Abstract
Viral encephalitis markedly increases the risk for the development of epilepsy. The Theiler’s murine encephalomyelitis virus (TMEV)-induced model of seizures/epilepsy is a murine model of both viral-induced seizures/epilepsy and human Temporal Lobe Epilepsy. The inflammatory cytokines interleukin (IL)-6 and tumor necrosis factor (TNF)-α have been shown to play a role in seizure development in the TMEV-induced model of seizures/epilepsy, and infiltrating macrophages along with microglia have been shown to be major producers of these cytokines. The metabotropic glutamate receptor 5 (mGluR5) is a G-protein coupled receptor that has been shown to reduce IL-6 and TNF-α and to provide neuroprotection in other disease models. Therefore, we hypothesized that stimulation of mGluR5 would not only reduce seizures but attenuate IL-6 and TNF-α production in microglia and macrophages in the TMEV model. We found that pharmacological stimulation of mGluR5 with the selective positive allosteric modulator VU0360172 not only reduced acute seizure outcomes, but also reduced the percent of microglia and macrophages producing TNF-α 3 days post infection. Furthermore, treatment with VU0360172 did not alter the level of viral antigen, compared to controls, showing that this treatment does not compromise viral clearance. These results establish that mGluR5 may represent a therapeutic target in the TMEV-induced model of seizures/epilepsy.
Keywords: Metabotropic glutamate receptor 5, Theiler’s murine encephalomyelitis virus, Seizures, Epilepsy, Temporal lobe epilepsy, Viral encephalitis, Tumor necrosis factor, Picornavirus, VU0360172
Introduction
Viral infection of the central nervous system (CNS) has been shown to increase the risk of developing seizures and epilepsy, with 4–20% of patients who have recovered from viral encephalitis going on to develop some form of seizures (Getts et al., 2008). Moreover, there are over 100 different neurotropic viruses that can cause encephalitis in humans, some of which have been implicated in the development of seizures and epilepsy [(Misra et al., 2008), further reviewed in (Libbey and Fujinami, 2011)]. Our lab has previously developed and characterized a model of viral-induced seizures/epilepsy using the Daniels (DA) strain of Theiler’s murine encephalomyelitis virus (TMEV), providing the first viral infection-driven model of seizures/epilepsy (Libbey et al., 2008). In the TMEV-induced seizure model, C57BL/6J mice are intracerebrally (i.c.) infected with the DA strain of TMEV at day 0. The DA strain of TMEV has a tropism for the hippocampus and other limbic regions, spreading to these structures bilaterally (Stewart et al., 2010a). Approximately 50% of the infected C57BL/6J mice develop behavioral seizures. These acute seizures start as early as 3 days post infection (DPI) and end by 10 DPI, with the majority of seizures occurring between 5 and 7 DPI. At 14 DPI there is little to no TMEV antigen positive cells detectable in the brain, and mice go on to clear the virus (Libbey et al., 2008; Kirkman et al., 2010). The mice then enter a latent period, of roughly 2 months, in which there are no detectable behavioral seizures, after which approximately 50% of the mice that had acute seizures go on to develop spontaneous recurrent seizures – epilepsy (Stewart et al., 2010b). In addition to seizures, mice display pathologies in the hippocampus, such as astrogliosis, microglial activation, and neuronal loss, all phenotypes shared with human Temporal Lobe Epilepsy (TLE) (Loewen et al., 2016). Therefore, the TMEV-induced seizure model represents not only a model of viral-induced seizures/epilepsy, but also of human TLE. The TMEV-induced model of seizures/epilepsy is further reviewed here (DePaula-Silva et al., 2017).
Previous work has shown the importance of CNS inflammation in the development of seizures in the TMEV-induced seizure model (Cusick et al., 2013; Kirkman et al., 2010; Patel et al., 2017) along with a variety of other models (Bertani et al., 2017; Pauletti et al., 2017; Vezzani et al., 2015). Two inflammatory cytokines shown to be important in this model are interleukin (IL)-6 and tumor necrosis factor (TNF)-α. Not only are TNF-α and IL-6 mRNAs increased in the brains of TMEV-infected mice experiencing seizures, but significantly fewer mice have seizures when deficient in TNF-α receptor-1 or IL-6 (Kirkman et al., 2010). Furthermore, a study looking specifically at TNF-α levels in the hippocampus showed that both mRNA and protein levels of this cytokine were significantly increased in TMEV-infected animals at 5 and 14 DPI (Patel et al., 2017).
Another important component to TMEV infection and seizure development is resident and infiltrating immune cells. It is already known that TMEV infection results in immune cell infiltration into the brain (Cusick et al., 2013; Kirkman et al., 2010), and that this infiltration is concentrated in the hippocampal formation (Howe et al., 2012). When macrophage infiltration was reduced through treatment with drugs such as wogonin or minocycline, two known anti-inflammatories, significantly fewer mice had seizures (Cusick et al., 2013; Libbey et al., 2011a). Furthermore, seizures were significantly reduced in TMEV-infected mice which had either resident brain cells deficient in IL-6, or IL-6-deficient infiltrating cells (Libbey et al., 2011b). Congruent with this work, depletion of monocytes through the use of the drug clodronate significantly reduced both infiltrating macrophages in the brain and TMEV-induced seizures (DePaula-Silva et al., 2018). These studies show that inflammation plays a large role in the development of seizures which are not solely dependent on viral infection alone. Additionally, it has been shown that even though there is extensive loss of neurons in the CA1 region of the hippocampus in a TMEV-infected C57BL/6J mouse, only 10% of those neurons are positive for TMEV antigen (Buenz et al., 2009), further demonstrating that inflammation and non-cell-autonomous mechanisms are critical factors in this model.
Studies evaluating resected tissue from human TLE patients have shown the metabotropic glutamate receptor 5 (mGluR5) to be upregulated in the hippocampus (Kandratavicius et al., 2013; Notenboom et al., 2006). Traumatic brain injury research has also shown that mGluR5 stimulation with a selective positive allosteric modulator, known as VU0360172, can reduce lesion volume, hippocampal cell loss and the production of IL-6 and TNF-α in the brain (Loane et al., 2014; Zhang et al., 2015). Experiments using an immortalized microglial cell line, BV2 cells, have also shown that VU0360172 reduces TNF-α production in lipopolysaccharide-stimulated cells (Loane et al., 2014). This collection of work makes it reasonable to hypothesize that mGluR5 stimulation with VU0360172 could produce neuroprotective and anti-inflammatory affects that result in decreased seizures. We therefore investigated whether mGluR5 represents a potential therapeutic target in the TMEV-induced model of viral-induced epilepsy.
Materials and Methods
Animals.
C57BL/6J inbred mice, 4-weeks old and male, were obtained from the Jackson Laboratory (Bar Harbor, ME). All experiments involving animals, along with their care, were reviewed and approved by the University of Utah Institutional Animal Care and Use Committee (Protocol #15–08004) and were conducted in accordance with guidelines prepared by the Committee on Care and Use of Laboratory Animals, Institute of Laboratory Animals Resources, National Research Council. Also, all animal studies complied with the ARRIVE guidelines. All efforts were made to minimize suffering and mice were euthanized through an overdose of isoflurane.
TMEV infection.
On Day 0, animals were infected as previously described (Libbey et al., 2011b). Briefly, 5- to 6-week old C57BL/6J mice were anesthetized with isoflurane by inhalation and infected i.c. with either 4 × 104 plaque forming units (PFUs) of the DA strain of TMEV, or mock infected with phosphate-buffered saline (PBS). The DA strain of TMEV was propagated as previously described (Zurbriggen and Fujinami, 1989).
Western blot.
Brains were obtained from TMEV-infected and mock-infected (PBS-injected) mice for 1–2 DPI, and for 3–14 DPI time points brains were obtained from mock-infected and TMEV-infected mice displaying seizures. Hippocampi were dissected, weighed, and then homogenize on ice in Syn-PER reagent (ThermoFisher Scientific, Waltham, MA). In order to obtain a synaptosomal fraction, the homogenized samples were centrifuged at 1200 × g for 10 minutes. The supernatants were collected and centrifuged at 15,000 × g for 20 minutes. The supernatants were discarded and the final pellets were resuspended in 100 μL Syn-PER reagent. Gel electrophoresis of the synaptosomal samples was performed using Criterion TGX 4–15% precast polyacrylamide gels (BioRad, Hercules, CA), and transfer of proteins to nitrocellulose was carried out using a semi-dry transfer system (BioRad). Nitrocellulose membranes were then stained using anti-mGluR5 (ABCAM, Cambridge, MA) and anti-β actin (SigmaAldrich, St. Louis, MO), and secondary antibodies conjugated with horseradish peroxidase (Jackson ImmunoResearch Laboratories Inc., West Grove, PA) were used to identify protein bands with chemiluminescence reagents. Analyses of western blots were performed using ImageJ software, normalizing all samples to β-actin as a loading control. Samples were eliminated if there were clear and visible artifacts, loading, or transfer errors.
Immunofluorescence.
For immunofluorescence imaging, whole brains were collected from mice displaying seizures and control mice. Brains were frozen in Tissue-Tek O.C.T. Compound (VWR, Radnor, PA) and 8 μm slices were obtained. Slices were stained with anti-mGluR5 primary antibodies (ABCAM) and visualized using Alexa Fluor 488 anti-rabbit secondary antibodies (ABCAM). Images were obtained using a confocal laser scanning microscope at the Fluorescence Microscopy Core Facility, a part of the Health Sciences Cores at the University of Utah. Images for analysis were obtained using 40× z-stack images taken in the CA1, CA3, and dentate regions of the hippocampus, along with cortex as an internal control. All images were taken on the left side of the tissue, contralateral to the injection site to avoid injection artifacts. All images underwent a background subtraction, and mGluR5 was quantified by multiplying the area of positive pixels by the average pixel intensity (IntDen). Representative mosaic images were constructed by stitching together 20× images of the hippocampus from representative slides.
Drug treatment.
For experiments investigating positive allosteric modulation of mGluR5, we used the drug VU0360172 (Tocris, Minneapolis, MN) suspended in PBS with 10% Tween-80, as per previous reports (Loane et al., 2014; Zhang et al., 2015). TMEV-infected mice were injected intraperitoneally (i.p.) with either 50 mg/kg VU0360172 (N=18–20 mice per group) or vehicle (N=19–20 mice per group), all in 200 μL, at 6 hours, 1, 2, and 3 DPI and mice were observed for behavioral seizures up to 10 DPI. Long term drug treatment included the same initial injection schedule and continued daily through 8 DPI. To block mGluR5 signaling, 3-((2-Methyl-4-thiazolyl)ethynyl)pyridine (MTEP) (Tocris) was solubilized in PBS and administered to mice at 10 mg/kg at 6 hours, 1, 2, and 3 DPI (N=24 mice); control mice were injected with 200 μL PBS as vehicle (N=25 mice).
Flow cytometry and direct intracellular cytokine staining (ICS).
Direct ICS was performed as previously described (Cusick et al., 2013; Liu and Whitton, 2005). Briefly, animals were retro-orbitally injected with 250 μg Brefeldin A (SigmaAldrich), an inhibitor of protein transport, 6 hours before being sacrificed. Brains were immediately collected and processed on ice. Extracellular staining was carried out using anti-CD45-V500 (BD Biosciences, San Jose, CA) and anti-CD11b-BV421 (BioLegend, San Diego, CA) in order to distinguish microglial (CD45low/intCD11b+), macrophage (CD45hiCD11b+), and lymphocyte (CD45hiCD11b−) cell types. Cells were then treated with cytoperm/cytofix (BD Biosciences) and stained with anti-TNF-α-Pe-Cy7 (BioLegend) and anti-IL-6-PE (eBioscience). Gating for all flow cytometry experiments was determined by Fluorescence-minus-one (FMO) for CD45, CD11b, TNF-α, and IL-6 antibodies.
Seizure scoring.
Seizure monitoring and scoring was performed as previously described (Libbey et al., 2011b). Briefly, mice were observed for 2 hours a day and graded using the Racine scale: stage 1, mouth and facial disturbances; stage 2, head nodding; stage 3, forelimb clonus; stage 4, rearing; stage 5, rearing and falling (Racine, 1972; Cusick et al., 2013). The percentage of mice with seizures was calculated as follows: (number of mice with seizures/total number of mice infected) × 100. Average cumulative seizure burden was determined by totaling the daily Racine scores for each mouse and then, using these totals, an average score for each experimental group was calculated.
Immunohistochemistry.
Mice were euthanized and perfused with PBS, followed by 4% paraformaldehyde solution. Brains were harvested, divided into 5 coronal slabs, embedded in paraffin and cut into 4 μm thick tissue sections. Immunohistochemistry was performed as previously described (Kirkman et al., 2010; Tsunoda et al., 2001). DA viral antigen positive cells were detected on paraffin embedded sections using anti-TMEV antibodies and secondary antibodies conjugated to horseradish peroxidase. Slides were then developed using the avidin-biotin peroxidase complex technique with 3,3’-diaminobenzidine tetrahydrochloride (SigmaAldrich) in 0.01% hydrogen peroxide (SigmaAldrich) in PBS. Quantification of DA viral antigen positive cells was performed in a blinded fashion with a light microscope using one slide per brain and evaluating tissue sections from all five coronal slabs represented per slide (5 animals per group). The following brain regions were observed during the quantification process: frontal lobe, septum, caudoputamen, hippocampus, thalamus, midbrain, cortex, and cerebellum.
Statistical analysis.
Prism v.7 (Graphpad software, La Jolla, CA) was used for statistical analysis and the creation of all graphs. The significance of the percent of mice having seizures per day was determined using a Fisher’s exact test. Western blot optical density, immunofluorescence, daily average clinical score, and seizure burden were analyzed using a Mann-Whitney U test. Area under the curve analysis was also performed on daily average clinical score data and the significance between curve areas was determined by a Student’s T-test. Outliers were identified by a Grubb’s test. Results with p<0.05 were considered statistically significant.
Results
mGluR5 expression is decreased in the hippocampus during acute seizures
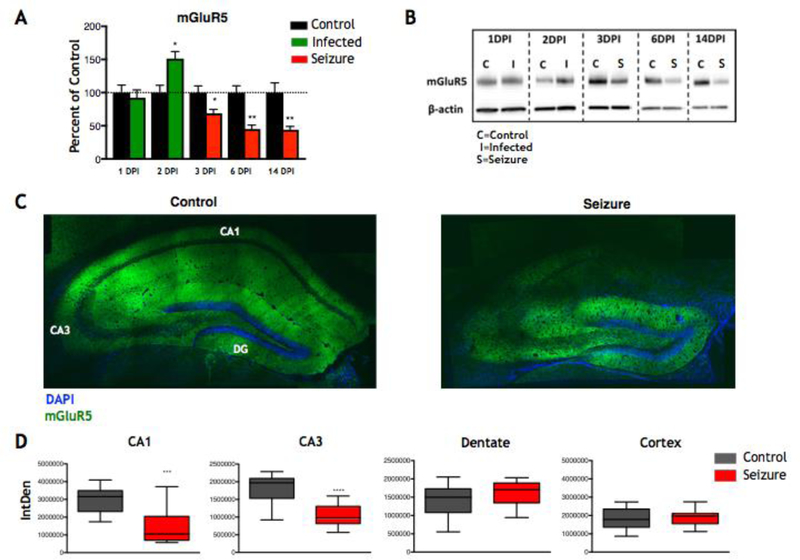
Previous work in human TLE showed that mGluR5 levels were increased in established TLE patients compared to controls (Kandratavicius et al., 2013; Notenboom et al., 2006). Before we started our investigation as to whether mGluR5 could serve as a therapeutic target in our model, we sought to determine whether there were changes in mGluR5 expression in animals having seizures compared to controls. We used western blot analysis to investigate whether mGluR5 levels were increased in our mouse model of human TLE. Hippocampi were collected from TMEV-infected mice with seizures, and from PBS mock-infected controls, at 1, 2, 3, 6, and 14 DPI. We found that mGluR5 levels were decreased at the start of the acute seizure phase (3 DPI) (Control: N=5, Seizure: N=4; p≤0.05, Mann-Whitney U test) (Fig. 1A–B) and remained decreased when measured at 6 DPI, the height of seizure activity (Control: N=5, Seizure: N=10; p≤0.01, Mann-Whitney U test) (Fig. 1A–B). Expression of mGluR5 was also observed to be reduced in the mice that had experienced seizures at 14 DPI (Control: N=5, Seizure: N=10; p≤0.01, Mann-Whitney U test) (Fig. 1A–B), the time at which seizures have stopped and the amount of TMEV antigen is either low, or undetectable (Kirkman et al., 2010). Just prior to the onset of seizures, an increase in expression level of mGluR5 was observed at 2 DPI (Control: N=3, Seizure: N=7; p≤0.05, Mann-Whitney U test) (Fig. 1A–B) in TMEV-infected mice as compared to PBS mock-infected controls. No significant differences were observed at 1 DPI (Control: N=5, Seizure: N=9) (Fig. 1A–B).
Figure 1.
mGluR5 expression during acute seizures. A) Quantification of western blots showing mGluR5 expression levels in TMEV-infected mice (1 and 2 DPI, green bars) and TMEV-infected mice experiencing seizures (3, 6, and 14 DPI, red bars) compared to mock-infected control mice (black bars) [*p≤0.05, **p≤0.01, Mann-Whitney U test; data presented as mean + standard error of the mean (SEM)]. B) Representative western blots of mGluR5 at each time point in (A) with β-actin used as a loading control. C) Representative 20× mosaic images of the hippocampus of control mice (left) and TMEV-infected mice experiencing seizures (right) at 6 DPI. CA1, CA3, and Dentate Gyrus (DG) regions are labeled in white in the left panel. D) Quantification of 40× images of the CA1, CA3, and Dentate regions of the hippocampus and the Cortex region of the brain from control mice (gray boxes) and TMEV-infected mice experiencing seizures (red boxes) at 6 DPI (***p≤0.001, ****p≤0.0001, Mann-Whitney U test).
We wanted to determine whether the decrease in mGluR5 was specific to a hippocampal region or if this change was global within the hippocampus. Therefore, we used immunofluorescence staining for mGluR5 and confocal imaging of brain slices from animals with seizures and mock-infected control animals at 6 DPI. Immunofluorescence confocal imaging showed a significant decrease in mGluR5 immunoreactivity in the CA1 (p≤0.001, Mann-Whitney U test) and CA3 (p≤0.0001, Mann-Whitney U test) regions of the hippocampus with no significant changes seen in the dentate or cortex (control brain region) in TMEV-infected mice with seizures compared to controls (3–4 slices per animal; Control: N=4, Seizure: N=5) (Fig. 1C–D). Therefore, following an initial increase in mGluR5 at 2DPI, mGluR5 was then decrease over the time course of acute seizures (3, 6, and 14 DPI) and this decrease was localized to the CA1 and CA3 regions of the hippocampus.
Treatment with the mGluR5 positive allosteric modulator VU0360172 changes seizure outcomes
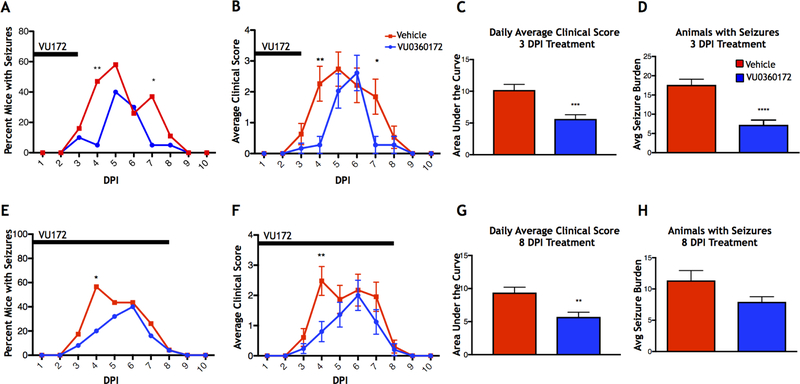
In order to test whether the observed changes in mGluR5 were merely a consequence of seizures, or whether mGluR5 could represent a potential therapeutic target, we used the mGluR5 positive allosteric modulator VU0360172. Animals were treated 6 hours after TMEV infection and 1, 2, and 3 DPI with either VU0360172 or vehicle control (short term treatment). The group treated with VU0360172 showed a significant decrease in the percent of mice with seizures at 4 (p≤0.01, Fisher’s exact test) and 7 DPI (p≤0.05, Fisher’s exact test), compared to controls (Vehicle: N=19, VU0360172: N=18) (Fig. 2A). When observing average clinical score, which equates to the average Racine scale seizure score, per group per day, the average clinical score was significantly lower in VU0360172-treated mice at 4 (p≤0.01, Mann-Whitney U test) and 7 DPI (p≤0.05, Mann-Whitney U test) (Fig. 2B), and there was a significantly smaller area under the curve for VU0360172-treated mice compared to vehicle-treated controls (p≤0.001, Student’s T-test) (Fig. 2C). Additionally, we measured the average cumulative seizure burden of the mice that experienced seizures in each experimental group and found that the mice treated with VU0360172 had a significantly lower average cumulative seizure burden than that of control mice (p≤0.0001, Mann-Whitney U test; Vehicle: N=11, VU0360172: N=14) (Fig. 2D). Thus, treatment with VU0360172 for up to 3 DPI resulted in attenuation of seizures.
Figure 2.
Effects of VU0360172 treatment on seizures. TMEV-infected mice were treated intraperitoneally with either VU0360172 (VU172) or vehicle for 3 DPI, (A-D) short term VU0360172 treatment, or for 8 DPI, (E-H) long term VU0360172 treatment. (A,E) Daily seizure rate (Racine scale, stages 3–5) for short term (A) and long term (E) VU0360172 treatment (*p≤0.05, **p≤0.01, Fisher’s exact test). (B,F) Average daily clinical score for short term (B) and long term (F) VU0360172 treatment (*p≤0.05, **p≤0.01, Mann-Whitney U test; data presented as mean ± SEM). (C,G) Area under the curve analysis of short term (C) and long term (G) VU0360172 treatment average daily clinical scores (**p≤0.01, ***p≤0.001, Student’s T-test). (D,H) Average seizure burden of mice with seizures for short term (D) and long term (H) VU0360172 treatment (****p≤0.0001, Mann-Whitney U test).
It was possible that the seizures were only delayed and not completely ameliorated due to the cessation of VU0360172 treatment after day 3. In order to test whether extended treatment could stop seizures altogether, we repeated VU0360172 treatment of TMEV-infected animals and continued administration of the drug through 8 DPI (long term treatment). Again, there was a significant decrease in the percent of mice experiencing seizures at 4 DPI, compared to vehicle-treated controls (p≤0.05, Fisher’s exact test; Vehicle: N=23, VU0360172: N=25), but not at 7 DPI (Fig. 2E). For average daily clinical score, again the average clinical score was significantly lower in VU0360172-treated mice at 4 DPI (p≤0.01, Mann-Whitney U test), but not at 7 DPI (Fig. 2F), and the area under the curves were significantly different between the VU0360172-treated animals and vehicle-treated controls (p≤0.01, Student’s T-test) (Fig. 2G). Finally, although there was a decrease in the average cumulative seizure burden of mice with seizures in VU0360172-treated mice compared to controls (Vehicle: N=19, VU0360172: N=18), this effect was not significant (Fig. 2H). These findings suggest that there is a critical window for treatment with VU0360172 between infection and 3 DPI and after that point treatment is no longer effective at delaying or limiting seizures. Additionally, this lack of further reduction in seizures with long term VU0360172 treatment suggests that this drug does not possess direct anti-seizure effects.
Inhibiting mGluR5 does not exacerbate seizures
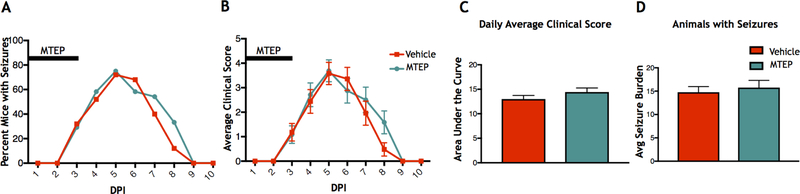
Since we observed that treatment with VU0360172, a positive allosteric modulator of mGluR5, had a positive effect on seizure outcomes as compared to controls, we then investigated whether decreasing mGluR5 activity would worsen the seizure phenotype in these animals. In order to block mGluR5 activity we administered MTEP, a selective allosteric antagonist of mGluR5. TMEV-infected mice were treated with either MTEP or vehicle control 6 hours after infection and 1, 2, and 3 DPI. No significant effects were observed on the percent of mice having seizures when comparing the MTEP-treated mice to controls (Vehicle: N=24, MTEP: N=25) (Fig 3A). Additionally, no differences between groups were observed in the average daily clinical score (Fig. 3B), the area under the curve for average clinical scores (Fig. 3C), or the average cumulative seizure burden in the mice with seizures (Vehicle: N=22, MTEP: N=22) (Fig. 3D). Therefore, blocking mGluR5 activity did not make the seizure phenotype worse.
Figure 3.
Effects of MTEP treatment on seizures. TMEV-infected mice were treated intraperitoneally with either MTEP or vehicle for 3 DPI. (A) Daily seizure rate (Racine scale, 3–5). (B) Average daily clinical score. (C) Area under the curve analysis of average daily clinical scores. (D) Average seizure burden of mice with seizures.
mGluR5 stimulation does not decrease macrophage infiltration into the brain
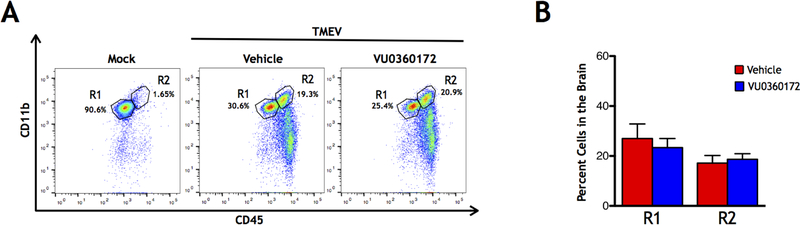
Previous work in the TMEV-induced seizure model has shown that infection leads to macrophage (CD45hiCD11b+) and lymphocyte (CD45hiCD11b−) infiltration into the brain (Cusick et al., 2013; DePaula-Silva et al., 2018; Libbey et al., 2011b). Furthermore, the use of minocycline and wogonin, two drugs that have been shown to reduce seizures in the TMEV model, reduced macrophage infiltration into the brain at 3 DPI (Cusick et al., 2013). It is also known that mGluR5 has anti-inflammatory actions (Byrnes et al., 2009; Loane et al., 2009; Zhang et al., 2015). Therefore, we tested whether short term VU0360172 administration to activate mGluR5 would limit infiltration of immune cells into the brain at 3 DPI. Whole brains were collected from TMEV-infected VU0360172-treated animals along with TMEV-infected vehicle-treated controls. Flow cytometry was performed on cells isolated from brains and gating, using CD11b and CD45 markers, was performed to isolate the single cell R1 (ramified microglia: CD45lo/intCD11b+) and R2 (macrophages and activated microglia: CD45hiCD11b+) populations (Fig. 4A). No significant reduction was seen in the percent of either the R1 or R2 cell populations in the brain at 3 DPI in the VU0360172-treated mice compared to the vehicle-treated controls (Control: N=10, VU0360172: N=10) (Fig. 4B). Therefore, activation of mGluR5 did not limit the infiltration of immune cells into the brain.
Figure 4.
Cell Infiltration in the brains of VU0360172-treated TMEV-infected mice. (A) Representative flow cytometry plots of mock-infected control mice (left panel), and TMEV-infected mice treated with either Vehicle (center panel) or VU0360172 (right panel) at 3 DPI. (B) Quantification of ramified microglia (R1 = CD45lo/intCD11b+) and infiltrating macrophages and activated microglia (R2 = CD45hiCD11b+) cell populations.
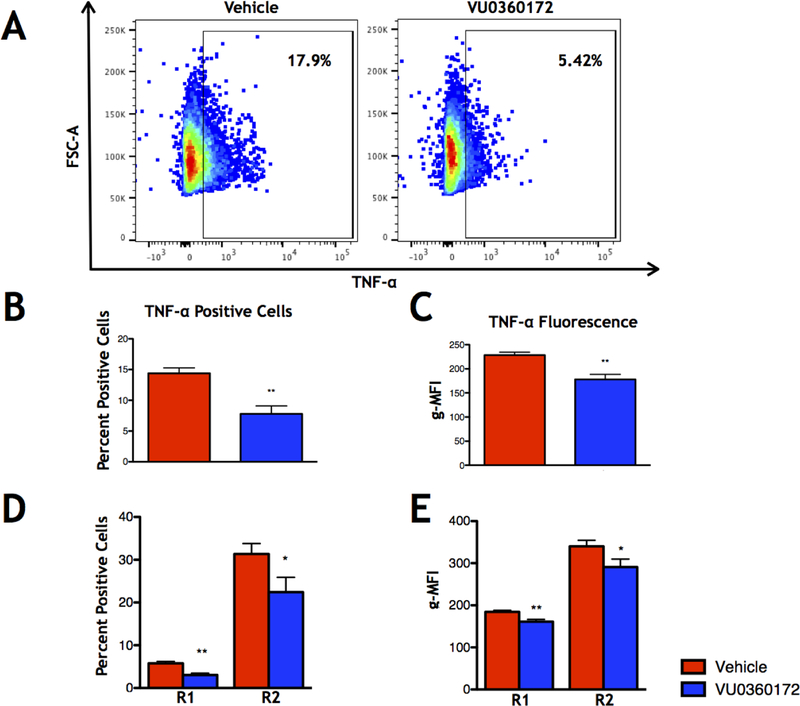
VU0360172 treatment reduces TNF-α production by immune cells in vivo at 3 DPI
Although VU0360172 treatment did not significantly alter immune cell infiltration into the brain, we sought to test whether the infiltrating macrophages and resident microglia produced significantly less TNF-α or IL-6, two proinflammatory cytokines previously shown to play a role in seizure development in the TMEV-induced seizure model (Kirkman et al., 2010; Libbey et al., 2011b). In order to measure changes in the production of these two cytokines specifically in microglia and macrophages at 3 DPI, we performed direct ICS and flow cytometry on isolated immune cells from brains of TMEV-infected, short term VU0360172-treated mice and vehicle-treated control mice (Fig. 5A). We found that the geometric mean fluorescence intensity (g-MFI) of the TNF-α+ cells, along with the percent of TNF-α+ cells, isolated from the brain was significantly decreased in VU360172-treated mice as compared to vehicle-treated controls (p≤0.01 for both measures, Mann-Whitney U test; Control: N=10, VU0360172: N=10) (Fig 5B–C). Furthermore, TNF-α g-MFI and the percent of TNF-α+ cells was significantly decreased in both the R1 and R2 cell populations (R1: p≤0.01, R2: p≤0.05, Mann-Whitney U test) (Fig 5D–E). In contrast, IL-6 was significantly increased in the percent of IL-6+ cells in the R2 cell population (p≤0.05, Mann-Whitney U test; Control: N=10, VU0360172: N=10). However, this effect was not reflected in the IL-6 g-MFI of this cell population (Supplemental Fig. 1). Thus, VU0360172 treatment reduces TNF-α production without affecting IL-6 production at 3 DPI.
Figure 5.
Direct intracellular cytokine staining (ICS) for TNF-α at 3 DPI. (A) Representative flow cytometry plots of TNF-α+ single cells isolated from the brain in Vehicle- (left) and VU0360172-treated (right) animals. (B) Percent TNF-α+ cells in whole brain. (C) Geometric mean fluorescence intensity (g-MFI) of the TNF-α+ cells in whole brain. (D) TNF-α+ cells in the R1 and R2 cell populations. (E) g-MFI of the TNF-α+ cells in the R1 and R2 cell populations. Data presented as means + SEM. *p≤0.05, **p≤0.01, Mann-Whitney U test.
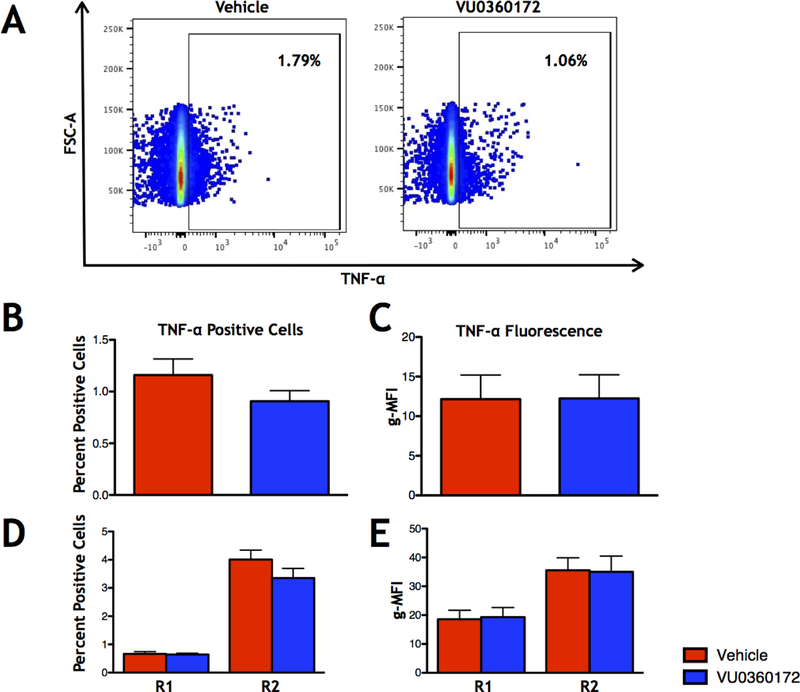
We repeated this experiment at 6 DPI to determine the effects of short term VU0360172 treatment on cytokine production in the whole brain, R1, and R2 cell populations during the height of acute seizures. At this time point we found that there were no significant changes in TNF-α (Fig. 6) or IL-6 (Supplemental Fig. 2) for percent positive cells or g-MFI for either the whole brain, R1, or R2 cell populations (Control: N=10, VU0360172: N=10). These results show that VU0360172 treatment reduces the TNF-α+ microglia and macrophages at 3 DPI, however, this effect is lost at 6 DPI, a time point in our model where most of the mice that will have seizures have already had observed seizures (peak of seizures).
Figure 6.
Direct ICS for TNF-α at 6 DPI. (A) Representative flow cytometry plots of TNF-α+ single cells isolated from the brain in Vehicle- (left) and VU0360172-treated (right) animals. (B) Percent TNF-α+ cells in whole brain. (C) g-MFI of the TNF-α+ cells in whole brain. (D) TNF-α+ cells in the R1 and R2 cell populations. (E) g-MFI of TNF-α+ cells in the R1 and R2 cell populations. Data presented as means + SEM.
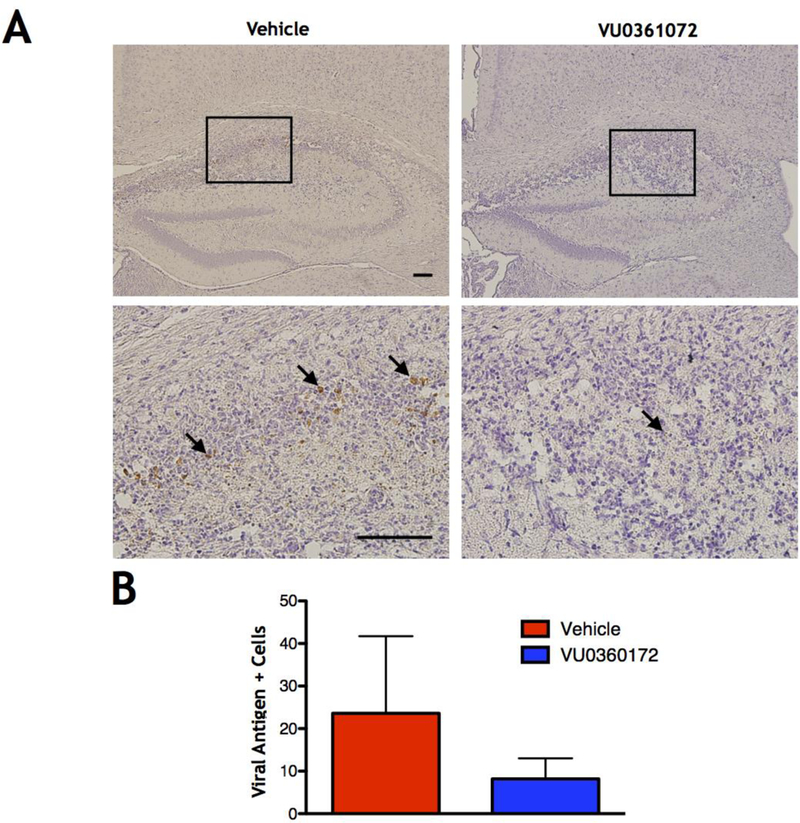
VU0360172 treatment does not alter clearance of viral antigen at 14 DPI
Our data show that VU0360172 treatment can modulate the immune response in our TMEV-induced seizure model, and it is known that TNF-α can have important anti-viral effects (Chyuan et al., 2015; Shrestha et al., 2008). Therefore, it is possible that the clearance of TMEV in animals treated with VU0360172 could be compromised. In order to determine whether short term treatment with VU0360172 compromised clearance of TMEV in our model, we stained for TMEV antigen on paraffin embedded brain tissue slices from vehicle- and VU0360172-treated mice at 14 DPI (Fig. 7A), the time point at which little to no TMEV antigen should be detectable (Kirkman et al., 2010). We found that there was no significant difference in the number of antigen positive cells in VU0360172-treated mice compared to vehicle-treated controls (Control: N=5, VU0360172: N=5) (Fig. 7B). An outlier in the vehicle group was detected using the Grubb’s test (p≤0.05). However, the presence or absence of this data point did not affect the significance of the results, and therefore it was not removed from the final data set or graphical representation (Fig. 7). The lack of significant changes in the number of antigen positive cells shows that VU0360172-treatment did not compromise viral clearance.
Figure 7.
TMEV antigen clearance at 14 DPI. (A) Representative images of TMEV antigen staining (arrows) at 14 DPI in the hippocampus of Vehicle-treated animals (left panels), at 10× (top left) and 40× (bottom left) magnification, and VU0360172-treated animals (right panels) at 10× (top right) and 40× (bottom right) magnification (scale bars=100 μm). (B) Quantification of TMEV antigen staining throughout the brain in Vehicle- versus VU0360172-treated mice. An outlier in the Vehicle-treated group was detected using the Grubb’s test (p<0.05). However, the presence or absence of this data point did not affect the significance of the results and therefore it was not removed from the final data set.
Discussion
Previous work has shown that TNF-α and IL-6 production by resident microglia and infiltrating macrophages, respectively, plays a role in the development of acute seizures in the TMEV-induced model of seizures/epilepsy (Cusick et al., 2013). It has also been established that mGluR5 stimulation with the positive allosteric modulator VU0360172 can reduce IL-6 and TNF-α production, along with having neuroprotective effects, in other models of brain injury (Loane et al., 2009; Zhang et al., 2015). Therefore, the present work was initiated to explore the potential of using VU0360172-induced mGluR5 stimulation as a means to reduce the production of IL-6 and TNF-α, and attenuate seizures, therefore exploring its use as a potential therapeutic target in the TMEV model.
Upon examination of mGluR5 expression within the hippocampus over the course of seizures, we described a decrease in mGluR5 protein levels in the hippocampus of mice that displayed seizures starting at 3 DPI which was observed out to 14 DPI (Fig. 1A–B). This reduction in mGluR5 was localized to the CA1 and CA3 regions of the hippocampus (Fig. 1C–D), two regions known to develop pathologies, to include cell death, gliosis, and network changes, in our model (Buenz et al., 2009; Libbey et al., 2008; Loewen et al., 2016; Smeal et al., 2015, 2012). For the experiments investigating mGluR5 levels at 3–14 DPI, we only used mock infected controls and TMEV-infected mice that displayed seizures. Since we wanted to compare mice with seizure pathology to those without, we did not include TMEV-infected mice that did not display seizures. Additionally, we did not include this TMEV-infected, non-seizure group since we could not rule out the possibility of sub-clinical seizures, or seizures that happened between observation periods. However, due to this fact, currently we cannot parse out a potential relation of the decrease in mGluR5 to TMEV infection alone. It should be noted that previous work in the TMEV model has shown that TMEV-infected mice that do not display seizures have less cell loss and pyknosis in the hippocampus, along with less TNF-α and IL-6 mRNA in the brain than TMEV-infected mice with seizures (Libbey et al., 2008; Kirkman et al., 2010). The differences between TMEV-infected mice with and without seizures can be reviewed further here (Libbey and Fujinami, 2011). Therefore, it is reasonable to hypothesize that the levels of mGluR5 will not show the same reduction in expression in TMEV-infected mice that did not display seizures as was seen in mice with seizures.
Our observed decrease in mGluR5 differs from previous reports examining mGluR5 expression in human TLE (Kandratavicius et al., 2013; Notenboom et al., 2006). One distinction between the studies, however, is that our experiments are measuring mGluR5 levels during the acute phase of our TMEV-induced seizure model. This acute phase is early in the course of disease when the animals have seizures in tandem with viral infection. In contrast, the mentioned studies of human TLE did not occur during known viral encephalitic infections and were conducted after the TLE was well established in the patients. Studies looking at the mGluR5 expression levels in mice at later time points, such that they have cleared the virus and have developed spontaneous and recurrent seizures (epilepsy phase), could better reflect the time point of human TLE examined in the previous studies. An additional focus for investigation at the acute phase and later time points would include cell type specific expression of mGluR5; this is especially relevant as other TLE models, such as the low dose kainate model, show an upregulation of mGluR5 in astrocytes after status epilepticus (Umpierre et al., 2016).
This study shows that mGluR5 stimulation with VU0360172, starting on day 0 and continuing through 3 DPI (short term treatment), changes seizure outcomes in TMEV-infected mice (Fig. 2A–D). We observed that TMEV-infected mice had significantly fewer seizures at 4 and 7 DPI, along with significantly lower average clinical scores for those days. Our data also showed that this short term treatment reduced seizure burden in the mice experiencing seizures. Long term VU0360172 treatment (through 8 DPI) did not totally eliminate or even reduce seizures as compared to mice only treated through 3 DPI (Fig. 2E–H). This suggests that there is a critical window for treatment in the first three days of infection. During long term VU0360172 treatment, a decrease in seizure burden for mice experiencing seizures was observed in the VU0360172-treated mice as compared to controls, but this effect was not significant. It is possible that long term treatment with VU0360172 could have diminishing returns or, eventually, adverse effects, and further experiments are needed to determine whether this might be the case. The lack of further improvement of seizure outcomes with the longer VU0360172 treatment also suggests that the drug may be more protective against the development of seizures as opposed to having any direct anti-seizure effect. It is also possible that any direct effects of VU0360172 on cells particularly in the CA1 and CA3 regions of the hippocampus may be lost around the time seizures take hold since we show here that mGluR5 is reduced in these regions starting around 3 DPI and continuing through 14 DPI.
A possible mechanism by which short term VU0360172 treatment may improve seizure outcomes in TMEV-infected mice is through its ability to reduce the amount of TNF-α produced in immune cells (microglia and macrophages) isolated from the brains of VU0360172-treated mice as compared to vehicle-treated controls at 3 DPI (Fig. 5). This same reduction was not seen with the proinflammatory molecule IL-6 (Supplemental Fig. 1), and both cytokines showed no difference in VU0360172-treated animals as compared to controls at 6 DPI (Fig. 6 & Supplemental Fig. 2). These experiments show that mGluR5 stimulation suppresses the production of TNF-α at the start of seizure development. It is already known that TNF-α plays a role in the development of seizures in the TMEV-induced seizure model (Kirkman et al., 2010). However, targeting of TNF-α directly, as a possible treatment, poses some difficulties as monoclonal antibody treatments used to inhibit TNF-α do not cross the blood brain barrier (Tracey et al., 2008), and many patients on TNF-α inhibitors are reported to experience adverse neurological events (Kaltsonoudis et al., 2014; Solomon et al., 2011). Additionally, Xpro1595, a blood brain barrier permeable, dominant-negative selective inhibitor of soluble TNF-α, was ineffective at blocking acute seizures in the TMEV-induced seizure model when administered peripherally or to the CNS directly (Patel et al., 2017), further underlining the potential difficulty of TNF-α inhibition as a treatment in this model. Our results show that VU036172 may represent a new way to target TNF-α production in the early stages of seizure development. Previous work in the TMEV model has also shown that infection leads to an upregulation of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor subunits GluA1 and GluA2, an effect that was suggested to be driven in part by TNF-α signaling (Patel et al., 2017). This could be a potential mechanism, and experiments are underway to investigate whether VU0360172 decreases the level of AMPA receptors and whether this is mediated through TNF-α.
While short term VU0360172 treatment had positive effects on TMEV-infected mice and reduced TNF-α+ microglia and macrophages, we wanted to make sure that drug treatment did not compromise the clearance of TMEV. Therefore, we investigated whether VU0360172 treatment altered the amount of viral antigen in the brain at 14 DPI. We found that the treatment did not compromise clearance of viral antigen as compared to vehicle-treated controls (Fig. 7). This is advantageous since inflammation and seizures can be modulated by this drug without compromising the immune system in a way that leads to viral persistence or prolonged viral infection.
Since we observed an improvement in seizure outcomes with positive mGluR5 modulation, we sought to investigate whether the inverse was also true. Therefore, in order to potentially observe a worsening of seizure outcomes compared to controls, we treated TMEV-infected mice with the negative allosteric antagonist MTEP, which has been shown to be highly selective and successful at penetrating the brain upon systemic injection (Cosford et al., 2003; Loane et al., 2014; Rodriguez et al., 2010). We found that there were no differences between groups (Fig. 3), demonstrating that limiting mGluR5 signaling does not result in more seizures in the acute phase. Since inflammation plays a role in the development of acute seizures in our model (Cusick et al., 2013; Kirkman et al., 2010; Patel et al., 2017), and we show here that short term VU0360172 treatment is likely working by limiting the production of proinflammatory cytokines, it is possible that the failure of MTEP to worsen the disease is due to the fact that mGluR5 antagonism does not increase TNF-α, or that any seizure outcomes in these mice are not exacerbated by a further increase in TNF-α. Moreover, we cannot completely rule out the fact that reducing mGluR5 signaling does not have adverse effects, as it is also possible that mGluR5 activity is already so low at this time that MTEP does not provide any further inhibition, and therefore no worsening in seizure outcomes is observed. To this point, with the percent of mice with seizures at almost 80% at 5 DPI, it is possible that these mice are reaching a ceiling of seizure symptoms and thereby potentially masking any adverse effects of MTEP. It is also possible that only treating mice with MTEP from 0–3 DPI may not have been enough time, or the right time period, to produce an observable effect. Treating mice with MTEP during the time in which they are having acute seizures (3–10 DPI) could result in worsening of seizure severity or increase cumulative seizure burden. Therefore, we cannot rule out the fact that longer treatment with this drug could result in a worsening of seizure outcomes. It should also be noted that even though the dose used here has been well established in the literature (Loane et al., 2014; Rodriguez et al., 2010), it is possible that, due to the half-life of MTEP, the drug may not be having the optimal effect, and therefore, additional injection time points, or doses, could be used in future studies to address this.
Long term studies are needed not only to observe the effects of TMEV infection on mGluR5 expression in later stages, but whether VU0360172 produces better outcomes past the acute phase of the TMEV-induced seizure model. It is known that TMEV-infected mice with seizures have increased anxiety-like behavior and impairment in episodic and spatial memory (Umpierre et al., 2014). Furthermore, when TMEV-infected animals are given a sub-chronic dose of minocycline, 50 mg/kg once daily, there is no significant change in acute seizures, however, there are improved long-term behavioral outcomes; the inverse was true for valproic acid which showed decreased seizures but poor behavioral outcomes (Barker-Haliski et al., 2016). This work highlights the fact that seizure phenotype alone is not necessarily a good predictor of chronic outcomes in the TMEV-induced seizure model. Therefore, it would be beneficial to investigate the effects of VU0360172 on the long term behavioral outcomes in TMEV-infected mice.
The present work demonstrates the potential of using mGluR5 as a therapeutic target in the TMEV-induced model of seizures/epilepsy, a mouse model of human TLE. We have found that not only does pharmacological stimulation of mGluR5, through administration of VU0361072, have potential as a treatment for acute phase seizures, but is also a means of attenuating TNF-α levels in the brain during the start of this period. This is of particular interest since, as previously stated, monoclonal antibodies used to inhibit TNF-α do not cross the blood brain barrier, and furthermore, complete inhibition of TNF-α may have adverse effects (Kaltsonoudis et al., 2014; Solomon et al., 2011; Tracey et al., 2008). Now, VU0360172 presents a way to attenuate TNF-α production in microglia and macrophages, which means that signaling is most likely reduced without being completely abolished. In conclusion, the work here shows that mGluR5 stimulation can indeed reduce seizure outcomes in TMEV-infected mice, along with attenuating TNF-α levels, providing a target for further research in the TMEV model.
Supplementary Material
Highlights.
VU0360172 reduced seizure outcomes in the virus-induced seizure model
VU0360172 reduced TNF-α+ microglia and macrophages in early seizure development
Positive mGluR5 modulation did not affect viral clearance in treated animals
Acknowledgements:
We would like to thank Mitchell A. Wilson, BS, Mike O’Connell, Kelley M. Ingram and Samantha P. Duzy, BS, for excellent technical assistance, and John Sanchez, BS, for many helpful discussions. Microscopy equipment was obtained using a NCRR Shared Equipment Grant # 1S10RR024761–01. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Funding: This work was supported by the National Institutes of Health 5R01NS065714.
Abbreviations:
- TMEV
Theiler’s murine encephalomyelitis virus
- IL
Interleukin
- TNF
Tumor necrosis factor
- mGluR5
metabotropic glutamate receptor 5
- CNS
Central nervous system
- i.c.
Intracerebrally
- DPI
Days post infection
- PFUs
Plaque forming units
- PBS
Phosphate buffered saline
- i.p.
Intraperitoneally
- MTEP
3-((2-Methyl-4-thiazolyl)ethynyl)pyridine
- ICS
Intracellular staining
- g-MFI
geometric mean fluorescence intensity
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: The authors declare that they have no conflicts of interest.
References
- 1.Barker-Haliski ML, Heck TD, Dahle EJ, Vanegas F, Pruess TH, Wilcox KS, White HS, 2016. Acute treatment with minocycline, but not valproic acid, improves long-term behavioral outcomes in the Theiler’s virus model of temporal lobe epilepsy. Epilepsia 57, 1958–1967. https://doi.org/10.1111/epi.13577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bertani I, Iori V, Trusel M, Maroso M, Foray C, Mantovani S, Tonini R, Vezzani A, Chiesa R, 2017. Inhibition of IL-1β Signaling Normalizes NMDA-Dependent Neurotransmission and Reduces Seizure Susceptibility in a Mouse Model of Creutzfeldt–Jakob Disease. J. Neurosci 37, 10278–10289. https://doi.org/10.1523/JNEUROSCI.1301-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buenz EJ, Sauer BM, Lafrance-Corey RG, Deb C, Denic A, German CL, Howe CL, 2009. Apoptosis of hippocampal pyramidal neurons is virus independent in a mouse model of acute neurovirulent picornavirus infection. Am. J. Pathol 175, 668–684. https://doi.org/10.2353/ajpath.2009.081126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Byrnes KR, Stoica B, Loane DJ, Riccio A, Davis MI, Faden AI, 2009. Metabotropic glutamate receptor 5 activation inhibits microglial associated inflammation and neurotoxicity. Glia 57, 550–560. https://doi.org/10.1002/glia.20783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chyuan I-T, Tsai H-F, Tzeng H-T, Sung C-C, Wu C-S, Chen P-J, Hsu P-N, 2015. Tumor necrosis factor-alpha blockage therapy impairs hepatitis B viral clearance and enhances T-cell exhaustion in a mouse model. Cell. Mol. Immunol 12, 317–325. https://doi.org/10.1038/cmi.2015.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cosford NDP, Tehrani L, Roppe J, Schweiger E, Smith ND, Anderson J, Bristow L, Brodkin J, Jiang X, McDonald I, Rao S, Washburn M, Varney MA, 2003. 3-[(2-Methyl-1,3-thiazol-4-yl)ethynyl]-pyridine: a potent and highly selective metabotropic glutamate subtype 5 receptor antagonist with anxiolytic activity. J. Med. Chem 46, 204–206. https://doi.org/10.1021/jm025570j [DOI] [PubMed] [Google Scholar]
- 7.Cusick MF, Libbey JE, Patel DC, Doty DJ, Fujinami RS, 2013. Infiltrating Macrophages Are Key to the Development of Seizures following Virus Infection. J. Virol 87, 1849–1860. https://doi.org/10.1128/JVI.02747-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DePaula-Silva AB, Hanak TJ, Libbey JE, Fujinami RS, 2017. Theiler’s murine encephalomyelitis virus infection of SJL/J and C57BL/6J mice: Models for multiple sclerosis and epilepsy. J. Neuroimmunol 308, 30–42. https://doi.org/10.1016/j.jneuroim.2017.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DePaula-Silva AB, Sonderegger FL, Libbey JE, Doty DJ, Fujinami RS, 2018. The immune response to picornavirus infection and the effect of immune manipulation on acute seizures. J. Neurovirol https://doi.org/10.1007/s13365-018-0636-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Getts DR, Balcar VJ, Matsumoto I, Müller M, King NJC, 2008. Viruses and the immune system: their roles in seizure cascade development. J. Neurochem 104, 1167–1176. https://doi.org/10.1111/j.1471-4159.2007.05171.x [DOI] [PubMed] [Google Scholar]
- 11.Howe CL, Lafrance-Corey RG, Sundsbak RS, Lafrance SJ, 2012. Inflammatory monocytes damage the hippocampus during acute picornavirus infection of the brain. J. Neuroinflammation 9, 50 https://doi.org/10.1186/1742-2094-9-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaltsonoudis E, Zikou AK, Voulgari PV, Konitsiotis S, Argyropoulou MI, Drosos AA, 2014. Neurological adverse events in patients receiving anti-TNF therapy: a prospective imaging and electrophysiological study. Arthritis Res. Ther 16, R125 https://doi.org/10.1186/ar4582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kandratavicius L, Rosa-Neto P, Monteiro MR, Guiot M-C, Assirati JA, Carlotti CG, Kobayashi E, Leite JP, 2013. Distinct increased metabotropic glutamate receptor type 5 (mGluR5) in temporal lobe epilepsy with and without hippocampal sclerosis. Hippocampus 23, 1212–1230. https://doi.org/10.1002/hipo.22160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirkman NJ, Libbey JE, Wilcox KS, White HS, Fujinami RS, 2010. Innate but not Adaptive Immune Responses Contribute to Behavioral Seizures Following Viral Infection. Epilepsia 51, 454–464. https://doi.org/10.1111/j.1528-1167.2009.02390.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Libbey JE, Fujinami RS, 2011. Neurotropic viral infections leading to epilepsy: focus on Theiler’s murine encephalomyelitis virus. Future Virol. 6, 1339–1350. https://doi.org/10.2217/fvl.11.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Libbey JE, Kennett NJ, Wilcox KS, White HS, Fujinami RS, 2011a. Once Initiated, Viral Encephalitis-Induced Seizures are Consistent No Matter the Treatment or Lack of Interleukin-6. J. Neurovirol 17, 496–499. https://doi.org/10.1007/s13365-011-0050-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Libbey JE, Kennett NJ, Wilcox KS, White HS, Fujinami RS, 2011b. Interleukin-6, Produced by Resident Cells of the Central Nervous System and Infiltrating Cells, Contributes to the Development of Seizures following Viral Infection. J. Virol 85, 6913–6922. https://doi.org/10.1128/JVI.00458-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Libbey JE, Kirkman NJ, Smith MCP, Tanaka T, Wilcox KS, White HS, Fujinami RS, 2008. Seizures following picornavirus infection. Epilepsia 49, 1066–1074. https://doi.org/10.1111/j.1528-1167.2008.01535.x [DOI] [PubMed] [Google Scholar]
- 19.Liu F, Whitton JL, 2005. Cutting edge: re-evaluating the in vivo cytokine responses of CD8+ T cells during primary and secondary viral infections. J. Immunol. Baltim. Md 1950 174, 5936–5940. [DOI] [PubMed] [Google Scholar]
- 20.Loane DJ, Stoica BA, Pajoohesh-Ganji A, Byrnes KR, Faden AI, 2009. Activation of Metabotropic Glutamate Receptor 5 Modulates Microglial Reactivity and Neurotoxicity by Inhibiting NADPH Oxidase. J. Biol. Chem 284, 15629–15639. https://doi.org/10.1074/jbc.M806139200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loane DJ, Stoica BA, Tchantchou F, Kumar A, Barrett JP, Akintola T, Xue F, Conn PJ, Faden AI, 2014. Novel mGluR5 Positive Allosteric Modulator Improves Functional Recovery, Attenuates Neurodegeneration, and Alters Microglial Polarization after Experimental Traumatic Brain Injury. Neurotherapeutics 11, 857–869. https://doi.org/10.1007/s13311-014-0298-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loewen JL, Barker-Haliski ML, Dahle EJ, White HS, Wilcox KS, 2016. Neuronal Injury, Gliosis, and Glial Proliferation in Two Models of Temporal Lobe Epilepsy. J. Neuropathol. Exp. Neurol 75, 366–378. https://doi.org/10.1093/jnen/nlw008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Misra UK, Tan CT, Kalita J, 2008. Viral encephalitis and epilepsy. Epilepsia 49 Suppl 6, 13–18. https://doi.org/10.1111/j.1528-1167.2008.01751.x [DOI] [PubMed] [Google Scholar]
- 24.Notenboom RGE, Hampson DR, Jansen GH, van Rijen PC, van Veelen CWM, van Nieuwenhuizen O, de Graan PNE, 2006. Up-regulation of hippocampal metabotropic glutamate receptor 5 in temporal lobe epilepsy patients. Brain J. Neurol 129, 96–107. https://doi.org/10.1093/brain/awh673 [DOI] [PubMed] [Google Scholar]
- 25.Patel DC, Wallis G, Dahle EJ, McElroy PB, Thomson KE, Tesi RJ, Szymkowski DE, West PJ, Smeal RM, Patel M, Fujinami RS, White HS, Wilcox KS, 2017. Hippocampal TNFα Signaling Contributes to Seizure Generation in an Infection-Induced Mouse Model of Limbic Epilepsy. eNeuro 4 https://doi.org/10.1523/ENEURO.0105-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pauletti A, Terrone G, Shekh-Ahmad T, Salamone A, Ravizza T, Rizzi M, Pastore A, Pascente R, Liang L-P, Villa BR, Balosso S, Abramov AY, van Vliet EA, Del Giudice E, Aronica E, Antoine DJ, Patel M, Walker MC, Vezzani A, 2017. Targeting oxidative stress improves disease outcomes in a rat model of acquired epilepsy. Brain J. Neurol 140, 1885–1899. https://doi.org/10.1093/brain/awx117 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27.Racine RJ, 1972. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr. Clin. Neurophysiol 32, 281–294. [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez AL, Grier MD, Jones CK, Herman EJ, Kane AS, Smith RL, Williams R, Zhou Y, Marlo JE, Days EL, Blatt TN, Jadhav S, Menon UN, Vinson PN, Rook JM, Stauffer SR, Niswender CM, Lindsley CW, Weaver CD, Conn PJ, 2010. Discovery of Novel Allosteric Modulators of Metabotropic Glutamate Receptor Subtype 5 Reveals Chemical and Functional Diversity and In Vivo Activity in Rat Behavioral Models of Anxiolytic and Antipsychotic Activity. Mol. Pharmacol 78, 1105–1123. https://doi.org/10.1124/mol.110.067207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shrestha B, Zhang B, Purtha WE, Klein RS, Diamond MS, 2008. Tumor Necrosis Factor Alpha Protects against Lethal West Nile Virus Infection by Promoting Trafficking of Mononuclear Leukocytes into the Central Nervous System. J. Virol 82, 8956–8964. https://doi.org/10.1128/JVI.01118-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smeal RM, Fujinami R, White HS, Wilcox KS, 2015. Decrease in CA3 inhibitory network activity during Theiler’s virus encephalitis. Neurosci. Lett 609, 210–215. https://doi.org/10.1016/j.neulet.2015.10.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smeal RM, Stewart K-A, Iacob E, Fujinami RS, White HS, Wilcox KS, 2012. The activity within the CA3 excitatory network during Theiler’s virus encephalitis is distinct from that observed during chronic epilepsy. J. Neurovirol 18, 30–44. https://doi.org/10.1007/s13365-012-0082-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Solomon AJ, Spain RI, Kruer MC, Bourdette D, 2011. Inflammatory neurological disease in patients treated with tumor necrosis factor alpha inhibitors. Mult. Scler. Houndmills Basingstoke Engl. 17, 1472–1487. https://doi.org/10.1177/1352458511412996 [DOI] [PubMed] [Google Scholar]
- 33.Stewart K-AA, Wilcox KS, Fujinami RS, White HS, 2010a. Theiler’s virus infection chronically alters seizure susceptibility. Epilepsia 51, 1418–1428. https://doi.org/10.1111/j.1528-1167.2009.02405.x [DOI] [PubMed] [Google Scholar]
- 34.Stewart K-AA, Wilcox KS, Fujinami RS, White HS, 2010b. Development of Post-infection Epilepsy Following Theiler Virus Infection of C57BL/6 Mice. J. Neuropathol. Exp. Neurol 69, 1210–1219. https://doi.org/10.1097/NEN.0b013e3181ffc420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tracey D, Klareskog L, Sasso EH, Salfeld JG, Tak PP, 2008. Tumor necrosis factor antagonist mechanisms of action: A comprehensive review. Pharmacol. Ther 117, 244–279. https://doi.org/10.1016/j.pharmthera.2007.10.001 [DOI] [PubMed] [Google Scholar]
- 36.Tsunoda I, Wada Y, Libbey JE, Cannon TS, Whitby FG, Fujinami RS, 2001. Prolonged Gray Matter Disease without Demyelination Caused by Theiler’s Murine Encephalomyelitis Virus with a Mutation in VP2 Puff B. J. Virol 75, 7494–7505. https://doi.org/10.1128/JVI.75.16.7494-7505.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Umpierre AD, Bennett IV, Nebeker LD, Newell TG, Tian BB, Thomson KE, White HS, White JA, Wilcox KS, 2016. Repeated low-dose kainate administration in C57BL/6J mice produces temporal lobe epilepsy pathology but infrequent spontaneous seizures. Exp. Neurol 279, 116–126. https://doi.org/10.1016/j.expneurol.2016.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Umpierre AD, Remigio GJ, Dahle EJ, Bradford K, Alex AB, Smith MD, West PJ, White HS, Wilcox KS, 2014. Impaired cognitive ability and anxiety-like behavior following acute seizures in the Theiler’s virus model of temporal lobe epilepsy. Neurobiol. Dis 64, 98–106. https://doi.org/10.1016/j.nbd.2013.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vezzani A, Lang B, Aronica E, 2015. Immunity and Inflammation in Epilepsy. Cold Spring Harb. Perspect. Med 6, a022699 https://doi.org/10.1101/cshperspect.a022699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Z-Y, Sun B-L, Liu J-K, Yang M-F, Li D-W, Fang J, Zhang S, Yuan Q-L, Huang S-L, 2015. Activation of mGluR5 Attenuates Microglial Activation and Neuronal Apoptosis in Early Brain Injury After Experimental Subarachnoid Hemorrhage in Rats. Neurochem. Res 40, 1121–1132. https://doi.org/10.1007/s11064-015-1572-7 [DOI] [PubMed] [Google Scholar]
- 41.Zurbriggen A, Fujinami RS, 1989. A neutralization-resistant Theiler’s virus variant produces an altered disease pattern in the mouse central nervous system. J. Virol 63, 1505–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.