Abstract
BACKGROUND:
It has been demonstrated that fecal immunochemical test (FIT) mailing programs are effective for increasing colorectal cancer (CRC) screening. The objectives of the current study were to assess the magnitude of uptake that could be achieved with a mailed FIT program in a federally qualified health center and whether such a program can be implemented at a reasonable cost to support sustainability.
METHODS:
The Washington State Department of Health’s partner HealthPoint implemented a direct-mail FIT program at 9 medical clinics, along with a follow-up reminder letter and automated telephone calls to those not up-to-date with recommended screening. Supplemental outreach events at selected medical clinics and a 50th birthday card screening reminder program also were implemented. The authors collected and analyzed process, effectiveness, and cost measures and conducted a systematic assessment of the short-term cost effectiveness of the interventions.
RESULTS:
Overall, 5178 FIT kits were mailed with 4009 follow-up reminder letters, and 8454 automated reminder telephone calls were made over 12 months. In total, 1607 FIT kits were returned within 3 months of the end of the implementation period: an overall return rate of 31% for the mailed FIT program. The average total intervention cost per FIT kit returned was $39.81, and the intervention implementation cost per kit returned was $18.76.
CONCLUSIONS:
The mailed FIT intervention improved CRC screening uptake among HealthPoint’s patient population. This intervention was implemented for less than $40 per individual successfully screened. The findings and lessons learned can assist other clinics that serve disadvantaged populations to increase their CRC screening adherence.
Keywords: cancer screening, colorectal cancer, community health centers, health care economics and organizations, reminder system, Washington
INTRODUCTION
Mortality from colorectal cancer (CRC) could be reduced through the use of CRC screening tests, such as fecal immunochemical tests (FITs) and colonoscopies, to detect early cancers.1 In Washington State, the 2014 incidence rate of CRC was 36.3 per 100,000, and the mortality rate was 11.7 per 100,000.2 According to the 2013 Behavioral Risk Factor Surveillance System, 67% of Washington residents aged 50 to 75 were up to date with CRC screening. Screening prevalence was substantially lower among American Indians and Alaska Natives and Hispanics, residents who were uninsured, and individuals who reported annual incomes at or below $25,000.3
Federally qualified health centers (FQHCs) provide comprehensive primary care and preventative health services in the community. In 2016, 27 FQHCs served more than a million patients in low-income and medically underserved communities across Washington State.4 The Uniform Data System, which is based on data collected from health centers and FQHCs, reports that only 46% of FQHC patients between ages of 50 and 75 years were up to date with recommended CRC screening in 2016,4 which is far below the 2015 state and the national screening rates of 67% and 62%, respectively.5 Although the national screening rate, which is based on households interviewed for the National Health Interview Survey, is not directly comparable to the Uniform Data System, these data still reflect the disparities observed among low-income populations who receive care at FQHCs.
The Washington State Department of Health (WASDOH) was awarded funds from the Centers for Disease Control and Prevention in July 2015 to increase CRC screening using a health systems strategy. The WASDOH partnered with large FQHC health systems, like HealthPoint in King County, to implement evidence-based interventions (EBIs). In 2016, HealthPoint served 84,646 clients, 93.6% of whom were below the 200% Federal Poverty Level,4 and served clients from a variety of racial and ethnic backgrounds. Historically, HealthPoint has promoted CRC screening by offering the FIT at no cost to patients during office visits. In 2014, HealthPoint’s quality-improvement (QI) team noted that only about one-half of HealthPoint patients were up to date with recommended CRC screening. A review of clinical practices revealed that timely and consistent screening promotion was hindered by the limited time providers had with patients and by structural barriers such as language and transportation. HealthPoint’s leadership decided to implement a mailed FIT program (MailedFIT) to eligible clients, with follow-up reminder letters and telephone calls, to reduce structural barriers and improve screening uptake.
A few comparative effective trials and pilot studies have indicated that MailedFIT can increase CRC screening rates among populations seeking care at safety-net clinics.6,7 These effectiveness impacts were measured under research conditions, and a key gap in the current literature is evidence on optimal approaches to scale up promising interventions in the real-world setting. The objectives of the current study were to evaluate the return rate that can be achieved with a MailedFIT intervention under the real-world implementation conditions at HealthPoint and whether the intervention can be implemented at a reasonable cost to enhance long-term sustainability. Although FQHCs may receive incentive payments for achieving screening goals and payments for the clinical cost of screening tests for insured patients, they do not receive reimbursement for screening promotion interventions. Therefore, it is essential to assess the promotion cost of the interventions, because high-cost interventions likely will not be adopted.
MATERIALS AND METHODS
Intervention Development and Partnerships
In partnership with the WASDOH, HealthPoint increased CRC screening among clients by reducing structural barriers. The primary intervention was a direct-mail FIT program using the Consult Diagnostics immunochemical fecal occult blood test (McKesson Medical-Surgical, Richmond, VA) at HealthPoint’s 9 medical clinics. MailedFIT was selected based on prior success with this approach in research studies, and HealthPoint chose to implement supplemental activities to assess whether these interventions could complement the MailedFIT program. Supplementary outreach events included distributing FIT kits during flu and mammography campaigns at selected medical clinics and 50th birthday mailings with information CRC screening.
HealthPoint was already implementing 2 EBIs in the form of provider reminders and provider assessment and feedback. The provider assessment and feedback is publicly posted on a board that shares the screening numbers and the percentage screened; it is placed in daily services-due reports and discussed during monthly care team meetings that address quality metrics, including CRC screening. No changes were made to these interventions, and the MailedFIT intervention was not integrated with these existing interventions.
HealthPoint received technical assistance from the WASDOH and external stakeholders—including the Alliance for Reducing Cancer Northwest, Dr. Gloria Coronado (from the Screen to Prevent [STOP] CRC project at the Kaiser Permanente Center for Health Research), and the Washington Association of Community and Migrant Health Centers. These partners shared their CRC screening promotion materials (eg, letters to patients, use of wordless instructions) and best practices to aid in program and small media development. The Alliance for Reducing Cancer Northwest developed a data tracking tool used by HealthPoint to track their CRC screening efforts and outcomes.
The HealthPoint team consisted of the medical director, health center managers, medical assistant supervisors, laboratory medical assistants, and the QI team. The team provided input on process flows, communication with patients, and in-clinic efforts to promote CRC screening; and they worked to identify and develop processes that could be standardized across all 9 clinics. Centralized screening was established to ensure consistent implementation across the clinic sites.
Intervention Implementation
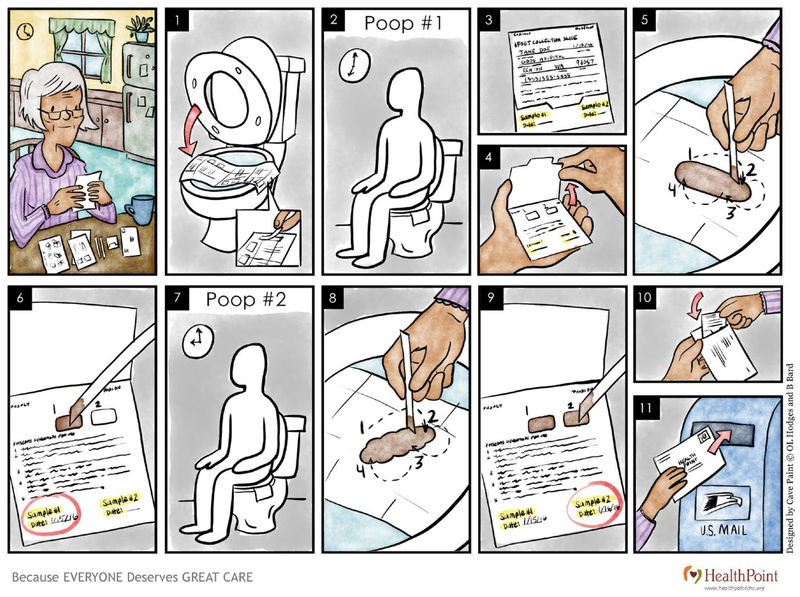
Average-risk patients who were not up to date with CRC screening were identified through a combination of automated electronic medical record extraction and manual review. Patients were eligible for the FIT mailing if they had not received a colonoscopy in the past 10 years or a fecal test in the past year. The team spent approximately 3 months developing and piloting both written and wordless illustrations of the instructions for the test (see Fig. 1). Prior qualitative assessment indicated that wordless instructions were preferred to instructions that included just word descriptions to explain the procedures for FIT testing.8 The wordless instructions were specifically selected for this intervention, because HealthPoint patients generally have low literacy levels and often do not speak English. All written materials were in English and were translated using a verified translation company into 12 of the most common languages among HealthPoint patients aged 50 to 75 years: Amharic, Arabic, Farsi, Hindi, Korean, Nepali, Punjabi, Russian, Somali, Spanish, Tagalog, and Vietnamese. Wordless instructions and other materials were created with input from QI coordinators and pilot tested in 2 clinics with patients before the first mailing. The feedback received was used to tailoring the materials, making the individuals depicted appear more representative of the target population. Written communications were created using tested messaging published in the 80% by 2018 Communications Guidebook from the National Colorectal Cancer Roundtable.9 Patients received all communications in their preferred language, as determined at their initial intake visit at HealthPoint.
FIGURE 1.
These are wordless instructions for the fecal immunochemical test.
The HealthPoint team first mailed an introductory letter, including answers to frequently asked questions, to all patients who needed a CRC screening appointment. These questions were determined with input from clinical staff who commonly received questions about CRC screening either during appointments or by telephone call. The letter also provided a web link to an online portal to opt out of receiving the FIT kit by mail or to request an FIT kit (in case they misplaced their original kit). Letters were mailed in clinic envelopes monthly during the implementation period, and staff attempted to update incorrect patient addresses that were returned. Approximately 2 weeks after the initial mailing, patients who had not opted out and had a valid address were mailed a FIT kit with the easy-to-read instructions, which included pictures and text in each patient’s language. Each of the MailedFIT kits was labeled with patient identification information (name, medical record number, date of birth) to enable HealthPoint staff to track kits that were returned as a result of this intervention. The cover letter provided patients with the telephone number of HealthPoint’s call center in case they had any questions. The kits were postage paid, labeled with the clinic’s address for easy return, and labeled with key patient information (name, medical record number, and date of birth). FIT kits that were not returned were followed with 2 automated reminder calls and a reminder letter. The first call was placed approximately 1.5 weeks after the kit was mailed, the second call was placed approximately 1 week after the first call, and the reminder letter was mailed approximately 1 week after the second automated reminder call. The FIT mailings and reminders continued from July 2015 to June 2016.
HealthPoint also conducted outreach at flu shot clinics (FluFIT) and mammography screenings (MammoFIT) for eligible patients who needed screening. The HealthPoint team attended the designated flu shot or mammogram clinic days from July 2015 to June 2016 to provide patients with a free FIT kit, instructions, and additional support.
HealthPoint also developed a 50th birthday screening reminder program to reach clients for CRC screening. The purpose of the cards was to provide education on the recommended screening age for CRC and sensitize patients to their need for screening. The card provided contact information for the clinic and a web link to an online portal to request a mailed FIT screening test. The birthday cards were mailed monthly between March and June 2016 to all patients who were turning 50 and were translated into the 12 languages common to the population served.
Activity-Based Cost Data Collection
A tailored Excel-based cost data-collection instrument was developed to obtain information on labor and nonlabor resources that supported implementation of the EBIs. This instrument was based on previously published methods of collecting cost data for economic evaluation of cancer screening programs.10–13 The project team collected costs related to activities in 4 categories: intervention development, intervention implementation, data quality assessment, and administration and management. For each of these broad categories, a detailed list of subactivities could be selected to document the procedures and processes used during implementation. For example, the intervention implementation included subactivities for initial patient mailings, FIT mailings, patient tracking, and follow-up reminders. Data were collected retrospectively to capture resource use and cost information for both baseline activities related to planning of the intervention and implementation.
To estimate costs associated with labor, we requested that all project and partner staff who developed and implemented the intervention provide information on their salaries (which were verified by the HealthPoint project coordinator), including fringe, and report the number of hours spent supporting the detailed list of activities. We also systematically collected nonlabor expenditures related to travel and the patient mailings. Laboratory processing costs were not included in the cost of implementation, because the focus of this study was on the intervention-specific costs and not clinical delivery of services. To ensure a comprehensive representation of all costs involved, we separately report the costs of purchasing and processing the FIT kits.
Study Measures and Data Analysis
To track implementation of the 3 CRC interventions, we report process measures, including the number of FIT kits mailed or distributed and the number of FIT kits returned. In addition, we report the number of reminder mailings and automated telephone calls. The key outcome measure is the percentage of FIT kits returned. Process and outcome measures are reported individually for each of the 9 participating HealthPoint clinics to explore the impact of the interventions at the site level. The overall total and average process and outcome measures across the sites also are reported.
We calculated the staff cost of each activity and then aggregated all labor and nonlabor costs to derive the activity-based costs. The cost estimates are presented for the development phase, implementation phase, administration activities, and data quality-assessment procedures. We also report the cost per FIT kit returned for each of these broad program-level activities to evaluate the overall cost effectiveness of the interventions.
RESULTS
The characteristics of HealthPoint’s patients who were not up to date with CRC screening and were targeted for the study intervention are provided in Table 1. HealthPoint has 9 locations in urban areas, and greater than 5178 patients aged 50 to 75 years were not up to date with CRC screening. Of these, 82.1% were ages 50 to 64 years, and 40.3% were men. Types of insurance varied across patients: 17.3% had Medicare, 20.7% were uninsured, 20.7% were self-pay, and 40.6% had other insurance. The preferred language of communication was English (68.1%), Spanish (15.0%), and other languages (16.9% spoke a range of languages, including those from Asia and the Middle East).
TABLE 1.
HealthPoint Patient Characteristics: Not Up to Date With CRC Screening
| Characteristic | Frequency |
|---|---|
| No. of clinic sites | 9 |
| No. of patients aged 50–75 y not up-to-date with CRC screening | 5178 |
| Patients aged 50–64 y, % | 82.1 |
| Men, %a | 40.3 |
| Insurance status, % | |
| Uninsured (requiring free care) | 20.7 |
| Medicare patients | 17.3 |
| Self-pay patients | 20.7 |
| Other Insured patients | 40.6 |
| Unknown insurance status | 0.7 |
| Preferred language, % | |
| English | 68.1 |
| Spanish | 15 |
| Punjabi | 3.2 |
| Somali | 1.3 |
| Other (Arabic, Ethiopian, Hindi, Farsi, Russian, etc) | 12.4 |
Estimated up-to-date screening rates were not stratified by sex.
Table 2 presents the interventions implemented at the clinic sites. All clinics participated in the MailedFIT and 50th birthday card interventions, and 4 clinics participated in the FluFIT and MammoFIT interventions. Overall, MailedFIT was the largest of all interventions, and the other activities only targeted small cohorts to increase CRC screening uptake. Therefore, most of the FIT kits returned were facilitated through the MailedFIT program.
TABLE 2.
Interventions and Process Measures by Site
| Variable | Site 1 | Site 2 | Site 3 | Site 4 | Site 5 | Site 6 | Site 7 | Site 8 | Site 9 | Overall |
|---|---|---|---|---|---|---|---|---|---|---|
| Interventions | ||||||||||
| Main intervention | ||||||||||
| Mailed FIT (July 2015 to June 30, 2016) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 9 |
| Other interventions implemented alongside mailed FIT | ||||||||||
| 50th Birthday cards | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 9 |
| FluFIT and MammoFIT | ✓ | ✓ | ✓ | ✓ | 4 | |||||
| Existing interventions and initiatives | ||||||||||
| Provider reminder | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 9 |
| Provider assessment and feedback | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 9 |
| Process measures for mailed FIT | ||||||||||
| FIT kit mailings | ||||||||||
| No. of FIT kits mailed | 536 | 599 | 716 | 862 | 424 | 590 | 597 | 491 | 363 | 5178 |
| No. of FIT kits returned | 170 | 214 | 212 | 310 | 117 | 159 | 186 | 146 | 93 | 1607 |
| Rate of FIT kits returned, % | 31.7 | 35.7 | 29.6 | 36.0 | 27.6 | 26.9 | 31.2 | 29.7 | 25.6 | 31.0 |
| Patient reminders | ||||||||||
| No. of reminders mailed | 417 | 420 | 565 | 675 | 315 | 466 | 473 | 382 | 296 | 4009 |
| No. of automated phone calls placed | 903 | 909 | 1197 | 1413 | 663 | 985 | 953 | 822 | 609 | 8454 |
Abbreviation: FIT, fecal immunochemical test; FluFIT, flu shot clinics at which free FIT kits were distributed; MammoFIT, mammography screenings at which free FIT kits were distributed.
In total, as indicated in Table 2, 5178 FIT kits were mailed, with a range from 363 to 862 mailings across the clinic sites. In addition, 4009 reminder letters were mailed, and 8454 automated reminder telephone calls were placed to those who received the mailed FITs. In total, 1607 FIT kits were returned within 3 months of the end of the implementation period, for a return rate of 31.0%. Overall, 5.4% of patients had positive (abnormal FIT) findings, and they received a referral to undergo colonoscopy outside of HealthPoint (data not shown in Table 2). Seventy percent underwent a diagnostic colonoscopy based on information available in the patient medical record.
Across all clinics, 243 birthday cards were mailed to patients turning 50, and 30 of these individuals (12.4%) completed CRC screening within 3 months of receiving the card. During the implementation period, 5 FluFIT events and 3 MammoFIT events were held at 4 clinics. Project staff met with 35 patients to provide CRC screening education and distributed 21 FIT kits, and 12 kits were returned (57.1% rate of return).
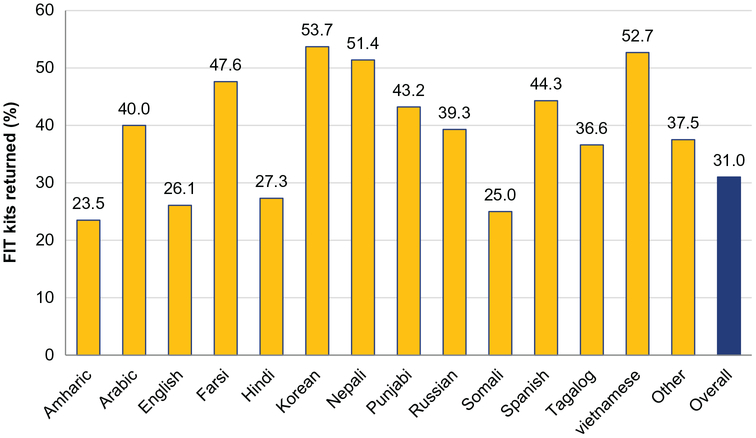
Figure 2 provides the return rate for the FIT kits stratified by language. Return rates among patients who received materials translated into Asian languages were higher (Korean, 53.7%; Nepali, 51.4%; Vietnamese, 52.7%). Return rates were lowest among patients who received African-language materials, such as Amharic (23.5%) and Somali (25.0%). The return rates also were lower among English-speaking patients at 26.1%, whereas Spanish speakers had a higher rate of 44.3%.
Figure 2.
This chart illustrates the percentage of fecal immunochemical test (FIT) kits returned by language used for the mailed FIT intervention. Note that some return rates are based on small numbers, and results may not be stable.
Table 3 presents the costs of the interventions in key categories and the cost per kit returned. The total cost of planning and implementing the MailedFIT, 50th birthday card, FluFIT, and MammoFIT interventions was $63,978 across all sites. This total included all key program components, which ranged from $5134 for activities related to data quality assessment to $30,148 for implementation. We calculated an average total intervention cost of $39.81 per FIT kit returned and an intervention implementation cost of $18.76 per kit returned. The clinical cost of the MailedFIT intervention was $6.25 to purchase a kit and $9.38 for laboratory procedures to process each returned kit.
TABLE 3.
Activity-Based Costs and Cost per Fecal Immunochemical Test Kit Returneda
| Total Costs and Cost per Kit Returned | Cost, $ | Cost per FIT Kit Returned, $ |
|---|---|---|
| Intervention development phase (create process to identify eligible patients, develop and translate materials, train staff, create processes for mailing and processing FIT kits) | 21,910 | 13.63 |
| Intervention implementation phase (select patients and mail FIT kits and 50th birthday letters, conduct FluFIT and MammoFIT sessions, track returned FIT kits, refer for diagnostic follow-up) | 30,148 | 18.76 |
| Administration and management (execute contracts, conduct oversight meetings, hire or recruit staff, generate reports on intervention progress) | 6785 | 4.22 |
| Data quality assessment (conduct initial quality assessment, including review of patient charts; provide technical assistance to improve data; monitor data quality) | 5134 | 3.19 |
| Total | 63,978 | 39.81 |
Abbreviation: FIT, fecal immunochemical test; FluFIT, flu shot clinics at which free FIT kits were distributed; MammoFIT, mammography screenings at which free FIT kits were distributed.
Note that clinical costs were $6.25 to purchase a kit and $9.38 for laboratory procedures to process each kit.
DISCUSSION
In this study, we report findings from the implementation of the MailedFIT program with supporting interventions in 9 FQHCs serving primarily low-income, medically underserved populations in Washington State. Overall, 31.0% of those who received the MailedFIT kits completed the screening, and this increase in screening uptake among those previously not up to date with screening recommendations was reported consistently across all participating clinics. A randomized study on the reach of MailedFIT kits among those seeking services at FQHCs reported a screening uptake of 32.7%, which is similar to the uptake observed in our real-world implementation study. In other studies performed in non-FQHC settings, the screening uptake of MailedFIT kits was higher, ranging from 37.0% to 48.7%.14,15
MailedFIT was the main intervention and had the most impact in increasing screening; FluFIT, MammoFIT, and 50th birthday mailings had a limited reach to smaller patient cohorts. The supplemental offering of FIT kits during flu shot clinics and mammography screening events resulted in more than one-half of individuals returning the kits and could be a cost-effective approach.16,17
Overall, nearly one-third of patients who were due for screening successfully participated in the FIT program. Despite potential facilitators, such as receiving mailings in their preferred language and wordless instructions, most of those who received the FIT kits did not complete the screenings. Prior studies have identified several potential barriers, including fear about what the test may indicate, the cost of follow-up colonoscopy, the potential risk of perforation, embarrassment of a fecal collection procedure, concerns about mailing fecal matter, and being busy or forgetful.18–21 Future efforts to improve the uptake of CRC screening in MailedFIT programs should address these concerns.
The variation we observed in the FIT kit return rate by patient language should be further evaluated. The lower return rate by English speakers compared with Spanish speakers is consistent with a prior study, which reported FIT kit return rates of 43% to 48% for Spanish speakers and 22% to 33% for English speakers.7 These rates are almost identical to the 44% and 26% return rates observed in the current study for Spanish and English speakers, respectively. The use of preferred language materials may have encouraged foreign language speakers to return FIT kits; therefore, in relative terms, the rates among English speakers (for instance) may be lower.
The cost information presented can help other health systems plan the implementation of MailedFIT programs. The total cost of the program across all clinics was less than $65,000; programs would need approximately $22,000 to plan the implementation process and develop the required package of materials for the MailedFIT program. The total cost per FIT kit returned, including planning costs, was less than $40, and this represents the additional cost per person screened. When only implementation costs are considered, the cost per additional person screened decreased to approximately $19. A study implemented in the Iowa City Veterans Affairs Health Care System to increase FIT screenings reported a cost per FIT kit returned of $27 or $45, depending on the intensity of the mail and telephone interventions.22 In another study that conducted simulations based on prior randomized trials, the cost per completed FIT ranged from $45 to $74.23 By comparison, the costs presented in the current study offer a similar or better value for the resources expended. In addition, we would expect the implementation planning costs to decrease substantially in the future, because there is often a steep learning curve associated with the development of any intervention. However, the actual cost borne in replicating the interventions presented in our study would depend on the infrastructure already in place, pre-exiting materials and instructions for mailings, and the cost of living, which would impact staff salaries and other nonlabor costs.
The current findings can help guide planning and implementation of MailedFIT programs to increase screening uptake among individuals seeking care at FQHCs. Several design and methodological limitations need to be acknowledged. First, the current results indicate that the MailedFIT program successfully reached one-third of those who were not up to date with CRC screenings, but external factors not known to the program team could have affected the return rates. Second, we were unable to verify with accuracy the number of those with positive FITs who completed follow-up colonoscopies. HealthPoint refers patients to external providers to undergo diagnostic colonoscopies and does not actively track the completion of these procedures. HealthPoint clinics are working on procedures to improve the tracking of colonoscopy referrals. In addition, we did not capture health outcomes or costs beyond the screening episode. Third, all cost data were collected retrospectively, so the cost estimates and activity-based cost assignments could be subject to recall bias. The intervention implementation was mostly conducted by HealthPoint, and we are confident that these costs have been accurately captured. Fourth, although we attempted to include a comprehensive list of activities in the intervention planning, we may not have captured all costs involved. There may be additional costs involved with ensuring follow-up colonoscopy completion because of the generally low rates of completion among those seeking care at FQHCs,24,25 which were not captured in the current study. The objective of this study was to report intervention costs; therefore, we do not report the cost of follow-up colonoscopies. Fifth, HealthPoint clinics provide care to migrant, low-income, and/or underserved populations, and the patient cohort at a given clinic may fluctuate. Patients who received mailed FIT kits may not have stayed with HealthPoint during the entire implementation and follow-up periods, thus potentially impacting the true return rates. HealthPoint did not track the characteristics of patients who returned FIT kits, except for language preference, and additional details could have provided more information on patient characteristics that were associated with FIT screenings.
The cost per returned kit and the variation in kits returned by preferred languages have implications for program improvements. HealthPoint is currently evaluating the sustainability of the program, and the cost assessment performed in the current study is an important component of their decision-making process. Although the cost per kit is likely to decrease for this health system, because as startup costs will not be needed, the costs related to implementation still must be considered. The WASDOH is reporting the experiences of all their partners, including HealthPoint, as a part of the Centers for Disease Control and Prevention’s Colorectal Cancer Control Program to develop and implement cost-effective EBIs across health systems.
Acknowledgments
FUNDING SUPPORT
Support for this study was provided by the Centers for Disease Control and Prevention to RTI International (contract 200-2014-61263, Task 4). The provision of data by grantees was supported through funding under a cooperative agreement with the Centers for Disease Control and Prevention.
Washington State Department of Health acknowledges the following individuals for their contributions to this research: Thuy Vu (Alliance for Reducing Cancer, Northwest, Health Promotion, Research Center, University of Washington); Gloria Coronado, PhD (Epidemiologist, Senior Investigator, and “Mitch Greenlick Fellow,” Center for Health Research at Kaiser Permanente in Oregon); and Jodi Olson (Seattle King County Public Health; and Ashley Grant (Washington Association for Community and Migrant Health Centers).
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
REFERENCES
- 1.US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC et al. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2016;315:2564–2575. [DOI] [PubMed] [Google Scholar]
- 2.Washington State Department of Health. Washington State Cancer Registry. Cancer by Site. Tumwater, WA: Washington State Department of Health; 2014. Available at: https://fortress.wa.gov/doh/wscr/WSCR/PDF/14Report/CancerBySite14.pdf. Accessed February 2, 2018. [Google Scholar]
- 3.Washington State Department of Health. Health of Washington State: Colorectal Cancer. Tumwater, WA: Washington State Department of Health; 2013. Available at: https://www.doh.wa.gov/Portals/1/Documents/1500/CD-CCN2013.pdf. Accessed March 31, 2015. [Google Scholar]
- 4.US Department of Health and Human Services, Health Resources and Services Administration. 2016. Health Center Data: Washington Program Grantee Data. Rockville, MD: US Department of Health and Human Services; Available at: https://bphc.hrsa.gov/uds/data-center.aspx?q=d&year=2016&state=WA#glist. Accessed October 3, 2017. [Google Scholar]
- 5.White A, Thompson TD, White MC, et al. Cancer screening test use—United States, 2015. MMWR Morb Mortal Wkly Rep. 2017;66:201–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singal AG, Gupta S, Skinner CS, et al. Effect of colonoscopy outreach vs fecal immunochemical test outreach on colorectal cancer screening completion: a randomized clinical trial. JAMA. 2017;318:806–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coronado GD, Vollmer WM, Petrik A, et al. Strategies and opportunities to STOP colon cancer in priority populations: pragmatic pilot study design and outcomes [serial online]. BMC Cancer. 2014;14:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coronado GD, Sanchez J, Petrik A, Kapka T, DeVoe J, Green B. Advantages of wordless instructions on how to complete a fecal immunochemical test: lessons from patient advisory council members of a federally qualified health center. J Cancer Educ. 2014;29:86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Colorectal Cancer Roundtable, American Cancer Society. 80% by 2018 Communications Guidebook: Recommended Messaging to Reach the Unscreened. Atlanta, GA: National Colorectal Cancer Roundtable, American Cancer Society; 2017. Available at: https://nccrt.org/resource/2017-80-2018-communications-guidebook-recommended-messaging-reach-unscreened. Accessed April 10, 2018. [Google Scholar]
- 10.Subramanian S, Ekwueme DU, Gardner JG, Trogdon J. Developing and testing a cost-assessment tool for cancer screening programs. Am J Prev Med. 2009;37:242–247. [DOI] [PubMed] [Google Scholar]
- 11.Subramanian S, Tangka FK, Hoover S, et al. Costs of planning and implementing the CDC’s Colorectal Cancer Screening Demonstration Program. Cancer. 2013;119(suppl 15):2855–2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tangka FK, Subramanian S, Bapat B, et al. Cost of starting colorectal cancer screening programs: results from 5 federally funded demonstration programs [serial online]. Prev Chronic Dis. 2008;5:A47. [PMC free article] [PubMed] [Google Scholar]
- 13.Tangka FK, Subramanian S, Hoover S, et al. Costs of promoting cancer screening: evidence from CDC’s Colorectal Cancer Control Program (CRCCP). Eval Program Plann. 2017;62:67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daskalakis C, Vernon SW, Sifri R, et al. The effects of test preference, test access, and navigation on colorectal cancer screening. Cancer Epidemiol Biomarkers Prev. 2014;23:1521–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levy BT, Daly JM, Xu Y, Ely JW. Mailed fecal immunochemical tests plus educational materials to improve colon cancer screening rates in Iowa Research Network (IRENE) practices. Am Board Fam Med. 2012;25:73–82. [DOI] [PubMed] [Google Scholar]
- 16.Potter MB, Phengrasamy L, Hudes ES, McPhee SJ, Walsh JM. Offering annual fecal occult blood tests at annual flu shot clinics increases colorectal cancer screening rates. Ann Fam Med. 2009;7:17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hillyer GC, Basch CE, Schmitt KM, Neugut AI. Feasibility and efficacy of pairing fecal immunochemical testing with mammography for increasing colorectal cancer screening among uninsured Latinas in northern Manhattan. Prev Med. 2011;53:194–198. [DOI] [PubMed] [Google Scholar]
- 18.Kimura A, Sin MK, Spigner C, Tran A, Tu SP. Barriers and facilitators to colorectal cancer screening in Vietnamese Americans: a qualitative analysis. J Cancer Educ. 2014;29:728–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arnold CL, Rademaker A, Bailey SC, et al. Literacy barriers to colorectal cancer screening in community clinics. J Health Commun. 2012;17(suppl 3):252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coronado GD, Schneider JL, Sanchez JJ, Petrik AF, Green B. Reasons for non-response to a direct-mailed FIT kit program: lessons learned from a pragmatic colorectal-cancer screening study in a federally sponsored health center. Transl Behav Med. 2015;5:60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones RM, Devers KJ, Kuzel AJ, Woolf SH. Patient-reported barriers to colorectal cancer screening: a mixed-methods analysis. Am J Prev Med. 2010;38:508–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schlichting JA, Mengeling MA, Makki NM, et al. Increasing colorectal cancer screening in an overdue population: participation and cost impacts of adding telephone calls to a FIT mailing program. J Community Health. 2014;39:239–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liss DT, French DD, Buchanan DR, et al. Outreach for annual colorectal cancer screening: a budget impact analysis for community health centers. Am J Prev Med. 2016;50:e54–e61. [DOI] [PubMed] [Google Scholar]
- 24.Oluloro A, Petrik AF, Turner A, et al. Timeliness of colonoscopy after abnormal fecal test results in a safety net practice. J Community Health. 2016;41:864–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin J, Halm EA, Tiro JA, et al. Reasons for lack of diagnostic colonoscopy after positive result on fecal immunochemical test in a safety-net health system. Am J Med. 2017;130:93.e1–93.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]