Abstract
Background:
Most absorbed lead ends up in the bone, where it can be measured as a biomarker of cumulative exposure, elevations of which have been shown to predict a higher risk of coronary heart disease (CHD). Knowledge about the role of dietary patterns is critical to the development of effective interventions for the cardiovascular toxicity of cumulative lead exposure.
Methods:
594 men, free of CHD at baseline, were followed from August 1991 to June 2011 in the Normative Aging Study. Bone lead concentrations were measured by K-shell-X-ray fluorescence. Dietary patterns were identified using principal components analysis. Two dietary patterns were identified: a ‘prudent’ pattern characterized by high intake of fruit, vegetables, legumes, tomatoes, poultry, and seafood; and a ‘Western’ pattern, with high intake of red meat, processed meat, refined grains, high-fat dairy products, high-energy drinks, fries, butter and eggs. Cox proportional hazard models were used to compute hazard ratios (HRs) and 95% confidence intervals (CIs) for incident CHD. Effect modification on the multiplicative scale was examined through cross-product interaction terms.
Results:
137 men developed incident CHD events during 5,071 person-years of follow-up. After adjusting for age, body mass index, total energy intake, smoking status, total cholesterol to high-density lipoprotein ratio, education and occupation, an HR of incident CHD was 1.64 (95% CI: 1.27–2.11) with each doubling in patella lead concentration in the low prudent diet group (<median prudent score); and the HR decreased to 1.07 (95% CI: 0.86–1.34) in the high prudent diet (≥median prudent score) (p-for-interaction=0.01), suggesting protective effects of prudent diet against lead-related CHD. By contrast, the association between tibia lead and CHD was non-significantly larger in the low Western diet group (HR=1.43, 95% CI: 1.14–1.80) compared with the high Western diet group (HR=1.08, 95% CI: 0.86–1.34) (p-for-interaction=0.06). No significant effect modifications were detected by Western diet in the patella lead-CHD association and by prudent diet in the tibia lead-CHD association.
Conclusions:
Prudent diet may reduce the risk of development of CHD in relation to patella lead. However, these findings need to be interpreted with caution, given the modest sample size.
Keywords: Patella lead, Tibia lead, Diet patterns, Coronary heart disease
Graphical Abstract:
A potential cardio-protective role of prudent diet, characterized by high consumption of fruits and vegetables, against toxicity of patella lead on incident coronary heart disease among middle-aged and older men in the Normative Aging Study with 20-year follow-up from 1991–2011.
©Pixabay and MindtheGraph
INTRODUCTION
Lead exposure is known to have lasting adverse effects on coronary heart disease (CHD) (Jain et al., 2007; Navas-Acien et al., 2007; Weisskopf et al., 2015, 2009). Primary prevention strategies that control or eliminate lead sources before exposure remain the preeminent public health approach to the problem of lead poisoning (e.g. phase-out of leaded gasoline and paint). Unfortunately, as a potential route of lead exposure, water has not received as much attention. It is estimated that tragedies due to lead in water have taken place in nearly 3,000 U.S. neighborhoods, with lead poisoning rates even greater than those recently seen in Flint, Michigan (Pell and Schneyer, 2016). This lead could accumulate in the osseous tissues and re-enter the circulatory system throughout a person’s lifetime.
Unlike blood lead, with a half-life of approximately 30 days, lead in the bone has a half-life ranging from years to decades. As an indicator of long-term exposure, bone lead is considered an endogenous source of lead in the human body, and has been identified as a better biomarker for examining chronic health outcomes (Hu et al., 1998a; Nie et al., 2009). Impaired renal function, systemic oxidative stress, and inflammation may serve as potential mechanisms underlying the association between bone lead and CHD (Gonick et al., 1997; Rodríguez-Iturbe et al., 2005; Willerson and Ridker, 2004).
Dietary strategies provide the possibility of effective and affordable ways to deal with side effects caused by lead. A growing body of evidence indicates that deficiencies of essential metals (such as zinc, calcium, selenium, iron) could enhance lead absorption and retention (Bridges and Zalups, 2005; Flora and Tandon, 1990; Liu et al., 2013; Nemsadze et al., 2009; Prasanthi et al., 2010); and that vitamins, including vitamins C, E, B1, and B6, could play important roles as antioxidants against lead toxicity (Al-Attar, 2011; Bakulski et al., 2014; Bratton et al., 1981; Calabrese et al., 1987; Dawson et al., 1999; Ghanwat, 2016; Goyer and Cherian, 1979; Hsu and Guo, 2002; Reddy et al., 2010; Rendón-Ramírez et al., 2014; Sasser et al., 1984; Simon and Hudes, 1999; Tandon et al., 1984).
However, studies based on single nutrients or dietary components have several limitations as they are highly interdependent and can have additive, synergistic or antagonistic effects which may not be captured when single nutrients are examined (Stradling et al., 2014). For instance, red meat consumption is correlated with higher intake of saturated fat and lower intake of vegetables and has been linked to cancer and cardiovascular disease (Bronzato and Durante, 2017; Turner and Lloyd, 2017). Vitamin C can enhance absorption of non-heme iron (Hallberg et al., 1987), which can in turn influence the lead toxicokinetics. It is, therefore, inappropriate to make public health recommendations to address lead issues from studies that solely examined single nutrients or dietary components. Dietary patterns have been widely examined during the past decade to represent the overall combination of foods and nutrients consumed and account for quality and diversity of diets (Newby and Tucker, 2004; Wirfält et al., 2013). To the best of our knowledge, there is little information currently available regarding the influence of dietary patterns on lead-related CHD.
To address this issue, we examined effect modification by dietary patterns in the association between bone lead and CHD among participants in the Normative Aging Study (NAS), a prospective cohort of community-dwelling middle-aged to elderly men. We aimed to investigate whether the derived dietary patterns alter individual susceptibility to lead toxicity. In a previous study, we found that a Western diet, characterized by high intake of processed meat, red meat, and high-fat dairy products, was associated with increased bone lead concentrations (Wang et al., 2017). The present study was designed to further explore the role of dietary patterns in susceptibility to lead toxicity and CHD risk.
METHODS
Ethics
The current study was reviewed and approved by the Institutional Review Boards of each participating Institute, the University of Michigan School of Public Health, the Harvard School of Public Health and the Department of Veterans Affairs Boston Healthcare System. All the participants provided written informed consent. Dr. Sung Kyun Park, Ning Ding and Xin Wang had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analyses.
Study Population
The NAS cohort was initiated in 1963 with the enrollment of 2280 healthy white males from Boston, Massachusetts. All were free of chronic medical conditions, including cancer, diabetes, hypertension, heart disease, recurrent asthma, peptic ulcer, gout, bronchitis, or sinusitis. Participants returned for examination every 3 to 5 years, including physical examination, laboratory tests, dietary intake questionnaire, and other factors that might influence health.
Between August 1st, 1991 and August 8th, 2002, 871 consecutive NAS participants underwent patella and tibia bone lead measurements using K-shell-X-ray fluorescence (KXRF). After excluding 226 participants with prevalent CHD at baseline (angina pectoris, myocardial infarction or CHD death), 645 men were at risk of developing CHD. In addition, we excluded 4 participants who did not return their food frequency questionnaires (FFQs), 8 with high bone lead uncertainties, and 39 with missing values for potential covariates (28 for HDL cholesterol, 1 for total cholesterol, 27 for education level, 1 for BMI and 8 for variables used in the application of inverse probability weighting method), leaving a final sample of 594 men with 2098 observations for the analyses (Figure 1).
Figure 1.
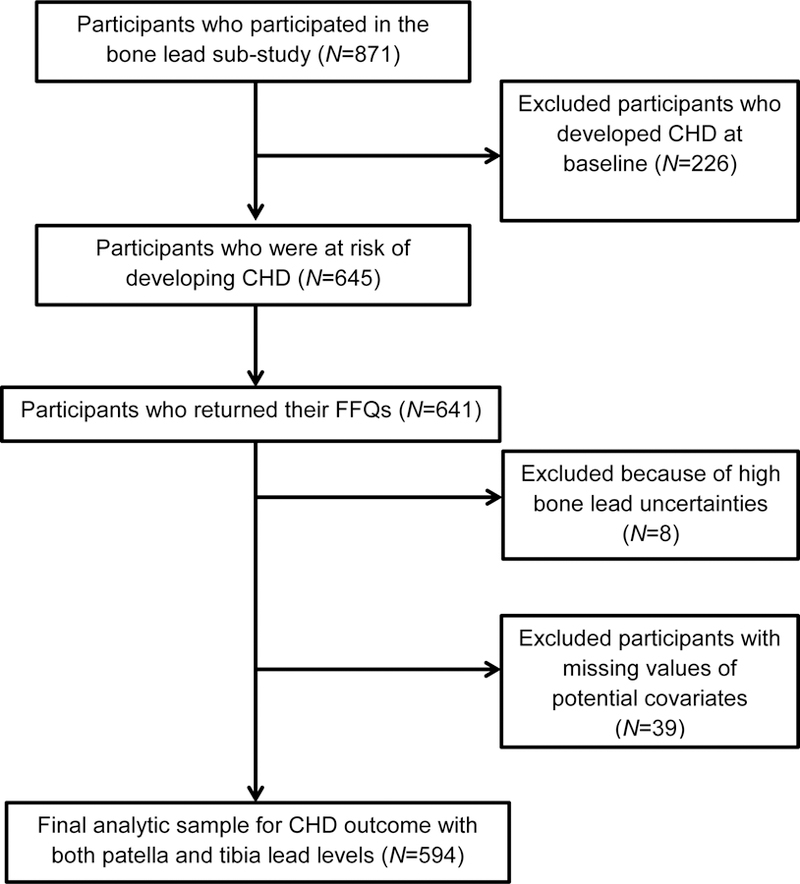
Flow diagram of the VA Normative Aging Study 1991–2011 cohort and analytic sample. CHD, coronary heart disease; FFQ, food frequency questionnaire.
Case Ascertainment
Coronary heart disease (CHD) cases include myocardial infarction, angina pectoris, and CHD deaths. Myocardial infarction was diagnosed by changes in pathological Q waves, elevation of serum enzyme levels along with chest discomfort, or autopsy. Angina pectoris was defined as recurrent chest discomforts lasting up to 15 minutes. CHD death was confirmed based on the 9th revision of International Classification of Disease (ICD-9) codes 410 to 414 and 429.2. Each participant provided their medical records at their regular visits. Every possible report of a CHD event was confirmed by a board-certified cardiologist.
Bone Lead Measurement
K-shell-X-ray fluorescence (KXRF) (ABIOMED, Inc.) was used to measure bone lead concentration for 30 min each at the patella and mid-tibia shaft, as previously described (Aro et al., 1994). Patella bone consists primarily of trabecular bone, while tibia bone consists primarily of cortical bone. Lead accumulation in cortical bone possesses a half-life of decades. In contrast, lead retained in trabecular bone mobilizes more rapidly, with a half-life of a few years. Thus, in general, tibia bone lead has been viewed as an indicator of lifetime cumulative exposure to lead, while patella bone lead has been identified as current and mobilizable lead reserves (Hu et al., 1998b). The K-XRF instrument provides an unbiased estimate of bone lead concentration (standardized by bone mineral content) with a unit of μg/g bone mineral, and an estimate of uncertainty. Participants with patella and tibia lead measurements with high uncertainty, >15 and >10 μg/g, respectively, were excluded, as these uncertainties usually reflect excessive movement during bone lead measurement.
Semi-quantitative Food Frequency Questionnaire
Dietary intake was assessed with a validated, semi-quantitative FFQ adapted from the one used in the Nurses’ Health Study (Willett et al., 1985). Each questionnaire assessed consumption of 135 food items during the past year. Starting from 1991, the FFQs were mailed on average every 4 years to NAS participants before their examination visits and checked for completeness during examinations. For the purpose of this analysis, we only used FFQs collected right before or within 1 year after bone lead measurements. Each food item intake of the FFQ was measured by checking one of 9 frequency categories, ranging from “never or less than 1 time per month” to “six or more times per day”. Food intake was converted to a total amount based on serving size per day times the daily intake frequency, and converted to nutrients by linking with a nutrient database at the Harvard School of Public Health. The reproducibility and validity of this FFQ has been reported satisfactory elsewhere (Wang et al., 2017; Willett et al., 1985). We estimated dietary intake of essential minerals and vitamins that related to lead toxicity: zinc, calcium, selenium, iron, vitamins C, B1, and B6.
Dietary Pattern Analysis
To identify dietary patterns, individual food items were first aggregated into 40 pre-defined food groups, to minimize within-person variation (Wang et al., 2017). The classification of foods or food items was based on similarities in nutrient profile and culinary preference, as previously described (Wang et al., 2017). We derived dietary patterns using principal components factor analysis with orthogonal rotation, as described elsewhere (O’Rourke and Hatcher, 2013). The number of dietary patterns (factors) was decided based on Kaiser-Guttman rule (eigenvalue>1), Scree test and interpretability.
Dietary pattern scores were calculated by summing the standardized values of food groups with weights derived from factor analysis. A positive factor loading indicates a positive association with the factor; while a negative factor loading shows a negative relationship. A higher score indicates a higher adherence to dietary pattern. We identified two dietary patterns, labeled “prudent” and “Western” patterns. The consistency and robustness of data-driven dietary patterns were examined using maximum likelihood rather than the principal components method, oblique instead of orthogonal transformation, as well as 135 food items other than 40 pre-defined food groups, according to our previous study (Wang et al., 2017). Pearson’s correlation coefficients were calculated between dietary pattern scores and single nutrient density. The analyses were conducted by using PROC FACTOR in SAS, version 9.4 (SAS Institute, Inc., Cary, North Carolina).
Covariates
Self-administered questionnaires were used to obtain demographic and socio-economic information during regular follow-up visits. Total energy intake was obtained using semi-quantitative FFQ based on food intake. Serum high-density lipoprotein (HDL) and total cholesterol concentrations were analyzed using blood samples during laboratory examinations. Body mass index (BMI) was calculated from height and weight obtained during the physical examination. Smoking status was categorized as never, former, or current cigarette smoking. Education levels were identified as less than high school, high school, some college, or undergraduate or higher degree. Occupation was expressed as white collar, or non-white collar work.
Statistical Analyses
Hazard ratios (HRs) and 95% confidence intervals (CIs) of patella and tibia bone lead were estimated by Cox proportional hazards regression to account for long-term follow up until the first CHD event. Participants contributed follow-up time from the date of KXRF bone lead measurement to the date of first CHD event or CHD death, whichever occurred first for CHD cases; or the end of study (June 2011) for non-cases. In the multivariate analyses, HRs were adjusted for age, BMI, total energy intake, smoking status, ratios of total cholesterol to HDL cholesterol, education, and occupation. Patella and tibia bone lead concentrations were log-transformed with base 2 based on the evidence of log-linear relationships (Ding et al., 2016). To facilitate interpretation of the results, HRs and 95% CIs were interpreted as effects of a two-fold increase in bone lead concentration.
In assessing potential interactions between bone lead and diet, we first analyzed prudent and Western diets, separately, in the models, for the ease of illustration. Diet scores higher than their medians were identified as high adherence, while those lower than their medians were labeled as low adherence, with cutoffs at their medians (−0.16 for prudent diet and −0.14 for Western diet). We then divided our study population into four dietary groups to examine the health effects of bone lead in the sub-populations: 1) participants with low adherence to both Western and prudent diet, 2) low adherence to prudent diet and high adherence to Western diet, 3) high adherence to prudent diet and low adherence to Western diet, and 4) high adherence to both Western and prudent diet.
For the first approach, we included a cross-product term of bone lead concentrations with binary indicator of prudent or Western dietary pattern, separately, as shown in the models below.
Where λ(t) is the hazard of CHD at time t and λ0(t) is the baseline hazard; Lead is log-transformed concentration of either patella lead or tibia lead; High prudent (or High Western) includes men with high adherence to prudent (or Western) diet, while those with low adherence to prudent (or Western) diet were identified as the reference group; Covariates include age, BMI, total energy intake, smoking status, ratios of total cholesterol to HDL cholesterol, education, and occupation.
For the second approach, four dietary groups were compared: 1) participants with low adherence to both Western and prudent diets, 2) low adherence to prudent diet and high adherence to Western diet, 3) high adherence to prudent diet and low adherence to Western diet, and 4) high adherence to both Western and prudent diets.
Main effects of dietary groups and their interaction with log-transformed bone lead concentration in our final model as shown below.
Where λ(t) is the hazard of CHD at time t and λ0(t) is the baseline hazard; Lead is log-transformed concentration of either patella lead or tibia lead; Dietary group2 includes men with low adherence to Western diet and high adherence to prudent diet. Dietary group3 includes men with high adherence to Western diet and low adherence to prudent diet. Dietary group4 includes men with high adherence to both Western and prudent diets; Covariates include age, BMI, total energy intake, smoking status, ratios of total cholesterol to HDL cholesterol, education, and occupation. More details of significance testing for effect modification used in our study can be found in appendix.
It is possible that participants’ enrollment into the KXRF bone lead sub-study were affected by their bone lead concentrations, dietary patterns or other health factors at the time of enrollment. To mitigate potential selection bias, we assigned weights to participants based on inverse probability weighting (IPW), to create a pseudo population representing the original cohort, as per Weisskopf et al (Weisskopf et al., 2015). Briefly, a single logistic regression model was constructed to predict the probability of sub-study entrance, with one record per study visit at the time of bone lead measurement for those participants in the sub-study; for those without bone lead measured, we utilized the last visit before 2002 (the last year of bone lead measurements in our study). The C statistic from this model was 0.87. All the predictors incorporated in the logistic regression model are presented in our previous studies (Ding et al., 2016; Wang et al., 2017).
To verify analytical consistency, we conducted additional analyses to evaluate multiplicative interaction through cross-product terms between bone lead concentrations and continuous dietary pattern scores in the multivariate Cox proportional hazard regression models. Moreover, we assessed correlation between nutrients and dietary pattern scores, as well as effect modification by single nutrients. All the statistical analyses were conducted by SAS, version 9.4 (SAS Institute, Inc., Cary, North Carolina). All the statistical tests were two-sided, with a type I error rate of 0.05.
RESULTS
Among 594 NAS participants with bone lead measurements and dietary history information, 137 developed CHD during 5066 person-years of follow-up, with an average of 8.52 years per participant (SD= 5.75 years). Summary statistics of the key study variables at baseline are listed in Table 1 for CHD incident cases and non-cases. The average patella bone lead concentration was 32.2 μg/g (SD=18.9 μg/g) for CHD cases, and 29.4 μg/g (SD=18.9 μg/g) for non-cases. Tibia bone lead concentration was 22.6 μg/g (SD=13.5 μg/g), on average, for CHD cases, and 20.9 μg/g (SD=13.2 μg/g) for non-cases. The mean baseline age of participants who developed CHD events was 65.5 years (SD=6.2 years), younger than non-cases with 66.5 years (SD=7.5 years) (P= 0.13). Traditional CHD risk factors, such as BMI and ratio of total to high-density lipoprotein cholesterol (HDL) in the CHD incidence group were significantly higher than in non-cases. The mean BMI was 28.7 kg/m2 in cases and 27.6 kg/m2 in non-cases (P= 0.003). The ratio of total to HDL cholesterol was 5.2 among cases and 4.9 among non-cases (P= 0.001). Moreover, CHD cases tended to be former smokers, to have lower level of education, and greater total energy intake, although these differences did not reach statistical significance.
Table 1.
Baseline characteristics (mean ± SD or %) of according to CHD status, the VA Normative Aging Study (n=594).
| Baseline characteristics | Incident CHD (n=137) | Non-CHD (n=457) |
|---|---|---|
| Age, years | 65.5 ± 6.2 | 66.5 ± 7.5 |
| Follow-up time, years | 5.7 ± 4.7 | 9.3 ± 5.9 |
| Body mass index, kg/m2 | 28.7 ± 4.0 | 27.6 ± 3.7 |
| Smoking | ||
| Never | 27.0% | 32.2% |
| Former | 66.4% | 57.8% |
| Current | 6.6% | 10.0% |
| Total cholesterol, mg/dL | 234 ± 40.0 | 226 ± 35.5 |
| High-density lipoprotein | 47.1 ± 11.7 | 49.4 ± 13.5 |
| (HDL), mg/dL | ||
| Total cholesterol/HDL ratio | 5.2 ± 1.4 | 4.9 ± 1.4 |
| Total energy intake, kCal | 2110 ± 893 | 1989 ± 676 |
| Education | ||
| <high school | 13.1% | 10.3% |
| High school | 35.0% | 34.1% |
| Some college | 24.8% | 24.5% |
| ≥4-year college | 27.0% | 31.1% |
| Occupation | ||
| White collar | 33.6% | 36.5% |
| Non-white collar | 66.4% | 63.5% |
| Patella lead, μg/g | 32.2 ± 18.9 | 29.4 ± 18.9 |
| Tibia lead, μg/g | 22.6 ± 13.5 | 20.9 ± 13.2 |
| Food intakes (servings/d) | ||
| Fruit | 1.6 ± 1.2 | 1.4 ± 1.2 |
| Legumes | 0.4 ± 0.4 | 0.4 ± 0.3 |
| Vegetables | 2.1 ± 3.1 | 1.7 ± 1.3 |
| Tomatoes | 0.6 ± 0.9 | 0.6 ± 0.5 |
| Seafood | 0.4 ± 0.3 | 0.4 ± 0.3 |
| Poultry | 0.4 ± 0.4 | 0.4 ± 0.3 |
| Condiments | 0.5 ± 0.7 | 0.5 ± 0.6 |
| Processed meat | 0.4 ± 0.5 | 0.3 ± 0.3 |
| Red meat | 0.5 ± 0.4 | 0.5 ± 0.3 |
| Refined grains | 1.6 ± 1.4 | 1.4 ± 1.1 |
| Butter | 0.3 ± 0.7 | 0.3 ± 0.7 |
| High-fat dairy products | 1.2 ± 1.6 | 1.1 ± 1.3 |
| Eggs | 0.2 ± 0.3 | 0.2 ± 0.3 |
| Fries | 0.1 ± 0.1 | 0.1 ± 0.1 |
| High-energy drinks | 0.4 ± 0.7 | 0.3 ± 0.6 |
| Dietary patterns | ||
| Prudent diet score | 0.2 ± 1.3 | −0.1 ± 0.8 |
| Western diet score | 0.1 ± 1.1 | −0.1 ± 0.8 |
| Dietary intake of selected nutrientsa | ||
| Calcium, mg | 810 ± 303 | 811 ± 315 |
| Iron, mg | 15.3 ± 6.0 | 14.8 ± 4.7 |
| Zinc, mg | 12.0 ± 3.9 | 11.5 ± 3.3 |
| Vitamin C, mg | 171 ± 81 | 162 ± 85 |
| Vitamin B1, mg | 1.7 ± 0.5 | 1.6 ± 0.4 |
| Vitamin B2, mg | 2.0 ± 0.6 | 2.0 ± 0.6 |
| Vitamin B6, mg | 2.4 ± 0.8 | 2.3 ± 0.7 |
Selected nutrients were adjusted for total energy intake.
Two major patterns were identified through principal components analysis (Table 2). The first factor was named a prudent dietary pattern, loaded heavily with fruit, legumes, whole grains, tomatoes, seafood, poultry, cruciferous vegetables, dark-yellow vegetables, leafy vegetables, and other vegetables. The second factor, labeled as a Western dietary pattern, shows high loadings for intakes of processed meat, red meat, refined grains, butter, high-fat dairy products, eggs, and fries.
Table 2.
Factor loading matrixa for the dietary patterns derived from the FFQ in the VA Normative Aging Study (n=594).
| Foods or food groups | Prudent dietary pattern | Western dietary pattern |
|---|---|---|
| Fruit | 0.55 | — |
| Legumes | 0.47 | — |
| Cruciferous vegetables | 0.70 | — |
| Dark-yellow vegetables | 0.62 | — |
| Leafy vegetables | 0.64 | — |
| Other vegetables | 0.76 | — |
| Tomatoes | 0.65 | — |
| Seafood | 0.39 | — |
| Poultry | 0.53 | — |
| Condiments | 0.40 | — |
| Processed meat | — | 0.59 |
| Red meat | — | 0.56 |
| Refined grains | — | 0.36 |
| Butter | — | 0.53 |
| High-fat dairy products | — | 0.39 |
| Eggs | — | 0.41 |
| Fries | — | 0.39 |
| High-energy drinks | — | 0.34 |
Absolute values <0.30 were not shown in the table for simplicity.
Table 3 shows the associations of bone lead with incident CHD among participants with high or low adherence to prudent or Western diet, respectively. After adjusting for age, BMI, total energy intake, smoking status, ratio of total cholesterol to HDL, education and occupation, participants with low adherence to a prudent diet had a HR of 1.64 (95% CI: 1.27–2.11) per doubling increase in patella lead concentration, whereas those with high adherence to a prudent diet had a HR of 1.07 (95% CI: 0.86–1.34) (p-for-interaction=0.01). There was no significant difference in the patella lead-incident CHD associations by Western diet (p-for-interaction=0.71). No statistically significant effect modification by dietary pattern in the association between tibia lead and incident CHD was observed although effect modification by Western diet was borderline significant (p-for-interaction=0.90 by prudent diet, p-for-interaction=0.06 by Western diet). Unlike patella lead, the association between tibia lead and CHD was larger in the low Western diet group (HR=1.43, 95% CI: 1.14–1.80) compared with the high Western diet group (HR=1.08, 95% CI: 0.86–1.34).
Table 3.
Adjusted estimatesa of CHD per 2-fold increase in bone lead concentration, stratified by Western and prudent dietary scores, respectively (n=594).
| Patella lead | Tibia lead | ||||
|---|---|---|---|---|---|
| No. of cases /person-year |
Hazard Ratio (95% CI) |
P for interaction |
Hazard Ratio (95% CI) |
P for interaction |
|
| Association in total population |
137/5017 | 1.30 (1.09, 1.56) |
1.25 (1.06, 1.48) |
||
| Prudent Diet | |||||
| Low | 60/2415 | 1.64 (1.27, 2.11) |
0.01 | 1.24 (0.96, 1.59) |
0.90 |
| High | 77/2602 | 1.07 (0.86, 1.34) |
1.26 (1.02, 1.55) |
||
| Western Diet | |||||
| Low | 69/2638 | 1.35 (1.05, 1.72) |
0.71 | 1.43 (1.14, 1.80) |
0.06 |
| High | 68/2379 | 1.27 (0.96, 1.61) |
1.08 (0.86, 1.34) |
||
Model was adjusted for age, BMI, total energy intake, smoking status (current/former/never), total cholesterol to HDL cholesterol ratio, education level and occupation.
Figure 2 and Figure 3 present effect modification by four dietary groups, according to the combination of the categories of dichotomized dietary pattern scores. Within the low Western diet, those in the low prudent diet showed a HR of 1.73 (95% CI: 1.14–2.62) per doubling increase in patella lead whereas those in the high prudent diet had a non-significant association (HR=1.16, 95% CI: 0.86–1.56). Similarly, within the high Western diet, those in the low prudent diet showed a HR of 1.57 (95% CI: 1.14–2.17) per doubling increase in patella lead whereas those in the high prudent diet had a non-significant association (HR=0.98, 95% CI: 0.72–1.33) (P-for-interactions=0.03 for prudent diet×patella lead and prudent diet×Western diet×patella lead; 0.68 for Western diet×patella lead and prudent diet×Western diet×patella lead). The interactions between tibia lead and dietary patterns were less pronounced and not statistically significant (P-for-interactions=0.51 for prudent diet×tibia lead and prudent×Western diet×tibia lead; 0.07 for Western diet×tibia lead and prudent×Western diet×tibia lead.). However, the trends were opposite as seen above in Table 3. Within the high prudent diet, a larger association was observed in the low western diet (HR=1.49, 95% CI: 1.15–1.94) whereas a null association was observed in the high western diet (HR=0.94, 95% CI: 0.68–1.29). We did not find differences in the associations between low vs. high western diet groups within the low prudent diet.
Figure 2.
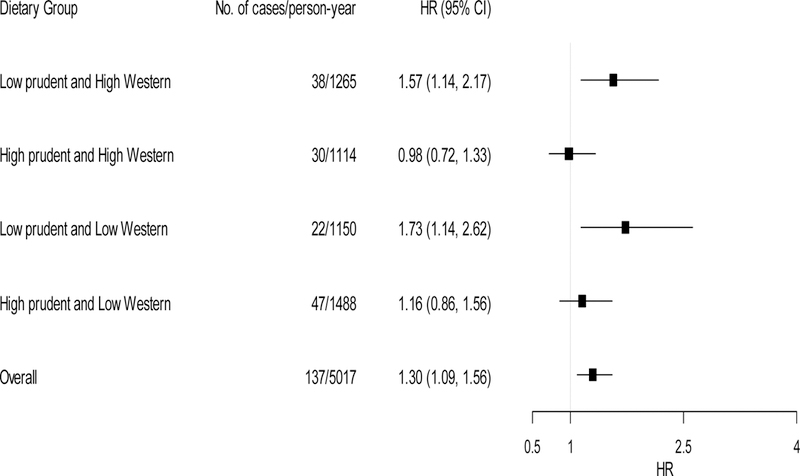
Adjusted hazard ratios (HRs) of incident CHD per 2-fold increase in patella lead concentration by combined dietary groups (n=594). Model was adjusted for age, BMI, total energy intake, smoking status (current/former/never), total cholesterol to HDL cholesterol ratio, education level and occupation. Low and high dietary groups were based on each dietary score cut at the median. Tests for effect modification were based on likelihood ratio tests comparing the full model (cross-product terms of Western diet×prudent diet, Western diet×patella lead, prudent diet×patella lead and a three-way interaction of Western diet×prudent diet×patella lead) vs. the reduced model (the full model minus an interaction term of prudent diet×patella lead or Western diet×patella lead). P-for-interactions were 0.03 for prudent diet×patella lead and prudent diet×Western diet×tibia lead; 0.68 for Western diet×patella lead and prudent diet×Western diet×tibia lead.
Figure 3.
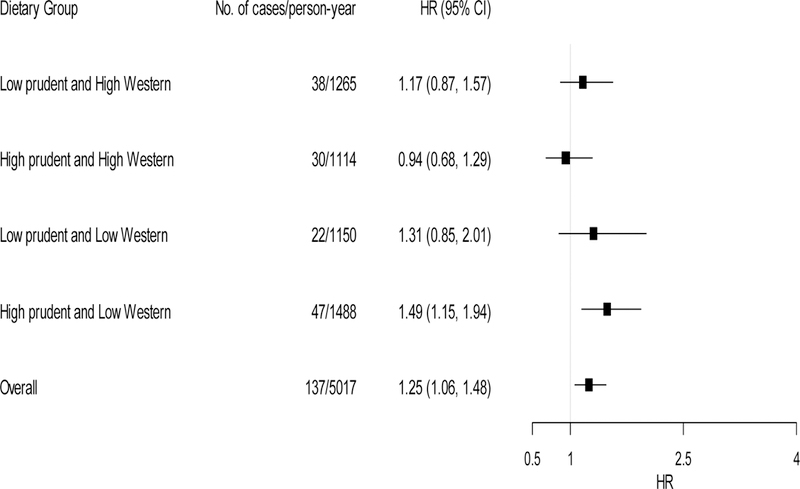
Adjusted hazard ratios (HRs) of incident CHD per 2-fold increase in tibia lead concentration by combined dietary groups (n=594). Model was adjusted for age, BMI, total energy intake, smoking status (current/former/never), total cholesterol to HDL cholesterol ratio, education level and occupation. Low and high dietary groups were based on each dietary score cut at the median. Tests for effect modification were based on likelihood ratio tests comparing the full model (cross-product terms of Western diet×prudent diet, Western diet×tibia lead, prudent diet×tibia lead and a three-way interaction of Western diet×prudent diet×tibia lead) vs. the reduced model (the full model minus an interaction term of prudent diet×tibia lead or Western diet×tibia lead). P-for-interactions were 0.51 for prudent diet×tibia lead and prudent diet×Western diet×tibia lead; 0.07 for Western diet×tibia lead and prudent diet×Western diet×tibia lead.
The prudent pattern was positively correlated with dietary intake of calcium, iron, zinc, and vitamins C, B1, B2, and B6 (Supplemental Table A.1). In contrast, the Western pattern was negatively associated with intakes of minerals and vitamins. Compared with dietary patterns, single nutrients had moderate magnitude and/or significance in the detection of modification effects with bone lead on CHD (Supplemental Table A.2). Iron, zinc, and vitamin B1had statistically significant impact on the association between patella lead and CHD, with higher nutrient levels associated with lower risks of CHD. Meantime, calcium and iron were found to have significant interaction with tibia lead. Higher levels of iron and lower levels of calcium were associated with lower risks of CHD. Note that nutrient levels were solely estimated from food consumption without nutritional supplements.
DISCUSSION
CHD is a multifactorial chronic disease that develops from the interplay of environmental toxicants, lifestyle, and many other factors. An understanding of the interaction between these risk factors is important to identify susceptible groups as targets for CHD prevention. In our previous study (Wang et al., 2017), dietary patterns were found to affect bone lead concentration among middle-aged to elderly men. In this study, we evaluated potential effect modification by dietary patterns on the association between bone lead and incident CHD events. Among subjects with low adherence to the prudent diet, higher patella lead concentrations were associated with higher risks of CHD incidence; while there was no association between patella lead and incident CHD among those with high adherence to the prudent diet. These results suggest that consumption of a prudent diet, made up primarily of fruit, legumes, whole grains, tomatoes, seafood, poultry, and various kinds of vegetables, may reduce lead toxicity on the risk of CHD development. However, our findings need to be interpreted with a caution given the limited power.
We did not detect statistically significant interactions between bone lead and Western diet. It is hard to come to a conclusion about the role of Western diet in the association between lead and CHD risk. Our previous study showed that the Western diet, and not the prudent diet, was associated with higher concentrations of blood and bone lead markers (Wang et al., 2017). Lead absorption has been shown to increase with a high-fat and high-cholesterol diet, after exposing rats to lead for 48 hours (Barltrop and Khoo, 1975). Hence, the previous literature and our present findings suggest that the Western diet may play a more important role in lead toxicokinetics and influence cumulative lead dose and lead body stores, whereas the prudent diet may play a critical role in lead toxicodynamics and the adverse health effects of lead.
A possible explanation for the discrepancies in results between patella and tibia lead is the difference between these two distinct measures of bone lead exposure in terms of their compositions and magnitude of health effects. The patella is composed mostly of trabecular bone, which has a higher resorption rate; in contrast, the tibia constitutes more cortical bone with a slow rate of bone turnover and a longer half-life (Hu, 1998; Hu et al., 2007). Trabecular bone typically has more active metabolism than cortical bone (Clarke, 2008). Previous findings have shown that rates of turnover activity for trabecular bone are around 2% per year and 8% for trabecular lead (Rabinowitz, 1991).
Therefore, trabecular bone lead, represented here by patella lead, is considered the major skeletal source of circulating lead and a moving average of environmental lead exposure in the past 10 years. On the contrary, cortical bone lead, represented by tibia lead, is an indicator of lifetime exposure even during a period when exogenous exposure decline substantially. It is expected that patella lead may be influenced by lifestyle factors and other predictors, more than tibia lead. This is consistent with previous results that cigarette smoking was linked to increments in patella lead concentrations; however, no significant differences by smoking status were detected for tibia lead (Wilker et al., 2011).
Dietary patterns in general play a role in bone resorption and formation. A healthy diet with high consumption of fruit and vegetables has contributed to increased bone mineral density (BMD) and decreased bone resorption rates, particularly in old age (Hardcastle et al., 2011; Tucker et al., 2002). Previous animal and human studies have suggested differential effects of dietary patterns on trabecular vs. cortical bone (Kim and Park, 2013; Laudermilk et al., 2011; Wray et al., 2011). In Sprague-Dawley rats, a low calcium diet led to decreased trabecular BMD.(Kim and Park, 2013) Moreover, high vitamin A (VA) intake affected trabecular bone more than cortical bone, while the effects of a VA-marginal diet did not impact tibia bone mineral content (BMC) in old rats.(Wray et al., 2011) In another study, dietary protein intake was related to trabecular but not cortical BMC and bone strength.(Laudermilk et al., 2011) However, it is still unclear whether different dietary patterns affect trabecular and cortical bones differentially.
The observed findings of the prudent diet are consistent with many studies in animals and humans, which have shown that a deficiency in essential minerals, such as zinc, calcium, and iron, can contribute to greater lead burdens and toxicity. (Bridges and Zalups, 2005; Flora and Tandon, 1990; Liu et al., 2013; Nemsadze et al., 2009; Prasanthi et al., 2010) Zinc, calcium, and iron have similar chemical and physical characteristics to lead, and compete for binding sites of metal absorptive and enzymatic proteins.(Bridges and Zalups, 2005) Additionally, zinc supplementation has been shown to increase urinary lead elimination and reduce adverse effects of lead on delta-aminolevulinic acid dehydratase (ALAD) activity in rats. (Flora and Tandon, 1990) Zinc and calcium supplementation could protect against lead-induced oxidative damage on proteins, lipids, and DNA(Prasanthi et al., 2010); while iron deficiency can result in enhanced lead toxicity to the heme-synthetic system.(Nemsadze et al., 2009)
Moreover, high consumption of foods rich in vitamin C, vitamin E, vitamin B1 and vitamin B6, may counteract oxidative damage.(Al-Attar, 2011; Bakulski et al., 2014; Bratton et al., 1981; Calabrese et al., 1987; Dawson et al., 1999; Ghanwat, 2016; Goyer and Cherian, 1979; Hsu and Guo, 2002; Reddy et al., 2010; Rendón-Ramírez et al., 2014; Sasser et al., 1984; Simon and Hudes, 1999; Tandon et al., 1984) Vitamin C and vitamin E can act as low molecular mass antioxidants that scavenge lead-related reactive oxygen species (ROSs) by rapid electron transfer and inhibit lipid peroxidation.(Al-Attar, 2011; Calabrese et al., 1987; Dawson et al., 1999; Ghanwat, 2016; Goyer and Cherian, 1979; Hsu and Guo, 2002; Rendón-Ramírez et al., 2014; Simon and Hudes, 1999) Vitamin B6 has been reported to reduce lead-associated increases in homocysteine, a known risk factor for cardiovascular disease, among middle-aged to elderly men.(Bakulski et al., 2014) Vitamin B1 and vitamin B6 have ring structures with nitrogen atoms, which mediate interactions with lead,(Reddy et al., 2010; Tandon et al., 1984) and these vitamins have been shown to be effective in decreasing lead concentrations in the liver, kidneys, bone and blood in animal studies.(Bratton et al., 1981; Reddy et al., 2010; Sasser et al., 1984)
To our knowledge, this is the first study to evaluate effect modification on lead toxicity by data-driven dietary patterns. Previous studies have focused mainly on single nutrients related to lead toxico-kinetics and toxico-dynamics. Although several nutritional factors have been found to counteract or exacerbate lead toxicity, it is not sufficient to consider individual nutrients to represent overall dietary exposure. Thus, we derived dietary patterns to achieve better translatability into public health intervention. To verify analytical consistency, we compared our pattern results with those obtained using the maximum likelihood method, orthogonal vs. oblique transformation, as well as factor identification from 135 food items instead of 40 pre-defined food groups, and we found similar patterns and factor loadings (data not shown).
The main strength of the present study was the use of a prospective, longitudinal cohort with bone lead measured before the development of CHD. Unlike blood lead concentration, which reflects only recent exposure to lead, bone lead is an endogenous reservoir of body lead and captures long-term lead exposure during a person’s lifetime. Given that bone lead may be a better indicator of cumulative lead exposure than blood lead, it could be the best biomarker to examine effect modification by diet on the chronic toxicity by bone lead.
Some limitations need to be addressed in this study. First, statistical methods used to derive dietary patterns, including food grouping and principal component factor analysis, involve some subjective decisions. However, our previous study has already shown the high reproducibility of these data-driven dietary patterns.(Wang et al., 2017) In addition, participants in our study were predominantly white, middle-aged to elderly men, 95% of whom were of European descent, so the present findings may not be generalizable to women, to other racial/ethnic groups, or to younger individuals. Our results may be subject to selection bias at enrollment into the bone lead sub-study or with selective dropouts during follow-up. To minimize the possibility of bias in effect estimates, we utilized inverse probability weighting approach and found similar results with or without incorporation of weights into the models. However, a competing risk situation might still arise among participants with high Western pattern scores or “unhealthy” diet.
In conclusion, our study suggests that a prudent diet with a combination of natural antagonists to lead toxicity may reduce the risk of development of CHD. However, these findings need to be interpreted with a caution given the modest sample size. More epidemiologic studies with a larger sample size and animal studies directly evaluating the role of dietary patterns in lead toxicity are needed to confirm our findings. If confirmed, such information will be crucial to identify susceptible populations that are at greater risk for CHD and to effectively prevent CHD events and premature mortality.
Significance Testing for Effect Modification
In order to disentangle effect modification by prudent and Western diet, likelihood ratio tests were utilized to examine significance of interactions, with a full model compared to two reduced models. The full model included cross-product terms between Western dietary scores and bone lead, between prudent dietary scores and bone lead, between Western and prudent dietary scores, and with a three-way interaction term among bone lead, Western and prudent dietary score, as shown in the model below. This model is mathematically equivalent to the model with four separate dietary groups.
Where λ(t)is the hazard of CHD at time t and λ0(t)is the baseline hazard; Lead is log-transformed concentration of either patella lead or tibia lead; High prudent includes men with high adherence to prudent diet, while those with low adherence to prudent diet were identified as the reference group; High Western includes men with high adherence to Western diet, while those with low adherence to Western diet were used as the reference; Covariates include age, BMI, total energy intake, smoking status, ratios of total cholesterol to HDL cholesterol, education, and occupation.
These two reduced models were shown below:
to examine effect modification by Western diet; while,
to evaluate effect modification by prudent diet.
Supplementary Material
HIGHLIGHTS.
A prudent diet may reduce lead toxicity on the risk of CHD development.
Diet could impact toxicokinetics and toxicodynamics of bone lead.
Future studies directly evaluating the role of dietary patterns in lead toxicity are needed.
Acknowledgments
SOURCES OF FUNDING
This work was supported by grants from the National Institute of Environmental Health Sciences (NIEHS) R01-ES005257, K01-ES016587 and P30-ES017885. Dr. David Sparrow was supported by a VA Research Career Scientist Award. The VA Normative Aging Study is supported by the Cooperative Studies Program/Epidemiology Research and Information Centers of the U.S. Department of Veterans Affairs and is a component of the Massachusetts Veterans Epidemiology Research and Information Center, Boston, Massachusetts.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interest:
All authors declare no financial and personal relationships with other people or organizations that could inappropriately influence the work.
REFERENCES
- Al-Attar AM, 2011. Antioxidant effect of vitamin E treatment on some heavy metals-induced renal and testicular injuries in male mice. Saudi J. Biol. Sci 18, 63–72. https://doi.org/10.1016/j.sjbs.2010.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aro ACA, Todd AC, Amarasiriwardena C, Hu H, 1994. Improvements in the calibration of 109Cd K x-ray fluorescence systems for measuring bone lead in vivo. Phys. Med. Biol 39, 2263–71. https://doi.org/10.1088/0031-9155/39/12/009 [DOI] [PubMed] [Google Scholar]
- Bakulski KM, Park SK, Weisskopf MG, Tucker KL, Sparrow D, Spiro A, Vokonas PS, Nie LH, Hu H, Weuve J, 2014. Lead exposure, B vitamins, and plasma homocysteine in men 55 years of age and older: the VA normative aging study. Environ. Health Perspect 122, 1066–74. https://doi.org/10.1289/ehp.1306931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barltrop D, Khoo HE, 1975. The influence of nutritional factors on lead absorption. Postgrad. Med. J 51, 795–800. https://doi.org/10.1136/pgmj.51.601.795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratton GR, Zmudzki J, Bell MC, Warnock LG, 1981. Thiamin (vitamin B1) effects on lead intoxication and deposition of lead in tissues: Therapeutic potential. Toxicol. Appl. Pharmacol 59, 164–172. https://doi.org/10.1016/0041-008X(81)90464-6 [DOI] [PubMed] [Google Scholar]
- Bridges CC, Zalups RK, 2005. Molecular and ionic mimicry and the transport of toxic metals. Toxicol. Appl. Pharmacol 204, 274–308. https://doi.org/10.1016/j.taap.2004.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronzato S, Durante A, 2017. A Contemporary Review of the Relationship between Red Meat Consumption and Cardiovascular Risk. Int. J. Prev. Med 8, 40 https://doi.org/10.4103/ijpvm.IJPVM_206_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese EJ, Stoddard A, Leonard DA, Dinardi SR, 1987. The effects of vitamin C supplementation on blood and hair levels of cadmium, lead, and mercury. Ann. N. Y. Acad. Sci 498, 347–53. [DOI] [PubMed] [Google Scholar]
- Clarke B, 2008. Normal bone anatomy and physiology. Clin. J. Am. Soc. Nephrol https://doi.org/10.2215/CJN.04151206 [DOI] [PMC free article] [PubMed]
- Dawson EB, Evans DR, Harris WA, Teter MC, McGanity WJ, 1999. The effect of ascorbic acid supplementation on the blood lead levels of smokers. J. Am. Coll. Nutr 18, 166–170. https://doi.org/10.1080/07315724.1999.10718845 [DOI] [PubMed] [Google Scholar]
- Ding N, Wang X, Weisskopf MG, Sparrow D, Schwartz J, Hu H, Park SK, 2016. Lead-Related Genetic Loci, cumulative lead exposure and incident coronary heart disease: The normative aging study. PLoS One 11, 1–18. https://doi.org/10.1371/journal.pone.0161472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flora SJS, Tandon SK, 1990. Beneficial effects of zinc supplementation during chelation treatment of lead intoxication in rats. Toxicology 64, 129–139. https://doi.org/10.1016/0300-483X(90)90130-9 [DOI] [PubMed] [Google Scholar]
- Ghanwat G, 2016. Effect of Vitamin C Supplementation on Blood Lead Level, Oxidative Stress and Antioxidant Status of Battery Manufacturing Workers of Western Maharashtra, India. J. Clin. Diagnostic Res 10, 8–11. https://doi.org/10.7860/JCDR/2016/15968.7528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonick HC, Ding Y, Bondy SC, Ni Z, Vaziri ND, 1997. Lead-Induced Hypertension. Hypertension 30. [DOI] [PubMed] [Google Scholar]
- Goyer RA, Cherian MG, 1979. Ascorbic acid and EDTA treatment of lead toxicity in rats. Life Sci 24, 433–438. https://doi.org/10.1016/0024-3205(79)90215-7 [DOI] [PubMed] [Google Scholar]
- Hallberg L, Brune M, Rossander-Hulthén L, 1987. Is there a physiological role of vitamin C in iron absorption? Ann. N. Y. Acad. Sci 498, 324–32. [DOI] [PubMed] [Google Scholar]
- Hardcastle AC, Aucott L, Fraser WD, Reid DM, MacDonald HM, 2011. Dietary patterns, bone resorption and bone mineral density in early post-menopausal Scottish women. Eur. J. Clin. Nutr 65, 378–385. https://doi.org/10.1038/ejcn.2010.264 [DOI] [PubMed] [Google Scholar]
- Hsu PC, Guo YL, 2002. Antioxidant nutrients and lead toxicity. Toxicology 180, 33–44. https://doi.org/10.1016/S0300-483X(02)00380-3 [DOI] [PubMed] [Google Scholar]
- Hu H, 1998. Bone lead as a new biologic marker of lead dose: recent findings and implications for public health. Environ. Health Perspect 106, 961–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Rabinowitz M, Smith D, 1998a. Bone lead as a biological marker in epidemiologic studies of chronic toxicity: Conceptual paradigms. Environ. Health Perspect 106, 1–8. https://doi.org/10.1289/ehp.981061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Rabinowitz M, Smith D, 1998b. Bone lead as a biological marker in epidemiologic studies of chronic toxicity: Conceptual paradigms. Environ. Health Perspect 106, 1–8. https://doi.org/10.1289/ehp.981061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Shih R, Rothenberg S, Schwartz BS, 2007. The epidemiology of lead toxicity in adults: Measuring dose and consideration of other methodologic issues. Environ. Health Perspect https://doi.org/10.1289/ehp.9783 [DOI] [PMC free article] [PubMed]
- Jain NB, Potula V, Schwartz J, Vokonas PS, Sparrow D, Wright RO, Nie H, Hu H, 2007. Lead Levels and Ischemic Heart Disease in a Prospective Study of Middle-Aged and Elderly Men: The VA Normative Aging Study. Environ. Health Perspect 115, 871–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Park D, 2013. The effect of restriction of dietary calcium on trabecular and cortical bone mineral density in the rats. J. Exerc. Nutr. Biochem 17, 123–31. https://doi.org/10.5717/jenb.2013.17.4.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudermilk MJ, Vassallo DM, Manore MM, Going SB, 2011. Usual dietary protein intake is related to trabecular but not cortical bone mineral content, density and strength measured by pQCT in young girls. FASEB J. Conference, Eermenta
- Liu MC, Xu Y, Chen YM, Li J, Zhao F, Zheng G, Jing JF, Ke T, Chen JY, Luo WJ, 2013. The effect of sodium selenite on lead induced cognitive dysfunction. Neurotoxicology 36, 82–88. https://doi.org/10.1016/j.neuro.2013.03.008 [DOI] [PubMed] [Google Scholar]
- Navas-Acien A, Guallar E, Silbergeld EK, Rothenberg SJ, 2007. Lead Exposure and Cardiovascular Disease: A Systematic Review. Environ. Health Perspect 115, 472–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemsadze K, Sanikidze T, Ratiani L, Gabunia L, Sharashenidze T, 2009. Mechanisms of lead-induced poisoning. Georgian Med News 92–6. [PubMed]
- Newby PK, Tucker KL, 2004. Empirically derived eating patterns using factor or cluster analysis: a review. Nutr. Rev 62, 177–203. [DOI] [PubMed] [Google Scholar]
- Nie H, Sánchez BN, Wilker E, Weisskopf MG, Schwartz J, Sparrow D, Hu H, 2009. Bone lead and endogenous exposure in an environmentally exposed elderly population: the normative aging study. J. Occup. Environ. Med 51, 848–57. https://doi.org/10.1097/JOM.0b013e3181aa0106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Rourke N, Hatcher L, 2013. A Step-by-Step Approach to Using SAS for Factor Analysis and Structural Equation Modeling, Second Edition. https://doi.org/10.1111/insr.12111_2
- Pell MB, Schneyer J, 2016. Reuters finds lead levels higher than Flint’s in thousands of locales [WWW Document]. Reuters URL http://www.reuters.com/investigates/special-report/usa-lead-testing/ (accessed 6.26.17).
- Prasanthi RPJ, Devi CB, Basha DC, Reddy NS, Reddy GR, 2010. Calcium and zinc supplementation protects lead (Pb)-induced perturbations in antioxidant enzymes and lipid peroxidation in developing mouse brain. Int. J. Dev. Neurosci 28, 161–167. https://doi.org/10.1016/j.ijdevneu.2009.12.002 [DOI] [PubMed] [Google Scholar]
- Rabinowitz MB, 1991. Toxicokinetics of bone lead. Environ. Health Perspect 91, 33–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy SY, Pullakhandam R, Kumar BD, 2010. Thiamine reduces tissue lead levels in rats: Mechanism of interaction. BioMetals 23, 247–253. https://doi.org/10.1007/s10534-009-9282-8 [DOI] [PubMed] [Google Scholar]
- Rendón-Ramírez A-L, Maldonado-Vega M, Quintanar-Escorza M-A, Hernández G, Arévalo-Rivas B-I, Zentella-Dehesa A, Calderón-Salinas J-V, 2014. Effect of vitamin E and C supplementation on oxidative damage and total antioxidant capacity in lead-exposed workers. Environ. Toxicol. Pharmacol 37, 45–54. https://doi.org/10.1016/j.etap.2013.10.016 [DOI] [PubMed] [Google Scholar]
- Rodríguez-Iturbe B, Sindhu RK, Quiroz Y, Vaziri ND, 2005. Chronic Exposure to Low Doses of Lead Results in Renal Infiltration of Immune Cells, NF-κB Activation, and Overexpression of Tubulointerstitial Angiotensin II. Antioxid. Redox Signal 7, 1269–1274. https://doi.org/10.1089/ars.2005.7.1269 [DOI] [PubMed] [Google Scholar]
- Sasser LB, Hall GG, Bratton GR, Zmudzki J, 1984. Absorption and tissue distribution of lead in thiamin-replete and thiamin-deficient rats. J. Nutr 114, 1816–1825. [DOI] [PubMed] [Google Scholar]
- Simon JA, Hudes ES, 1999. Relationship of Ascorbic Acid to Blood Lead Levels. Jama 281, 2289 https://doi.org/10.1001/jama.281.24.2289 [DOI] [PubMed] [Google Scholar]
- Stradling C, Hamid M, Taheri S, Thomas GN, 2014. A review of dietary influences on cardiovascular health: part 2: dietary patterns. Cardiovasc. Hematol. Disord. Drug Targets 14, 50–63. [DOI] [PubMed] [Google Scholar]
- Tandon SK, Flora SJ, Singh S, 1984. Influence of vitamin B-complex deficiency on lead intoxication in young rats. Indian J Med Res 80, 444–448. https://doi.org/10.2486/indhealth.25.93 [PubMed] [Google Scholar]
- Tucker KL, Chen H, Hannan MT, Cupples LA, Wilson PWF, Felson D, Kiel DP, 2002. Bone mineral density and dietary patterns in older adults: the Framingham Osteoporosis Study. Am J Clin Nutr 76, 245–52. https://doi.org/10.1093/ajcn/76.1.245 [DOI] [PubMed] [Google Scholar]
- Turner ND, Lloyd SK, 2017. Association between red meat consumption and colon cancer: A systematic review of experimental results. Exp. Biol. Med 242, 813–839. https://doi.org/10.1177/1535370217693117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Ding N, Tucker KL, Weisskopf MG, Sparrow D, Hu H, Park SK, 2017. A Western Diet Pattern Is Associated with Higher Concentrations of Blood and Bone Lead among Middle-Aged and Elderly Men. J. Nutr. jn249060 https://doi.org/10.3945/jn.117.249060 [DOI] [PMC free article] [PubMed]
- Weisskopf MG, Jain N, Nie H, Sparrow D, Vokonas P, Schwartz J, Hu H, 2009. A Prospective Study of Bone Lead Concentration and Death From All Causes, Cardiovascular Diseases, and Cancer in the Department of Veterans Affairs Normative Aging Study. Circulation 120, 1056–1064. https://doi.org/10.1161/CIRCULATIONAHA.108.827121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisskopf MG, Sparrow D, Hu H, Power MC, 2015. Biased exposure-health effect estimates from selection in cohort studies: Are environmental studies at particular risk? Environ. Health Perspect 123, 1113–1122. https://doi.org/10.1289/ehp.1408888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilker E, Korrick S, Nie LH, Sparrow D, Vokonas P, Coull B, Wright RO, Schwartz J, Hu H, 2011. Longitudinal Changes in Bone Lead Levels. J. Occup. Environ. Med 53, 850–855. https://doi.org/10.1097/JOM.0b013e31822589a9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willerson JT, Ridker PM, 2004. Inflammation as a Cardiovascular Risk Factor. Circulation 109, II-2–II-10. https://doi.org/10.1161/01.CIR.0000129535.04194.38 [DOI] [PubMed] [Google Scholar]
- Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE, 1985. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am. J. Epidemiol 122, 51–65. https://doi.org/10.1093/acprof [DOI] [PubMed] [Google Scholar]
- Wirfält E, Drake I, Wallström P, 2013. What do review papers conclude about food and dietary patterns? Food Nutr. Res 57, 20523 https://doi.org/10.3402/fnr.v57i0.20523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray AE, Okita N, Ross AC, 2011. Cortical and trabecular bone, bone mineral density, and resistance to ex vivo fracture are not altered in response to life-long vitamin A supplementation in aging rats. J. Nutr 141, 660–6. https://doi.org/10.3945/jn.110.132126 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


