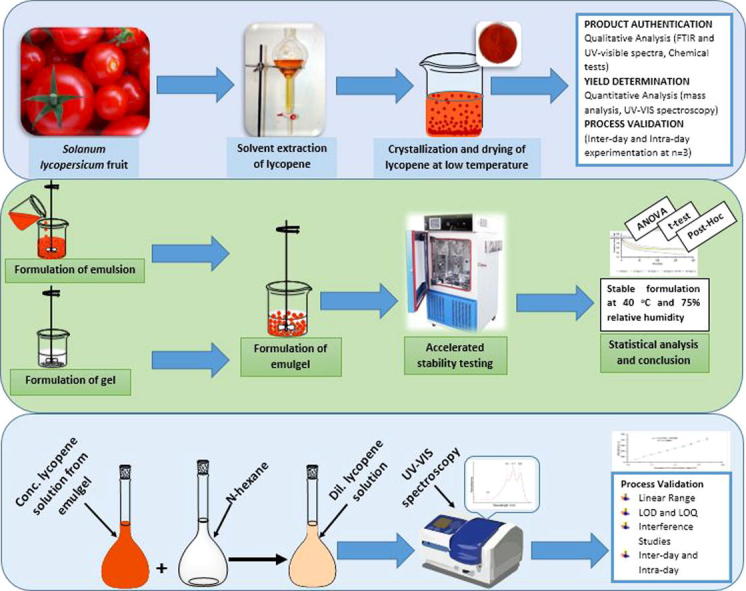
Graphical abstract
Keywords: Lycopene, Emulgel, Extraction, Analysis, ANOVA, T-test
Abstract
Focus of the study was to design a novel and cost effective extraction technique for the lycopene from Lycopersicum esculentum L. fruit and to develop and characterize a stable emulgel formulation containing lycopene as an active ingredient as well as to design an analytical method to determine lycopene concentration in emulgel. Emulgel formulation was prepared and evaluated for its stability at different storage conditions, 8 °C, 25 °C, 40 °C, 40 °C + 75% relative humidity (RH) and 50 °C, for 6 months. Results were statistically analyzed using two way ANOVA, Post-Hoc test and paired sample t-test at 5% significance level. Designed extraction technique presented comparable yield, 154.83 mg/Kg of tomato fruit, with all recoveries in the range of 145–156 mg/Kg of tomato. “P-values” calculated for different levels of stability parameters were <0.05, except at 50 °C and time points of 60th day and later. Analytical method designed was having linear range of lycopene 1–10 µg/mL with limit of detection 0.11 µg/mL and limit of quantification 0.34 µg/mL. All inter-day and intra-day recoveries were in the range of 94–105% while in all measurements RSD % was ≤5.36. It can be concluded that the extraction technique was cost effective with comparable results and analytical method was simple, robust, specific and sensitive enough to be used for lycopene concentration determination in emulgel formulation. Furthermore, designed formulation was stable even at high temperature of 40 °C and RH 75%.
1. Introduction
Solanum lycopersicum (tomato) fruit is used as a valuable active ingredient in different topical preparations e.g. emulsions. Here, it serves as an anti-aging and pigment-lightening constituent due to its free radical scavenging property (Cefali et al., 2015a, Cefali et al., 2015b). Lycopene is one of the important constituents of tomato fruit which acts as a potent antioxidant (Cheng et al., 2017). It belongs to the family of cyclic carotenoids (Cefali et al., 2015a, Cefali et al., 2015b). Tomato fruit contains 0.03–0.14 mg/g of lycopene content (Ma et al., 2016). Biological functions attributed to carotenoids include prevention of certain types of cancer, cardiovascular diseases, hyperlipidemia, etc. (Sheriff et al., 2017) and these are independent of the provitamin-A activity and are due to antioxidant property of the carotenoids through singlet oxygen quenching and deactivation of free radicals. Moreover, lycopene has a direct impact to prevent oxidative damage related cardiovascular diseases (Song et al., 2017) and topical ailments (Ascenso et al., 2013).
A number of procedures for the extraction of lycopene from tomato fruit are available. Some are time consuming, others require high expertise and a few demands expensive equipment. Low yield of the product is also a limitation in some cases. A procedure with maximum features is required to be designed for the stated purpose.
Various types of dosage forms are used to deliver lycopene but topical drug delivery system has several advantages such as delivery of drug selectively to a specific site, avoidance of gastro-intestinal incompatibility & metabolic degradation associated with oral administration (Choudhury et al., 2017, Paolicelli et al., 2017). Furthermore, topical delivery provides an increased bioavailability by avoiding metabolism by liver and a consistent delivery for an extended period (Singh Malik et al., 2016). Topical products are diverse in nature regarding formulation and having range in terms of consistency from liquid to powder but the most popular products are semisolid preparations (Garg et al., 2015). Within the major group of semisolid preparations, the use of transparent gels has gained importance both in cosmetics and pharmaceutical preparations. Gels are a relatively novel class of dosage forms prepared by entrapping high amount of aqueous or hydro alcoholic liquid in a network of colloidal solid particles. Gel formulations provide faster drug release as compared to ointments and creams. In spite of many advantages of gels, a major problem is the delivery of hydrophobic drugs. To overcome this limitation, emulgels are prepared. When emulsion and gels are combined together, the formulation is termed as emulgel (Bera et al., 2017). The emulgel for dermatological use has various favorable properties such as being thixotropic, greaseless, easily spreadable, easily removable, emollient, non-staining, water soluble, longer shelf life, bio-friendly, pleasing appearance and better stability (Kute and Saudagar, 2013). Currently, Scientists are facing problems during development of new drug delivery systems as many drugs coming directly from synthesis or from high throughput screening, have poor water solubility (Gürbüz et al., 2017). Based on IN VITRO solubility and IN VIVO permeability data, biopharmaceutical classification system divides drugs into four classes. Among the four classes, class II drugs show poor solubility and high permeability. It is obvious that for class II drugs the poor aqueous solubility is the most prominent limitation factor to their overall rate and extent of absorption. The next significant factor affecting the bioavailability, after dissolution step, is the permeability (Alam et al., 2015). Therefore, when one is concerned with topical delivery of poorly water-soluble drugs, emulgels are the option of choice. Emulsified gel has proven a stable one and best vehicle for the delivery of class II drugs (Jagdale and Pawar, 2017). Luckily, lycopene is well absorbed when applied topically (e.g. in a cream or lotion) because of its lipophilic nature and small size. Dietary intake is not sufficient to maximize skin benefits of lycopene. When taken orally, lycopene is distributed throughout the entire body and only small amount finds its way to the skin. Lycopene has been proven a potent physical quencher of singlet oxygen but its stability is relatively low (Okonogi and Riangjanapatee, 2015). Researchers are trying to find a best carrier system for topical delivery of lycopene which will retard its degradation.
The present research was aimed to design a cost effective and simple technique with high yield for extraction of the lycopene from tomato fruit, to formulate and characterize a stable emulgel of extracted lycopene for topical applications. Furthermore, purpose of this work was to design a simple, cost effective, reproducible and specific method to quantitatively analyze lycopene in emulgel formulation.
2. Materials and methods
2.1. Materials
Solanum lycopersicum L. (Local Market of Bahawalpur Pakistan), acetone (BDH, England), n-hexane, sodium nitrate and sulphuric acid (Franken Chemicals, Gebinde Germany), ethyl acetate, liquid Paraffin, propylene glycol, triethanolamine and methanol (Merck KGaA Darmstadt, Germany), lycopene standard (Shaanxi Kingsci Biotechnology, China), carbopol 940, span 20 and tween 20 (Sigma, USA), methyl paraben (Acros Organics, USA), purified distilled water (Cosmaceutical Lab. IUB, Pakistan).
2.2. Equipment
Centrifuge Machine (Hettich EBA 20, Germany), cold incubator (Sanyo MIR-153, Japan), hot incubator (Sanyo MIR-162, Japan), refrigerator (Orient technology Pakistan), electrical balance (Precisa BJ-210, Switzerland), high shear homogenizer (Euro-Star, IKA D 230, Germany), pH meter (WTW pH-197i, Germany), rotary evaporator (Eyela, Co. Ltd. Japan), dual-beam UV–VIS spectrophotometer (Uvikon XL, Bio-Tek Instruments, Bad Friedrichshall, Germany), water bath (HH. S21 4 China), FTIR spectrophotometer (Aligent Technologies USA), micro plate-reader (Synergy HT BioTek USA).
2.3. Software
SPSS 19.0, graph pad prism 5, MS-excel 2013 and endnote X7.
2.4. Extraction of lycopene
Method was designed on the basis of properties of lycopene, the most important of which is the solubility in various solvents i.e. soluble in chloroform, n-hexane, benzene, carbon disulfide, acetone and petroleum ether while insoluble in water, ethanol and methanol (Chauhan et al., 2011), as lycopene is a non-polar compound. The principal used in this experiment was the solvent extraction technique and is a modification in the method described by MH Chapman et al (2009). Briefly, 100 g of tomato fruit without seeds was sliced and extracted thrice with 100 ml portions of water soluble solvent, acetone. Residue was collected and dried after filtration. Finally dried residue was extracted thrice with 100 ml portions of water immiscible solvent, ethyl acetate and n-hexane mixture in the ratio of 3:17 by volume, respectively, stirred for 5–10 min each time and filtered. Filtrate was concentrated using rotary evaporator at 40 ± 1 °C temperature and 60 rpm speed to the final volume of 20 ml (Narendran et al., 2013a, Narendran et al., 2013b). 1 ml of extract was diluted to 100 ml with n-hexane and then 2 ml from Dilution 1 was diluted to 100 ml with n-hexane. Extract was analyzed by UV–Visible spectrophotometer and lycopene was precipitated by mixing the extract with anti-solvent, methanol, at low temperature to reduce the solubility. Crystalline product was filtered and dried. Procedure for extraction was repeated to check inter-day and intra-day variation at n = 3 (three sampling times, in case of intra-day this referred to 3 times with equal intervals on the same day while in inter-day, it was three different days).
2.5. Characterization of lycopene
The color change of the solution of sample in acetone was observed by successive addition of a 5% solution of sodium nitrate in water and 1 N solution of sulphuric acid in water (Meeting, 2006).
Solution of Lycopene in n-hexane was prepared and scanned using UV–VIS spectrophotometer to read λmax in the wavelength of 300–600 nm, as reported λmax for solution of lycopene in n-hexane is 472 nm (Meeting, 2006).
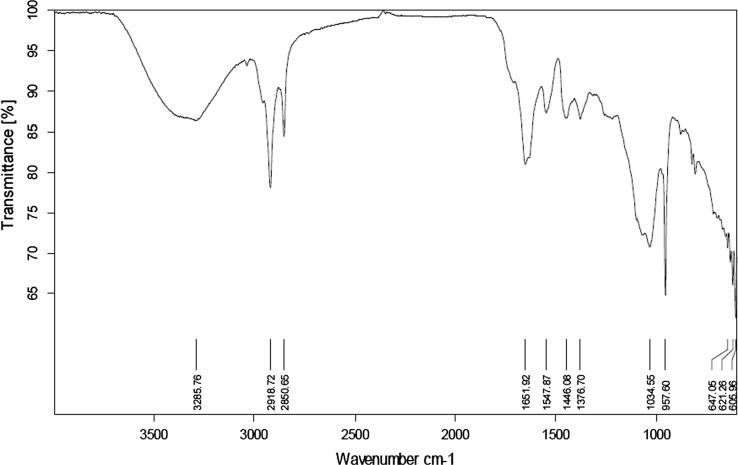
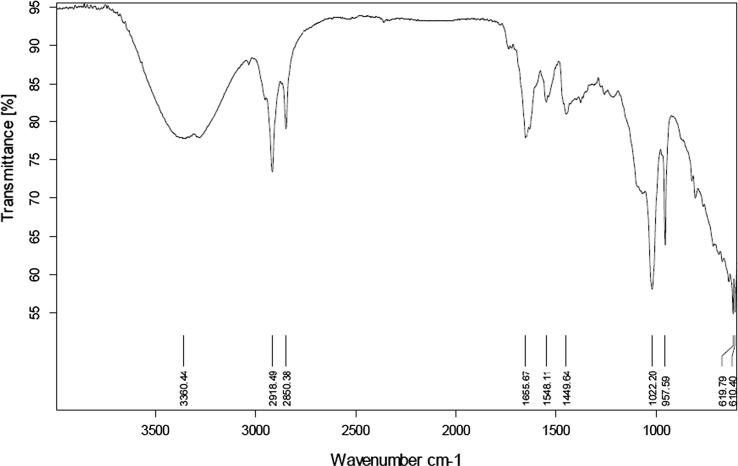
FTIR spectrum of lycopene was taken by FTIR-Spectrophotometer. Lycopene was scanned between wave number ranges of 4000 cm−1 to 650 cm−1. Major peaks of the spectra were interpreted to determine the respective functional groups present. To authenticate the spectrum, FTIR spectrum of the extracted lycopene was compared with spectrum of imported lycopene, spectrum given by Lopez-Cervantes et al. (2014) and also with the salient peaks described by Kamil et al. (2011).
After that, yield of lycopene was calculated using Beer Lambert Law and direct mass analysis (Yang et al., 2005, Ishigaki et al., 2017).
Beer Lambert law can be stated as;
Here, A = absorption of sample at 472 nm, Ɛ = molar extinction coefficient for lycopene solution in n-hexane at 472 nm and it value is 185 × 103 L mol−1 cm−1, calculated by using E (1%, 1 cm) which is 3450 (Luengo et al., 2014), b is path length, its value is 1 cm and c is concentration of sample solution in mol/L.
For mass analysis, crystals of lycopene were filtered, dried and their mass was determined.
2.6. Formulation of emulgel
Emulgel was formulated with modifications in the method described by Narendran et al., 2013a, Narendran et al., 2013b. Following Steps were involved;
STEP 1: Preparation of emulsion
Stable oil in water type emulsion was prepared according to the previously described methods with some changes (Khullar et al., 2012, Narendran et al., 2013a, Narendran et al., 2013b).
In short, to prepare test formulation, ingredients of oil phase i.e. liquid paraffin 7.5 g, span20 1 g, lycopene 0.03 g were measured in a beaker and that of aqueous phase, tween20 0.5 g, propylene glycol 5 g, methyl paraben 1.5 g, distilled water 84.47 g, were taken in a separate beaker. Both the phases were heated at a temperature of 70–80 °C, separately. Then oil phase was added to the aqueous phase drop by drop along with continuous stirring at 2000 rpm by the mechanical stirrer for 15 min.
After continuous stirring of 15 min at 2000 rpm, the speed of the mixer was reduced to 1000 rpm for 5 min to homogenize. The speed of the mixer was then further reduced to 500 rpm for further 5 min and then the emulsion was let to cool down to room temperature.
Control and experimental formulations were prepared in the same way with the exception of addition of lycopene in the test formulation only and quantity of water in control formulation was increased by 0.03 g.
STEP 2: Preparation of gel
1 g of carbopol 940 was dispersed in water at room temperature with the help of mechanical stirrer at a speed of 1000 rpm until no lump of carbopol left. After complete dispersion of carbopol, triethanolamine was added drop wise and mixed thoroughly and after each addition, pH was checked until it reached in the range of 6–6.5.
STEP 3: Incorporation of emulsion into gel
Finally, prepared emulsion was dispersed drop by drop in prepared gel in 1:1 using mechanical stirrer at a speed of 1000 rpm for 15 min. Same procedure was adapted for control as well as for test formulation.
Moreover, in this study 25 emulgel formulae were tried with varying quantities of different ingredients in emulsion phase (liquid paraffin, span20, liquid paraffin 7.5 g, span20) and gel phase (carbopol 940) and kept for stability studies at accelerated temperature conditions (40 °C, 40 °C + 75% RH and 50 °C) for a period of 30 days. Phase separation and organoleptic evaluation were performed after 30th day. The finally selected formula used in the current study was found to be most stable and selected for the preparation of control and test formulations.
2.7. Stability studies
Accelerated stability studies were performed on control and test formulations for a period of 6 months. Emulgel was evaluated for the stability of formulation as well as for the stability of lycopene in formulation. Tests for stability were performed considering different storage conditions of temperature and humidity i.e. 8 °C, 25 °C, 40 °C, 40 °C + 75% relative humidity (RH) and 50 °C.
For organoleptic evaluation, emulgel was physically analyzed for any change in color, smell, liquefaction and appearance of microbial culture.
After the preparation of emulgel, centrifugation tests were carried out to check the phase separation and repeated at sampling intervals after application of the storage conditions. These tests were performed by taking 5g of sample in disposable stoppered centrifuge tube and centrifuged at 25 °C at a speed of 5000 rpm for 10 min.
By using digital pH meter, pH values of freshly prepared emulgel and emulgel kept at different storage conditions, were determined.
Standard curve for lycopene solution in n-hexane was prepared after measuring absorbance at 472 nm using UV–VIS spectrophotometer. To determine the stability of active moiety in freshly prepared formulation and formulations stored at various conditions, method designed to measure lycopene in emulgel formulation, was used.
All test were performed at freshly prepared formulations and sampling time points of 24 h, 48 h, and 3rd, 7th, 14th, 21st, 30th, 45th, 60th, 90th, 120th, 150th, 180th day after storage at said conditions. Moreover, all readings were taken in triplicates.
2.8. Analytical method development to determine lycopene in emulgel formulation
Based on UV–Visible spectroscopy, analytical method was designed to determine lycopene concentration in emulgel formulation. Method was validated with respect to linear range of lycopene, precision, accuracy, specificity and sensitivity. Following procedure was adapted for the said purpose;
1 g of test emulgel was taken in a beaker and 10 ml of ethanol was added with continuous stirring for 10 min, using magnetic stirrer, to disrupt the gel structure. After that, 70 ml of n-hexane was added and stirred for further 10 min to extract the lycopene from the emulgel. N-hexane phase was separated, filtered and its volume was made up to 100 ml with n-hexane. Finally, absorbance of resulting solution was measured at 472 nm. Blank solution was prepared in the same way using 1 g of the control formulation and concentration of lycopene was calculated with the help of the standard straight line equation. Designed method was a modified method described by Loveleen Preet Kaur (Preet, 2013). The author of the stated reference recommended to weigh 1 g of the emulgel and mixing it with 100 ml of a proper solvent. Finally, measuring the concentration of the active ingredient by linear line equation. The best strategy used in this experiment was the disruption of the gel structure and then extraction of the drug by solvent extraction technique, prior to determine the concentration.
3. Results and discussion
3.1. Extraction of lycopene
Screening of plants to discover important antioxidant phytoconstituents may guide to explore compounds with more safety and effectiveness, as agents of synthetic origin may have various side effects (Fazli et al., 2014). Extraction of individual chemical compounds from natural sources is of great importance and has been used widely. Although various synthetic techniques are available to prepare compounds but still a variety of chemical compounds are obtained from the natural products and these are extensively used in different fields e.g. lycopene extracted from tomato fruit is widely used as an antioxidant. Extraction procedure is mostly adapted to obtain individual compounds from natural sources. A cost effective extraction method with comparable yield, was successfully developed for lycopene extraction. Results verified the said purpose.
3.2. Qualitative and quantitative analysis of lycopene
Qualitative analysis of lycopene included identification test for carotenoids, measurement of λmax, determination of FTIR spectrum and its comparison with spectrum of standard powder of lycopene and literature.
Identification test for carotenoids was performed and the result was described as the disappearance of the color of solution of lycopene in acetone by successive addition of solution of sodium nitrate in water and solution of sulphuric acid in water.
Results of UV scan of the solution of lycopene in n-hexane gave a λmax value of 471.8 nm.
Fig. 1, Fig. 2 are presenting FTIR spectra of standard and extracted lycopene, respectively, while Table 1 shows the prominent peaks of functional groups of extracted lycopene, standard lycopene and those explained by Lopez-Cervantes et al. and Kamil et al.
Fig. 1.
FTIR spectrum of standard lycopene.
Fig. 2.
FTIR spectrum of extracted lycopene.
Table 1.
Interpretation of FTIR spectra of lycopene from different sources.
| Reported by Kamil et al. | Reported by Lopez-Cervantes et al. | Standard Lycopene | Extracted Lycopene | Functional Group Determination |
|---|---|---|---|---|
| 3200–3450 cm−1 | 3356 cm−1 | 3285 cm−1 | 3360 cm−1 | CH (Stretching) |
| 2918 cm−1 | 2922 cm−1 | 2918 cm−1 | 2918 cm−1 | CH (Stretching) |
| 1643 cm−1 | 1652 cm−1 | 1652 cm−1 | 1656 cm−1 | C=C (Stretching) |
| 1101.07 cm−1 | 1088 cm−1 | 1035 cm−1 | 1022 cm−1 | CH (Trans) |
| 960 cm−1 | 965 cm−1 | 958 cm−1 | 958 cm−1 | R–CH=CH–R |
FTIR analysis was performed to authenticate the major functional groups present in the extracted lycopene. Transmittance versus wave number FTIR spectrum of extracted product when compared with the spectrum obtained by scanning standard and with those explained by Lopez-Cervantes et al. (Lopez-Cervantes et al., 2014) and Kamil et al. (Kamil et al., 2011) gave the authentication of lycopene. Some other minor peaks might be due to the solvent or small amount of any impurity present.
Quantity of lycopene per kilogram of tomato fruit was determined by using two techniques, Beer Lambert Law and measurement of the mass of the product, the lycopene, and results obtained were 154.83 mg/Kg of tomato fruit (equivalent to 1.44 × 10−6 mol/L of lycopene in solution) and 149.8 mg/Kg of tomato fruit, respectively.
Inter-day and intra-day experiments (n = 3) showed that yield, by both methods, was within the range of 145–156 mg/Kg of tomato.
3.3. Development of emulgel
Cream of the lycopene is available but stability of the formulation is the major persistent problem. Prominent stability problem is the temperature stability. The viscosity of the cream is decreased as the temperature is elevated. This decline in viscosity presents the problems of phase separation, creaming and coalescence leading to the destruction of formulation and degradation of active moiety. Moreover, increase in fluidity may result in physical and chemical interactions among ingredients, alteration in physical appearance render it to be unacceptable by the consumers etc. Emulgel formulations are stable at high temperature due to polymer structure of the gel which do not allow the phases of the emulsion to get separated and presents the formulation devoid of the stated problems. As lycopene is insoluble in water but soluble in organic compounds, so gel formulation cannot be prepared due to problem of liberation of lycopene and in turn the absorption. Hence, there was a need to develop a dosage form for topical delivery of lycopene with high stability and comparable liberation of the lycopene for maximum effects. Focus of the current research was the problem indicated and results were an evidence to answer the stated problem. Furthermore, the novel stable emulgel developed was supposed to be very useful to serve the purpose of topical delivery of lycopene in future.
3.4. In vitro evaluation of stability of lycopene emulgel
3.4.1. Organoleptic evaluation and thermal stability
There was no change in color or change the odor till last observation except for the formulation stored at 50 °C. A slight change in color from orange to yellowish orange was observed in case of test formulation at 50 °C on 60th day but no specific odor was felt in any of the sample. Moreover, no growth of any microbe was observed as well as there was no liquefaction or phase separation till the end of the study period in any sample. These results depicted that formulation was stable even at high humidity and temperature of 75% and 40 °C, respectively. Color change in case of 50 °C was an indication of lycopene degradation at high temperature. Liquefaction is an indication of mild level phase separation but formulated emulgel was devoid of this feature. Centrifugation test demonstrated a high degree of stability even at high temperature unlike the cream formulations, which show a change in viscosity and physical state from semisolid to liquid.
Creaming occurs because of the differences in density of two phases under the influence of gravity which results in phase separation. Creaming can be reduced/prevented by reducing the globule size. No phase separation was observed in any of the sample of control and test emulgels kept at stated conditions throughout the study period of 180 days. It shows that all formulations were stable at all storage conditions considering no creaming, as a parameter of stability. It may be attributed to a number of stability factors mainly the proper agitation speed which decreased the coalescence and prevented the components of the formulation to break and separate.
3.4.2. Centrifugation test
Centrifugation is an important tool for evaluating and predicting the shelf life of semisolid formulations like emulsion. The centrifugation test (stress testing) is usually used to evaluate the physical stability of semisolid formulation stored at different temperatures in terms of phase separation (Jadhav et al., 2010).
Formulations didn’t show any sign of phase separation throughout the study period. It was also a verification of stability.
3.4.3. Variation in physical characteristics of emulgel formulation with respect to time and temperature
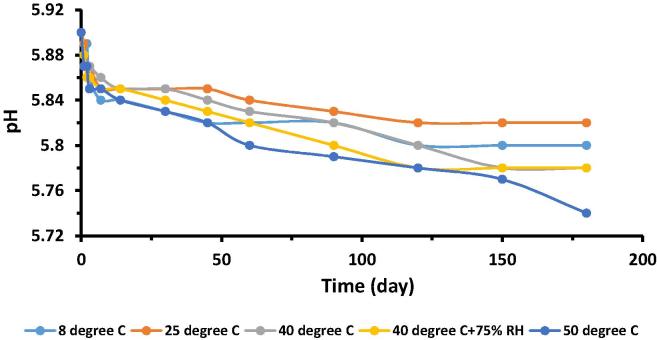
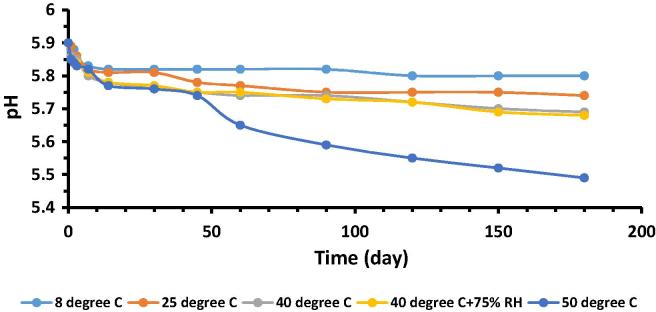
Regarding stability studies, the pH values of all control and test formulations, kept at different storage conditions i.e. 8 °C, 25 °C, 40 °C, 40 °C + 75% RH and 50 °C, were measured at various reading intervals and plotted against time, as shown in Fig. 3, Fig. 4. Results demonstrated that variation in pH of both the formulations were in the range of 5.49–5.9, during the study period. Moreover, variation was more at 50 °C after 45th day and this change was a little more in case of test formulation as compared to control but within reported range as skin pH values are variable with a broad range of 4.0–7.0 (Lambers et al., 2006). Phenomenon of oxidation was supposed to be a cause of this happening. In short, presence of lycopene did not affect the pH significantly.
Fig. 3.
Variation of pH of control formulation with respect to time kept at different storage conditions.
Fig. 4.
Variation of pH of test formulation with respect to time kept at different storage conditions.
3.5. Analytical method development
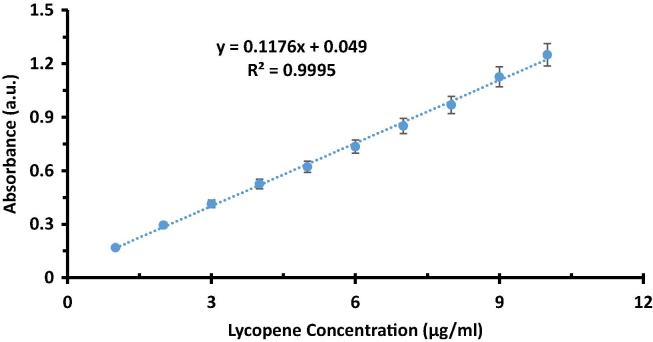
Fig. 5 shows the standard curve for lycopene constructed at λmax of 472 nm using n-hexane as solvent.
Fig. 5.
Standard lycopene curve at 472 nm.
To validate the method, linear range of lycopene, precision, accuracy as well as sensitivity were determeined. Linear range of lycopene for the assay was 1–10 µg/mL. To estimate the sensitivity LOD (limit of detection) and LOQ (limit of quantification) were calculated. Formulae for LOD and LOQ were 3.3SD/b and 10SD/b, respectively. Here, SD is standard deviation of response (absorbance) and b is slope of the linear straight line. LOD was 0.11 µg/mL while LOQ was 0.34 µg/mL.
Specificity of method was judged by interference studies. For this purpose, common excipients of emulsion and gel formulations as well as common metal ions were added to the lycopene solution as interfering agents. Excipients used were liquid paraffin, span 20, tween 20, propylene glycol, methyl paraben, carbopol 940, triethanolamine while common metal ions used were Na+, K+, Ca2+, Mg2+, Al3+, Ag2+, Fe2+, Fe3+, Cu2+ and Zn2+. Percentage change in absorbance after addition of interfering agent in all the cases was less than 5%. Moreover, all excipients were taken in much higher concentrations than their amount present in formulation and tolerable concentration of metal ions were also many folds higher than their expected concentration as contaminants.
Relative standard deviation % and percentage of recovery, interday and intraday, were determined at n = 3 level as shown in Table 2. In this experiment, all recoveries were in the range of 94–105% while in all measurements RSD % was ≤5.36.
Table 2.
Precision and accuracy of the method for determination of lycopene concentration in emulgel (n = 3).
| Concentration of lycopene (µg/mL) | Interday |
Intraday |
||
|---|---|---|---|---|
| Mean recovery (%) ± SEM | RSD (%) | Mean recovery (%) ± SEM | RSD (%) | |
| 2 | 101 ± 1.15 | 1.99 | 98 ± 0.5 | 0.86 |
| 5 | 99 ± 0.47 | 0.80 | 101 ± 0.2 | 0.39 |
| 8 | 102 ± 1.5 | 2.56 | 103 ± 1.5 | 2.1 |
3.6. Effect of storage conditions on concentration of lycopene in formulation
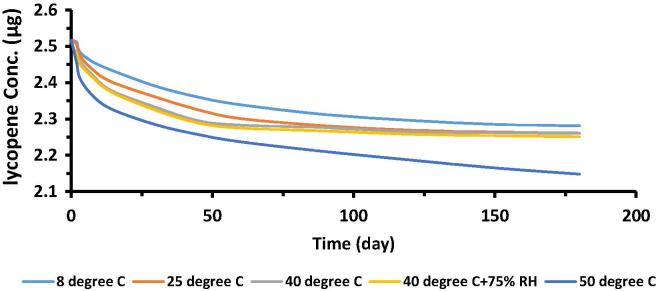
Fig. 6 depicts the variation in concentration of lycopene in emulgel with respect to time, stored at different conditions of temperature and humidity. It is clear from the figure that variation range of lycopene concentration was narrow on all storage conditions except 50 °C at 60th day and later. Oxidation was supposed to be an expected reaction at this high temperature as oxidation of lycopene is temperature dependent (John et al., 2002, Shi et al., 2003).
Fig. 6.
Variation in lycopene concentration in emulgel with respect to time at various storage conditions.
UV Analysis is extensively used to analyze the compounds which absorb light in a specific region of the wavelength, more specifically the Ultraviolet and visible region. Moreover, it is a quantitative as well as qualitative technique. Like other compounds, dilute solution of lycopene in n-hexane when scanned in a range of wavelengths between 200 and 800 nm, it showed maximum absorbance at 472 nm. Purpose to design the method was to determine the concentration of lycopene in emulgel formulation. Liquid-Liquid extraction technique and the knowledge of distribution law was applied to solve this problem as lycopene in the formulation was first extracted, then its concentration was measured by using standard curve. Necessity of the said procedure was the use of this technique to check the stability of the product developed, emulgel, with respect to lycopene, an active principle of the product. Furthermore, to measure the effects of various environmental conditions on the concentration of lycopene in the product were analyzed. Method provided a beneficial facility for the explained purpose which will be further helpful for other products of the same qualities.
Although cosmetic formulations appear as stable products when prepared but time and temperature-dependent processes occur during storage (Moulai Mostefa et al., 2006). The sources of physical instability of the emulgel are common to all dispersed systems, such as creaming (or sedimentation), reversible aggregation (flocculation) and/or irreversible aggregation (coalescence) of oil droplets (Tromeur et al., 2003).
Final acceptance of a product depends on the stability and appearance of the formulation. No quick and sensitive method for determining potential instability in an emulgel are available. Stress conditions are normally applied for evaluating the stability of the product including temperature, centrifugation etc. Other methods can also conceivably be used to predict stabilities such as pH tests, electrical conductivity determination and/or rheological parameters etc. (Muehlbach et al., 2006)
3.7. Statistical analysis
Linear regression analysis at 5% level of significance was performed at the data of standard straight line. Determination coefficient estimated was 0.9995 while slope was 0.1176. Standard deviation of response was found to be 0.00397.
Following hypothesis was designed and tested by suitable statistical tests;
Null hypothesis (µo)
-
I.
pH values of the control as well as test formulation significantly vary with respect to accelerated storage conditions, temperature and humidity, and time.
-
II.
There is significance difference between mean pH values of control and test formulation stored at accelerated conditions of temperature and humidity, with respect to time.
-
III.
Concentration of lycopene in formulation vary significantly with respect to accelerated storage conditions and time.
-
IV.
Significant variation exists in the results of inter-day and intra-day experimentation related to analytical method development.
Alternative hypothesis (µ1)
-
I.
Variation in pH values of control and test formulation are not significant with respect to storage conditions and time.
-
II.
Mean pH values of control and test formulation do not vary significantly with respect to storage conditions and time.
-
III.
Lycopene concentration in formulation do not vary significantly with time at different storage temperatures and high humidity level.
-
IV.
There is no significant variation in the results of inter-day and intra-day experiments.
To test the first statement of null hypothesis i.e. pH values of the control as well as test formulation significantly vary with respect to accelerated storage conditions, temperature and humidity, and time, two way ANOVA (analysis of variance) and Post Hoc tools at 5% significance level were used. “P-values” for both control and test formulations with respect to storage conditions as well time were <0.05, except at 50 °C and time points of 60th day and later where a significant variation in pH values observed for lycopene formulation, as p > 0.05.
Paired sample t-test at 5% level of significance was performed to compare the mean pH values of control and test formulation stored at different accelerated conditions of temperature and humidity with respect to time. It was the second statement of null hypothesis. “P-values” calculated for different levels of these parameters varied but variation was not statistically significant as p < 0.05, except at 50 °C and time points of 60th day and later. This verified that presence of lycopene had no significant effect on pH of formulation with an exception of 50 °C at time points 60th day and later.
Third statement of null hypothesis was related to the stability of active moiety in emulgel. Two way ANOVA and Post Hoc tests at significance level of 5% were performed for this purpose. Results of statistical analysis verified that there is no significance difference in lycopene concentration in the formulation at almost all levels of all parameters, p < 0.05, except at time level of 60th day and after that at 50 °C. These results narrated that lycopene formulation was completely stable even at 40 °C and 75% relative humidity throughout the study period i.e. 6 months. LSD calculations for temperature 50 °C at time point 60th day and later, presented p > 0.05. So, lycopene concentration in emulgel varied significantly at these levels.
The last statement of the null hypothesis was tested using t-test at 95% level of confidence. The p-values were <0.05 which resulted in rejection of the 4th statement of the null hypothesis and authenticated that the differences among the results of inter-day and intra-day experiments were not significant.
4. Conclusion
Results of analysis illustrated that the designed extraction technique was simple with respect to equipment, procedure, labor and time. Furthermore, it was cost effective as well as yield was comparable to that of already available methods. As a conclusion of this work, an O/W based stable emulgel containing lycopene, a carotenoid extracted from Solanum lycopersicum L. was successfully developed. Formulation developed was stable with respect to phase separation as well as active moiety even at high temperature of 40 °C and relative humidity level of 75%. Furthermore, analytical method designed for lycopene emulgel analysis was specific, reproducible and sensitive enough to be used for determination of the lycopene content in emulgel formulation.
Conflict of interest
There is no conflict of interest in any part of this experiment or any section of this manuscript.
Acknowledgement
This work was funded by faculty of pharmacy and alternative medicines, the Islamia University Bahawalpur, Pakistan.
Footnotes
Peer review under responsibility of King Saud University.
References
- Alam B.M., Aouak T., Alandis N.M., Alam M.M. Synthesis, characterization, drug solubility enhancement, and drug release study of poly (methacrylic acid-graft-simvastatin) Int. J. Polym. Mater. Polym. Biomater. 2015;64(5):229–241. [Google Scholar]
- Ascenso, A., Pinho, S.n., Eleutério, C., Praça, F.G., Bentley, M.V.r.L.B., Oliveira, H., Santos, C.a.o., Silva, O., Simões, S., 2013. Lycopene from tomatoes: vesicular nanocarrier formulations for dermal delivery. J. Agric. Food Chem. 61(30):7284–7293. [DOI] [PubMed]
- Bera H., Nadimpalli J., Kumar S., Vengala P. Kondogogu gum-Zn+ 2-pectinate emulgel matrices reinforced with mesoporous silica for intragastric furbiprofen delivery. Int. J. Biol. Macromol. 2017;104:1229–1237. doi: 10.1016/j.ijbiomac.2017.07.027. [DOI] [PubMed] [Google Scholar]
- Cefali L.C., Cazedey E.C.L., Souza-Moreira T.M., Correa M.A., Salgado H.R.N., Isaac V.L.B. Antioxidant activity and validation of quantification method for lycopene extracted from tomato. J. AOAC Int. 2015;98(5):1340–1345. doi: 10.5740/jaoacint.14-151. [DOI] [PubMed] [Google Scholar]
- Cefali L.C., Souza-Moreira T.M., Corrêa M.A., Salgado H.R.N., Isaac V.L.B. Development and evaluation of an emulsion containing lycopene for combating acceleration of skin aging. Brazil. J. Pharm. Sci. 2015;51(3):579–590. [Google Scholar]
- Chapman M.H., Ishida B.K., Randhava S.S., Randhava S.S. Google Patents; 2009. Extraction of carotenoids from plant material. [Google Scholar]
- Chauhan K., Sharma S., Agarwal N., Chauhan B. Lycopene of tomato fame: its role in health and disease. IJPSR. 2011;10:99–115. [Google Scholar]
- Cheng H.M., Koutsidis G., Lodge J.K., Ashor A., Siervo M., Lara J. Tomato and lycopene supplementation and cardiovascular risk factors: a systematic review and meta-analysis. Atherosclerosis. 2017;257:100–108. doi: 10.1016/j.atherosclerosis.2017.01.009. [DOI] [PubMed] [Google Scholar]
- Choudhury H., Gorain B., Pandey M., Chatterjee L.A., Sengupta P., Das A., Molugulu N., Kesharwani P. Recent update on nanoemulgel as topical drug delivery system. J. Pharm. Sci. 2017;106(7):1736–1751. doi: 10.1016/j.xphs.2017.03.042. [DOI] [PubMed] [Google Scholar]
- Fazli, K., I, Z., K, A., Zakiullah, S, Y., A, L., N, F., H, M., Ismail, W.A., 2014. Evaluation of anti-inflammatory activity of selected medicinal plants of Khyber Pakhtunkhwa. Pakistan. Pak. J. Pharm. Sci. 27, 365–368. [PubMed]
- Garg T., Rath G., Goyal A.K. Comprehensive review on additives of topical dosage forms for drug delivery. Drug Delivery. 2015;22(8):969–987. doi: 10.3109/10717544.2013.879355. [DOI] [PubMed] [Google Scholar]
- Gürbüz M.U., Ertürk A.S., Tülü M. Synthesis of surface-modified TREN-cored PAMAM dendrimers and their effects on the solubility of sulfamethoxazole (SMZ) as an analog antibiotic drug. Pharm. Dev. Technol. 2017;22(5):678–689. doi: 10.1080/10837450.2016.1221425. [DOI] [PubMed] [Google Scholar]
- Ishigaki M., Meksiarun P., Kitahama Y., Zhang L., Hashimoto H., Genkawa T., Ozaki Y. Unveiling the aggregation of lycopene in vitro and in vivo: UV–Vis, resonance raman, and raman imaging studies. J. Phys. Chem. B. 2017 doi: 10.1021/acs.jpcb.7b04814. [DOI] [PubMed] [Google Scholar]
- Jadhav K., Shetye S., Kadam V. Design and evaluation of microemulsion based drug delivery system. Int. J. Adv. Pharm. Sci. 2010;1:156–166. [Google Scholar]
- Jagdale S., Pawar S. Gellified emulsion of ofloxacin for transdermal drug delivery system. Adv. Pharm. Bull. 2017;7(2):229. doi: 10.15171/apb.2017.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John S., Ying W., Mike B., Maguer L.M. Oxidation and isomerization of lycopene under thermal treatment and light irradiation in food processing. Prev. Nutr. Food Sci. 2002;7(2):179–183. [Google Scholar]
- Kamil M.M., Mohamed G.F., Shaheen M.S. Fourier transformer infrared spectroscopy for quality assurance of tomato products. J. Am. Sci. 2011;7:558–572. [Google Scholar]
- Khullar R., Kumar D., Seth N., Saini S. Formulation and evaluation of mefenamic acid emulgel for topical delivery. Saudi Pharm. J. 2012;20(1):63–67. doi: 10.1016/j.jsps.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kute S., Saudagar R. Emulsified gel A Novel approach for delivery of hydrophobic drugs: an overview. J. Adv. Pharm. Educ. Res. Oct-Dec. 2013;3(4) [Google Scholar]
- Lambers H., Piessens S., Bloem A., Pronk H., Finkel P. Natural skin surface pH is on average below 5, which is beneficial for its resident flora. Int. J. Cosmet. Sci. 2006;28(5):359–370. doi: 10.1111/j.1467-2494.2006.00344.x. [DOI] [PubMed] [Google Scholar]
- Lopez-Cervantes J., Sanchez-Machado D.I., Valenzuela-Sanchez K.P., Nunez-Gastelum J.A., Escarcega-Galaz A.A., Rodriguez-Ramirez R. Effect of solvents and methods of stirring in extraction of lycopene, oleoresin and fatty acids from over-ripe tomato. Int. J. Food Sci. Nutr. 2014;65(2):187–193. doi: 10.3109/09637486.2013.839630. [DOI] [PubMed] [Google Scholar]
- Luengo E., Álvarez I., Raso J. Improving carotenoid extraction from tomato waste by pulsed electric fields. Front. Nutr. 2014;1 doi: 10.3389/fnut.2014.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma T., Deng Z., Liu T. Microbial production strategies and applications of lycopene and other terpenoids. World J. Microbiol. Biotechnol. 2016;32(1):15. doi: 10.1007/s11274-015-1975-2. [DOI] [PubMed] [Google Scholar]
- Meeting, J. F. W. E. C. o. F. A., 2006. Compendium of Food Additive Specifications: Joint FAO/WHO Expert Committee on Food Additives: 67th Meeting 2006, Food & Agriculture Org.
- Moulai Mostefa N., Hadj Sadok A., Sabri N., Hadji A. Determination of optimal cream formulation from long-term stability investigation using a surface response modelling. Int. J. Cosmet. Sci. 2006;28(3):211–218. doi: 10.1111/j.1467-2494.2006.00305.x. [DOI] [PubMed] [Google Scholar]
- Muehlbach M., Brummer R., Eggers R. Study on the transferability of the time temperature superposition principle to emulsions. Int. J. Cosmet. Sci. 2006;28(2):109–116. doi: 10.1111/j.1467-2494.2006.00284.x. [DOI] [PubMed] [Google Scholar]
- Narendran H., Koorapati S., Mamidibathula L. Formulation and evaluation of aceclofenac-lycopene transemulgel. World J. Pharm. Res. 2013;2(4):1036–1045. [Google Scholar]
- Narendran H., Koorapati S., Mamidibathula L. Formulation and evaluation of aceclofenac –lycopene transemulgel. World J. Pharm. Res. 2013;2(4):1036–1045. [Google Scholar]
- Okonogi S., Riangjanapatee P. Physicochemical characterization of lycopene-loaded nanostructured lipid carrier formulations for topical administration. Int. J. Pharm. 2015;478(2):726–735. doi: 10.1016/j.ijpharm.2014.12.002. [DOI] [PubMed] [Google Scholar]
- Paolicelli P., Varani G., Pacelli S., Ogliani E., Nardoni M., Petralito S., Adrover A., Casadei M.A. Design and characterization of a biocompatible physical hydrogel based on scleroglucan for topical drug delivery. Carbohydr. Polym. 2017;174:960–969. doi: 10.1016/j.carbpol.2017.07.008. [DOI] [PubMed] [Google Scholar]
- Preet L. Topical gel: a recent approach for novel drug delivery. Asian J. Biomed. Pharm. Sci. 2013;3(17):1–5. [Google Scholar]
- Sheriff S.A., Shaik Ibrahim S., Devaki T., Chakraborty S., Agarwal S., Pérez-Sánchez H. Lycopene prevents mitochondrial dysfunction during d-galactosamine/lipopolysaccharide-induced fulminant hepatic failure in albino rats. J. Proteome Res. 2017;16(9):3190–3199. doi: 10.1021/acs.jproteome.7b00176. [DOI] [PubMed] [Google Scholar]
- Shi, J., Maguer, M., Bryan, M., Kakuda, Y., 2003. Kinetics of lycopene degradation in tomato puree by heat and light irradiation. J Food Process Eng 25(6), 485–498.
- Singh Malik D., Mital N., Kaur G. Topical drug delivery systems: a patent review. Expert Opin. Therap. Patents. 2016;26(2):213–228. doi: 10.1517/13543776.2016.1131267. [DOI] [PubMed] [Google Scholar]
- Song, B., Liu, K., Gao, Y., Zhao, L., Fang, H., Li, Y., Pei, L. Xu, Y., 2017. Lycopene and risk of cardiovascular diseases: a meta-analysis of observational studies. Mol. Nutr. Food Res. [DOI] [PubMed]
- Tromeur E., Garnier E., Sagaut P., Basdevant C. Large eddy simulations of aero-optical effects in a turbulent boundary layer*. J. Turbul. 2003;4(5):1–22. [Google Scholar]
- Yang X., Li P., Dai S., Wu D., Li R., Yang J., Xiao H. The measurement and analysis of visible-absorption spectrum and fluorescence spectrum of lycopene. Guang pu xue yu guang pu fen xi= Guang pu. 2005;25(11):1830–1833. [PubMed] [Google Scholar]