Abstract
Research demonstrates that the majority of alarms derived from continuous bedside monitoring devices are non-actionable. This avalanche of unreliable alerts causes clinicians to experience sensory overload when attempting to sort real from false alarms, causing desensitization and alarm fatigue, which in turn leads to adverse events when true instability is neither recognized nor attended to despite the alarm. The scope of the problem of alarm fatigue is broad, and its contributing mechanisms are numerous. Current and future approaches to defining and reacting to actionable and non-actionable alarms are being developed and investigated, but challenges in impacting alarm modalities, sensitivity and specificity, and clinical activity in order to reduce alarm fatigue and adverse events remain. A multi-faceted approach involving clinicians, computer scientists, industry, and regulatory agencies is needed to battle alarm fatigue.
INTRODUCTION
ALARM FATIGUE
Definition
Bedside monitoring is mandatory in intensive care units, and ubiquitous on hospital step-down units and wards. Such monitors are meant to provide continuous oversight of parameters associated with the patient’s physiologic state such as heart rate (HR) and cardiac arrhythmias, respiratory rate (RR) indirect measurement of blood oxygen saturation via peripheral pulse oximetry (SpO2), noninvasive and invasive blood pressure (BP) and invasive monitoring pressure parameters of the arterial, central venous and intracranial systems. Monitors are equipped with alarms meant to call clinician attention to abnormal physiologic states. Alarms are specifically designed to cause cognitive distress [1] and capture the attention of clinicians’ caring for multiple patients to a change warranting clinician awareness, closer assessment, and supportive intervention. In the current monitor paradigm with existing widely distributed technology, clinicians must interrupt a task when an alarm activates, identify the patient and device alarming, determine if it is actionable or non-actionable, and the type of action required [1]. Alarm fatigue occurs when non-actionable alarms are in the majority, and clinicians develop decreased reactivity, causing them to “tune out’’ or ignore the alarms. A schematic in Figure 1 demonstrates that monitoring alarms may be clinically non-actionable or actionable, and the layers of action required. Actionable alarms are those that are due to a valid physiologically abnormal state which requires both clinical awareness and interaction. Mild abnormalities may require only focused assessment or correction with nursing interventions (e.g. repositioning, application of standing medical orders such as increasing oxygen flow, providing “as needed” ordered medications). However, some actionable alarms require that nurses escalate care to a medical provider, in either a routine or emergent manner. In contrast, non-actionable alarms may be due to invalid alarms such as monitoring artifact, or a true alarm detecting an abnormality unworthy of clinician awareness (e.g. overly sensitive or fleeting deviation beyond a normality threshold) [2]. There are some non-actionable alarms clinicians want to be aware of but not immediately do anything about, for example, a subtle abnormality that may require evaluation for progression and later action if persistent in duration or magnitude [3]. Actionable alarms account for only 5–13% of alarms in current monitoring systems [4], although reports vary widely [5]. When the number of non-actionable alarms that are invalid or not worthy of clinician awareness or intervention become excessive, alarm fatigue ensues. Alarm fatigue has two components: alarm desensitization from sensory overload and blunting of responsiveness, and alarm apathy wherein alarms that are oversensitive and under-specific cause lack of trust in their veracity [6]. This resultant desensitization and apathy cause valid alarms in need of awareness and intervention to be missed.
Figure 1.
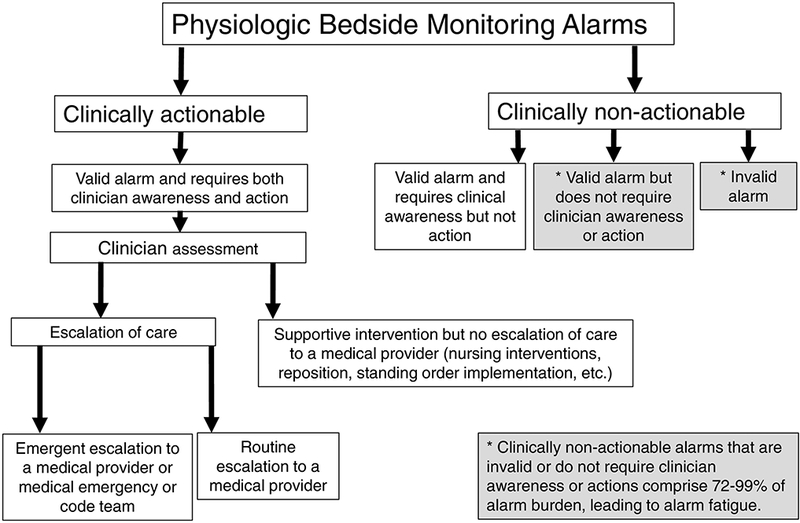
Schematic of alarm types generated by bedside physiologic monitors.
Causes
There are numerous causes of alarm fatigue along the monitoring care continuum—the patient, monitoring system, alarm pathway, individual clinician, and the organization (Figure 2). At the patient level, the American Heart Association has narrowed indications for continuous electrocardiographic (ECG) monitoring [7], thereby decreasing alarms by decreasing the number of monitored patients. However, this approach is self-limited and opposite to the concern that all hospitalized patients require continuous monitoring to prevent missed instability in the absence of a validated triage system for monitoring need [8]. Some patient characteristics (e.g. age >70 years, confused mental state, cardiovascular or respiratory diagnosis, mechanical ventilation, wide QRS or low amplitude, ventricular arrhythmias) are associated with false alarms [9]. Patient movement is a predominant cause for invalid alarms due to waveform disturbance interpreted as parameter threshold violation or arrhythmia [10]. Additionally, most false alarms are generated by only a few patients, with one-quarter of monitored patients responsible two-thirds of all alarms, [11], and as few as 2% of patients responsible for 77% of false alarms [9]. Alarm frequency is also related to the monitored parameter, and arrhythmia and SpO2 alarms seem to predominate [10, 11].
Figure 2.
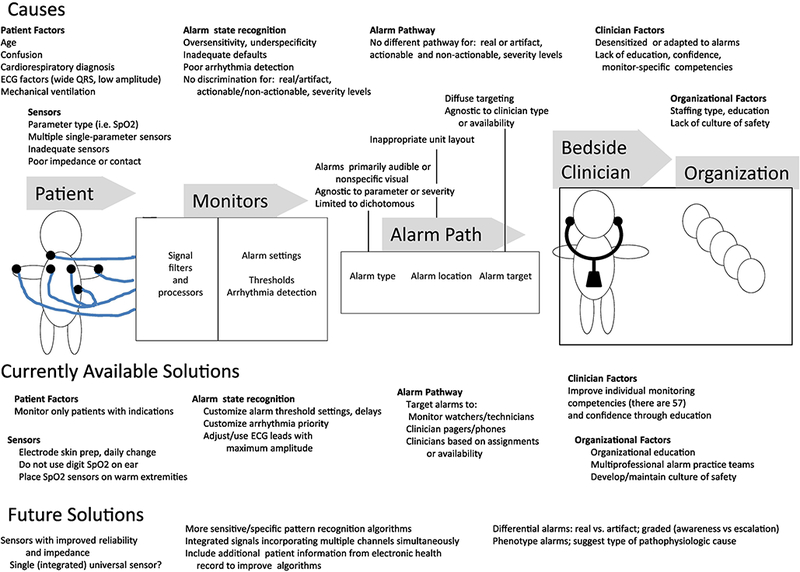
Summary of the causes of alarm fatigue along the monitoring care continuum of patient, monitor, alarm pathway, clinician and organization, and current and future potential solutions.
Characteristics of monitoring systems themselves give rise to alarm fatigue. Manufacturer’s default alarm settings are purposively set to not miss true events, resulting in overly low specificity and positive predictive value [12], and when implemented across multiple parameters compounds the problem. Alarm sounds themselves can contribute to alarm fatigue when tones and pitch do not differentiate between alarm source and priority [12], or are dichotomous rather than suggesting graded levels of severity [13].
Aspects of clinician work and workload also play a role in alarm fatigue. Nurses integrate information from multiple sources in a heuristic fashion when deciding about their response to alarms [14], and perceive their ability to respond to critical alarms as impaired when engaged in patient care or medication preparation [14] due to cognitive distribution across multiple critical tasks simultaneously. Finally, alarm fatigue causes extend to organizational aspects of unit environment and layout, workflow and process, and safety culture [15], all of which are potentially modifiable.
Thus the causes of alarm fatigue are complex. Although the current standard for medical alarms IEC 60601 1–8 attempts to specify basic safety and performance requirements [1], and the Food and Drug Administration provides device alarm guidance, they offer no penalty for high sensitivity of sensors with low specificity of alarm conditions, and thus fall short in eliminating this serious and complex problem, which requires multi-part solutions not limited to the technical alone.
Consequences
The consequences of alarm fatigue upon patients range widely. Excess unnecessary audible alarms cause patients and families to have disrupted communication and disturbed sleep [16]. More serious patient consequence is when clinicians experiencing alarm desensitization results in missed patient instability. Briefly missed periods of instability may be unimportant to patients with sufficient physiologic reserve. But missing longer periods of instability prevents needed intervention, leading to progressive physiologic stress and metabolic impairment, exhaustion of compensatory mechanisms, and eventually acid-base disturbance, arrhythmia, end-organ impairment, need for late rescue strategies such as unplanned intubation or admission to the intensive care unit, and even death. All of these are potentially avoidable if instability is recognized earlier and interventions applied more promptly when lower level corrective strategies suffice.
There seems to be a dose-response relative to alarm exposure and fatigue. As the number of non-actionable alarms increases, there is an incremental increase in clinician response delay [14, 17]. Bonafide et al. [17] demonstrated that 87% of alarms in a pediatric intensive care units and 99% of alarms on pediatric wards were non-actionable, and as the number of non-actionable alarms nurses were exposed to in the preceding two hours increased, their response time to subsequent alarms incrementally increased, and this result was most prominent on the ward. Alarm response rates also lengthen as time on duty increases, and each hour elapsed in anurse’s shift is associated with a 15% longer response time [18]. In addition to consequences for patients, adverse events resulting from alarm fatigue and missed instability also impact caregivers. Clinicians involved in serious adverse events become “second victims”, suffering collateral damage ranging from self-blame and intense guilt, seeking reassignment to less critical areas, post-traumatic stress, and even leaving the profession [19].
CURRENT SOUTIONS
Clinical Action
Clearly, strategies to decrease non-actionable alarms are paramount. Some sources recommend improving sensor reliability by adequate skin preparation, changing electrodes daily, and using disposable rather than reusable wiring and sensors [5, 12, 16, 20]. Customizing alarm settings frequently around a patient’s baseline or changing baseline are strategies to limit alarms to announce only changes from an already known state [12, 20]. Widening alarm threshold parameters, and incorporating delay between the time that a threshold violation is detected and announced are two current strategies to make alarms less sensitive to minor deviations from normality, or fleeting deviations (due to real instability or monitoring artifact) which self-correct [5, 6, 21]. Some studies reported no untoward outcomes with this strategy, while others reported missed instability resulting in fewer rescue events and deeper physiologic abnormality when rescue was initiated [5].
Other strategies are related to environment, education, and organization. Evaluating unit layout and placement of central monitors can improve sightlines and minimize unnecessary response distances. Educating staff on how monitors, sensors and alarms function specific to the product manufacturer and especially arrhythmia alarms is important. Sowan et al. [22] determined that there are 59 nurse competencies related to physiologic monitoring use, with subscales of appropriate monitoring, admission/discharge transfer, alarm management, hardware and connectivity, and advanced/specialized functions. They evaluated nurse’s perceptions of their achievement of these competencies, and found that 3–23% reported lack of skills or knowledge in at least one area, 25% lacked of confidence in essential functions, and 30% had never heard of at least one function. They advocated for educating staff in alarm competencies on an initial and ongoing basis, and development of a cadre of super-users to support and refresh knowledge and expertise in appropriate monitoring practices including alarm settings and adjustment [22]. Another recommended organizational strategy is establishing an interprofessional team to gather data and address issues related to alarms, and use team consensus to develop unit-specific clinical indications for monitoring, default parameters, and alarm management policies [20]. Another proposed organizational strategy is to implement “monitor watchers” whose role is to observe a centralized bank of monitors and contact nurses with only actionable events via mobile technologies [23], but this approach has not yet been proven to definitively decrease mortality [12], and does nothing to decrease the number of non-actionable alarms, but merely shifts their burden.
Technologic
Some current technologically focused strategies to reduce alarm fatigue include directed alerting or secondary notification. Directed alerting sends alerts to specific individuals, rather than deploying a non-specific area alarm This is accomplished by directing alarms to a small subset of locations where individuals responsible for responding are located, or directly to a phone or pager [24, 25]. Another strategy is the implementation of a secondary device notification system. Here, alarms from a primary device are transmitted to a middleware system, which uses predefined rules to suppress, translate, escalate and/or communicate these alarms to a secondary device [26]. These wireless secondary devices, such as a pager, cell phone or tablet, can be carried constantly by a particular caregiver, or handed off in situations where the primary caregiver is unavailable [5, 26]. The difference between directed alarms and secondary device notification is that the latter utilizes more embedded rules determining which alarms are sent and to whom (for example an alarm for leads-off may go to a technician, but an arrhythmia to a nurse), providing graded rather than dichotomous alarms, escalation of unacknowledged alarms, or applying additional delays beyond those applied at the bedside [26]. These strategies decrease the number of alarms generally announced, but their impact on alarm fatigue, safety and efficacy is still not well established.
FUTURE SOLUTIONS
Varieties of solutions are being developed for future use, and are currently in laboratory stages or in early clinical usage not yet widespread and so categorized as a future solution.
Alarm Suppression and Artifact Discrimination
Alarm suppression can be accomplished with a variety of suppression algorithms, including statistical metrics, wavelet signal analysis, and spectral regression [16]. These are most effective in discerning single parameter alerts due to monitoring artifact. Suppression can also be achieved by evaluating two parameters simultaneously. For example, a vital sign parameter with large oscillation simultaneous with other stable vital sign patterns can likely be suppressed. Both approaches decrease the rate of false alarms, but also miss true alerts to varying degrees [16].
Discriminating between alerts due to true instability or monitoring artifact can be accomplished by using machine learning approaches to featurize the vital sign parameters (both the parameter in an abnormal state and the simultaneous additional vital parameters), and then apply algorithms to differentiate between feature patterns due to true instability or artifact [27]. Chen et al. [28] used a random forest classification model to discriminate between real and artifact alerts as they evolved online, with an area under the curve (AUC) of 0.79 for SpO2 at the instant the vital sign first crossed threshold, while BP performed at an AUC of 0.87 and RR at an AUC of 0.85. Such an algorithm, if applied to bedside monitors in real time, could differentiate between real instability and artifact alerts at a clinically meaningful rate. Nevertheless, when suppressing or discriminating artifact, the problem still remains as to what type of action should next be taken. Simply not announcing an alarm due to artifact eliminates a false alarm, but while the vital sign parameter is in an artifact state, it is not discerning the true underlying physiologic state, which may be of clinical concern and physiologic consequence. This goes back to the need for better sensors and signal capture which are not prone to monitoring artifact in the first place.
Smart Alarms
There is movement away from monitoring alarm strategies based on basic arrhythmia recognition and high and low threshold parameters, to make abnormality recognition more discerning and alarms “smarter”. For example, single parameter alarms could dynamically apply adaptive delays (shorter delay for a greater excursion above thresholds, longer delays for milder excursions) [29].
There are a variety of approaches for development of integrated signals at varying levels of complexity. In the most basic example, signals can be evaluated simultaneously but without any further signal processing. For example, ventricular tachycardia alarms can be prioritized based on the level of hemodynamic impairment displayed by the arterial waveform [30]. In a more complex scenario, multiple vital signs can be evaluated simultaneously, and a single integrated instability index value developed. Hravnak et al. [31] evaluated such an integrated monitoring system displaying a single risk score generated using neural networking based on a probabilistic model of instability if a single vital sign deviated from normality by ±3 standard deviations, or by smaller amounts of deviation for multiple parameters. Individual parameters may have all still been within accepted normality thresholds when the pooled risk instability. When this system was used in clinical care to more sensitively and specifically alert clinicians to true instability and presumably promote intervention earlier, the cumulative number of instability episodes decreased 58%, and the cumulative duration of time patients were in an unstable state decreased 60%.
Real Instability Nowcasting and Forecasting with Machine Learning Approaches
Another approach is to use machine learning to featurize information obtained from traditional numeric or waveform parameters to provide hundreds of features from vital sign time series data predicting risk (e.g. slope, standard deviation, delta standard deviation, range, ratio, etc.). Such features are extracted from single parameters, or from multiple parameters as they relate to each other, and then algorithms applied to predict feature patterns associated with true instability—when it is subtly manifesting but not yet clinically discernible (nowcasting), or even before it manifests (forecasting).
In one approach, [32] featurizing HR, RR, BP and SpO2 from continuously noninvasive bedside monitoring used a supervised machine learning approach to compare the risk of instability between cases (patients who ever developed instability) and controls (patients who were never were unstable), the algorithm detected an increasing risk for cases about 90–120 minutes before an abnormal vital sign threshold was crossed, while the instability risk remained stable for controls. Further, when examining the first four hours of admission, the cases that would later develop instability had a significantly higher risk score at admission than those who would never be unstable, thereby forecasting the risk of developing instability in future. The implication this has for bed and resource triage is great. Lastly, it was possible to discern that patients become unstable along a limited number of risk trajectories, with some demonstrating prolonged stability and then an acute rise in risk, while others evolve their risk in a steady fashion [32, 33], presumably due to different underlying disease processes.
The above information demonstrates that moving from individual vital sign threshold or wave template monitoring to dynamic risk scoring of multiple vital signs may address the problem of alarm fatigue seen in the current single-parameter monitoring paradigm, but more work needs to be done before widespread adoption in clinical care.
New Instability Parameters?
All strategies above rely on use of the traditional vital signs of HR, RR, BP, SpO2 and invasive parameters. However, ability to assess vital signs with non-traditional methods is emerging. For example, respiratory rate monitoring can be accomplished with alternative approaches such as acoustic monitoring, piezoelectric membranes, fiberoptics, photoplesmography, and thermodynamics [34]. However, new holistic stability parameters are needed which do not rely on vital signs, but focus on markers of physiologic decompensation. The ideal solution would be development a single parameter to assess early and subtle changes in cellular metabolism and stress via a noninvasive sensor.
CONLUSIONS
Physiologic monitoring alarms are meant to alert clinicians to present patient instability, but the preponderance of clinically non-actionable alarms reported overwhelm clinicians and the care delivery system, resulting in missed instability and threatens patient safety. Solving alarm fatigue is not simple, since non-actionable alarms and impaired alarm response arise from a variety of technical, patient, clinician and organizational factors. Therefore, approaches to alleviate alarm fatigue must occur on all these fronts. Some current solutions exist, and future ones are evolving. To assess solution impact, it is also crucial that reliable metrics to measure alarm fatigue be developed. Presently, strategies to decrease alarm fatigue are evaluated by comparing numbers of alarms before and after the intervention. Some alarm fatigue surveys are available, and based primarily on nurse perceptions [3, 23, 35, 36]. More accurate and unbiased alarm fatigue metrics must be developed. Making alarms less sensitive by raising thresholds or imposing delay should be balanced against the potential pathophysiologic cost of longer duration of patient time enduring arrhythmia, bradycardia, hypoxemia, or hypotension [37]. Therefore metrics judging alarm improvement strategies must also incorporate safety, impact on the quality and process of care, and human factors outcomes [37, 38], since there is no guarantee that decreasing the number of non-actionable alarms will cure this dangerous but ubiquitous problem. Finally regulatory agencies must acknowledge that alarms which are overly sensitive and non-specific, while erring on the side of not missing real instability, promote alarm fatigue and threaten public safety, and strengthened guidance is required. New paradigms in monitoring instability and alerting mechanisms are needed.
Acknowledgments
FINANCIAL SUPPORT: This paper was supported with NIH NINR funding RO1 NR13912; NSF awards 0911032 and 1320347
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ruskin KJ, Hueske-Kraus D. Alarm fatigue: impacts on patient safety. Curr Opin Anaesthesiol. 2015;28(6):685–90. [DOI] [PubMed] [Google Scholar]
- 2.Karnik A, Bonafide CP. A framework for reducing alarm fatigue on pediatric inpatient units. Hosp Pediatr. 2015;5(3):160–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruppel H, Funk M, Clark JT, Geras I, David Y, Bauld TJ, Coss P, Holland ML. Attitudes and practices related to clinical alarms: a follow-up survey. Am J Crit Care. 2018;27(2):114–123. [DOI] [PubMed] [Google Scholar]
- 4.Ruppel H, Funk M, Whittemore R. Measurement of physiological monitor alarm accuracy and clinical relevance in intensive care units. Am J Crit Care. 2018;27(1):11–21. [DOI] [PubMed] [Google Scholar]
- 5.Paine CW, Goel VV, Ely E, Stave CD, Stemler S, Zander M, Bonafide CP. Systematic review of physiologic monitor alarm characteristics and pragmatic interventions to reduce alarm frequency. J Hosp Med. 2016;11(2):136–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turmell JW, Coke L, Catinella R, Hosford T, Majeski A. Alarm fatigue: Use of an evidence-based alarm management strategy. J Nurs Care Qual. 2017;32(l):47–54. [DOI] [PubMed] [Google Scholar]
- 7.Sandau KE, Funk M, Auerbach A, Bareness GW, Blum K, Cvach M, Lampert R, et al. Update to practice standards for electrocardiographic monitoring in hospital settings: a scientific statement from the American Heart Association. Circulation. 2017; 136:e273–e344. https ://doi.org/10.1161/CIR.0000000000000527 [DOI] [PubMed] [Google Scholar]
- 8.DeVita MA, Smith GB, Adam SK, Adams-Pizarro I, Buist M, Bellomo R, Bonello R, Cerchiari E, Farlow B, Goldsmith D, Haskell H, Hillman K, Howell M, Hravnak M, Hunt EA, Hvarfner A, Kellett J, Lighthall GK, Lippert A, Lippert FK, Mahroof R, Myers JS, Rosen M, Reynolds S, Rotondi A, Rubulotta F, Winters B. “Identifying the hospitalised patient in crisis”— a consensus conference on the afferent limb of rapid response systems. Resuscitation. 2010;81(4):375–82. [DOI] [PubMed] [Google Scholar]
- 9.Harris PR, Zègre-Hemsey JK, Schindler D, Bai Y, Pelter MM, Hu X Patient characteristics associated with false arrhythmia alarms in intensive care. Ther Clin Risk Manag. 2017;13:499–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drew BJ, Harris P, Zègre-Hemsey JK, Mammone T, Schindler D, Salas-Boni R, Bai Y, Tinoco A, Ding Q, Hu X. Insights into the problem of alarm fatigue with physiologic monitor devices: a comprehensive observational study of consecutive intensive care unit patients. PLoS One. 014;9(10):e110274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schondelmeyer AC, Brady PW, Goel VV, Cvach M, Blake N, Mangeot C, Bonafide CP. Physiologic monitor alarm rates at 5 children’s hospitals. J Hosp Med. 2018;13(6):396–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cvach M Monitor alarm fatigue: an integrative review. Biomed Instrum Technol. 2012;46(4):268–77. [DOI] [PubMed] [Google Scholar]
- 13.Honan L, Funk M, Maynard M, Fahs D, Clark JT, David Y. Nurses’ perspectives on clinical alarms. Am J Crit Care. 2015;24(5):387–95. [DOI] [PubMed] [Google Scholar]
- 14.Joshi R, Mortel HV, Feijs L, Andriessen P, Pul CV. The heuristics of nurse responsiveness to critical patient monitor and ventilator alarms in a private room neonatal intensive care unit. PLoS One. 2017;12(10):e0184567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilken M, Hüske-Kraus D, Klausen A, Koch C, Schlauch W, Röhrig R. Alarm fatigue: Causes and effects. Stud Health Technol Inform. 2017;243:107–111. [PubMed] [Google Scholar]
- 16.Winters BD, Cvach MM, Bonafide CP,Hu X, Konkani A, O’Connor MF, Rothschild JM, Selby NM, Pelter MM, McLean B, Kane-Gill SL; Society for Critical Care Medicine Alarm and Alert Fatigue Task Force. Technological distractions (part 2): a summary of approaches to manage clinical alarms with intent to reduce alarm fatigue. Crit Care Med. 2018;46(1):130–137. [DOI] [PubMed] [Google Scholar]
- 17.Bonafide CP, Lin R, Zander M, Sarkis Graham C, Paine CW, Rock W, Rich A, Roberts KE, Fortino M, Nadkarni VM, Localio AR, Keren R. Association between exposure to non-actionable physiologic monitor alarms and response time in a children’s hospital. J Hosp Med. 2015;10(6): 345–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonafide CP, Localio AR, Holmes JH, Nadkami VM, Stemler S, MacMurchy M, Zander M, Roberts KE, Lin R, Keren R. Video analysis of factors associated with response time to physiologic monitor alarms in a children’s hospital. JAMA Pediatr. 2017;171(6):524–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mira JJ, Lorenzo S, Carrillo I, Ferrús L, Silvestre C, Astier P, Iglesias-Alonso F, Maderuelo JA, Pérez-Pérez P, Torijano ML, Zavala E, Scott SD; Research Group on Second and Third Victims. Lessons learned for reducing the negative impact of adverse events on patients, health professionals and healthcare organizations. Int J Qual Health Care. 2017;29(4):450–460. [DOI] [PubMed] [Google Scholar]
- 20.American Association of Critical Care Nurses. Managing alarms in acute care across the life span: electrocardiography and pulse oximetry. Crit Care Nurse. 2018;38(2):el6–e20. [DOI] [PubMed] [Google Scholar]
- 21.Lansdowne K, Strauss DG, Scully CG. Retrospective analysis of pulse oximeter alarm settings in an intensive care unit patient population. BMC Nurs. 2016;15:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sowan AK, Vera AG, Fonseca El, Reed CC, Tarriela AF, Brndt AE. Nurse competence on physiologic monitors use: toward eliminating alarm fatigue in intensive care units. Open Med Inform J. 2017;11:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Funk M, Ruppel H, Blake N, Phillips J. Research: use of monitor watchers in hospitals: characteristics, training, and practices. Biomed Instrum Technol. 2016;50(6):428–438. [DOI] [PubMed] [Google Scholar]
- 24.Allan SH, Doyle PA, Sapirstein A, Cvach M. Data-driven implementation of alarm reduction interventions in a cardiovascular surgical ICU. Jt Comm J Qual Patient Saf. 2017;43(2):62–70. [DOI] [PubMed] [Google Scholar]
- 25.Cvach MM, Frank RJ, Doyle P, Stevens ZK. Use of pagers with an alarm escalation system to reduce cardiac monitor alarm signals. J Nurs Care Qual. 2014;29(1):9–18. [DOI] [PubMed] [Google Scholar]
- 26.Jacques S Factors that affect design of secondary alarm notification systems. Biomed Instrum Technol. 2017;51(s2):16–20. [DOI] [PubMed] [Google Scholar]
- 27.Hravnak M, Chen L, Dubrawski A, Bose E, Clermont G, Pinsky MR. Real alerts and artifact classification in archived multi-signal vital sign monitoring data: implications for mining big data. J Clin Monit Comput. 2016;30(6):875–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.hen L, Dubrawski A, Wang D, Fiterau M, Guillame-Bert M, Bose E, Kaynar AM, Wallace DJ, Guttendorf J, Clermont G, Pinsky MR, Hravnak M. Using supervised machine learning to classify real alerts and artifact in online multisignal vital sign monitoring data. Crit Care Med. 2016;44(7):e456–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmid F, Goepfert MS, Franz F, Laule D, Reiter B, Goetz AE, Reuter DA. Reduction of clinically irrelevant alarms in patient monitoring by adaptive time delays. J Clin Monit Comput. 2017;31(1):213–219. [DOI] [PubMed] [Google Scholar]
- 30.Desai K, Lexa M, Matthews B, Genc S. Hemodynamic-impact-based prioritization of ventricular tachycardia alarms. Conf Proc lEEE Eng Med Biol Soc. 2014;2014:3456–9. doi:10.1109/EMBC.2014.6944366. [DOI] [PubMed] [Google Scholar]
- 31.Hravnak M, DeVita MA, Clontz A, Edwards L, Valenta C, Pinsky MR. Cardiorespiratory instability before and after implementing an integrated monitoring system. Crit Care Med. 2011; 39(1): 65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen L, Ogundele O, Clermont G, Hravnak M, Pinsky MR, Dubrawski AW. Dynamic and personalized risk forecast in step-down units. Implications for monitoring paradigms. Ann Am Thorac Soc. 2017;14(3):384–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen L, Dubrawski A, Clermont G, Hravnak M, Pinsky MR. Modelling risk of cardio-respiratory instability as a heterogeneous process. AMIA Annu Symp Proc. 2015;2015:1841–50. [PMC free article] [PubMed] [Google Scholar]
- 34.Preiss D, Drew BA, Gosnell J, Kodali BS, Philip JH,Urman RD. Linshom thermodynamic sensor is a reliable alternative to capnography for monitoring respiratory rate. J Clin Monit Comput. 2018;32(1):133–140. [DOI] [PubMed] [Google Scholar]
- 35.Petersen EM, Costanzo CL. Assessment of clinical alarms influencing nurses’ perceptions of alarm fatigue. Dimens Crit Care Nurs. 2017;36(l):36–44. [DOI] [PubMed] [Google Scholar]
- 36.Torabizadeh C, Yousefinya A, Zand F, Rakhshan M, Fararooei M. A nurses’ alarm fatigue questionnaire: development and psychometric properties. J Clin Monit Comput. 2017;31(6):1305–1312. [DOI] [PubMed] [Google Scholar]
- 37.Johnson KR, Hagadom JI, Sink DW. Alarm safety and alarm fatigue. Clin Perinatal. 2017;44: 713–728. [DOI] [PubMed] [Google Scholar]
- 38.Kobayashi L, Gosbee JW, Merck DL. Development and application of a clinical microsystem simulation methodology for human factors-based research of alarm fatigue. HERD. 2017;10(4):91–104. [DOI] [PubMed] [Google Scholar]


