Abstract
The roots of Salvia miltiorrhiza (“Danshen”) have been used in Chinese herbal medicine for centuries for a host of different conditions. While the exact nature of the active components of this material are unknown, large amounts of tanshinones are present in extracts derived from these samples. Recently, the tanshinones have been demonstrated to be potent human carboxylesterase (CE) inhibitors, with the ability to modulate the biological activity of esterified drugs. During the course of these studies, we also identified more active, irreversible inhibitors of these enzymes. We have purified, identified and synthesized these molecules, and confirmed them to be the anhydride derivatives of the tanshinones. These compounds are exceptionally potent inhibitors (Ki <1nM) and can inactivate human CEs both in vitro and in cell culture systems and can modulate the metabolism of the esterified drug oseltamivir. Therefore, the co-administration of Danshen extracts with drugs that contain the ester chemotype should be minimized since, not only is transient inhibition of CEs observed with the tanshinones, but also prolonged irreversible inhibition arises via interaction with the anhydrides.
Graphical Abstract

INTRODUCTION
Numerous clinically used agents are esterified, in part because the ester chemotype significantly improves the water solubility and bioavailability of these compounds. However, this modification increases the likelihood that such molecules will be substrates for carboxylesterase (CE). To date, no endogenous substrates have been identified for CEs, and since they are primarily expressed in the epithelia of organs that are likely to be exposed to xenobiotics (e.g., liver, lung, kidney, gut), it is thought that these enzymes represent a first line defense for the detoxification of such compounds.1–4 This hydrolysis reaction can result in the activation of prodrugs (e.g., the anticancer agent irinotecan5–7 or the antiviral neuraminidase inhibitor oseltamivir8), or the inactivation of parent molecules (e.g., methylphenidate, used to treat attention deficit disorder and attention deficit hyperactivity disorder9, and the opioid pain reliever meperidine10). We have previously demonstrated that the inhibition of CEs results in the modulation of irinotecan hydrolysis, and as a consequence, a reduction in its cytotoxic activity.11,12 Clearly therefore, any agents that alter CE activity will impact clinical use of these compounds, and may, unwittingly, result in reduced biological activity.
Using a defined pharmacophore for human CE inhibition based upon the prototypical compound benzil and a series of other small molecules containing the ethane-1,2-dione scaffold, we identified the tanshinones as potent inhibitors of these enzymes.13 Tanshinones are present in a variety of Salvia, and one such species Salvia miltiorrhiza (“Danshen”; Figure 1A), and the red roots obtained from this plant (Figure 1B), have been used in traditional Chinese medicine for decades. It is claimed that extracts of the root material of Danshen have antitumor, anti-inflammatory, anti-microbial and cytoprotective activities14, and as a consequence, this medicine is extensively used in the Asian community. Recently, a variety of clinical trials have been initiated in the US using formulations containing Danshen (Danshen Dripping Pills, Danshen Decoction, Danshen Gegen capsules, etc) for a host of maladies including coronary heart disease, dysmenorrhea, pulmonary hypertension, and ischemic stroke15. The actual active molecule(s) in these preparations is unknown, but due to the abundance of the tanshinones in these samples, it is thought that these compounds are the major contributors to the biological activity of Danshen.14 We undertook chromatography of Danshen extracts and confirmed that several abietane diterpenoids present within these samples could modulate CE activity. This included the related compounds tanshinone I, tanshinone IIA, dihydrotanshinone, cryptotanshinone and miltirone. Incubation of cells expressing CEs with these molecules resulted in reduced irinotecan metabolism, and a significant reduction in the cytotoxic activity of this drug.13
Figure 1.
Salvia miltiorrhiza.
A. An image indicating the pale blue flowers of the plant (courtesy of Wikipedia, CC by 3,0)
B. The dried roots of the plant (“Danshen”).
However, during the course of these studies, we identified components of Danshen extract that were considerably more potent at CE inhibition than the tanshinones. Here we detail the chromatographic separation of these compounds, their purification to homogeneity and a determination of their chemical structure. Using this information, we have chemically synthesized these molecules, demonstrated that they can inhibit human CEs with picomolar Ki values and result in irreversible inactivation of the enzymes. Finally, we have confirmed that these agents bind within the active site of the human liver CE hCE1 (EC 3.1.1.1; CES1), and prevent the hydrolysis of oseltamivir. Since these inhibitors act in an irreversible fashion, we anticipate that their biological activity would be considerably prolonged as compared to the tanshinones. Furthermore, their presence within Danshen extracts indicate that these agents are likely consumed on a regular basis by individuals who use this Chinese herbal medicine and may therefore modulate the metabolism of any co-administered esterified agents.
RESULTS AND DISCUSSION
Identification of novel carboxylesterase inhibitors in Danshen extracts
Recently, we have identified and evaluated the ability of natural products containing the ethane-1,2-dione moiety to inhibit human CEs.13,16 This has included compounds obtained from Danshen root, principally the tanshinones. However, during the course of these studies, it was apparent that there were potent CE inhibitors in extracts of this material that were more active than the previously isolated molecules. To identify these compounds in extracts of Danshen, material was chromatographed and individual fractions were assessed for their ability to inhibit hCE1 using o-nitrophenyl acetate (o-NPA) as a substrate. These fractions were also analyzed for the presence of tanshinone I (1), tanshinone IIA (3), dihydrotanshinone (5) and cryptotanshinone (7; Figure 2A), compounds that we have previously identified as human CE inhibitors.13 However as indicated in the enzyme inhibition curves, significant activity was observed in fractions that lacked these small molecules (e.g., fractions 2–7, 26–35). Further chromatography using reverse phase preparative HPLC yielded two homogenously pure compounds (A and B) with masses that were distinct from the previous natural products. A typical example is demonstrated in Figure 2B, where fractions 4–6 (containing compound A) have been subjected to preparative HPLC to isolate a pure sample of an unknown compound, with a high resolution mass of 311.1291Da (Figure 2B inset). In total, 2 novel molecules were identified using this approach, and their biological and physical parameters are shown in Table 1.
Figure 2.
Purification and identification of tanshinone anhydrides from Danshen extract.
A. Chromatographic traces of levels of tanshinone 1 (1; orange), tanshinone IIA (3; purple), dihydrotanshinone (5; red) and cryptotanshinone (7; green) in Danshen extract. The inhibition of hCE1 is indicated by the black line. Unknown hCE1 inhibitors are present in fractions: 2–7, compound A; 27–30, compound B; and 61–66.
B. Preparative HPLC (main panel), UPLC (large inset) and MS (small inset) of material isolated from fractions 4–6, compound A.
C. Deduced structures of compounds A (4) and B (6) determined from NMR, HMBC, HSQC and HRMS.
Table 1.
Biological and physical properties of unknown CE inhibitors present within Danshen extracts.
| ID | Isolated from fractions | UPLC retention time (min) | hCE1 IC50 (ng/ml) | HRMS [M + H]+ (Da) | Predicted molecular formula |
|---|---|---|---|---|---|
| A | 4–6 | 4.29 | 0.328 ± 0.056 | 311.1291 | C19H18O4 |
| B | 28–30 | 4.03 | 0.417 ± 0.15 | 313.1441 | C19H20O4 |
Identification of tanshinone anhydrides as potent inhibitors of human CEs
Having obtained chromatographically pure, novel CE inhibitors of known mass from Danshen root (compounds A and B), a variety of different NMR approaches (1H, 13C, HMBC, HSQC, − see Supporting Information) were used to identify the respective compounds. This information, coupled with the predicted molecular formulas allowed us to assign the following structures to the unknown molecules (see Figure 2C; Table 2): A − tanshinone IIA anhydride (4); and B − cryptotanshinone anhydride (8). Subsequent to the identification of tanshinone IIA anhydride and cryptotanshinone anhydride, our NMR data for these isolated compounds was found to be in complete agreement with previous reports of the structures of these molecules.17
Table 2.
Structures and CE inhibition data for tanshinones and their respective synthetic anhydrides.
| ID | Compound | Structure | hCE1 (o-NPA) | hiCE (o-NPA) | IC50 (nM) ± SE | |||
|---|---|---|---|---|---|---|---|---|
| Ki ± SE (nM) | Fold increase in potencya | Ki ± SE (nM) | Fold increase in potencya | hAChE [% Inhibition at 10μM] |
hBChE [% Inhibition at 10μM] |
|||
| 1b | Tanshinone I |  |
26,250 ± 1,730 | NAc | 14,550 ± 1,560 | NA | >10,000 [10] |
>10,000 [6] |
| 2 | Tanshinone I anhydride |  |
2.4 ± 0.49 | 10,937 | 0.82 ± 0.16 | 17,744 | >10,000 [47] |
>10,000 [32] |
| 3b | Tanshinone IIA |  |
6,890 ± 424 | NA | 2,450 ± 369 | NA | >10,000 [4] |
>10,000 [1] |
| 4 | Tanshinone IIA anhydride |  |
1.9 ± 0.41 | 3,626 | 1.4 ± 0.49 | 1,750 | 3,312 ± 715 [65] |
7,883 ± 2,237 [38] |
| 5b | Dihydro-tanshinone |  |
398 ± 66 | NA | 118 ± 21 | NA | 1,950 ±381 [84] |
>10,000 [5] |
| 6 | Dihydro-tanshinone anhydride |  |
3.6 ± 0.79 | 111 | 0.61 ± 0.14 | 193 | 10,132 ± 3,358 [66] |
7,823 ± 3,866 [61] |
| 7b | Crypto-tanshinone |  |
544 ± 64 | NA | 141 ± 23 | NA | 6,410 ± 1,160 [44] |
>10,000 [1] |
| 8 | Crypto-tanshinone anhydride |  |
2.8 ± 0.46 | 194 | 0.75 ± 0.16 | 188 | 3,213 ± 711 [68] |
2,844 ± 694 [69] |
– Increase in potency is compared to the parent tanshinone.
– The Ki values for 1, 3, 5 and 7 have been previously reported13, but are included here for comparative purposes..
– NA - Not applicable
Synthesis and validation of tanshinone anhydrides
Having identified tanshinone anhydrides in Danshen root extracts, we synthesized these molecules to provide independent confirmation of their biological activity. Attempts to reproduce the recently reported methods that employed a H2/air mixture in the presence of a palladium catalyst18 were unsuccessful and resulted in very poor yields. Therefore, we adapted protocols developed by previous investigators that used a modified Baeyer-Villiger oxidation reaction to generate the anhydrides from the tanshinones.19–22 This methodology involved direct oxidation of the natural products using m-chloroperoxybenzoic acid (mCPBA; see Supplementary Information Scheme 1) and following chromatography, resulted in acceptable yields (18–21%) with sufficient material for physical, chemical and biochemical characterization. For these studies, we generated the respective anhydrides of tanshinone I (2), tanshinone IIA (4), cryptotanshinone (6) and dihydrotanshinone (8), since the parent compounds have all demonstrated inhibitory activity towards the human CEs.13 The physical parameters obtained for the synthesized molecules (2, 4, 6 and 8) were: identical with previous reports;17 consistent with their chemical structures (see Supplementary Information); and identical to the data obtained from the molecules purified from Danshen root extract. Furthermore, all synthesized analogs co-eluted with the corresponding anhydrides identified from this material under identical chromatographic conditions (see Supporting Information).
Selective inhibition of carboxylesterases by tanshinone anhydrides
To further validate that the tanshinone anhydrides were indeed CE inhibitors, we assessed the inhibition of hCE1 and the human intestinal CE, hiCE (EC 3.1.1.1; CES2), as well as acetyl- and butyrlcholinesterases, (AChE and BChE, respectively) using o-NPA, acethylthiocholine (ATCh) or butyrylthiocholine (BTCh) as substrates. As indicated in Table 2, thee anhydrides were exceptionally potent, selective inhibitors of CEs, yielding Ki values as low as 600pM. All were considerably more active than the corresponding tanshinone, in some cases providing ~18,000-fold increase in potency (e.g., compare compounds 1 and 2 with hiCE). The compounds were weakly active towards the cholinesterases (see Table 2), with 8 demonstrating the greatest activity. However, even for this molecule, the Ki for CE inhibition was ~1000-fold lower than that observed for the cholinesterases. We also noticed that complete enzyme inhibition was not observed, even at high concentrations of inhibitor, for AChE and BChE (data not shown). Therefore, we assessed levels of cholinesterase enzyme inhibition at a fixed concentration of inhibitor (10μM; Table 2). Under these conditions, the highest level of inhibition that was observed for the anhydrides was 69% for 8 with BChE. Since this concentration is far greater than would be used in in vitro studies, we conclude that the CE selectivity observed for the anhydrides is sufficient for biochemical studies. Having demonstrated exceptional potency of inhibition of both hCE1 and hiCE, we assessed the ability of both the anhydrides purified from Danshen extracts and the synthetic molecules to inhibit the CEs irreversibly. In these studies, either hCE1 or hiCE was pre-incubated with inhibitor and following extensive dilution and size exclusion filtration, the protein was assayed for CE activity. As controls for these studies, we used benzil (a known reversible CE inhibitor) and DMSO (a solvent control). As indicated in Figures 3A and 3B, compounds A and B from the extract resulted in irreversible enzyme inhibition. Studies conducted with the synthetic compounds confirmed these results with molecules 2, 4, 6 and 8 resulting in irreversible inhibition of both human CEs (Figure 3C and 3D). Incubation of the inactivated protein in buffer for extended periods of time (up to 18hr) resulted in no recovery of enzymatic activity, and hence we presumed that the stable covalent bond was formed between the protein and the inhibitor. Esterases have a catalytic triad of amino acids (Ser, His, and Glu) that are required for catalytic activity, with the initial attack occurring by the serine Oy atom acting as a nucleophile towards the carbonyl carbon atom within the ester group. We surmised therefore, that since the anhydride structure was very similar to that of an ester, the enzyme would initiate attack at one of the carbonyl carbons, but ultimately cleavage of the adjacent C−O bond could not occur. The resulting intermediate would no longer be subject to attack by a water molecule that acts as an intermediate nucleophile in the esterase catalytic cycle.
Figure 3.
Irreversible inhibition of hCE1 by purified compounds isolated from Danshen extract and tanshinone anhydrides.
A. Inhibition of hCE1 by compounds A and B;
B. Inhibition of hiCE by compounds A and B;
C. Inhibition of hCE1 by molecules 2, 4, 6 and 8;
D. Inhibition of hiCE by molecules 2, 4, 6 and 8;
In all cases, numbers above the bars indicate the levels of enzyme activity following pre-incubation with the sample, after extensive chromatography to remove the small molecule.
Detection of tanshinone anhydrides in Danshen extract
To assess the levels of the tanshinone anhydrides in acetone extracts of Danshen, we used the synthesized molecules as standards for quantitation of the compounds in root material. As indicated in Figure 4A, 4, 6 and 8 were readily identified in these samples, although 2, if present, was below the limit of detection. It should be noted that the trace indicating the inhibition of hCE1 in these samples, correlates well with the corresponding elution profiles for the tanshinone anhydrides, arguing that these molecules effect the majority of CE inhibition in Danshen root extracts. To evaluate whether the anhydrides were consistently present within this material, we determined the levels of 2, 4, 6 and 8 in 5 independent samples of Danshen. Figure 4B confirms that 4, 6 and 8 are present, at levels up to 7mg/g. While an exact calculation of the relative contribution of each molecule (including both the parent tanshinones and their anhydrides) towards CE inhibition is difficult to establish, due to the significant increase in potency of the anhydrides, we argue that, in all likelihood, these compounds are the primary active compounds in Danshen.
Figure 4.
Quantitation of levels of tanshinone anhydrides in Danshen extracts.
A. Chromatographic traces of compounds 4 (purple line), 6 (red line) and 8 (green line) in an acetone extract of Danshen root extract. The black line indicates levels of hCE1 inhibition.
B. Quantitation of compounds 2, 4, 6 and 8 in extracts obtained from 5 independent Danshen samples.
Tanshinone anhydrides form covalent complexes with hCE1 active site serine
To validate our hypothesis that a covalent interaction was formed between the small molecule and the active site serine, we undertook MS analysis of protein samples, both before and after incubation with 4. As indicated in Figure 5 and Table 3, three different masses were present in the purified hCE1 protein sample. Based upon their mass differences, we presumed that these were due to differences in the glycosylation patterns of the hCE1 species.23 Upon incubation of hCE1 with 10μM 4, an increase in mass of 308–312Da was observed in all three different enzyme species, consistent with the formation of a single adduct (Figure 5A and Table 3). As a control for these studies, we used phenylmethanesulfonyl fluoride (PMSF) that can irreversibly inactivate esterases by covalent attachment to the active site serine to yield the sulfonate derivative. As expected, this compound yielded an increase in the masses by 154–157Da (Figure 5C). To determine whether 4 reacted with the catalytic serine residue, we pre-incubated hCE1 with PMSF (100μM) followed by the anhydride (10μM), and then determined the masses of the resulting proteins. Under these conditions, the only mass change seen was 154Da, consistent with the reaction of the sulfonyl fluoride moiety with the active site serine (Figure 5B). No larger adducts were seen in these samples. Therefore, in the absence of the catalytic serine, 4 does not adduct to hCE1, confirming that the anhydride reacts with this residue within the enzyme. Consistent with the above results, incubation of hCE1 with 6, also resulted in increases in the masses of the proteins due the addition of one small molecule to each species (Table 3).
Figure 5.
Mass spectrum traces of hCE1 protein following incubation with different compounds.
Three different protein species are seen for each sample due to differences in glycosylation and the mass difference following adduction of inhibitor is indicated above each peak.
A. After incubation with 4 alone (purple line).
B. Following pre-incubation with PMSF and then 4 (cyan line);
C. After incubation with PMSF alone (green line);
D. Following incubation with DMSO (red line);
Table 3.
Masses of hCE1 determined by MS following incubation with different molecules.
| Small molecule ID | Small molecule exact mass (m/z; Da) | Predicted mass of adducted moiety (Da) | Observed mass (Da) | Observed mass of adduct (Da) |
|---|---|---|---|---|
| None | NAa | NA | 61374 61537 61698 |
NA |
| 4 | 310.12 | 311.13 | 61,686 61,845 62,008 |
312 308 309.8 |
| PMSF | 174.02 | 155.02 | 61,528 61,691 61,855 |
153.8 153.6 157.2 |
| PMSF+4 | PMSF = 174.02 4 = 311.13 |
Unknownb | 61,528 61,691 61,852 |
153.6 153.7 153.7 |
| 6 | 294.09 | 295.10 | 61662 61826 61980 |
288 289 282 |
– NA - Not applicable
– The product of the reaction of hCE1 with PMSF followed by 4 would either be ~155Da or 311Da (or potentially, 466 Da - 155+311) depending upon the reactivity of the enzyme with these small molecules.
Molecular modeling of covalent anhydride adduct
Based upon the experimental evidence of covalent attachment to the serine residue, we undertook molecular modeling studies using the PDB coordinates for hCE1 to assess the structure of the adduct bound within the enzyme active site. In these studies, ICMPro software using the covalent docking option was employed, and two different reaction schemes were proposed: Nucleophilic attack at the carbonyl carbon atom adjacent to the benzene ring or; attack at the same moiety juxtaposed to the furan ring. Following the docking/minimization procedure, only the former adduct formation was favored, yielding three different conformations. The lowest energy conformation is shown in Figure 6.
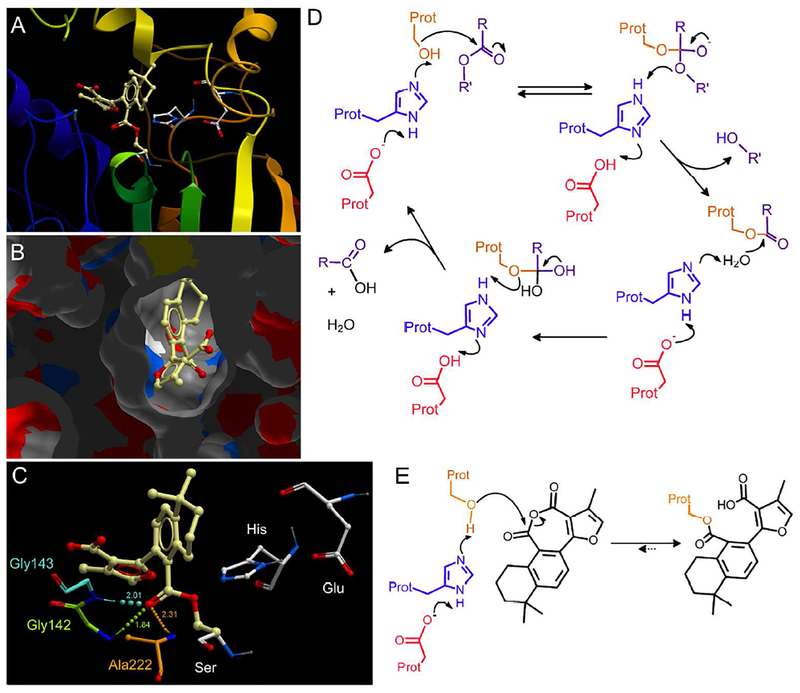
Figure 6.
Modeling of the tanshinone IIA anhydride (4)-serine adduct in hCE1. For these studies, the hCE1 1MX1 structure was used using the covalent docking module of ICMPro software.
A. Ribbon view of the enzyme with the covalent adduct bound to Ser221. The tanshinone ester is indicated in yellow with the catalytic amino acids (Ser221, His467 and Glu353) depicted in white.
B. View looking down into the active site gorge from the exterior of the protein.
C. A close up view of the tanshinone anhydride-serine adduct (yellow). Residues Gly142, Gly143 and Ala222, that form H-bonds with the carbonyl carbon atom of the ester group, are indicated in green, cyan and orange, respectively. H-bonds are indicated by the spherical dotted lines, with the lengths indicated in Ångstroms. The size of the spheres within the line represent the relative strength of the bond. For all molecules, O (red) and N (blue) atoms are displayed accordingly.
D. The esterase reaction cycle. The catalytic serine (Ser, brown), histidine (His, indigo) and glutamic acid (Glu, red) are indicated, with a generic ester depicted in purple. The water molecule that acts as an intermediate nucleophile towards the serine ester is shown in black.
E. The predicted reaction scheme and structure of the covalent adduct product (based upon the outcome of the modeling studies) following CE incubation with tanshinone IIA anhydride (4). A serine ester is generated by nucleophilic attack on the carbonyl carbon atom proximal to the benzene ring in the anhydride. The color scheme is the same as in panel D.
The predicted adduct existed as a serine ester and was readily accommodated by the hCE1 active site. This was exemplified by the view looking into the gorge (Figure 6B) from the exterior of the enzyme (the covalent linkage to the serine is occluded by the protein atoms). The stability of this particular conformation was likely due to the presence of three hydrogen bonds (~1.8 Å - 2.3Å) that are formed between the carbonyl carbon atom within the newly formed ester group, and residues within the hCE1 active site (Figure 6C). These bonds are generated from the amide protons of amino acids Gly142, Gly143 and Ala222. ICMPro software categorized the H-bonds with residues 142 and 222 as weaker (relative values 1.561 and 1.336 on a 0–2 arbitrary scale, with 2 being the strongest) than that seen for that with 143 (1.710 on the same scale). The two higher energy conformations of the modeled adduct did not demonstrate the presence of these H-bonds, and hence we hypothesize that this bonding is responsible for the observed orientation of the tanshinone serine ester (Figure 6C).
In the normal esterase reaction cycle (Figure 6D), a serine ester is formed with the ‘acid’ fragment of the substrate. This is then subjected to nucleophilic attack by an intermediate water molecule that results in the formation of a relatively unstable hydrate. This complex then collapses with the cleavage of the serine Oy-C bond resulting in the release of the carboxylic acid and the restoration of the enzyme in a state to allow further catalysis (Figure 6D). While our physical and modeling data suggests that reaction of the anhydrides with the enzyme yields a serine ester (Figure 6E), the latter apparently cannot undergo the second step of the reaction cycle with water. The reason for this is unclear, but it may be a steric issue where the water molecule cannot physically access the carbonyl carbon atom, or that it is not sufficiently activated by the histidine residue to be an effective or strong nucleophile. Current biophysical studies are underway to validate or disprove these hypotheses.
Tanshinone anhydrides modulate intracellular oseltamivir hydrolysis
To assess whether the anhydrides were cell permeable and could modulate CE activity intracellularly, we pre-incubated U373MG cells expressing hCE1 with selected inhibitors and then added oseltamivir (20μM). The levels of oseltamivir carboxylate (the product resulting from CE-mediated hydrolysis) that were formed were then determined in the culture media by UPLC/MS. As indicated in Figure 7, compounds 2, 4, 6 and 8 all reduced the levels of the active metabolite in the culture media, consistent with the hypothesis that these molecules were all able to effect intracellular inhibition of hCE1. Indeed, 6 was extremely potent, resulting in ~99% reduction in substrate hydrolysis. For molecules 2, 4 and 8, the absolute levels of oseltamivir carboxylate formed in these assays were greater than that seen for the prototypical selective CE inhibitor benzil, however for all molecules, a reduction in more than 70% of the active metabolite was observed. It should be noted however that the activity of the anhydrides in the cell culture assays was weaker than that observed for recombinant protein in vitro. This is likely due to multiple factors including: the permeability of these molecules towards the cell membrane; the binding of the anhydrides to proteins, both in the culture medium and in cells; and the saturation of potential transporters that may be used for the uptake of these compounds. Overall however, since a reduction in drug metabolism was observed in these assays, we conclude that the tanshinone anhydrides are cell permeable, can inhibit CEs intracellularly and can modulate enzyme-mediated hydrolysis of oseltamivir.
Figure 7.
Inhibition of intracellular oseltamivir hydrolysis by tanshinone anhydrides. Cells were pre-incubated with inhibitors (10μM) for 10 min and after addition of oseltamivir (20μM) for 30 min, levels of the active metabolite (oseltamivir carboxylate) were determined in the media by UPLC/MS. The percentage of metabolite formed, as compared to cells treated with DMSO, is indicated above each bar.
Conclusions
In summary, we have determined that the tanshinone anhydrides (2, 4, 6 and 8) are exceedingly potent, irreversible inhibitors of the human CEs. They demonstrate Ki values in the picomolar range, are selective for CEs over the human cholinesterases; generate adducts with the catalytic serine; and can modulate the cellular hydrolysis of the oseltamivir. While present in relatively small amounts in Danshen root, due to their very high potency, it is likely that individuals using alcoholic extracts of this material are ingesting these compounds. More importantly, since the anhydrides act in an irreversible fashion, prolonged absence of CE activity would be apparent, since new protein synthesis would be required to replace the inactivated enzyme. Furthermore, since CEs are expressed in tissues likely exposed to xenobiotics (epithelia of the gut, liver, kidney, etc), irreversible prolonged inhibition on these enzymes would result in significant modulation of the metabolism of compounds that contain the ester function. We continue to urge caution therefore, when Danshen root extracts are co-administered with esterified, clinically-used drugs.
EXPERIMENTAL SECTION
General experimental procedures
All synthetic reagents were of ACS grade or better and were used without further purification. Tanshinone I, tanshinone IIA, dihydrotanshinone, and cryptotanshinone were obtained from Bosche Scientific (New Brunswick, NJ, USA), Carbocore (The Woodlands, TX, USA), ChromaDex (Irvine, CA, USA), or LKT Laboratories (St. Paul, MN, USA). PMSF, a non-specific inactivator of serine hydrolases, was obtained from Sigma Aldrich (St. Louis, MO)
Human CEs (hCE1 and hiCE) were purified from serum-free media obtained from Spodoptera frugiperda cells infected with baculovirus engineered to express the respective secreted protein.23–25 Human glioblastoma cells (U373MG) that express these CEs have been described previously.11
Chromatography of extracts using silica cartridges and by UPLC/MS was achieved using a Biotage Isolera 4 system or a Waters Acquity instrument coupled to a Xevo G2 QToF mass spectrometer (Waters Technology Co., Milford, MA, USA), respectively. The identity of tanshinones and analogs present within these samples was determined by MS analysis, and by comparison to retention times for commercially available standards.
Salvia miltiorrhiza material
Dried roots of S. miltiorrhiza Bunge (provided by South Project Ltd., Hong Kong; lot number 6069902) were purchased from a Chinese supermarket in Memphis, Tennessee. A voucher specimen has been deposited in the Department of Chemical Biology and Therapeutics, St. Jude Children’s Research Hospital. The confirmation of identity of the sample was validated by genomic DNA sequencing (Authentechnologies, Petaluma, CA).
Extract preparation and compound isolation
Ground S. miltiorrhiza root material (10g) was extracted with 250ml acetone using a Soxhlet device in the dark. After 4 hr, the solvent was removed under reduced pressure and the solids re-dissolved in DMSO. Components were then separated using silica cartridges (Biotage SNAP Ultra HP-Sphere columns) using a hexane/ethyl acetate gradient. Following drying, samples were assessed for CE inhibition and those demonstrating activity were then subjected to further separation by preparative HLPC. The latter was accomplished using a Waters PrepLC system with a Symmetry C18 column (19 × 300 mm, 7 μm) and a water/acetonitrile gradient.
Enzyme inhibition
The inhibition of CEs was assessed using o-nitrophenyl acetate (o-NPA) as a substrate in a spectrophotometric assay. Routinely at least 8 concentrations of inhibitor were used and Ki values were determined as previously described.12,26–29
Inhibition of human acetyl- and butyrylcholinesterase (hAChE and hBChE, respectively) were undertaken as previously reported.30–32 In these assays, acetylthiocholine (ATCh) and butyrylthiocholine (BTCh) were used as substrates.
Chemical synthesis and compound characterization
General methods: Tanshinone anhydrides were synthesized by oxidation of the natural product using mCPBA.19–22 Typically, up to 100 mg of the respective tanshinone was stirred with mCPBA (1.5 eq.) and NaHCO3 (1.5 eq.) in dichloromethane and the reaction allowed to proceed for 1hr at 20C. Following quenching, the products were isolated using preparative silica and high performance chromatography. See Supporting Information for UPLC and NMR characterization of these compounds Tanshinone I anhydride (4,9-dimethylfuro[3,2-c]naphtho[2,1-e]oxepine-1,3-dione) (2): Tanshinone I anhydride was synthesized according to the general method using tanshinone I (1; 0.362 mmol) as the starting material. This reaction yielded a yellow-white solid (18.6mg, yield 18%). mp 185 −186 °C; 1H NMR (500 MHz, CDCl3) δ 8.29 (d, J = 8.9 Hz, 1H), 8.00 (d, J = 8.5 Hz, 1H), 7.97 (d, J = 8.9 Hz, 1H), 7.56 (dd, J = 8.6, 7.0 Hz, 1H), 7.47 (d, J = 6.8 Hz, 1H), 7.44 (q, J = 1.4 Hz, 1H), 2.75 (s, 3H), 2.33 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 162.02, 155.33, 154.72, 141.24, 135.03, 133.03, 130.00, 129.00, 128.75, 126.56, 123.96, 123.76, 123.38, 120.52, 114.59, 19.57, 8.99; HRMS m/z 293.0817 [M + H]+ (calcd for C18H13O4, 293.0814).
Tanshinone IIA anhydride (4,9,9-trimethyl-9,10,11,12-tetrahydrofuro[3,2-c]naphtho[2,1-e]oxepine-1,3-dione) (4): Tanshinone IIA anhydride was synthesized as described above using tanshinone IIA (3; 0.34 mmol) as the starting material. This reaction yielded a flaky, white solid (22.6mg, yield 21%). mp 155 °C; 1H NMR (500 MHz, CDCl3) δ 7.64 (ABq, J = 8.4 Hz, 2H), 7.33 (q, J = 1.3 Hz, 1H), 2.88 (t, J = 6.4 Hz, 2H), 2.27 (d, J = 1.3 Hz, 3H), 1.84 (tt, J = 3.0, 7.6 Hz, 2H), 1.71 (m, 2H), 1.33 (s, 6H). 13C NMR (126 MHz, CDCl3) δ 162.60, 155.62, 155.01, 149.72, 140.60, 135.74, 130.63, 128.61, 123.44, 122.78, 122.40, 38.16, 34.66, 31.69, 28.32, 19.02, 8.93. HRMS m/z 311.1277 [M + H]+ (calcd for C19H19O4, 311.1283).
Dihydrotanshinone anhydride (4,9-dimethyl-4,5-dihydrofuro[3,2-c]naphtho[2,1-e]oxepine-1,3-dione) (6): Dihydrotanshinone anhydride was synthesized according to the general method using dihydrotanshinone (5; 0.36 mmol) as the starting material. This yielded a white solid (19.4 mg, yield 18%). mp 152 −153 °C; 1H NMR (500 MHz, CDCl3) δ 8.26 (d, J = 9.0 Hz, 1H), 8.01 (d, J = 8.6 Hz, 1H), 7.87 (d, J = 8.9 Hz, 1H), 7.56 (dd, J = 8.6, 7.0 Hz, 1H), 7.56 (dd, J = 8.6, 7.0 Hz, 1H), 4.84 (dd, J = 10.0, 9.0 Hz, 1H), 4.29 (dd, J = 9.1, 7.3 Hz, 1H), 3.70 (dp, J = 10.1, 6.9 Hz, 1H), 2.74 (s, 3H), 1.41 (d, J = 6.7 Hz, 3H).. 13C NMR (126 MHz, DMSO) δ 162.81, 161.79, 154.78, 134.78, 132.83, 128.97, 128.75, 128.44, 127.94, 127.49, 122.67, 121.96, 120.75, 108.77, 77.53, 37.24, 18.45, 17.54.; HRMS m/z 295.0966 [M + H]+ (calcd for C18H15O4, 295.0970). Cryptotanshinone anhydride (4,9,9-trimethyl-4,5,9,10,11,12-hexahydrofuro[3,2-c]naphtho[2,1-e]oxepine-1,3-dione) (8): Cryptotanshinone anhydride was synthesized as described above using cryptotanshinone (7; 0.16 mmol) as the starting material. This reaction yielded an off-white solid (11.1mg, yield 20%). mp 172 −173 °C; 1H NMR (500 MHz, CDCl3) δ 7.59 (s, 2H), 4.75 (t, J = 9.5 Hz, 1H), 4.20 (dd, J = 9.0, 7.1 Hz, 1H), 3.61 (dp, J = 9.8, 6.8 Hz, 1H), 2.96 (ddd, J = 17.5, 8.2, 5.8 Hz, 1H), 2.78 (dt, J = 17.5, 5.8 Hz, 1H), 1.92 – 1.73 (m, 2H), 1.77 – 1.66 (m, 2H), 1.35 (d, J = 6.7 Hz, 3H), 1.32 (d, J = 10.8 Hz, 6H). 13C NMR (126 MHz, CDCl3) δ 163.80, 163.02, 156.56, 151.72, 135.41, 130.31, 130.15, 123.52, 122.22, 108.71, 78.04, 38.42, 38.11, 34.84, 31.77, 31.63, 19.01, 18.72; HRMS m/z 313.1440 [M + H]+ (calcd for C19H21O4, 313.1440).
MS analysis of carboxylesterase
MS analysis of CEs was accomplished by direct infusion of the sample into a Waters MS using an SQ Detector 2 (Waters Technology Co.). Samples were prepared in 3:2 water-acetonitrile (v/v) with 0.1% formic acid and data was accumulated for 2 minutes in ESI positive mode with a mass range of 600 to 2800 m/z using the following parameters: capillary voltage 3.29 kV; extractor voltage 3.00 V; source temperature 150 °C; desolvation temperature 450 °C; desolvation gas flow 800 L/hr; and cone gas flow 50 L/hr. The resulting spectra were then evaluated using MaxEnt software (Waters Technology Co.). For studies where proteins were incubated with inhibitors, the resulting samples were diluted and subjected to multiple rounds of size exclusion spin chromatography to eliminate residual unbound compounds. This approach was repeated until the final concentration of inhibitor present within the sample was less than 10pM.
Computer docking studies
Molecular models of the predicted adduct generated by tanshinone IIA anhydride (4) were visualized using the pdb coordinates for hCE1 (1MX1) with ICMPro software (Molsoft, LLC, San Diego, CA). Briefly, the covalent docking module was used with reaction mechanisms designed such that attack by the active site serine Oy atom occurred at either carbonyl group within the anhydride moiety. The resulting models were then minimized using the default parameters within the program. The lowest conformation model was used for all further analyses.
Intracellular inhibition of oseltamivir hydrolysis
The modulation of oseltamivir hydrolysis was accomplished by pre-incubating U373MG cells expressing hCE111 (~106 per well) with 10μM inhibitor for 10 min. At this time, oseltamivir (20μM final concentration) was added and after 30 minutes, the media was harvested and drug levels were determined using UPLC/MS. Briefly, an equal volume of acetonitrile was added to the samples and following centrifugation, the material was loaded onto a Waters Aquity Ultra performance LC (Waters Technology Co.). Following separation on an Acquity UPLC BEH C18 (2.1 × 50mm, 1.7 μm) column using a water:acetonitrile gradient, oseltamivir carboxylate levels were determined by MS using a Waters SQ Detector 2. Positive and negative controls for these assays used benzil11 and DMSO, respectively, and all data points were repeated in duplicate.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported in part by an NIH grant AT007531, a Cancer Center Core grant CA21765, and by ALSAC.
Footnotes
Publisher's Disclaimer: This document is confidential and is proprietary to the American Chemical Society and its authors. Do not copy or disclose without written permission. If you have received this item in error, notify the sender and delete all copies.
REFERENCES
- (1).Cashman J; Perroti B; Berkman C; Lin J, Pharmacokinetics and molecular detoxification. Environ. Health Perspect. 1996, 104, 23–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Potter PM; Wadkins RM, Carboxylesterases − detoxifying enzymes and targets for drug therapy. Curr. Med. Chem 2006, 13, 1045–1054. [DOI] [PubMed] [Google Scholar]
- (3).Redinbo MR; Potter PM, Mammalian Carboxylesterases: From drug targets to protein therapeutics. Drug Discov. Today 2005, 10, 313–325. [DOI] [PubMed] [Google Scholar]
- (4).Hatfield MJ; Umans RA; Hyatt JL; Edwards CC; Wierdl M; Tsurkan L; Taylor MR; Potter PM, Carboxylesterases: General detoxifying enzymes. Chem Biol Interact 2016, 259, 327–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Khanna R, Morton CL, Danks MK, and Potter PM, Proficient Metabolism of Irinotecan by a Human Intestinal Carboxylesterase. Cancer Res 2000, 60, 4725–4728. [PubMed] [Google Scholar]
- (6).Potter PM; Pawlik CA; Morton CL; Naeve CW; Danks MK, Isolation and partial characterization of a cDNA encoding a rabbit liver carboxylesterase that activates the prodrug Irinotecan (CPT-11). Cancer Res. 1998, 52, 2646–2651. [PubMed] [Google Scholar]
- (7).Tanizawa A; Fujimori A; Fujimori Y; Pommier Y, Comparison of topoisomerase I inhibition, DNA damage, and cytotoxicity of camptothecin derivatives presently in clinical trials. J. Natl. Cancer Inst. 1994, 86, 836–842. [DOI] [PubMed] [Google Scholar]
- (8).Shi D; Yang J; Yang D; LeCluyse EL; Black C; You L; Akhlaghi F; Yan B, Anti-influenza prodrug oseltamivir is activated by carboxylesterase human carboxylesterase 1, and the activation is inhibited by antiplatelet agent clopidogrel. J. Pharmacol. Exp. Ther 2006, 319, 1477–1484. [DOI] [PubMed] [Google Scholar]
- (9).Sun Z; Murry DJ; Sanghani SP; Davis WI; Kedishvili NY; Zou Q; Hurley TD; Bosron WF, Methylphenidate is stereoselectively hydrolyzed by human carboxylesterase CES1A1. J. Pharmacol. Exp. Ther 2004, 310, 469–76. [DOI] [PubMed] [Google Scholar]
- (10).Zhang J; Burnell JC; Dumaual N; Bosron WF, Binding and hydrolysis of meperidine by human liver carboxylesterase hCE-1. J. Pharmacol. Exp. Ther 1999, 290, 314–318. [PubMed] [Google Scholar]
- (11).Hyatt JL; Tsurkan L; Wierdl M; Edwards CC; Danks MK; Potter PM, Intracellular inhibition of carboxylesterases by benzil: Modulation of CPT-11 cytotoxicity. Mol. Cancer Ther. 2006, 5, 2281–2288. [DOI] [PubMed] [Google Scholar]
- (12).Wadkins RM; Hyatt JL; Yoon KJ; Morton CL; Lee RE; Damodaran K; Beroza P; Danks MK; Potter PM, Discovery of novel selective inhibitors of human intestinal carboxylesterase for the amelioration of irinotecan-induced diarrhea: synthesis, quantitative structure-activity relationship analysis, and biological activity. Mol. Pharmacol 2004, 65, 1336–1343. [DOI] [PubMed] [Google Scholar]
- (13).Hatfield MJ; Tsurkan LG; Hyatt JL; Edwards CC; Lemoff A; Jeffries C; Yan B; Potter PM, Modulation of Esterified Drug Metabolism by Tanshinones from Salvia miltiorrhiza (“Danshen”). J. Nat. Prod 2013, 76, 36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14.) Zhou L; Zuo Z; Chow MS, Danshen: an overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. J Clin Pharmacol 2005, 45, 1345–1359. [DOI] [PubMed] [Google Scholar]
- (15).Search of www.clinicaltrials.gov conducted August 2018.
- (16).Hatfield MJ; Chen J; Fratt EM; Chi L; Bollinger JC; Binder RJ; Bowling J; Hyatt JL; Scarborough J; Jeffries C; Potter PM, Selective inhibitors of human liver carboxylesterase based on a beta-lapachone scaffold: Novel reagents for reaction profiling. J Med Chem 2017, 60, 1568–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Chang HM; Cheng KP; Choang TF; Chow HF; Chui KY; Hon PM; Tan FWL; Yang Y; Zhong YP, Structure elucidation and total synthesis of new tanshinones isolated from Salvia miltiorrhiza Bunge (Danshen). J. Org. Chem 1990, 55, 3537–3543. [Google Scholar]
- (18).Zhang D-L; Zhou L-Y; Quan J-M; Zhang W; Gu L-Q; Huang Z-S; An LK, Oxygen insertion of o-quinone under catalytic hydrogenation conditions. Org Lett 2013, 15, 1162–1165. [DOI] [PubMed] [Google Scholar]
- (19).Marrero JG; Tejera LSA; Luis JG; Rodriguez ML, Unexpected oxidation of ortho-phenol groups of carnosol with m-chloroperbenzoic acid. Synlett 2002, 1517–1519. [Google Scholar]
- (20).Yang R-Y; Kizer D; Wu H; Volckova E; Miao X-S; Ali SM; Tandon M; Savage RE; Chan TCK; Ashwell MA, Synthetic methods for the preparation of ARQ 501 (b-Lapachone) human blood metabolites. Bioorg Med Chem 2008, 16, 5635–5643. [DOI] [PubMed] [Google Scholar]
- (21).Cambie RC; Grimsdale AC; Rutledge PS; Woodgate PD, Syntheses of confertifolin, winterin and isodrimenin congeners from podocarpic acid. Aus J Chem 1990, 43, 485–501. [Google Scholar]
- (22).Bendall JG; Cambie RC; Grimsdale AC; Rutledge PS; Woodgate PD, Synthesis of winterin From podocarpic acid. Aus J Chem 1992, 45, 1063–1067. [Google Scholar]
- (23).Bencharit S; Morton CL; Xue Y; Potter PM; Redinbo MR, Structural basis of heroin and cocaine metabolism by a promiscuous human drug-processing enzyme. Nat. Struct. Biol 2003, 10, 349–356. [DOI] [PubMed] [Google Scholar]
- (24).Morton CL; Potter PM, Comparison of Escherichia coli, Saccharomyces cerevisiae, Pichia pastoris, Spodoptera frugiperda and COS7 cells for recombinant gene expression: Application to a rabbit liver carboxylesterase. Mol. Biotechnol 2000, 16, 193–202. [DOI] [PubMed] [Google Scholar]
- (25).Hatfield MJ; Tsurkan L; Hyatt JL; Yu X; Edwards CC; Hicks LD; Wadkins RM; Potter PM, Biochemical and molecular analysis of carboxylesterasemediated hydrolysis of cocaine and heroin. Br. J. Pharmacol 2010, 160, 1916–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Wadkins RM; Hyatt JL; Wei X; Yoon KJ; Wierdl M; Edwards CC; Morton CL; Obenauer JC; Damodaran K; Beroza P; Danks MK; Potter PM, Identification and characterization of novel benzil (diphenylethane-1,2-dione) analogues as inhibitors of mammalian carboxylesterases. J. Med. Chem 2005, 48, 2905–2915. [DOI] [PubMed] [Google Scholar]
- (27).Webb JL, Enzyme and Metabolic Inhibitors Volume 1 General Principles of Inhibition. Academic Press Inc.: New York, 1963. [Google Scholar]
- (28).Akaike H In Information theory and an extension of the maximum likelihood principle, Second International Symposium on Information Theory, Budapest, 1973; Petrov BN; Csaki F, Eds. Akademiai Kiado: Budapest, 1973; pp 267–281. [Google Scholar]
- (29).Akaike H, A new look at the statistical model identification. IEEE Trans. Automatic Control 1974, AC-19, 716–723. [Google Scholar]
- (30).Doctor BP; Toker L; Roth E; Silman I, Microtiter assay for acetylcholinesterase. Anal. Biochem 1987, 166, 399–403. [DOI] [PubMed] [Google Scholar]
- (31).Ellman GL; Courtney KD; Anders V; Featherstone RM, A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol 1961, 7, 88–95. [DOI] [PubMed] [Google Scholar]
- (32).Morton CL; Wadkins RM; Danks MK; Potter PM, The anticancer prodrug CPT-11 is a potent inhibitor of acetylcholinesterase but is rapidly catalyzed to SN-38 by butyrylcholinesterase. Cancer Res 1999, 59, 1458–1463. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.