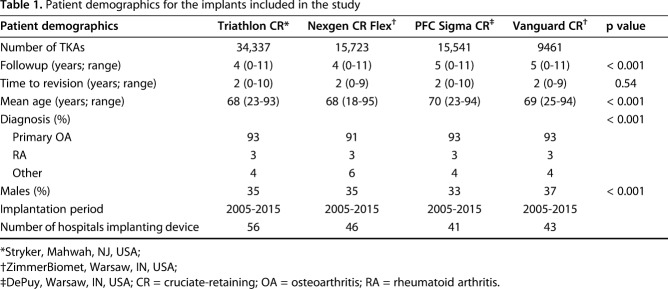
For this study, data from the four most common cemented CR TKA designs were assessed; 34,337 Triathlon® CR (Stryker, Mahwah, NJ, USA), 15,723 NexGen® CR Flex (ZimmerBiomet, Warsaw, IN, USA), 15,541 PFC® Sigma® CR (DePuy, Warsaw, IN, USA), and 9461 Vanguard® CR (ZimmerBiomet) TKAs were performed in Finland between January 2005 and December 2015. Mean followup was longest for patients treated with the PFC Sigma CR (5 years; range, 0-11 years). The proportion of male patients was largest in patients treated with Vanguard CR (37%) and the mean age was highest in the PFC Sigma CR group (70 years; range, 23-94 years) (Table 1). There were statistically significant differences in the demographics because the study groups were large; however, these differences were not viewed as clinically significant.
Table 1.
Patient demographics for the implants included in the study
Statistical Analysis
Implant survival of Triathlon CR, Nexgen CR Flex, PFC Sigma CR, and Vanguard CR was assessed by Kaplan-Meier (KM) analysis. Mortality during the study time was < 10% and therefore KM analysis was chosen over competing risk analysis. The Cox multiple regression model was used to study differences in revision rates of the devices and to adjust for hospital volume, sex, age group, and diagnosis.
Revisions were linked to the primary operation through the personal identification number. The survival endpoint was defined as revision when any component or the entire implant was removed or exchanged, including the tibial insert or patellar resurfacing alone. First revision for any reason and first revision in which an originally unresurfaced patella was resurfaced with or without other component exchange served as separate endpoints. There were some differences in the incidence of patellar resurfacing at the time of index arthroplasty (Nexgen CR Flex 18.7%, PFC Sigma CR 18.4%, Triathlon CR 11.3%, Vanguard CR 14.4%), which was controlled by the Cox model.
Kaplan-Meier survival data were used to construct the survival probabilities of implants with 95% confidence intervals (CIs). Patients who died during the followup period (until December 31, 2015) were censored at that point. The factors studied with the Cox model were hospital volume (< 50 TKAs per year, 50-100 TKAs per year, 100-200 TKAs per year, and > 200 TKAs per year), age group (18-55 years, 56-65, 66-75, and 76-100 years), sex, and preoperative diagnosis. The proportional hazards assumption of the Cox model was checked by inspecting the KM graphs. It was seen that the survival rates of Triathlon CR, Nexgen CR Flex, PFC Sigma CR, and Vanguard CR in the Finnish Register intersected at approximately 12 months of followup. For Cox analyses comparing these study devices, we divided the total followup time into two periods: 0 to 12 months and > 12 months because the proportional hazards assumption was not fulfilled for the total followup.
The Wald test was used to test the estimated hazard ratios. Differences among groups were considered to be statistically significant if the p values were < 0.05 in a two-tailed test.
The National Institute of Health and Welfare gave permission for this study (Dnro THL/506/5.05.00/16).
Results
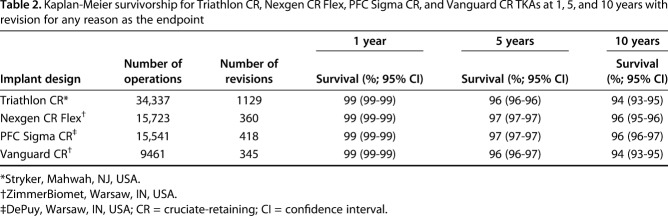
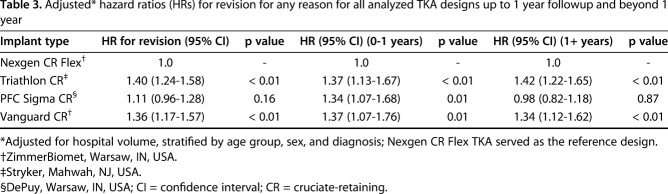
Ten-year KM survivorship was 96% (95% CI, 95-96) for Nexgen CR Flex, 96% (95% CI, 96-96) for PFC Sigma CR, 94% (95% CI, 93-95) for Triathlon CR, and 94% (95% CI, 93-95) for Vanguard CR (Table 2) with revision for any reason as the endpoint (Fig. 1). After controlling for potential confounding variables like age, sex, hospital volume, and primary diagnosis, both the Triathlon CR (1.4; 95% CI, 1.2-1.6; p < 0.01) and Vanguard CR (1.4; 95% CI, 1.2-1.6; p < 0.01) were found to have an increased hazard ratio for revision for any reason as compared with the reference device (Nexgen CR Flex). There was no difference in the hazard ratio for revision between PFC Sigma CR and Nexgen CR Flex (the reference device) (Table 3).
Table 2.
Kaplan-Meier survivorship for Triathlon CR, Nexgen CR Flex, PFC Sigma CR, and Vanguard CR TKAs at 1, 5, and 10 years with revision for any reason as the endpoint
Fig. 1.
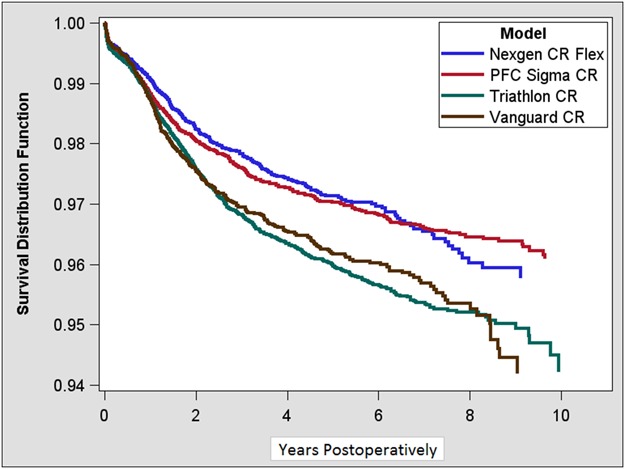
This figure presents the KM survival for all four study devices with any reason for revision as the endpoint.
Table 3.
Adjusted* hazard ratios (HRs) for revision for any reason for all analyzed TKA designs up to 1 year followup and beyond 1 year
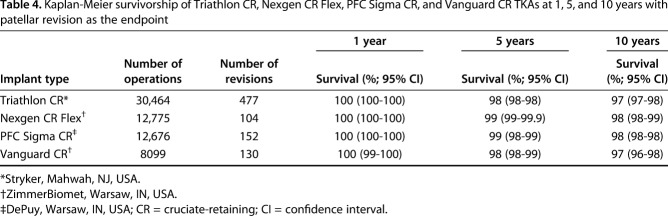
With patellar resurfacing as the endpoint after primary TKA with an unresurfaced patella, KM survivorship at 10 years was 98% (95% CI, 98-99) for Nexgen CR Flex, 98% (95% CI, 98-98) for PFC Sigma, 97% (95% CI, 97-98) for Triathlon CR, and 97% (95% CI, 96-98) for Vanguard CR (Table 4). There was some device-wise variability in survival (Fig. 2).
Table 4.
Kaplan-Meier survivorship of Triathlon CR, Nexgen CR Flex, PFC Sigma CR, and Vanguard CR TKAs at 1, 5, and 10 years with patellar revision as the endpoint
Fig. 2.
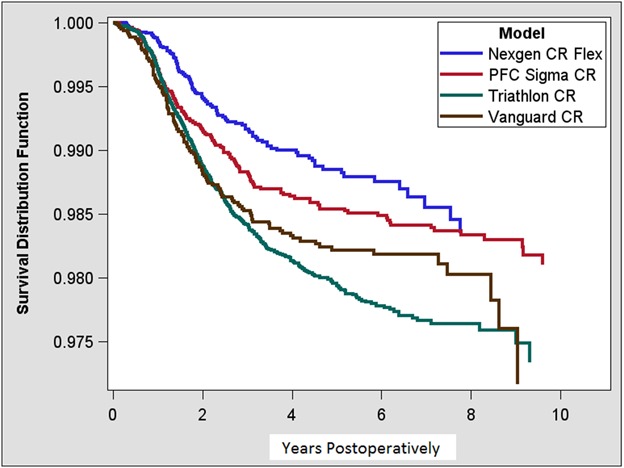
This figure presents the KM survival for all study devices with patellar revision as the endpoint.
After controlling for potential confounding variables like age, sex, and primary diagnosis, Nexgen Flex had a lower revision risk of secondary patellar resurfacing during the first postoperative year (hazard ratio [HR] for patellar revision, 1.8, 95% CI, 1.5-2.2, p < 0.01 for Triathlon CR; 1.6, 95% CI, 1.2-2, p < 0.01 for PFC Sigma; and 1.7, 95% CI, 1.3-2.2, p < 0.01 for Vanguard CR). However, from 1 year onward, only the Triathlon CR had a higher risk for patellar revision than Nexgen CR (HR, 1.7; 95% CI, 1.3-2.1; p < 0.01) (Table 5).
Table 5.
Adjusted* hazard ratios (HRs) for patellar revision for all studied TKA designs up to 1 year followup and beyond 1 year
Discussion
Cruciate-retaining TKAs are a mainstay of primary TKA, and so even small differences in survivorship among the available designs might have an important impact on the health of a population. Those differences might be a function of fixation, secondary resurfacing, or even hospital volume or surgeon relationships. However, prior reports on individual implants are difficult to compare with one another, and they do not paint a consistent picture in terms of the superiority of one CR TKA design over another [3, 9, 13]. We therefore performed a registry analysis to evaluate the survival of the most frequently used CR TKA devices based on data from the Finnish Arthroplasty Register to evaluate risk for revision and risk for later patellar resurfacing. In our material, all four studied implants had an acceptable survival at up to 10 years followup. However, the Nexgen CR Flex design had a lower revision risk when patellar resurfacing was studied as the endpoint compared with other devices and lower revision risk revision for any reason as the endpoint compared with Triathlon CR and Vanguard CR.
We acknowledge that our study had several limitations. First, as generally true in registry-based studies, implant survival was the only outcome we were able to assess; patient-reported outcome measures are not included in the Finnish Register database and it is possible that some of the patients may be symptomatic, although they have not been revised. Second, data regarding patients’ medical histories, comorbidities, or knee radiographs were not available. It is possible that there is selection bias toward using some implant designs in more severe cases that could have been detected from the history or radiographs. We also only included CR implants in our study. In Scandinavia, the clear majority of all primary TKAs are CR implants with the proportion of posterior-stabilized implants in primary TKA < 10% [13]. Based on the most recent data, this seems to be a justified trend [15]. Third, we were not able to assess individual reasons for revisions precisely, because the data concerning revision indications before the Finnish Register data content revision in May 2014 are incomplete. In theory it is possible that some of the devices studied have a higher risk for revision for some specific reason. Since this data content revision, the accuracy of revision indication data has improved, and quality checkups are currently systematically performed. Overall completeness of primary and revision TKA data in the Finnish Register is high, meaning that almost all primary TKAs (95%) and most revision TKAs (85%) are reported to the Finnish Register when compared with the National Discharge Register [5]. However, completeness of reporting revision surgery to the Finnish Register varies among hospitals, which may bias our results. For example, in 2015, revision completeness varied from 71% to 100% among hospitals. However, only seven hospitals had revision completeness < 80% and the majority of these hospitals were smaller providers that only perform a few revision operations yearly. In addition, surgeon-level data collection only started in May 2014 in the Finnish Registry. It is possible that some individual surgeons might have influenced implant survival in some hospitals. We do not believe that this significantly skewed our results because there are always several surgeons performing TKA at each hospital. Furthermore, there were some statistically significant differences in the demographic data and followup time among the studied devices. To ensure that this did not affect our results, we adjusted multiple regression analysis for age, sex, hospital volume, and primary diagnosis. Implant survival was studied using KM analysis, which evaluates survival up to a predefined time point and the mean followup time does not affect this result. Finally, inclusion of bilateral cases in survival analysis violates the basic assumption that all cases are independent. However, several reports have shown that the effect of including bilateral cases in studies of hip and knee prosthesis survival, as done in our study, is negligible [7, 12].
The overall survival for all studied implants in our analysis was good at up to 10 years followup. This is in accordance with reports from other national registries. In our study, the Nexgen CR Flex TKA 10-year survival rate was 96% (95% CI, 95%-96%), whereas 10-year revision rates for this implant design were 2.8% (95% CI, 2.3%-3.4%) in the Australian registry data and 3.6% (95% CI, 3.3%-3.8%) in the National Joint Registry for England and Wales (NJR) [3, 9]. Unlike the Finnish and Australian data that include only Nexgen CR Flex implants, data from the NJR include all different types of Nexgen CR devices, which might affect the results. Based on Australian data, 10-year revision rates for Triathlon CR and PFC Sigma CR were slightly higher than for Nexgen CR (3.8%, 95% CI, 3.2%-4.5% and 3.4%, 95% CI, 3.0%-3.9%, respectively), whereas in the NJR data, PFC Sigma CR had slightly lower revision rates than Nexgen CR (2.7, 95% CI, 2.6%-2.7%). Triathlon CR had similar revision rates as Nexgen (3.7%, 95% CI, 3.2%-4.2%) [3, 9]. Vanguard CR TKA has not yet reached 10-year followup in either the Australian registry or in the NJR. Revision rates at up to 5-year followup in these registries were 1.7% (95% CI, 0.9%-3.2%) and 2.0% (95% CI, 1.9%-2.3%), respectively [3, 9]. In the New Zealand Registry data, the PFC Sigma CR had the lowest risk for revision of the studied implants (0.39 revision/100 component-years compared with 0.43 for Triathlon, 0.53 for Nexgen, and 0.66 for Vanguard) [8]. Hazard ratios for revision risk for these implants are approximately in line with data from the Swedish Knee Arthroplasty Register [13]. Overall it seems that the rank order of KM estimates of common TKA devices varies among national registries.
Nexgen CR had a lower risk for patellar revision, both during the first postoperative year and from 1 year onward. It is relatively rare to resurface the patella at the primary operation in Nordic countries (14.7% incidence in our study). To study whether some implant designs might be more “patellar-friendly,” we studied revisions in which an originally unresurfaced patella was resurfaced in the revision operation. In earlier studies, a deeper femoral groove and optimal femoral rotation have been associated with better patellar performance and these features are present in the Nexgen CR Flex as well [6]. Nonetheless, this difference in patellar revision rate did not explain all of the difference in overall revision rates between the Triathlon CR and Nexgen CR Flex.
Triathlon CR is the current market leader in Finland with more than twice as many implantations as any other study device. One possible explanation for regional differences in a small homogenous country like Finland is that adoption of the Triathlon CR throughout the country may have been too rapid, and proper education in surgical technique might not have been available at all hospitals. There might also be variation in the threshold to perform revision surgery in different hospitals. For example, in an experienced surgical unit servicing a demanding patient population, there would more likely be surgeons willing to revise a suboptimal result such as a stiff TKA or a case of mild instability. This could mean that if an implant were used in a "high-volume" department, it would more likely be revised for such suboptimal results than in a more general unit. In addition, differences in completeness of reporting revision surgery to the Finnish Register vary between hospitals, which may bias our hospital-level results. Local hospital-level circumstances like infection rates may also explain some of the variation in differences in implant survival in our study.
In conclusion, all four of the most frequently used CR TKAs in Finland had an acceptable long-term survivorship rate. Nexgen CR Flex and PFC Sigma CR had the highest survival at up to 10 years followup. Nexgen CR Flex had a lower risk for revision than Triathlon CR and PFC Sigma CR in an adjusted regression model and the lowest risk for patellar revision. In the future, more large, registry-based studies with additional variables included are needed to confirm our results, especially concerning patellar revision rates.
Footnotes
One of the authors certifies that he (AE), or a member of his immediate family, has or may receive payments or benefits, during the study period, an amount of less than USD 10,000 from DePuy (Warsaw, IN, USA) not related to this study and has or may receive payments or benefits, during the study period, an amount of less than USD 10,000 from Stryker (Mahwah, NJ, USA) not related to this study. The institution of one of the authors (AE) has received research funding from DePuy and ZimmerBiomet (Warsaw, IN, USA) outside of this study.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed in the Finnish Arthroplasty Register, Helsinki, Finland; the Department of Orthopaedics and Traumatology, Turku University Hospital, Turku, Finland; the Department of Biostatistics, University of Turku, Turku, Finland; Coxa Hospital for Joint Replacement, Tampere, Finland; the National Institute for Health and Welfare, Helsinki, Finland; the Department of Orthopaedics and Traumatology, Oulu University Hospital, Oulu, Finland; the Department of Orthopaedics and Traumatology, Helsinki University Hospital, Helsinki, Finland; the Department of Orthopaedics and Traumatology, Kuopio University Hospital, Kuopio, Finland; and in Orton Hospital, Helsinki, Finland.
References
- 1.Ali A, Lindstrand A, Nilsdotter A, Sundberg M. Similar patient-reported outcomes and performance after total knee arthroplasty with or without patellar resurfacing. Acta Orthop. 2016;87:274–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anand R, Graves SE, de Steiger RN, Davidson DC, Ryan P, Miller LN, Cashman K. What is the benefit of introducing new hip and knee prostheses? J Bone Joint Surg Am. 2011;93(Suppl 3):51–54. [DOI] [PubMed] [Google Scholar]
- 3.AOANJRR. Annual Report 2016. Available at: https://aoanjrr.sahmri.com/annual-reports-2016. Accessed December 30, 2017.
- 4.Carr AJ, Robertsson O, Graves S, Price AJ, Arden NK, Judge A, Beard DJ. Knee replacement. Lancet. 2012;379:1331–1340. [DOI] [PubMed] [Google Scholar]
- 5.The Finnish Arthroplasty Register (FAR). Available at: www.thl.fi/far. Accessed December 30, 2017.
- 6.Johnson TC, Tatman PJ, Mehle S, Gioe TJ. Revision surgery for patellofemoral problems. Clin Orthop Relat Res. 2012;470:211–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lie SA, Engesæter LB, Havelin LI, Gjessing HK, Vollset SE. Dependency issues in survival analyses of 55,782 primary hip replacements from 47,355 patients. Stat Med. 2004;23:3227–3240. [DOI] [PubMed] [Google Scholar]
- 8.New Zealand Orthopedic Association Joint Registry. Annual Report 2017. Available at: http://nzoa.org.nz/nz-joint-registry. Accessed December 30, 2017.
- 9.NJR. NJR 13th Annual Report. 2016. Available at: http://www.njrcentre.org.uk/njrcentre/Reports,PublicationsandMinutes/Annualreports/tabid/86/Default.aspx. Accessed December 30, 2017.
- 10.Paavolainen P, Hämäläinen M, Mustonen H, Slätis P. Registration of arthroplasties in Finland. A nationwide prospective project. Acta Orthop Scand Suppl. 1991;241:27–30. [DOI] [PubMed] [Google Scholar]
- 11.Pavlou G, Meyer C, Leonidou A, As-Sultany M, West R, Tsiridis E. Patellar resurfacing in total knee arthroplasty: does design matter?–A meta-analysis of 7075 cases. J Bone Joint Surg Am. 2011;93:1301–1309. [DOI] [PubMed] [Google Scholar]
- 12.Robertsson O, Ranstam J. No bias of ignored bilaterality when analysing the revision risk of knee prostheses: analysis of a population based sample of 44,590 patients with 55,298 knee prostheses from the national Swedish Knee Arthroplasty Register. BMC Musculoskelet Disord. 2003;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The Swedish Knee Arthroplasty Register. Annual Report 2016. Available at: http://www.myknee.se/pdf/SVK_2016_Eng_1.0.pdf. Accessed December 30, 2017.
- 14.Vertullo CJ, Grimbeek PM, Graves SE, Lewis PL. Surgeon’s preference in total knee replacement: a quantitative examination of attributes, reasons for alteration, and barriers to change. J Arthroplasty. 2017;32:2980–2989. [DOI] [PubMed] [Google Scholar]
- 15.Vertullo CJ, Lewis PL, Lorimer M, Graves SE. The effect on long-term survivorship of surgeon preference for posterior-stabilized or minimally stabilized total knee replacement: an analysis of 63,416 prostheses from the Australian Orthopaedic Association National Joint Replacement Registry. J Bone Joint Surg Am. 2017;99:1129–1139. [DOI] [PubMed] [Google Scholar]
- 16.Wright G, Chitnavis J. Which design of TKR–does it matter? J Bone Joint Surg Br. 2011:1–3. Available at: http://www.boneandjoint.org.uk/sites/default/files/FocusOn_TKRdesign.pdf. Accessed January 8, 2018. [Google Scholar]