Abstract
Background:
Phthalates and bisphenol A (BPA) are environmental contaminants that may affect early embryonic development.
Objective:
To assess the association between phthalate metabolites and BPA with early pregnancy endpoints in a cohort of women followed from before conception.
Methods:
We quantified 11 phthalate metabolites and BPA in 137 conception cycles from naturally conceived clinical pregnancies. Phthalate metabolites and BPA concentrations were measured in a pooled sample of three daily morning urine specimens. Daily urinary hormone measurements had previously been used to define ovulation, implantation, and corpus luteum rescue. We assessed associations between conception cycle exposures (phthalate biomarkers and BPA) and 1) time from ovulation to implantation; 2) type of corpus luteum rescue (timing and pattern of rise in progesterone: early, late, or no rise); and 3) rate of initial rise in hCG.
Results:
Mono(3-carboxypropyl) phthalate (MCPP) and mono-isobutyl phthalate (MiBP) were associated with earlier implantation (6–8 days vs. 9 days (the most commonly observed); per natural log-unit, OR (95% CI) = 2.8 (1.2, 6.7) and OR (CI) = 2.1 (1.2, 3.7), respectively). Monoethyl phthalate (MEP) was associated with later implantation (10–12 days vs. 9 days); OR (CI) = 1.5 (1.0, 2.1). Compared with implantation on day 9, BPA was significantly associated with both earlier and later implantation (OR=2.2 for both). Women with concentrations above the median of monobenzyl phthalate (MBzP) (p=0.04) or above the median of the molar sum of four di(2-ethylhexyl) phthalate metabolites (∑DEHP) (p=0.08) had a slower initial rise in hCG. Increasing MCPP was associated with an increased odds of a late rise rescue (OR (CI) = 2.9 (1.0, 8.5); late rise vs. early rise), while increasing MEP was associated with a no rise rescue (OR (CI) = 1.6 (0.9, 2.8); no rise vs. early rise).
Conclusions:
The reported associations varied in their direction of effect, some potentially protective, others adverse. This may reflect the complexity with which these potential endocrine disrupting chemicals can be acting, but chance findings are also possible. Given that women continue to be exposed to these compounds (or their precursors), continued research on the effects they may have on pregnancy is warranted.
Keywords: early pregnancy, phthalates, BPA
Introduction
Phthalates are used to increase flexibility of plastics and are also found in many personal care products. Human exposure to phthalates is widespread and can occur through ingestion, dermal application, and inhalation (CDC 2015). Bisphenol A (BPA) is used widely as a component of hard plastics and epoxy resins (CDC 2015). Several phthalates and BPA are suspected endocrine disrupting chemicals that could plausibly affect reproduction. These compounds have been studied in relation to fertility, most widely in animal studies and associations have been found with phthalates and reduced fertility and litter size (Davis et al. 1994; Ema and Miyawaki 2001; Gray et al. 2006). Among women undergoing in vitro fertilization, exposure to certain phthalates was associated with decreased estradiol levels and oocyte yield (Ehrlich et al. 2012). Some phthalates have the potential to influence the normal activity of hormones during pregnancy. In a study of naturally conceiving pregnant women mono-isobutyl phthalate (MiBP), monobenzyl phthalate (MBzP), and some metabolites of di(2-ethylhexyl) phthalate (DEHP) measured during early pregnancy (first trimester) were positively associated with estrogen levels measured during the same time period while monocarboxynonyl phthalate (MCNP) and a metabolite of DEHP were inversely associated with testosterone levels, also measured at the same time (Sathyanarayana et al. 2017).
Changes in reproductive hormone levels are required to maintain early pregnancy. Human chorionic gonadotropin or hCG is produced by the conceptus and rises rapidly after implantation. The abrupt rise in this hormone in early pregnancy can be used to identify the timing of implantation of the embryo (Wilcox et al. 1999). This rise in hCG prompts the rescue of the ovarian corpus luteum, another critical pregnancy event. Rescue of the corpus luteum allows for an extended period of progesterone production by the ovary until the increasing placental production becomes sufficient (Csapo and Pulkkinen 1978).
Exposure to phthalates and BPA may influence the cascade of hormonal changes in early pregnancy, which has the potential to affect the long-term health of the pregnancy. To examine these outcomes, we conducted an exploratory analysis to assess associations between urinary phthalate metabolites and BPA concentrations and the time from ovulation to implantation, pattern of hCG rise, and timing of rise in progesterone (corpus luteum rescue) in a cohort of women planning a pregnancy who were followed from before conception.
Methods
The North Carolina Early Pregnancy Study (EPS) was a prospective cohort study conducted in 1982–1986 to estimate the incidence of early pregnancy loss (Wilcox et al. 1988). Women with no known fertility problems were enrolled at the time they discontinued birth control to become pregnant (n=221). Once women were enrolled, they kept daily diaries in which they recorded sexual intercourse and menstrual bleeding. The bleeding data were used to define menstrual periods (Wilcox et al. 1988). Participants were followed for up to six months for the occurrence of a clinical pregnancy. Daily first-morning urine specimens were collected in screw top polypropylene jars without preservative throughout the study. If women achieved a clinically recognized pregnancy, they reported it to the study staff and continued collecting daily urine specimens until gestational week 8. Urine specimens were stored in participants’ freezers for up to 2 weeks before collection by study staff. Urine specimens were used to measure reproductive hormones to characterize early events of pregnancy. The current study includes women who became pregnant whose pregnancies lasted at least 6 weeks. Pregnancy losses before six weeks were excluded because of distinct irregular hormone patterns in these conceptions (Baird et al. 2003; Weinberg et al. 1992; Wilcox et al. 1999). Further, early pregnancy loss has been examined previously and little evidence of phthalate/BPA-related increase in risk was found (Jukic et al. 2016). The study protocol was approved by the Institutional Review Board of the National Institute of Environmental Health Sciences.
Exposure assessment.
After urine samples were retrieved from participants’ homes they were stored at a central location at −20 °C. After pilot testing to assess the stability of the analytes during the storage period (Baird et al. 2010; Nepomnaschy et al. 2009), concentrations of phthalate metabolites and BPA were measured in the stored urine samples from each menstrual cycle. Because of the short half-life of these compounds (Koch et al. 2004; Volkel et al. 2002) and likely episodic nature of the exposures, multiple urine specimens for each woman were pooled to provide a more stable and representative estimate of exposure (Jukic et al. 2016). In most cases Monday samples were used for pooling because the study protocol requested that women collect more urine on Mondays. For the conception cycle pool, the exposure period assessed in this study, 3 samples were selected from the conception-cycle interval starting the day after the end of menses and ending on the day before implantation. If there were not 3 Mondays during this interval or a Monday sample was missing, a sample from a nearby day was used for pooling so that each woman contributed 3 urine specimens to her pooled sample.
We quantified twelve chemical biomarkers commonly evaluated in epidemiologic studies, eleven phthalate metabolites (mono-n-butyl phthalate (MBP), monoethyl phthalate (MEP), monobenzyl phthalate (MBzP), mono-2-ethylhexyl phthalate (MEHP), mono(2-ethyl-5-oxohexyl) phthalate (MEOHP), mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), and mono(2-ethyl-5-carboxypentyl) phthalate (MECPP), monocarboxynonyl phthalate (MCNP), monocarboxyoctyl phthalate (MCOP), mono(3-carboxypropyl) phthalate (MCPP), mono-isobutyl phthalate (MiBP)), and bisphenol A (BPA), as well as creatinine in all pooled urine samples. Laboratory analysis was conducted at the Centers for Disease Control and Prevention (CDC) using online solid-phase-extraction, high performance liquid chromatography-isotope dilution tandem mass spectrometry (Silva et al. 2007; Ye et al. 2005). All measures were standardized by the creatinine concentration measured in the pooled specimen ([biomarker concentration/creatinine concentration] × 100) and none were below the limit of detection. Concentrations of phthalate metabolites in our cohort (1982–86) were higher overall compared with women aged 20–44 years in the 2009–2010 cycle of NHANES, but BPA levels were similar across the time periods (Jukic et al. 2016) and Supplemental Table 1. The involvement of the CDC laboratory did not constitute engagement in human subjects research.
Outcome measures.
At the time of the original study (1982–1986), daily urine specimens were analyzed for reproductive hormones and these measures were used to identify the day of ovulation, day of implantation, type of corpus luteum rescue, presence of pregnancy, and pattern of early hCG rise (Supplemental Table 2, Supplemental Figure 1). Radioimmunoassay was used to measure the major metabolites of estrogen (estrone 3-glucuronide (E1G)) and progesterone (pregnanediol 3-glucuronide (PdG)). The day of ovulation was identified by a rapid decline in the ratio of estrogen to progesterone metabolites (Baird et al. 1991), a marker of day of ovulation that has been validated against both the luteinizing hormone rise and ultrasound evidence of ovulation (Ecochard et al. 2001).
Human chorionic gonadotropin (hCG) was measured using a highly sensitive immunoradiometric assay with a polyclonal antibody (Wilcox et al. 1985). Urinary hCG levels were not adjusted for creatinine, because the magnitude of rise in hCG was considerably greater than the variations in creatinine level, making adjustment nugatory (McChesney et al. 2005). A sustained rise in hCG was used to identify pregnancy and the day of implantation of the conceptus. An hCG level ≥0.025 ng/ml for 3 consecutive days identified pregnancies (Wilcox et al. 1988). Among identified pregnancies, the first day of sustained rise ≥0.015 ng/ml was assigned as the day of implantation (Wilcox et al. 1999). Time from ovulation to implantation was calculated in days by subtracting the date of ovulation (identified using the estrogen to progesterone metabolite ratio) from the date of implantation (first day of sustained rise in hCG among identified pregnancies). hCG rate of rise was based on the level of hCG on the day of implantation and levels on the following 6 days.
Rescue of the corpus luteum is essential to maintaining early pregnancy and was identified by a pronounced rise in progesterone above preimplantation levels (Baird et al. 2003) in the subsample of the clinical pregnancies that had luteal progesterone measurements. PdG concentrations on luteal days 5–7 were averaged to represent the preimplantation levels. The day of rescue was defined as the first 2-day sequence in which values for the progesterone metabolite were at least 31% higher than the preimplantation value (i.e., twice the median coefficient of variation for the preimplantation levels). The algorithm was applied to 2-day sequences starting on the day of implantation (Baird et al. 2003). This late-luteal rise in PdG is quite specific to pregnancy. The estimated false positive rate was 8% based on its application to 239 non-conception cycles (Baird et al. 2003). Type of corpus luteum rescue was then characterized by the pattern and timing of the resulting PdG rise. The three categories in our sample were: 1) the expected early rescue (PdG rise within 2 days after implantation), 2) late rescue (PdG rise from 3–6 days after implantation), and 3) rescue with no rise (no criteria rise during the first week of hCG rise).
Analysis.
Analyses were performed using SAS version 9.4 (SAS Inc., Cary, NC). Sample sizes varied among the different outcomes (Supplemental Figures 2, 3, and 5). Time from ovulation to implantation required hormone data to identify both day of ovulation and day of implantation. Rate of hCG rise required sufficient data on hCG concentrations to identify the day of implantation, but hCG could be missing on one of the following 6 days, though this was uncommon (3% missing overall). Type of corpus luteum rescue required implantation day and luteal urinary progesterone data. For all outcomes, we excluded one woman missing phthalate metabolite and BPA measurements and four women exposed to diethylstilbestrol (DES) in utero because DES had been examined previously and was found to be associated with irregular hormonal patterns in early pregnancy (Jukic et al. 2011).
Each chemical biomarker was assessed in a separate model for all outcomes. For the DEHP metabolites quantified (MEHP, MEHHP, MEOHP, MECPP), we also assessed associations between their sum, using the molar sum concentration (∑DEHP in nmol/mL = [MEHP × (1/278.34)] + [MEHHP × (1/294.34)] + [MEOHP × (1/292.33)] + [MECPP × (1/308.33)]) and each outcome of interest. Little data exist on these early pregnancy endpoints in a cohort of naturally conceiving women, limiting our ability to determine potential confounders from prior literature. Hence, potential covariates for analysis were identified from the participant characteristics collected in the study that were considered associated with the exposures and outcomes, exclusive of hypothesized mediators, based on prior analyses of these outcomes in this cohort (Jukic et al. 2011). We considered maternal age, smoking status, and body mass index (BMI) as covariates in models for all outcomes.
Time to implantation.
The time from ovulation to implantation ranged between 6 and 12 days. This period was categorized into 6–8, 9, and 10–12 days for the analysis. These categories were used as a polytomous outcome in logistic regression models to assess the association with each of the phthalate metabolites and BPA, which were natural log-transformed to reduce the influence of extreme values, and included in the model as continuous. A continuous exposure was modeled to assess whether an increase in biomarker concentrations corresponded to a change in the odds of having a short (6–8 days) or long (10–12 days) time from ovulation to implantation, compared with the more common 9 day interval.
hCG rise.
Linear mixed models were used to assess the association between individual phthalate metabolite and BPA concentrations and rate of rise of ln(hCG). We dichotomized biomarker concentrations at the median because we were interested in investigating differences in the pattern of rise among those with high concentrations compared to low concentrations. Because the rise in hCG is best described by a quadratic function in this study (Nepomnaschy et al. 2008) the model necessarily included interaction terms between the exposure biomarker and both time and the chemical biomarker and time squared. The model also incorporated a random effect for each woman, to account for the woman-specific effect on the initial value of hCG (the intercept) and a random coefficient for time to allow for variation in the woman-specific steepness in the rise in hCG. A two-degree of freedom likelihood ratio test (to assess the joint effects of the linear and quadratic terms) was used to assess the statistical significance of the influence of each chemical biomarker on the pattern of rise.
Corpus luteum rescue.
We assessed the associations between phthalate metabolite and BPA concentrations with type of corpus luteum rescue using polytomous logistic regression. We compared the corpus luteum rescue categories of late and no rise with early rise, which Baird et al., (Baird et al. 2003) hypothesized to be the optimal rescue type for the survival of the pregnancy based on data in non-human primates (Atkinson et al. 1975). Phthalate metabolite and BPA concentrations were included as predictors as linear on the natural-log scale. As with our assessment of time from ovulation to implantation, a continuous exposure was modeled to assess if an increase in biomarker concentrations was associated with late or no rise corpus luteum rescue compared with early.
Results
The median age of the women in the study was 29 years, most were white, college educated (73%), non-smokers (94%), and over half had at least one prior pregnancy at enrollment (Table 1). We considered maternal age, BMI, and current smoking status (n=7) as potential confounders, but none were associated with biomarker concentrations and including them in the models did not appreciably change the estimates, so we present unadjusted estimates.
Table 1.
Characteristics of the participants in the Early Pregnancy Study
| Characteristic | na | % |
|---|---|---|
| Age | ||
| <29 years | 68 | 50 |
| >29 years | 69 | 50 |
| Age at menarche | ||
| <12 years | 20 | 15 |
| 12–13 years | 81 | 59 |
| >13 years | 36 | 26 |
| BMI category | ||
| <18.5 (underweight) | 14 | 10 |
| 18.5–24.9 (normal weight) | 110 | 80 |
| >25 (overweight/obese) | 13 | 9 |
| Current smoker | ||
| Yes | 7 | 6 |
| No | 120 | 94 |
| Education | ||
| Some college or less | 37 | 27 |
| College degree | 53 | 39 |
| Some graduate school or graduate degree | 47 | 34 |
| Race | ||
| White | 130 | 95 |
| Non-white | 7 | 5 |
| Prior pregnancy | ||
| None | 46 | 34 |
| 1 prior pregnancy | 47 | 34 |
| 2 or more prior pregnancies | 44 | 32 |
Total number of women represents the analytic sample for the human chorionic gonadotropin (hCG) analysis, the largest sample size across the 3 outcomes examined in this study.
Time from ovulation to implantation.
Data were available for 136 women with clinical pregnancies (Supplemental Figure 2). The range for the interval between ovulation to implantation was 6–12 days with a median (also the mode) of 9 days. The odds ratios for short (6–8 days) or long (10–12 days) time from ovulation to implantation relative to the 9-day mode are shown in Table 2. An increased odds for both short and long time to implantation was seen for BPA (OR for short time: 2.17, 95% CI: 1.05, 4.47; OR for long time: 2.23, 95% CI: 1.15, 4.34). There was an increased odds of short time to implantation for women with higher MCPP and MiBP (MCPP OR: 2.77, 95% CI: 1.15, 6.67; MiBP OR: 2.09, 95% CI: 1.18, 3.69). Higher MEP was associated with longer time to implantation (OR: 1.45, 95% CI: 0.99, 2.14).
Table 2.
Odds ratios and 95% CI for time from ovulation to implantation (polytomous outcomes: 6–8 days, 9 days, 10–12 days) associated with a log-unit increase in biomarker concentrations measured during the conception cycle, n = 136.
| Biomarkera | Ovulation to implantation 6–8 days(n=34) 9 days (n=52) 10–12 days (n=50) |
OR | 95% CI |
|---|---|---|---|
| MBP | 6–8 days | 1.75 | (0.94, 3.24) |
| 9 days | 1.00 | ||
| 10–12 days | 1.03 | (0.56, 1.90) | |
| MEP | 6–8 days | 1.37 | (0.90, 2.10) |
| 9 days | 1.00 | ||
| 10–12 days | 1.45 | (0.99, 2.14) | |
| MBzP | 6–8 days | 1.02 | (0.55, 1.87) |
| 9 days | 1.00 | ||
| 10–12 days | 0.96 | (0.55, 1.68) | |
| MEHP | 6–8 days | 1.25 | (0.73, 2.12 |
| 9 days | 1.00 | ||
| 10–12 days | 1.10 | (0.68, 1.78) | |
| MEHHP | 6–8 days | 1.18 | (0.62, 2.25) |
| 9 days | 1.00 | ||
| 10–12 days | 1.20 | (0.67, 2.14) | |
| MEOHP | 6–8 days | 1.14 | (0.60, 2.18) |
| 9 days | 1.00 | ||
| 10–12 days | 1.12 | (0.63, 2.02) | |
| MECPP | 6–8 days | 1.02 | (0.49, 2.14) |
| 9 days | 1.00 | ||
| 10–12 days | 1.17 | (0.62, 2.23) | |
| MCNP | 6–8 days | 0.95 | (0.55, 1.63) |
| 9 days | 1.00 | ||
| 10–12 days | 0.93 | (0.57, 1.51) | |
| MCOP | 6–8 days | 1.14 | (0.48, 2.72) |
| 9 days | 1.00 | ||
| 10–12 days | 1.04 | (0.47, 2.28) | |
| MCPP | 6–8 days | 2.77 | (1.15, 6.67) |
| 9 days | 1.00 | ||
| 10–12 days | 0.79 | (0.35, 1.82) | |
| MiBP | 6–8 days | 2.09 | (1.18, 3.69) |
| 9 days | 1.00 | ||
| 10–12 days | 1.56 | (0.91, 2.68) | |
| ∑DEHPb | 6–8 days | 1.11 | (0.56, 2.23) |
| 9 days | 1.00 | ||
| 10–12 days | 1.18 | (0.63, 2.18) | |
| BPA | 6–8 days | 2.17 | (1.05, 4.47) |
| 9 days | 1.00 | ||
| 10–12 days | 2.23 | (1.15, 4.34) |
Continuous log biomarker creatinine adjusted (ng/mg creatinine) concentrations from the conception cycle (period starting the day after the end of the last menstrual period and ending on the day before implantation).
∑DEHP represents the molar sum of 4 metabolites of DEHP: MEHP, MEHHP, MEOHP, and MECPP creatinine adjusted (nmol/mg creatinine).
hCG rise.
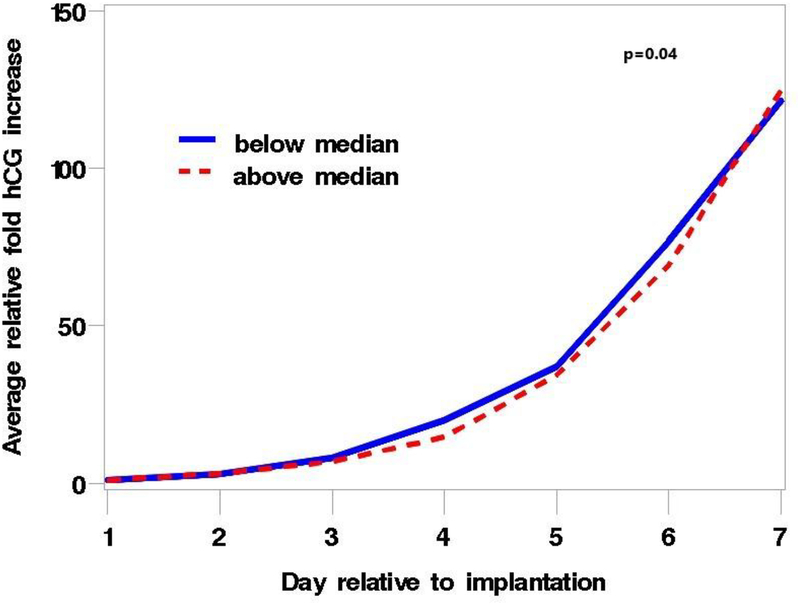
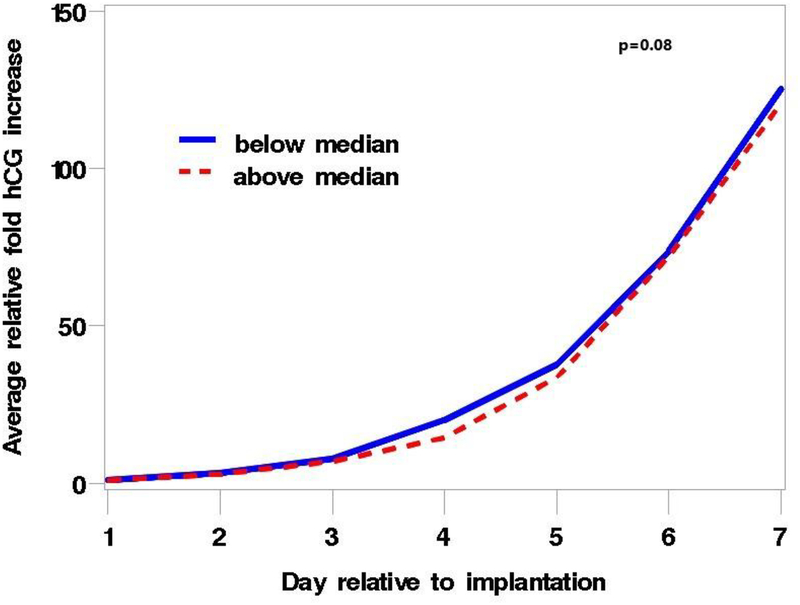
Data were available for 137 clinical pregnancies (Supplemental Figure 3). MBzP and ∑DEHP were associated with an altered pattern of rise in hCG during the first 6 days following implantation. Women with urinary MBzP concentrations above the median had a slower initial rate of rise compared with women with concentrations below the median, but this was followed by a faster rate that compensated for the initial lag by the end of the 6 days (p=0.04) (Figure 1). The pattern of rise for ∑DEHP was similar and the difference between women with high and low concentrations of ∑DEHP approached statistical significance (p=0.08) (Figure 2). The pattern of hCG rise observed for higher concentrations of MBzP and ∑DEHP is similar to that previously described in this cohort for women exposed in utero to DES who are not included in the analysis ((Jukic et al. 2011), published figure reproduced with permission in Supplemental Figure 4). The other phthalate metabolites and BPA were not associated with the hCG rise pattern (p-values all > 0.17).
Figure 1.
Plot of the average relative fold increase in hCG over the 6 days after implantation, stratified by median conception cycle MBzP concentration, p = 0.04. The blue line represents values below the median and the red line values above the median. The average relative fold increase was calculated as the average of the ratio of the hCG concentrations on each of the days 2–7 to hCG concentrations on the day of implantation (day 1) for each woman. P value represents a 2 degree of freedom likelihood ratio test to assess the statistical significance of the influence of MBzP on the rate of rise.
Figure 2.
Plot of the average relative fold increase in hCG over the 6 days after implantation, stratified by median conception cycle ∑DEHP concentration, p = 0.08. The blue line represents values below the median and the red line values above the median. The average relative fold increase was calculated as the average of the ratio of the hCG concentrations on each of the days 2–7 to hCG concentrations on the day of implantation (day 1) for each woman. P value represents a 2 degree of freedom likelihood ratio test to assess the statistical significance of the influence of ∑DEHP on the rate of rise. ∑DEHP represents the molar sum of 4 metabolites of DEHP: MEHP, MEHHP, MEOHP, and MECPP.
Corpus luteum rescue.
Data on corpus luteum rescue were available for only 74 clinical pregnancies because by design only a subsample of pregnancies had PdG measured outside of an ovulatory window (Supplemental Figure 5). The majority of pregnancies showed the expected early rise of progesterone in response to implantation (n=42, 57%). An equal number of pregnancies had a delayed rise or no rise during the 7-day observation period (n=16 in each group). Given the small sample, all associations between biomarker concentrations with corpus luteum rescue pattern were imprecise (Table 3). The strongest associations were seen for MEP, MCPP, and ∑DEHP. Compared with the early rise category, there was an increased odds of no rise with increasing MEP (OR per log unit: 1.61, 95% CI: 0.93, 2.79) and an increased odds of late rise with increasing concentrations of MCPP (OR: 2.85, 95% CI: 0.96, 8.47). There was a decreased odds of late rise with increasing ∑DEHP (OR: 0.36, 95% CI: 0.09, 1.43), as well as the 4 individually measured metabolites of DEHP (MEHP, MEHHP, MEOHP, MECPP).
Table 3.
Odds ratios and 95% CI for type of corpus luteum rescue (polytomous outcomes: early, late, no rise), associated with a log-unit increase in biomarker concentrations measured during the conception cycle, n = 74.
| Biomarkera | Type of rescue Early (n=42) Late (n=16) No rise (n=16) |
OR | 95% CI |
|---|---|---|---|
| MBP | Early | 1.00 | |
| Late | 1.02 | (0.48, 2.19) | |
| No rise | 1.27 | (0.64, 2.50) | |
| MEP | Early | 1.00 | |
| Late | 1.29 | (0.73, 2.27) | |
| No rise | 1.61 | (0.93, 2.79) | |
| MBzP | Early | 1.00 | |
| Late | 1.28 | (0.54, 3.00) | |
| No rise | 1.13 | (0.48, 2.66) | |
| MEHP | Early | 1.00 | |
| Late | 0.86 | (0.36, 2.05) | |
| No rise | 1.04 | (0.44, 2.48) | |
| MEHHP | Early | 1.00 | |
| Late | 0.41 | (0.12, 1.42) | |
| No rise | 0.93 | (0.31, 2.75) | |
| MEOHP | Early | 1.00 | |
| Late | 0.55 | (0.17, 1.75) | |
| No rise | 1.04 | (0.36, 3.02) | |
| MECPP | Early | 1.00 | |
| Late | 0.29 | (0.07, 1.26) | |
| No rise | 0.86 | (0.26, 2.91) | |
| MCNP | Early | 1.00 | |
| Late | 0.68 | (0.29, 1.59) | |
| No rise | 1.32 | (0.61, 2.87) | |
| MCOP | Early | 1.00 | |
| Late | 1.06 | (0.32, 3.52) | |
| No rise | 1.51 | (0.47, 4.90) | |
| MCPP | Early | 1.00 | |
| Late | 2.85 | (0.96, 8.47) | |
| No rise | 0.90 | (0.27, 2.94) | |
| MiBP | Early | 1.00 | |
| Late | 1.16 | (0.67, 2.02) | |
| No rise | 0.93 | (0.50, 1.73) | |
| ∑DEHPb | Early | 1.00 | |
| Late | 0.36 | (0.09, 1.43) | |
| No rise | 0.93 | (0.29, 2.97) | |
| BPA | Early | 1.00 | |
| Late | 0.77 | (0.34, 1.72) | |
| No rise | 1.53 | (0.70, 3.36) |
Continuous log biomarker creatinine adjusted (ng/mg creatinine) concentrations from the conception cycle (period starting the day after the end of the last menstrual period and ending on the day before implantation).
∑DEHP represents the molar sum of 4 metabolites of DEHP: MEHP, MEHHP, MEOHP, and MECPP creatinine adjusted (nmol/mg creatinine).
Discussion
In the current analysis of contaminant exposures, we found associations for MCPP, MEP, and ∑DEHP and the early pregnancy endpoints examined. MCPP, a non-specific metabolite of several phthalates, was associated with a borderline-significant increase in late corpus luteum rescue an outcome suggestive of poorer pregnancy health (Atkinson et al. 1975; Baird et al. 2003). MCPP was also associated with earlier implantation, the implications of which are not known in humans. MEP had borderline-significant associations with later implantation and a no rise rescue of the corpus luteum, both of which are associated with increased early pregnancy loss (Baird et al. 2003; Wilcox et al. 1999). ∑DEHP was associated with a decreased odds of late corpus luteum rescue suggestive of better pregnancy health (Baird et al. 2003), consistent with our prior finding of an association with reduced risk of early pregnancy loss (Jukic et al. 2016), but also a borderline-significant slower initial hCG rise. While the implications of a slower initial rise in hCG are not known, this pattern of rise is similar to that observed for women exposed to DES in utero in this cohort (Jukic et al. 2011), who are at elevated risk of unfavorable pregnancy outcomes (Hoover et al. 2011). Two additional metabolites were associated with single outcomes: MiBP was associated with earlier implantation and MBzP was associated with a slower initial hCG rise. None of the other phthalate metabolites were associated with any of the outcomes. BPA was associated with both earlier and later implantation. Given the prior finding of a shorter luteal phase but no increase in early loss with higher urinary concentrations of BPA (Jukic et al. 2016), one might expect more early implantations due to an earlier decline in progesterone levels required to maintain pregnancy, but an elevated risk of late implantations is difficult to explain.
Given the difficulty of studying early pregnancy events in natural conceptions, little research has been done, but the early events of placentation are widely accepted as important for pregnancy loss and preeclampsia risk (Norwitz et al. 2001; Steegers et al. 2010). Implantation within a narrow window of time is the norm in laboratory animals, and after this window the uterus becomes non-receptive to implantation (Cha et al. 2012). Our prior research in this cohort has shown that late implantation is associated with early pregnancy loss (Wilcox et al. 1999). In addition, those with a late corpus luteum rescue were more likely to be born earlier (12 day difference in median gestational length)(Jukic et al. 2013), and a slow initial rise in hCG was seen in women who had been exposed to DES in utero (Jukic et al. 2011), an exposure known to be associated with adverse reproductive and pregnancy outcomes (Hoover et al. 2011; Jukic et al. 2011; Troisi et al. 2007).
Phthalates and BPA have the potential to interfere with the hormonal changes that occur after conception and implantation (Ehrlich et al. 2012; Sathyanarayana et al. 2017), but another potential mechanism by which they may affect early pregnancy is through oxidative stress and inflammation. In data from the National Health and Nutrition Examination Survey (NHANES), investigators found certain phthalate metabolites to be associated with markers of oxidative stress and inflammation (Ferguson et al. 2012), processes that may adversely affect the maternal response to pregnancy or healthy development of the conceptus. While no other studies have examined phthalates and BPA exposure in relation to the early pregnancy endpoints assessed in our study (time from ovulation to implantation, pattern of hCG rise, and type of corpus luteum rescue), other reproductive and early pregnancy outcomes have been assessed in populations using assisted reproductive technologies (Hauser et al. 2016; Messerlian et al. 2016a; Messerlian et al. 2016b). Like in our study, associations were observed for the ∑DEHP, but unlike ours, the associations suggested adverse effects (Hauser et al. 2016; Messerlian et al. 2016a; Messerlian et al. 2016b). In a study of women using assisted reproductive technologies, authors found an association between increasing ∑DEHP, measured in two urine samples during an in vitro fertilization (IVF) cycle, and lower oocyte yield and reduced rates of clinical pregnancy and live birth (Hauser et al. 2016). Another study in the same cohort found an association between increasing ∑DEHP and decreased antral follicle count measured by transvaginal ultrasound on day 3 of the menstrual cycle and increased pregnancy loss based on decreasing β-hCG levels (Messerlian et al. 2016a; Messerlian et al. 2016b).
When BPA was examined in relation to early reproductive outcomes among women undergoing IVF, higher urinary concentrations during the IVF cycle were associated with decreased ovarian response to stimulation, as well as reduced quantity and quality of fertilized embryos (Ehrlich et al. 2012). This finding is consistent with a study of the effects of BPA in mice that showed inhibition of estradiol production and ovarian follicle growth (Peretz et al. 2011). In a more recent study, however, BPA was not associated with any of the early pregnancy outcomes assessed among a population of women undergoing in vitro fertilization (Minguez-Alarcon et al. 2015).
The women who participated in the EPS were healthy volunteers the majority of whom were white, college educated, and non-smokers, which may restrict the generalizability of our results. Further, the women who participated in the EPS did so during the late 1980s when exposure to certain phthalates and BPA might have been more prevalent than it is currently. Consumer awareness of the potentially endocrine disrupting properties of phthalates and BPA have led to a reduction in the use of these compounds in products, resulting in a decrease in exposure (Zota et al. 2014). Thus, the urinary concentrations of phthalate metabolites measured in the EPS women were higher overall compared with concentrations measured in the U.S. general population during the 1999–2000 NHANES (CDC 2015). Lastly, because of concerns regarding the potential contamination of the specimens with phthalates or BPA, we measured phthalate metabolites and not the parent compounds; for BPA, as described in detail before (Jukic et al. 2016), we ruled out systematic contamination or degradation of the urine specimen by confirming that most BPA was excreted as a conjugate. We also confirmed the stability of phthalate metabolites and BPA after long-term storage in pilot studies (Baird et al. 2010; Nepomnaschy et al. 2009).
Our study also had some limitations. The sample size was small, especially for the assessment of type of corpus luteum rescue. In addition, we performed many statistical analyses. We report the association between eleven biomarkers of phthalates and BPA with 3 outcomes (36 models fit). Given the exploratory nature of the study, we did not adjust for multiple comparisons and some of the observed associations likely occurred by chance.
Our study had several strengths. We had the ability to identify hormonally defined day of ovulation and hCG-defined day of implantation. Additionally, because we had daily urine samples from women during their conception cycle we were able to use concentrations of the chemical biomarkers in a pooled urine sample to provide a relatively stable estimate of exposure to phthalates and BPA, compounds which have rather short elimination half-lives. While more recent publications assessing the association between phthalate metabolites and pregnancy outcomes have moved away from using a single spot urine to assess exposure, those with multiple measures have focused on time points spread through pregnancy (Ferguson et al. 2014), and the use of multiple urine samples collected around conception is unique to our study. Our study adds conception-cycle specific biomarker measurements during early pregnancy to identify associations with early pregnancy endpoints that have the potential to influence the health of the fetus throughout pregnancy.
The results from this cohort of naturally conceiving women provided the opportunity to assess how exposure at the time of conception may affect critical early pregnancy hormonal changes and influence the sequalae of events that occur throughout pregnancy until birth.
Conclusions
We found potentially protective and adverse associations for certain individual urinary measures of phthalates and BPA with the early pregnancy endpoints examined. Compared with the most commonly observed 9-day implantation, BPA was associated with later implantation, an indicator of adverse health, but it was also associated with earlier implantation. MCPP was associated with later corpus luteum rescue, signifying poorer pregnancy health, as well as earlier implantation, the effects of which are unknown. MEP was associated with longer time to implantation and a no rise rescue of the corpus luteum suggesting poorer pregnancy health. Our results also suggested ∑DEHP was associated with a slower initial rise in hCG, which may be detrimental, but also with earlier corpus luteum rescue, which is associated with more favorable pregnancy outcomes. These associations merit further exploration, given that women of reproductive age continue to be exposed to phthalates and BPA.
Supplementary Material
Highlights:
Exposure to phthalates and bisphenol A may affect early pregnancy events
Changes in reproductive hormone levels are required to maintain pregnancy
Certain chemical biomarkers were associated with adverse early pregnancy endpoints
Acknowledgments:
This research was supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences, Z01ES049003–23. Greg Travlos and Ralph Wilson from the NIEHS Clinical Pathology Group in the Cellular and Molecular Biology Branch analyzed the urinary creatinine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer:
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the US Department of Health and Human Services.
Competing financial interests:
The authors declare that they have no actual or potential competing financial interests.
References:
- Atkinson LE, Hotchkiss J, Fritz GR, Surve AH, Neill JD, Knobil E. 1975. Circulating levels of steroids and chorionic gonadotropin during pregnancy in the rhesus monkey, with special attention to the rescue of the corpus luteum in early pregnancy. Biology of reproduction 12:335–345. [DOI] [PubMed] [Google Scholar]
- Baird DD, Weinberg CR, Wilcox AJ, McConnaughey DR, Musey PI. 1991. Using the ratio of urinary oestrogen and progesterone metabolites to estimate day of ovulation. Statistics in medicine 10:255–266. [DOI] [PubMed] [Google Scholar]
- Baird DD, Weinberg CR, McConnaughey DR, Wilcox AJ. 2003. Rescue of the corpus luteum in human pregnancy. Biology of reproduction 68:448–456. [DOI] [PubMed] [Google Scholar]
- Baird DD, Saldana TM, Nepomnaschy PA, Hoppin JA, Longnecker MP, Weinberg CR, et al. 2010. Within-person variability in urinary phthalate metabolite concentrations: Measurements from specimens after long-term frozen storage. Journal of exposure science & environmental epidemiology 20:169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. 2015. Fourth national report of human exposure to environmental chemicals. Atlanta, GA:Centers for Disease Control and Prevention. [Google Scholar]
- Cha J, Sun X, Dey SK. 2012. Mechanisms of implantation: Strategies for successful pregnancy. Nature Medicine 18:1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csapo AI, Pulkkinen M. 1978. Indispensability of the human corpus luteum in the maintenance of early pregnancy. Luteectomy evidence. Obstetrical & gynecological survey 33:69–81. [DOI] [PubMed] [Google Scholar]
- Davis BJ, Maronpot RR, Heindel JJ. 1994. Di-(2-ethylhexyl) phthalate suppresses estradiol and ovulation in cycling rats. Toxicology and applied pharmacology 128:216–223. [DOI] [PubMed] [Google Scholar]
- Ecochard R, Boehringer H, Rabilloud M, Marret H. 2001. Chronological aspects of ultrasonic, hormonal, and other indirect indices of ovulation. BJOG : an international journal of obstetrics and gynaecology 108:822–829. [DOI] [PubMed] [Google Scholar]
- Ehrlich S, Williams PL, Missmer SA, Flaws JA, Ye X, Calafat AM, et al. 2012. Urinary bisphenol a concentrations and early reproductive health outcomes among women undergoing ivf. Human reproduction (Oxford, England) 27:3583–3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ema M, Miyawaki E. 2001. Effects of monobutyl phthalate on reproductive function in pregnant and pseudopregnant rats. Reproductive toxicology (Elmsford, NY) 15:261–267. [DOI] [PubMed] [Google Scholar]
- Ferguson KK, Loch-Caruso R, Meeker JD. 2012. Exploration of oxidative stress and inflammatory markers in relation to urinary phthalate metabolites: Nhanes 1999–2006. Environmental science & technology 46:477–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, McElrath TF, Ko YA, Mukherjee B, Meeker JD. 2014. Variability in urinary phthalate metabolite levels across pregnancy and sensitive windows of exposure for the risk of preterm birth. Environment international 70:118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray LE Jr., Laskey J, Ostby J. 2006. Chronic di-n-butyl phthalate exposure in rats reduces fertility and alters ovarian function during pregnancy in female long evans hooded rats. Toxicological sciences : an official journal of the Society of Toxicology 93:189–195. [DOI] [PubMed] [Google Scholar]
- Hauser R, Gaskins AJ, Souter I, Smith KW, Dodge LE, Ehrlich S, et al. 2016. Urinary phthalate metabolite concentrations and reproductive outcomes among women undergoing in vitro fertilization: Results from the earth study. Environmental health perspectives 124:831–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover RN, Hyer M, Pfeiffer RM, Adam E, Bond B, Cheville AL, et al. 2011. Adverse health outcomes in women exposed in utero to diethylstilbestrol. The New England journal of medicine 365:1304–1314. [DOI] [PubMed] [Google Scholar]
- Jukic AM, Weinberg CR, Baird DD, Wilcox AJ. 2011. The association of maternal factors with delayed implantation and the initial rise of urinary human chorionic gonadotrophin. Human reproduction (Oxford, England) 26:920–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jukic AM, Baird DD, Weinberg CR, McConnaughey DR, Wilcox AJ. 2013. Length of human pregnancy and contributors to its natural variation. Human reproduction (Oxford, England) 28:2848–2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jukic AM, Calafat AM, McConnaughey DR, Longnecker MP, Hoppin JA, Weinberg CR, et al. 2016. Urinary concentrations of phthalate metabolites and bisphenol a and associations with follicular-phase length, luteal-phase length, fecundability, and early pregnancy loss. Environmental health perspectives 124:321–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch HM, Bolt HM, Angerer J. 2004. Di(2-ethylhexyl)phthalate (dehp) metabolites in human urine and serum after a single oral dose of deuterium-labelled dehp. Archives of toxicology 78:123–130. [DOI] [PubMed] [Google Scholar]
- McChesney R, Wilcox AJ, O’Connor JF, Weinberg CR, Baird DD, Schlatterer JP, et al. 2005. Intact hcg, free hcg beta subunit and hcg beta core fragment: Longitudinal patterns in urine during early pregnancy. Human reproduction (Oxford, England) 20:928–935. [DOI] [PubMed] [Google Scholar]
- Messerlian C, Souter I, Gaskins AJ, Williams PL, Ford JB, Chiu YH, et al. 2016a. Urinary phthalate metabolites and ovarian reserve among women seeking infertility care. Human reproduction (Oxford, England) 31:75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messerlian C, Wylie BJ, Minguez-Alarcon L, Williams PL, Ford JB, Souter IC, et al. 2016b. Urinary concentrations of phthalate metabolites and pregnancy loss among women conceiving with medically assisted reproduction. Epidemiology (Cambridge, Mass) 27:879–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minguez-Alarcon L, Gaskins AJ, Chiu YH, Williams PL, Ehrlich S, Chavarro JE, et al. 2015. Urinary bisphenol a concentrations and association with in vitro fertilization outcomes among women from a fertility clinic. Human reproduction (Oxford, England) 30:2120–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nepomnaschy PA, Weinberg CR, Wilcox AJ, Baird DD. 2008. Urinary hcg patterns during the week following implantation. Human reproduction (Oxford, England) 23:271–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nepomnaschy PA, Baird DD, Weinberg CR, Hoppin JA, Longnecker MP, Wilcox AJ. 2009. Within-person variability in urinary bisphenol a concentrations: Measurements from specimens after long-term frozen storage. Environmental research 109:734–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norwitz ER, Schust DJ, Fisher SJ. 2001. Implantation and the survival of early pregnancy. The New England journal of medicine 345:1400–1408. [DOI] [PubMed] [Google Scholar]
- Peretz J, Gupta RK, Singh J, Hernandez-Ochoa I, Flaws JA. 2011. Bisphenol a impairs follicle growth, inhibits steroidogenesis, and downregulates rate-limiting enzymes in the estradiol biosynthesis pathway. Toxicological sciences : an official journal of the Society of Toxicology 119:209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathyanarayana S, Butts S, Wang C, Barrett E, Nguyen R, Schwartz SM, et al. 2017. Early prenatal phthalate exposure, sex steroid hormones, and birth outcomes. The Journal of clinical endocrinology and metabolism 102:1870–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MJ, Samandar E, Preau JL Jr., Reidy JA, Needham LL, Calafat AM. 2007. Quantification of 22 phthalate metabolites in human urine. Journal of chromatography B, Analytical technologies in the biomedical and life sciences 860:106–112. [DOI] [PubMed] [Google Scholar]
- Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R. 2010. Pre-eclampsia. Lancet (London, England) 376:631–644. [DOI] [PubMed] [Google Scholar]
- Troisi R, Titus-Ernstoff L, Hyer M, Hatch EE, Robboy SJ, Strohsnitter W, et al. 2007. Preeclampsia risk in women exposed in utero to diethylstilbestrol. Obstetrics and gynecology 110:113–120. [DOI] [PubMed] [Google Scholar]
- Volkel W, Colnot T, Csanady GA, Filser JG, Dekant W. 2002. Metabolism and kinetics of bisphenol a in humans at low doses following oral administration. Chemical research in toxicology 15:1281–1287. [DOI] [PubMed] [Google Scholar]
- Weinberg CR, Hertz-Picciotto I, Baird DD, Wilcox AJ. 1992. Efficiency and bias in studies of early pregnancy loss. Epidemiology (Cambridge, Mass) 3:17–22. [DOI] [PubMed] [Google Scholar]
- Wilcox AJ, Weinberg CR, Wehmann RE, Armstrong EG, Canfield RE, Nisula BC. 1985. Measuring early pregnancy loss: Laboratory and field methods. Fertility and sterility 44:366–374. [PubMed] [Google Scholar]
- Wilcox AJ, Weinberg CR, O’Connor JF, Baird DD, Schlatterer JP, Canfield RE, et al. 1988. Incidence of early loss of pregnancy. The New England journal of medicine 319:189–194. [DOI] [PubMed] [Google Scholar]
- Wilcox AJ, Baird DD, Weinberg CR. 1999. Time of implantation of the conceptus and loss of pregnancy. The New England journal of medicine 340:1796–1799. [DOI] [PubMed] [Google Scholar]
- Ye X, Kuklenyik Z, Needham LL, Calafat AM. 2005. Automated on-line column-switching hplc-ms/ms method with peak focusing for the determination of nine environmental phenols in urine. Analytical chemistry 77:5407–5413. [DOI] [PubMed] [Google Scholar]
- Zota AR, Calafat AM, Woodruff TJ. 2014. Temporal trends in phthalate exposures: Findings from the national health and nutrition examination survey, 2001–2010. Environmental health perspectives 122:235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.