Abstract
Flavonoids are dietary compounds with potential anti-diabetes activities. Many flavonoids have poor bioavailability and thus low circulating concentrations. Unabsorbed flavonoids are metabolized by the gut microbiota to smaller metabolites, which are more bioavailable than their precursors. The activities of these metabolites may be partly responsible for associations between flavonoids and health. However, these activities remain poorly understood. We investigated bioactivities of flavonoid microbial metabolites [hippuric acid (HA), homovanillic acid (HVA), and 5-phenylvaleric acid (5PVA)] in primary skeletal muscle and β-cells compared to a native flavonoid ([(−)-epicatechin, EC]. In muscle, EC was the most potent stimulator of glucose oxidation, while 5PVA and HA simulated glucose metabolism at 25 μM, and all compounds preserved mitochondrial function after insult. However, EC and the metabolites did not uncouple mitochonndrial respiration, with the exception of 5PVA at10 μM. In β-cells, all metabolites more potently enhanced glucose-stimulated insulin secretion (GSIS) compared to EC. Unlike EC, the metabolites appear to enhance GSIS without enhancing β-cell mitochondrial respiration or increasing expression of mitochondrial electron transport chain components, and with varying effects on β-cell insulin content. The present results demonstrate the activities of flavonoid microbial metabolites for preservation of β-cell function and glucose utilization. Additionally, our data suggest that metabolites and native compounds may act by distinct mechanisms, suggesting complementary and synergistic activities in vivo which warrant further investigation. This raises the intriguing prospect that bioavailability of native dietary flavonoids may not be as critical of a limiting factor to bioactivity as previously thought.
Keywords: hippuric acid, homovanillic acid, 5-phenylvaleric acid, (−)-epicatechin, insulin, respiration
1. INTRODUCTION
Incidence rates of type-2 diabetes and obesity are rising worldwide. In adition to traditional medical interventions, complementary lifestyle strategies such as diet and exercise are needed to blunt this epidemic. Flavonoids from cocoa, fruit, tea and other sources have been identified as dietary bioactive compounds with potential anti-obesity and anti-diabetes activities. Many of these flavonoids, such as quercetin [1] and procyanidins [2], have poor oral bioavailability and thus low circulating concentrations. Non-extractable/bound flavonoids (from cocoa, etc.) and oxidized flavonoids, such as theaflavins and thearubigins from oolong and black teas, have extremely limited oral bioavailability [3,4] and vanishing low circulating concentrations. As an extreme example, consumption of 700 mg theaflavins (equivalent to ~30 cups of black tea), produced maximal blood concentrations of only 1 μg/L (~1.8 nM) in humans [3]. Therefore, circulating concentrations of the native species may represent a very small fraction of the ingested dose, whereas the majority reaches the colon unabsorbed. Unabsorbed flavonoids are extensively metabolized by the gut microbiota to a series of smaller metabolites such as valerolactones, phenylalkyl acids, and smaller aromatics (Figure 1A) [4–9]. While some metabolites are unique to individual flavonoid compounds or subclasses, dozens of metabolites are common to most flavonoids [10,11]. These metabolites are comparatively more bioavailable than their native flavonoid precursors, and in many cases represent the predominant circulating forms following flavonoid consumption [10]. For example, a recent study of pharmacokinetics following consumption of grape pomace demonstrated that anthocyanins and procyanidins were not detected in blood and catechins and their phase-II conjugates exhibited maximum blood levels of 7–136 nM (with only 1 compound reaching at least 100 nM), while microbial metabolites exhibited maximum blood levels of 3–1170 nM (with 8 compounds reaching at least 100 nM) [12]. In an extreme example, consumption of 6 cups of green or black tea resulted in circulating metabolite levels in the mM range (hippuric acid, HA, reached 2.3 mM) [13]. This highlights the comparative importance of these metabolites as potential bioactives in circulation following the consumption of flavonoids.
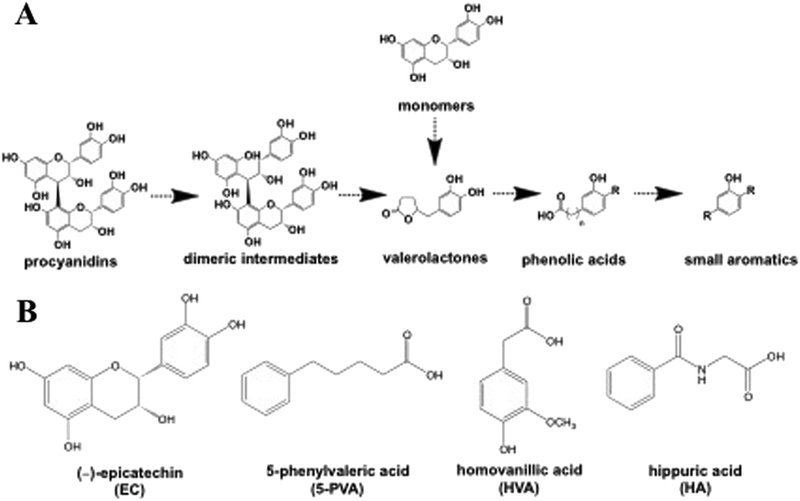
Figure 1.
A) Schematic showing representative sequential metabolism of representative flavonoids [a dimeric procyanidin, and (−)-epicatechin monomer] by the gut microbiota. B) Structures of (−)-epicatechin and the three representative flavonoid microbial metabolites employed in this present study.
Even flavonoids with comparatively high bioavailability (monomeric catechins, etc.) are only present in the bloodstream at nM to very low μM levels following consumption of typical doses in foods and supplements [14,15]. These doses are generally lower than the range of concentrations typically used to study mechanisms in cell culture models (1–100 μM, or sometimes higher). Despite poor bioavailability and low circulating concentrations, many of these compounds (and foods rich in them) appear to effectively prevent or ameliorate metabolic syndrome even at low dietary doses in animals [16] and humans [17]. Dietary efficacy, despite poor bioavailability and/or low circulating concentrations of the native forms, suggests three mechanisms by which ingested flavonoids exert their activities: 1) native flavonoids primarily exert their activities in the gut lumen (inhibition of digestive enzymes, alteration of microbiome composition and function, etc.) [18,19] and/or epithelium (improving barrier function, immune development, etc.) [20] where they are at highest concentrations (μM-mM range), 2) native flavonoids primarily exert their activity in peripheral tissues even at the very low (pM-low μM range) circulating levels achieved, or 3) microbial metabolites of flavonoids generated by commensal microbiota in the lower gut exert activities locally in the gut and systemically [21,22].
Considering the relatively high concentrations of microbial metabolites documented in plasma compared to the native compounds, it is plausible that these metabolites may be responsible, at least in part, for observed associations between dietary flavonoids and health outcomes. While all of the three possible scenarios identified above likely occur simultaneously, the potential anti-diabetic and anti-obesity activities of microbial metabolites formed from unabsorbed flavonoids remain poorly understood.
Recent provocative evidence has strengthened the argument that native flavonoids may exert their effects independent of systemic bioavailability: either directly on the microbiota, or by formation of bioavailable microbial metabolites that then act in peripheral tissues [23]. In vitro, 3-(3-hydroxyphenyl)propionoic acid (a microbial metabolite common to many flavonoids) prevented loss of insulin-stimulated nitric oxide synthesis and activity under high glucose concentrations in human aortic endothelial cells [24]. In human skeletal muscle myotubes, various microbial metabolites stimulated glucose and oleic acid uptake [25]. Recent studies demonstrated that phenylacetic and phenylpropionic acid have protective activities in pancreatic β-cells and islets [26,27] and protect hepatocytes from acetaminophen injury [28]. Two recent studies demonstrated that valerolactones inhibited monocyte adhesion to endothelial cells [29]. A key animal study demonstrated that administration of antibiotics (depletion of gut microbiota and their associated metabolites) abolished the ability of procyanidin-rich grape seed extract to prevent inflammation, insulin resistance, hyperglycemia and weight gain in a high-fat feeding mouse model [30]. Furthermore, antibiotic administration reversed the ability of blackcurrant anthocyanins to ameliorate diet-induced obesity in mice [31]. Finally, digestion and microbial metabolism of berry flavonoids did not diminish their protective activities against colon cancer [32]. While the in vivo studies did not measure metabolite production, they strongly suggest that these effects are mediated by the microbiota and/or their metabolites produced from the native dietary flavonoids. Perhaps the most well-known microbial metabolites, the phenylalkyl acids (phenylacetic, phenyl propionic, and phenylvaleric acids) have not been well studied, and the phenylvaleric acids have not been studied at all to our knowledge. Some compounds that are microbial metabolites have been studied, but only because they also exist as native compounds in foods, such as the cinnamic acids and small aromatics such as vanillic acid. These compounds have been shown to possess anti-diabetic and anti-obesity activities in β-cell, skeletal muscle, hepatocyte and adipose models (see Supplementary Information). Finally, some microbial metabolites of flavonoids have been shown to possess enhanced anti-tumor and anti-platelet agreggation activities compared to the native forms [33].
Despite these promising findings, relatively little work has been done to characterize the effects of these metabolites in cell or animal models, in comparison to the exhaustive body of literature on the bioactivities of native flavonoids. The majority of research that does exist on these metabolites has focused on their formation, but not their activities nor mechanisms of action. Our objectives were therefore to 1) investigate the anti-diabetic activities of microbial flavonoid metabolites (including a poorly-studied class, phenylvaleric acids) in β-cells and primary skeletal muscle cells, 2) compare these activities to those of a control native flavonoid, and 3) suggest potential mechanisms by which these activities may occur. Our findings demonstrate that these metabolites possess potent bioactivities, and may contribute to the observed peripheral tissue effects of dietary flavonoids.
2. MATERIALS AND METHODS
2.1. Materials
Three representative metabolites representative of three distinct classes of metabolites common to a variety of dietary flavonoids were selected for investigation: hippuric acid (HA, 98%), homovanillic acid (HVA), and 5-phenylvaleric acid (5PVA, 99%) were obtained from Sigma (St. Louis, MO). A native flavanol, (−)-epicatechin (EC, Sigma), was used as a positive control; note that the three selected metabolites can be obtained by metabolism of EC and related compounds [9]. Structures of these compounds are shown in Figure 1B. All compounds were tested over a range of 0–100 μM (depending upon the specific assay) in water or DMSO, with equal final concentrations of DMSO in cell media for all treatments. Generally, doses of 5–25 μM were employed, which are easily obtainable in circulation for metabolites but which represent the extreme upper end of what is attainable for native flavonoids [13,34]. Microbial metabolites, similar to those of native flavonoids, exhibit pharmacokinetic curves that depend on a variety of factors and circulating concentrations necessarily fluctuate over time based on consumption frequency. The levels employed herein are attainable following flavonoid consumption but are not continuously present, similar to those of native dietary flavonoids. Furthermore, while compounds and doses were uniform across experiments, differences in some aspects (treatment times, etc.) were necessary due to the use of established, robust experimental protocols for each model system.
2.2. Skeletal muscle experiments
Skeletal muscle metabolism experiments were conducted per previously published methods [35,36], with modifications. Primary human muscle cells were cultured for measuring palmitate and glucose oxidation. Cultures of primary human muscle cells were obtained from a singler subject who provided written informed consent under an approved protocol by Virginia Polytechnic Institute and State University Institutional Review Board (approval #11–770). The subject was a healthy Caucasian male, age 22 years, with a BMI of 23.6 and 20.9% body fat.
2.2.1. Skeletal muscle substrate metabolism
Cells were grown in low glucose DMEM supplemented with 10% fetal bovine serum and SkGM SingleQuots (Lonza, Walkersville, MD). Upon reaching ~80% confluence in standard 12-well plates, cells were differentiated for 7 days in 2% horse serum. All experiments were performed on day 7 of differentiation following overnight serum deprivation. The compounds tested were treated for 24 hours prior to assessment of substrate metabolism. Fatty acid oxidation was assessed by measuring and summing 14CO2 production (complete) and 14C-labeled acid-soluble metabolites (incomplete) from the oxidation of [1-14C] palmitic acid (American Radiolabeled Chemicals, St. Louis, MO). Briefly, cells were incubated in media containing radiolabeled substrate along with the compound at 5 or 10 μM, or vehicle only (0 μM, 0.1% DMSO) for 3 hours at 37ºC, 5% CO2. Following incubation media was removed and acidified with 45% perchloric acid to elute gaseous 14CO2. 14CO2 was trapped in 1M NaOH over the course of 1 hour. The NaOH was then placed in a liquid scintillation counter and counted. Data were expressed as means ± SEM and is normalized to total protein content. Glucose oxidation was assessed by measuring 14CO2 production from the oxidation of [U-14C] glucose (American Radiolabeled Chemicals, St. Louis, MO) in a manner similar to fatty acid oxidation expect for the substitution of glucose in place of palmitic acid. Compounds were tested at 10 and 25 μM.
2.2.2. Skeletal muscle cell respiration
Oxygen consumption rate (OCR) was measured with our established protocols [37] using a XF96 Seahorse Extracellular Flux Analyzer (Agilent Technologies, Santa Clara, California, USA). C2C12 myoblast studies are commonly used by our groups as a fast and practical model to screen for compound efficacy. Because differentiating cells into myotubes takes 7 days continuously in the SeaHorse plate, we utilized the myoblasts as a more feasible approach. Cultured C2C12 muscle cells were seeded at a density of 1.5 × 104 per well in supplemented DMEM media [4.5 g/L D-Glucose, L-Glutamine, and 110 mg/L Sodium Pyruvate supplemented with 10% Fetal Bovine Serum (FBS) and 1% Penicillin Streptomycin (PSA)] on a Seahorse XF96 Cell Culture Microplate. Cells were then incubated overnight at 37ºC in 5% CO2 to allow for adherence. Following adherence, cells were pretreated for 4 hours with 10% FBS/1% PSA DMEM containing the test compounds (5 and 10 μM) or vehicle only (≤0.1 % DMSO). After the 4-hour pretreatment, 500 μM H2O2 was added to injure the cells, and the microplate was subsequently incubated for an additional 4 hours. Following incubation, the cells were washed with supplemented XF media (XF base media plus 1 mM pyruvate, 2 mM glutamine, 10 mM glucose) twice before adding a final volume of 180 μL per well. A XF Cell Mitochondrial Stress Test was completed to assess the bioenergetic status of the cells by injecting ATP synthase inhibitor oligomycin (1 μg/mL), inner membrane uncoupler fluorocarbonyl cyanide (FCCP, 2 μM), and complex III inhibitor antimycin A (2 μM). Oxygen consumption rate data were normalized by subtracting non-mitochondrial rates of respiration (after antimycin A), and are expressed as pmol O2 per minute per 1.5 × 104 cells. Mitochondrial coupling efficiency was calculated by taking the ATP-dependent respiration (baseline-oligomycin) and dividing by the basal rates for internal normalization.
2.3. β-cell experiments
β-cell metabolism experiments were conducted per previously published methods, with modifications [38,39].
2.3.1. INS-1 832/13 β-cell culture
Cell culture was performed per our established protocols [40–44] The INS-1 derived 832/13 rat β-cell line was maintained in complete RPMI 1640 medium with L-glutamine and 11.2 mM glucose supplemented with 50 U/ml penicillin, 50 μg/ml streptomycin, 10 mM HEPES, 10% fetal bovine serum, and INS-1 supplement, as previously described. For all glucose-stimulated insulin secretion and respiration assays using the 832/13 β-cells, cells were plated at 0 hours, treated with test compounds at 24 hours, and harvested at 48 hours. Stock solutions of test compounds were made at 100mM, and diluted in media for assays at final concentrations of 0100 μM (0.1% DMSO in all treatments).
2.3.2. Glucose-stimulated insulin secretion
Glucose-stimulated insulin secretion (GSIS) was performed as previously described [40]. Briefly, INS-1 832/13 β-cells were plated and grown to confluency in standard 24-well plates. Upon reaching confluency, cell were cultured with test compounds at 0 −100 μM in complete media for 24 hours. Following the 24 hour treatment, cells were washed with PBS and preincubated in secretion assay buffer (SAB) for 1.5 hours (114 mM NaCl, 4.7 mM KCl, 1.2 mM KH2PO4 1.16 mM MgSO4, 20 mM HEPES, 2.5 mM CaCl2, 0.2% BSA, pH 7.2) containing 2.5 mM glucose. GSIS was performed by incubating quadruplicate replicate wells of cells previously cultured with test compounds in SAB containing 2.5 mM glucose for 1 hour (basal), followed by 1 hour in SAB with 16.7 mM glucose (glucose stimulation), followed by collection of the respective buffers, as previously described. For total insulin content, β-cells stimulated with 16.7 mM glucose for 1 hour were lysed in RIPA buffer with protease inhibitors (Life Technologies). Secreted insulin and total insulin was measured in SAB using a rat insulin RIA kit (MP Biomedicals), and normalized to total cellular protein concentration (determined by BCA assay), as previously described.
2.3.3. INS-1 832/13 β-Cell Oxygen Consumption Rate
Oxygen consumption rate (OCR) was measured using an XFp Extracellular Flux Analyzer (Agilent Technologies). INS-1 832/13 β-cells were seeded at 2.0 × 104 cells/well in complete 832/13 RPMI 1640 medium (L-glutamine, 11.2 mM glucose supplemented, 50 U/ml penicillin, 50 μg/ml streptomycin, 10 mM HEPES, 10% fetal bovine serum, and INS-1 supplement) on a Seahorse XFp Cell Culture Microplate. Cells were incubated overnight and then treated with test compounds at 10 μM, 5 μM or 0 μM in complete RPMI 1640 media. Following 24 hours of culture with the compounds, cells were incubated in 2.5mM glucose SAB for 3 hours. Following incubation, buffer was exchanged for 180 μL fresh pre-warmed 2.5mM glucose SAB per well. A XF Cell Mitochondrial Stress Test was completed to assess the bioenergetic status of the cells by injecting glucose (16.7mM, in order to examine respiration under glucose stimulation), oligomycin (4 μM), FCCP (2.5 μM), and antimycin A with rotenone (2.5 μM). Residual oxygen consumption was determined following inhibition of complex III with the addition of rotenone and antimycin A. This state of residual oxygen consumption served as a baseline correction for all of the other states. All data were normalized to protein content of each well, determined by BCA assay.
2.3.4. Western blotting
832/13 beta cells were plated in standard 6-welll plates, grown to confluency, and cultured overnight in media containing each test compound at 10 μM or vehicle control (0.1% DMSO in both). Cells were washed in PBS and harvested in RIPA buffer followed by sonication. Protein concentration was quantified by BCA, and 30 μg was run per sample. Western blotting and transfer was performed as previously described [38,40,41]. Blot were probed using the Anti Rt/Ms Total OxPhos Complex Kit (1:250, Life Technologies, Carlsbad, CA) which contains a cocktail of antibodies for the electron transport chain (ETC) components ATP5A (Complex V), UQCR2 (Complex III), MTCO1 (Complex IV), SDHB (Complex II) and NDUFB8 (Complex I). Blot was imaged in the linear range using a LI-COR Odyssey CLx (LI-COR Biotechnology, Lincoln, NE). Blotting was performed on triplicate samples.
2.4. Statistics
All results are expressed as mean ± SEM. For activity assays, data were analyzed by 1- or 2-way ANOVA as appropriate. For 2-way ANOVAs, if a significant main effect of treatment compound dose was detected, Dunnett’s post hoc test was performed within the high-glucose treatments to compare each dose to the vehicle (0 μM) control. For 1-way ANOVAs, if a significant treatment effect was detected, Dunnett’s post hoc test was performed within each compound to compare each dose to the vehicle controls. Significance was defined a priori as P < 0.05. Statistical anslyses were performed on Prism v6.0f (GraphPad, La Jolla, CA).
3. RESULTS AND DISCUSSION
3.1. Skeletal muscle
3.1.1. Skeletal muscle metabolism
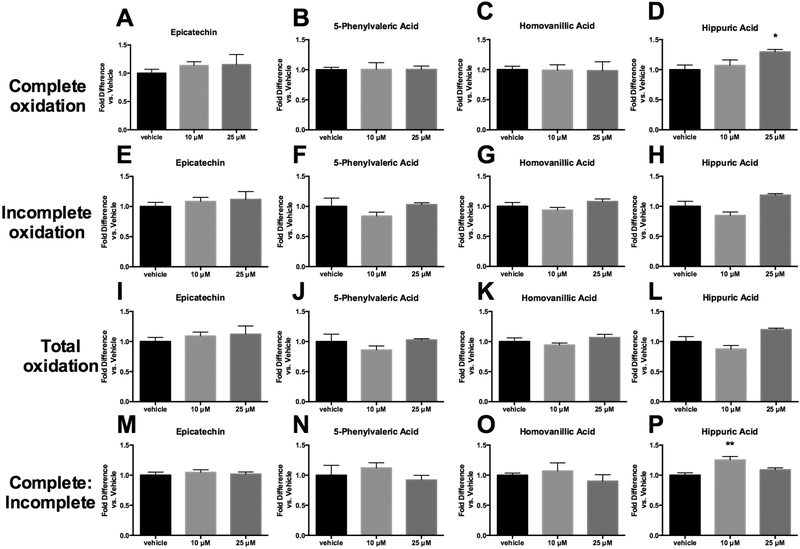
The ability of EC (+ control, native flavonoid) and 3 representative metabolites (HA, HVA and 5PVA) to influence fatty acid or glucose uptake and metabolism was examined in primary human skeletal muscle cells. As shown in Figure 2, these compounds exhibited minimal ability to alter fatty acid oxidation. The only statistically significant findings were that HA was able to increase complete fatty acid oxidation at 25 μM (Figure 2D) and increase the ratio of complete: incomplete oxidation at 10 μM (Figure 2P). While these results suggest that HA has more potent activities than EC, overall the enhancement of fatty acid oxidation does not seem to be a significant mechanism of action for these metabolites. These results suggest that, despite a reported finding that metabolites increased oleic acid uptake in human skeletal muscle myotubes [25], alteration of fatty acid oxidation in skeletal muscle may not be a primary mechanism by which flavonoid microbial metabolites exert anti-diabetic and anti-obesity activities.
Figure 2.
Fatty acid oxidation in primary human skeletal muscle cells treated with either hippuric acid, homovanillic acid, 5-phenylvaleric acid, or epicatechin. Complete oxidation represents evolution of 14CO2 from 14C-labeled palmitate. Incomplete oxidation represents production of 14C-labeled acid-soluble metabolites (ASM) from 14C-labeled palmitate. Total oxidation represents the sum of complete and incomplete oxidation. Values represent mean ± SEM from n=4 replicates, normalized to vehicle (vehicle expressed as 1). Data were analyzed by 1-way ANOVA. If a significant treatment effect was detected, Dunnett’s post hoc test was performed within each compound to compare each dose to the vehicle control. Significance vs. vehicle control is indicated by: *P ≤ 0.05, **P ≤ 0.01.
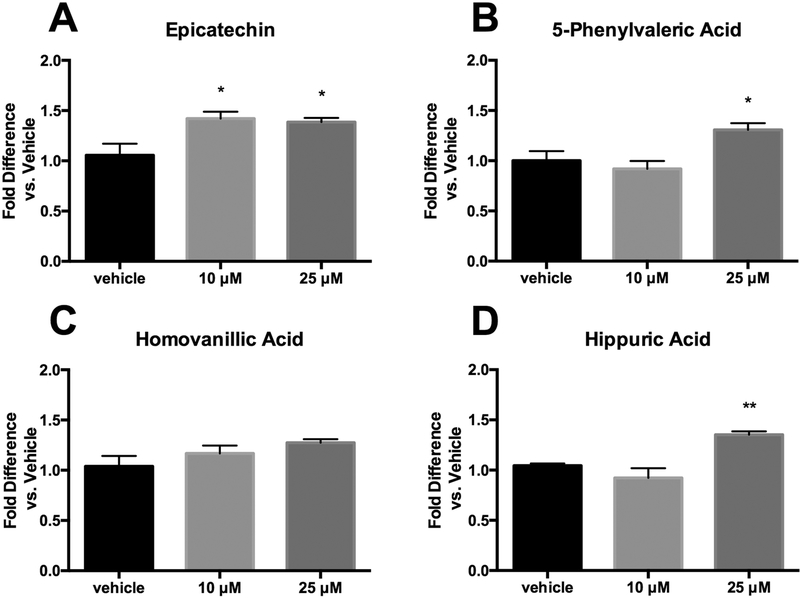
Glucose oxidation results (Figure 3) were more promising than fatty acid oxidation. EC appeared to be the most potent stimulator of glucose utilization, increasing activity at both 10 and 25 μM (Figure 3A). While HVA had no apparent activity, both 5PVA and HA were able to simulate glucose metabolism at 25 μM (Figures 3B-D). While the EC activity at lower concentrations suggests that it is more potent than the metabolites on an equal concentration basis, it is important to keep in mind that the metabolites tend to exist in circulation at higher levels than the native forms. Thus, the observed increase in glucose oxidation for 5PVA and HA, combined with previous reports that microbial metabolites stimulate glucose uptake [25], suggest promise for the ability of these metabolites to exert significant benefits on blood glucose levels in vivo.
Figure 3.
Glucose oxidation in primary human skeletal muscle cells treated with either hippuric acid, homovanillic acid, 5-phenylvaleric acid, or epicatechin. Oxidation represents evolution of 14CO2 from 14C-labeled glucose. Values represent mean ± SEM from n=4 replicates, normalized to vehicle (vehicle expressed as 1). Data were analyzed by 1-way ANOVA. If a significant treatment effect was detected, Dunnett’s post hoc test was performed within each compound to compare each dose to the vehicle control. Significance vs. vehicle control is indicated by: *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001.
3.1.2. Skeletal muscle cell respiration
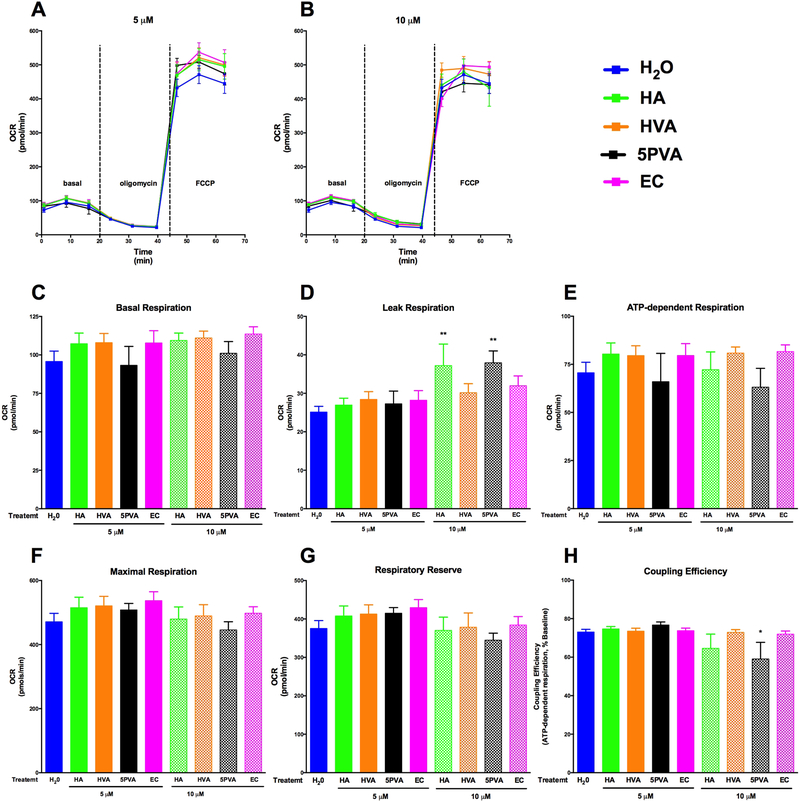
The effects of EC and the three metabolites on respiration in normal, uninjured C2C12 cells are shown in Figure 4. We utilized a peroxide stress paradigm since heightened mitochondrial ROS burdens are observed in skeletal muscle from humans and animal models of diabetes, often before the onset of overt systemic hyperglycemia [45]. Respiration curves for controls and each dose, including basal, leak (oligomycin) and maximal (FCCP) respiration, are shown in Figure 4A-B. None of the compounds tested significantly enhanced basal respiration (Figure 4C), ATP-dependent respiration (Figure 4E), maximal respiration (Figure 4F), or respiratory reserve (the difference between basal and maximal respiration, which reflects reserve bioenergetic capacity available to the cell, Figure 4G) compared to the control at either 5 or 10 μM compared to vehicle control. Coupling efficiency was not influenced by any of the compounds at any concentration, with the exception of 5PVA at 10 μM (Figure 4H). HA and 5PVA both modestly enhanced ‘leak’ respiration at 10 μM (Figure 4D), suggesting either slight mitochondrial injury (potentially due to minor pro-oxidant effects at these higher doses) or mitochondrial uncoupling. The data in uninjured cells generally suggest that EC and the metabolites do not alter skeletal muscle repiration under normal conditions at low doses, and indicate that do not appear to acutely uncouple mitochondria or partially inhibit the respiratory chain (both of which have been postulated as a strategy to treat obesity/diabetes for decades) with the possible exception of HA and 5PVA at high doses [46,47].
Figure 4.
Corrected mitochondrial respiration data for C2C12 cells cells cultured acutely (4h) in the presence of hippuric acid (HA), homovanillic acid (HVA), 5-phenylvaleric acid (5PVA), or epicatechin (EC): oxygen consumption rate (OCR) curves for treatments at 5 μM (A) and 10 μM (B), basal respiration (C), leak respiration (after oligomycin, D), ATP-dependent respiration (E),maximal respiration (after FCCP, F) respiratory reserve (maximal − basal, G), and coupling efficiency (ATP-dependent respiration/basal respiration, H). Oxygen consumption rate data were normalized by subtracting non-mitochondrial rates of respiration (after antimycin A, not shown), and are expressed as pmol O2 per minute per 1.5 × 104 cells. Values represent mean ± SEM from n=8 replicates. Data were analyzed by 1-way ANOVA. If a significant treatment effect was detected, Dunnett’s post hoc test was performed within each compound to compare each dose to the vehicle control (H2O). Significance vs. vehicle control is indicated by: *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001.
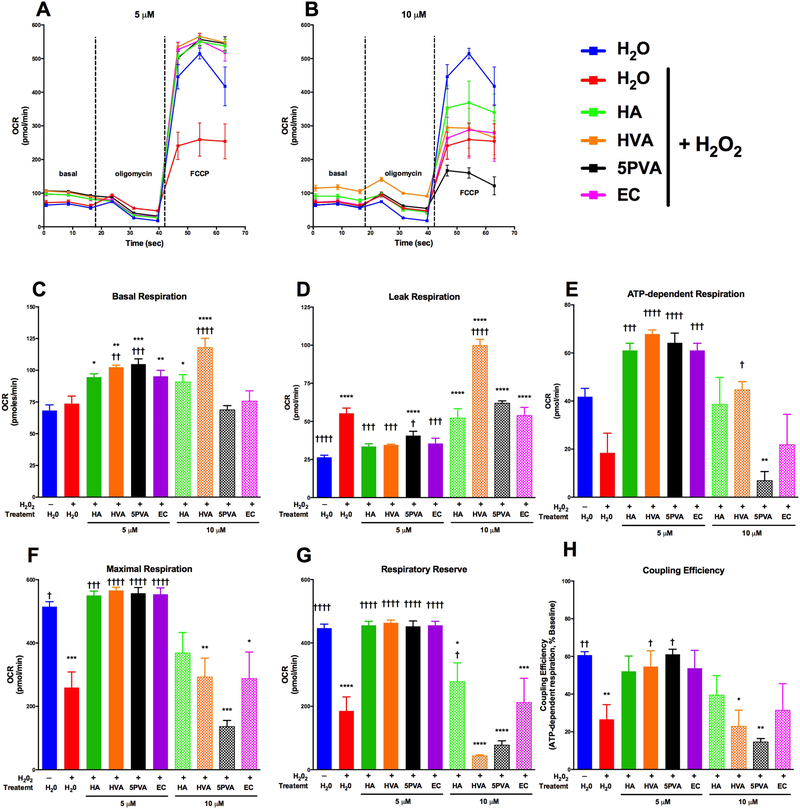
The effects of EC and the metabolites on C2C12 cells exposed to peroxide challenge (i.e. injured) are presented in Figure 5. Peroxide treatment induced mitochondrial injury as assessed by increased ‘leak’ respiration (respiration after oligomycin roughly doubled) (Figure 5D) and lower rates of maximal respiration (FCCP), ATP-dependent respiration (Figure 5E), respiratory reserve capacity (Figure 5G), and coupling efficiency (Figure 5H) for H2O2 treated cells (red bars) compared to control (blue bars). While there were some differences in basal respiration, this can be due to slight respiratory uncoupling due to the injury and should be interpreted with caution. Each of the compounds studied significantly protected against peroxide-mediated injury at 5 μM, reflected by reduced leak respiration, and preserved maximal respiration respiratory reserve and/or coupling efficiency at the same level as the uninjured control despite peroxide challenge (Figures 5D-H). As observed for uninjured cells, one metabolite actually worsed cell injury as measured by leak respiration, although in this case it was 10 μM HVA (as opposed to HA and PVA in uninjured cells), again suggesting either cellular injury or uncoupling. Interestingly, while HA and 5PVA increased leak respiration in the absence of H2O2, there was only a slight additional increase in leak respiration with H2O2 treatment. These data indicate that HA and 5PVA may have pro-oxidant effects similar to H2O2, but that these metabolites did not exacerbate leak respiration when combined with H2O2 stress. Future studies that further examine the effects of HA and 5PVA will advance our understanding of these compounds on mitochondrial bioenergetics. The 10 μM dose was generally ineffective for all compounds except HA, which partly preserved respiratory reserve (Figure 5E). These results suggest that EC and the flavonoid microbial metabolites preserve skeletal mitochondrial function after oxidative insult, notably at lower micromolar concentrations.
Figure 5.
Corrected mitochondrial respiration data for H2O2-injured C2C12 cells cultured acutely (4h) in the presence of hippuric acid (HA), homovanillic acid (HVA), 5-phenylvaleric acid (5PVA), or epicatechin (EC): oxygen consumption rate (OCR) curves for treatments at 5 μM (A) and 10 μM (B), basal respiration (C), leak respiration (after oligomycin, D), ATP-dependent respiration (E), maximal respiration (after FCCP, F) respiratory reserve (maximal – basal, G), and coupling efficiency (ATP-dependent respiration/basal respiration, H). Oxygen consumption rate data were normalized by subtracting non-mitochondrial rates of respiration (after antimycin A, not shown), and are expressed as pmol O2 per minute per 1.5 × 104 cells. Values represent mean ± SEM from n=8 replicates. Data were analyzed by 1-way ANOVA. If a significant treatment effect was detected, Dunnett’s post hoc test was performed within each compound to compare each dose to the vehicle control (H2O) as well as injury control (H2O + H2O2). Significance vs. vehicle control is indicated by: *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001; significance vs. injury control is indicated by: †P ≤ 0.05, ††P ≤ 0.01, †††P ≤ 0.001, ††††††P ≤ 0.0001.
3.2. β-cells
3.2.1. β-cell glucose-stimulated insulin secretion
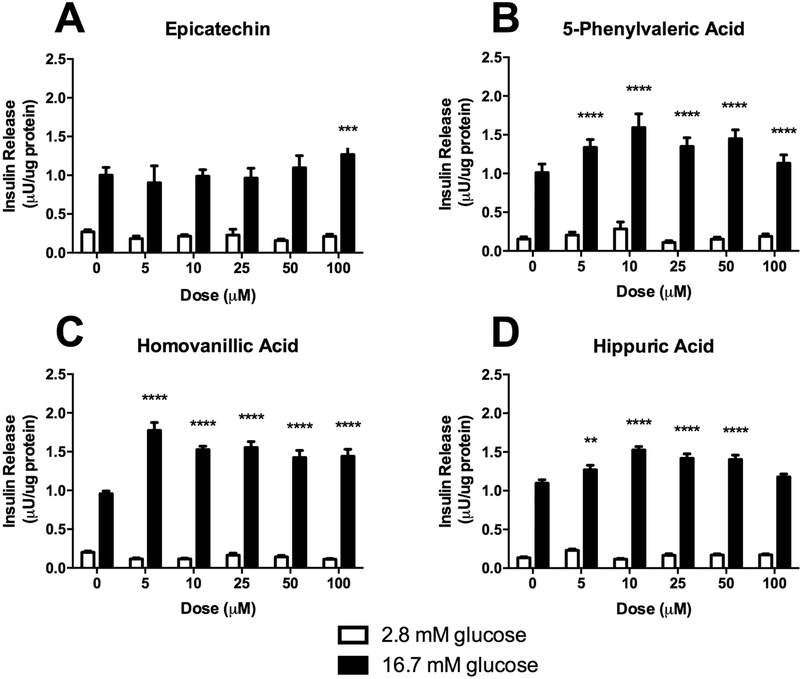
In addition to substrate utilization in skeletal muscle, β-cell function is a critical target at all stages of diabetes development. We sought to examine the impact of EC and representative flavonoid metabolites on GSIS in a β-cell model (Figure 6). We have previously demonstrated that the epicatechin-rich fraction from cocoa enhances β-cell GSIS at 25 μg/ml [38]. In the present experiment, EC was able to enhance GSIS in INS-1 832/13 β-cells but only at 100 μM (Figure 6A), which is not physiologically relevant, suggesting minimal relevance for activity in vivo. Interestingly, all three microbial metabolites demonstrated significant induction of GSIS at concentrations from 5–100 μM (Figure 6B-D) except HA, which induced GSIS at 5–50 μM but not 100 μM). These data demonstrate that the metabolites increase GSIS at much lower (and physiologically relevant) concentrations compared to EC, suggesting that the metabolites are more potent stimulators of GSIS than native EC. This fact, combined with the greater bioavailability of microbial metabolites than the parent compound, point towards the potential contribution of microbial metabolites to the observed effects of dietary flavanoids.
Figure 6.
Glucose-stimulated insulin secretion in INS-1 derived 832/13 rat β-cells treated with either hippuric acid, homovanillic acid, 5-phenylvaleric acid, or epicatechin. Values represent mean ± SEM from n=6 replicates. Data were analyzed by 2-way ANOVA. If a significant main effect of treatment compound dose was detected, Dunnett’s post hoc test was performed within the high-glucose treatments to compare each dose to the untreated (0 μM) control. Significance vs. untreated control is indicated by: *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001.
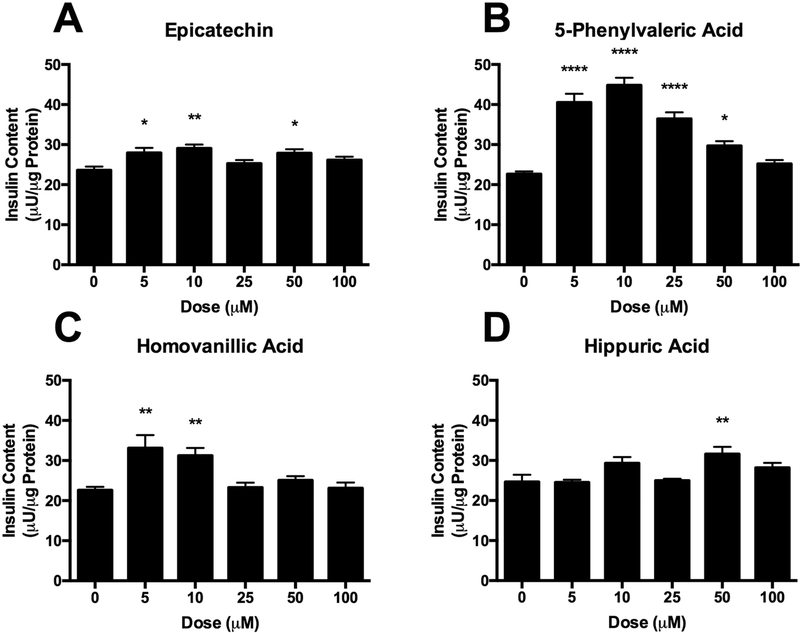
To further investigate the effects of these compounds on INS-1 832/13 β-cells, we examined the cellular insulin content under stimulatory conditions (16.7 mM glucose) to determine if treatment impacted insulin expression (Figure 7). An increase in insulin content, concomitant with an increase in insulin secretion would indicate greater insulin expression, while a decrease in insulin content with no change in insulin secretion would indicate an impediment in insulin production. EC exhibited small increases in insulin content (Figure 7A), but the effect was inconsistent across doses. Interestingly, increases in insulin content were vastly different across the metabolites (Figure 7B-D), despite similarities observed in GSIS. 5PVA and HVA stimulated greater insulin content, particularly at lower doses. HA exhibited a slight increase in insulin content at 50 μM. These results are intriguing, as they suggest distinct mechanism at play that impinges on β-cell insulin secretion. The results for EC are consistent with our previous results demonstrating increased insulin secretion at high doses, without concurrent increase in insulin content [38]. For 5PVA and HVA we observed increased GSIS and increased cellular insulin content. The increased insulin content could be due to greater insulin gene expression, enhanced insulin processing, or improved insulin stability. As has been previously shown, increased cellular insulin content can be sufficient to enhance GSIS [48]. Therefore, the enhanced insulin secretion from β-cells treated with these metabolites, particularly at lower doses, may be due to an increased insulin load, rather than modulation of the β-cell glucose sensing machinery. The GSIS observed by HA occurs with minimal changes to insulin content. The data suggest that flavonoid microbial metabolites may exert significant effects on β-cell function by increasing both β-cell insulin production and insulin secretion. These distinct mechanisms suggest complementary and synergistic activities of various metabolites present simultaneously following flavonoid consumption, and thus warrant further investigation in vitro and in vivo.
Figure 7.
Total insulin content of INS-1 derived 832/13 rat β-cells cultured in 16.7 mM glucose treated with either hippuric acid, homovanillic acid, 5-phenylvaleric acid, or epicatechin. Values represent mean ± SEM from n=6 replicates. Data were analyzed by 1-way ANOVA. If a significant treatment effect was detected, Dunnett’s post hoc test was performed to compare each dose to the untreated (0 μM) control. Significance vs. untreated control is indicated by: *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001.
3.2.2. β-cell respiration
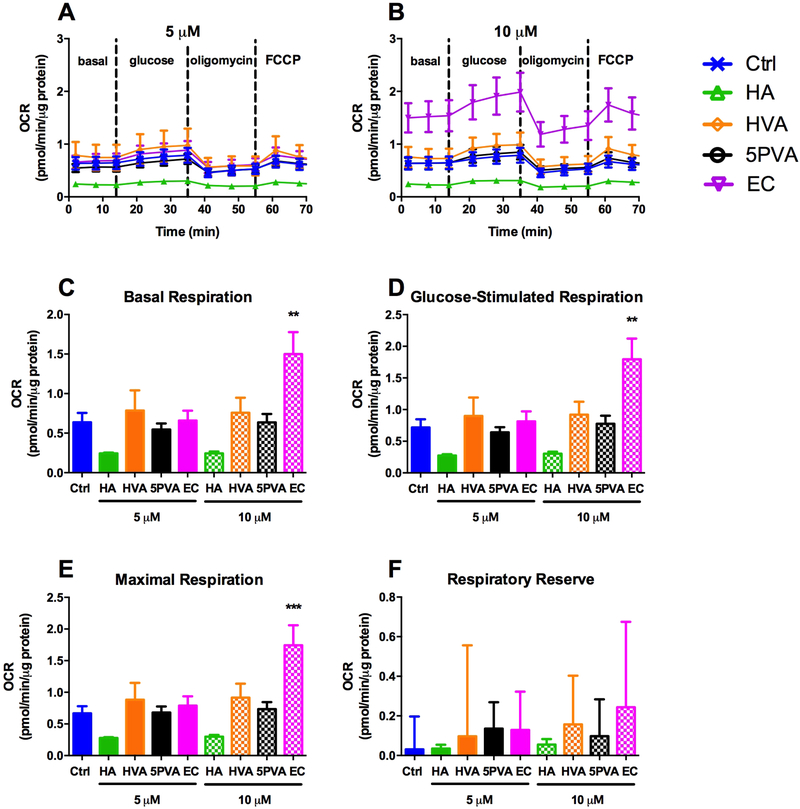
Given our previous data demonstrating enhanced β-cell mitochondrial respiration due to exposure to EC from cocoa [38,39], we sought to define the effect of culture in the presence of EC and microbial metabolites on β-cell mitochondrial respiration under basal conditions (low glucose) and glucose stimulation (Figure 8). Basal respiration rate was significantly increased by 10 μM EC, and appeared to be somewhat reduced (albeit not statistically significantly) by 5 and 10 μM HA (Figure 8C). The same results were also observed under glucose stimulation and maximal respiration (although the level of glucose induced respiration is surprisingly less that what has been observed in other studies) (Figure 8D-E). None of the compounds tested significantly affected respiratory reserve (Figure 8F). It is important to note that the low means and comparatively high SEMs for respiratory reserve in this case are indicative of the fact that these cells were essentially already operating near maximal respiration in the basal state (Figure 8A-B, F). Note that uncoupling and ATP-dependent respiration were not plotted individually from these data due to differences in the question being asked between the β-cells (do these compounds enhance respiration as a means to improve β-cell function?) vs. the skeletal muscle cells (do these compounds enhance respiration via uncoupling as a means to improve energy expenditure, and do they protect from injury?). The finding that EC enhances respiration is consistent with our previous data [38,39]. Coupled with the GSIS data (Figure 6), these respiration data suggest several novel findings. First, EC does not enhance GSIS except at extremely high doses despite enhacing β-cell respiration at lower doses. Second, HA enhances GSIS despite inhibition of β-cell respiration (although these reductions were not statistically significant, this trend appears to be of practical significance as suggested by Figures 8C-E). Third, HVA and 5PVA enhance GSIS despite not affecting β-cell respiration. Thus, these data demonstrate that while each of the epicatechin metabolites enhance GSIS; their individual mechanisms do not all increase insulin release through modulating mitochondrial respiration. Therefore, the mechanisms by which these compounds exert their effects are likely distinct and thus warrant further investigation.
Figure 8.
Corrected mitochondrial respiration measured after culturing INS-1 832/13 β-cells for 24 h in the presence of 0, 5 or 10 μM hippuric acid (HA), homovanillic acid (HVA), 5phenylvaleric acid (5PVA), or epicatechin (EC): A) 0 (Ctrl) and 5 μM, B) 0 (Ctrl) and 10 μM, C) Basal respiration (2 min), D) glucose-stimulated respiration (21 min), E) maximal respiration (61 min) and F) respiratory reserve (maxmal − basal). Oxygen consumption rate data were normalized by subtracting non-mitochondrial rates of respiration (after antimycin A, not shown), and are expressed as pmol O2 per minute, normalizer per μg protein. Values represent mean ± SEM from n=5 replicates. Significance vs. untreated control is indicated by: *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001.
3.2.3. Expression of ETC components
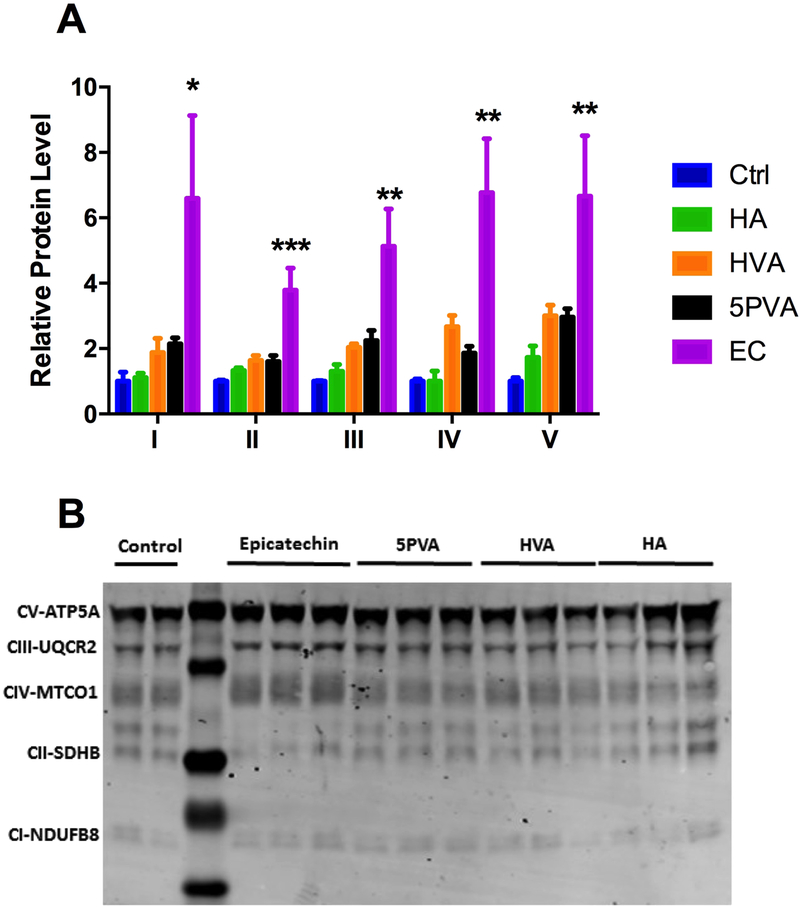
To validate the changes that we observed in β-cell respiration after treatment with EC or the gut metabolites, we measured protein levels of select ETC components (Figure 9). Similar to what was observed in our mitochondrial respiration studies, only treatment with EC changed protein levels of ETC components. These data validate our previous findings that while the metabolites do enhance glucose stimulated insulin secretion, it appears to be through extra mitochondrial modifications.
Figure 9.
A) Expression levels of electron transport chain components ATP5A (Complex V), UQCR2 (Complex III), MTCO1 (Complex IV), SDHB (Complex II) and NDUFB8 (Complex I) as quantified by Western blotting. Values are presented as mean ± SEM from n=3 replicates per condition. Data were analyzed by 1-way ANOVA. If a significant treatment effect was detected, Dunnett’s post hoc test was performed to compare each dose to the untreated (0 μM) control. Significance vs. untreated control is indicated by: *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001. B) Representative Western blot.
3.3. Discussion
The premise of this study was to explore the possibility that the unique activities of microbial flavonoid metabolites on peripheral tissues may contribute to the observed bioactivities of native dietary flavonoids. In other words, can dietary flavonoids exert significant bioactivities despite poor bioavailability, or is bioavailability of the native dietary species at peripheral target tissues indeed the primary limiting factor for bioactivity in vivo? Our central hypothesis, spanning this study and others in progress, is that the systemic, peripheral tissue activities of microbial metabolites may account for a significant portion of observed bioactivity following dietary flavonoid exposure in vivo.
The present results demonstrate the potent activities of flavonoid microbial metabolites, particularly for preservation of β-cell function, enhancement of skeletal muscle glucose utilization and protection of skeletal muscle respiratory function from oxidative injury, Therefore, these data suggest that further investigation of the anti-diabetic activities of flavonoid microbial metabolites is warranted. Additionally, our data suggest that metabolites and native compounds may act by distinct mechanisms, suggesting complementary and synergistic activities in vivo. Specifically, our data demonstrate that the gut metabolites enhance β-cell glucose stimulated insulin secretion more effectively than EC. Furthermore, unlike EC, these metabolites appear to do this without enhancing mitochondrial respiration or increasing expression of mitochondrial electron transport chain components, and with varying effects on β-cellinsulin content. Insulin secretion is dependent on ATP production in the β-cell due to glycolysis, TCA cycle and the ETC. In addition, the increases in ATP closes K+ channels which cause membrane depolarization and opening of Ca2+ channels which allow Ca2+ influx. The modulation of these two channels is an area of future interest in determine how the metabolites enhance glucose stimulated insulin secretion. In skeletal muscle, these compounds appear to enhance glucose utilization, but do not appear to enhance respiration under normal conditions. Therefore, mitochondrial uncoupling does not appear to be a mechanism by which these compounds can prevent obesity and glucose intolerance, with the exception of HA and 5PVA at high doses. However, they do appear to significantlyprotect respiratory function against oxidative injury. The objective of these respiration experiments was to evaluate the impacts of the selected compounds on overall respiration. Future mechanistic experiments, including use of ETC complex inhibitors as well as comparing intact cells, permeabilized cells and isolated mitochondria, will be useful to elucidate the specific mechanisms by which the microbial metabolites exert these effects on respiration. Future work will also provide new insight that address some of the current study limitations, such as examining compound efficacy in differentiated muscle myotubes from mouse and human (to compliment myoblast studies that were conducted herein).
The results presented here make significant additions to the small, yet growing, body of published data indicating that flavonoid microbial metabolites likely account for a significant fraction of many observed bioactivities of dietary flavonoids, particularly those with poor oral bioavailability of the native forms. These data help to explain epidemiological and experimental data suggesting that some dietary flavonoids (and potentially other classes of compounds, such as curcuminoids)possess potent bioactivities despite poor oral bioavailability. These results also suggest that the metabolites may be equally important to, if not more important than (in some cases), the native forms for in vitro mechanistic studies in cell culture models that attempt to recapitulate effects in peripheral tissues (hepatic, adipose, pancreatic, skeletal muscle, endothelial and other cell models). This is particularly true at compound doses in the mid to high μM range, which are commonly used for bioactives in cell culture but which are much more likely to be obtained by the microbial metabolites than the native dietary forms
Moving forward, there is a need to further identify the most active individual metabolites (or metabolite profiles) that confer systemic benefits, to understand the characteristics of the microbiome that facilitate generation of these profiles, and to understand how inter-individual variability in microbial metabolism affects subsequent metabolite profiles and bioactivities [49]. This knowledge will be critical for development of strategies to fully exploit the potential health benefits of dietary flavonoids. While initial studies have used antibiotics to eliminate the effect of the microbiome and microbiome-derived metabolites [30,31], germ-free and other gnotobiotic models will be instrumental in elucidation of the role of the microbiome in mediating the beneficial effects of poorly-bioavailable flavonoids. Furthermore, large-scale screening of several dozen (if not libraries of several hundred) microbial metabolites in peripheral tissue cell culture models will need to be performed in order to understand the tissue-specific mechanisms by which these compounds exert their activities. This will require advances in commercial availability of some metabolites, specifically the valerolactones, which to our knowledge are not currently available. It will also be important to conduct full dose-dependence studies of these metabolites. Furthermore, in vitro anaerobic fecal fermentations of flavonoids, with assessment of the bioactivity before and after fermentation in vitro and in vivo (via i.p. administration of filter-sterilized supernatants) will be useful to identify broad effects of microbial transformation.
It is important to note that we did not study valerolactones, which are among the early microbial metabolites of flavonoids. These compounds are present in high concentrations in circulation following flavonoid intake, and represent important compounds that may possess significant bioactivities. We did not study these compounds due to the lack of commercial availability, which is a significant obstacle for understanding their activities. Due to the provocative data in the present work, future work is needed to generate, isolate, and elucidate the activity of valerolactones. Two possible approaches include isolation from in vivo or ex vivo fecal fermentation mixtures, as well as synthetic approaches. These will need to be performed in order to complete our understanding of the potential bioactivities of flavonoid microbial metabolites.
It is also important to note that these microbial metabolites exist in circulation in the unconjugated forms studied, as well as Phase-II conjugates (sulfate, O-methyl and glucuronide forms) produced in enterocytes and hepatocytes following their absorption [50]. While the present work focused on the unconjugated forms, future work needs to be performed to elucidate the bioactivities of the conjugated forms. Such transformations can be performed using enterocytes, hepatocytes, liver microsomes, or isolated conjugating enzymes. Such studies will further advance the overall objective of the present work which is to understand the bioactivities of the actual circulating profile of compounds (unconjugated and phase-II conjugates of both native dietary flavonoids and their microbial metabolites) as opposed to just the native, unconjugated forms (i.e. the majority of existing studies).
4. CONCLUSION
In summary, our data demonstrate that flavonoid microbial metabolites stimulate β-cell function, as well as glucose utilization and mitochondrial respiration in skeletal muscle. These data support the hypothesis that dietary flavonoids may exert significant activity despite poor bioavailability via their microbial metabolites. This raises the intriguing prospect that bioavailability of native flavonoids may not be as critical of a limiting factor to bioactivity as previously thought. If, in fact, bioavailability of native flavonoids is not as crucial as currently thought, this would represent a paradigm shift in the thinking regarding how to exploit the activities of flavonoids in the diet. While development of strategies to enhance bioavailability of native compounds should not be discontinued, exploration of strategies that do not require bioavailability should receive extensive consideration as a parallel complementary approach to solving the same problem. Our overall logic for the proposed experiments moving forward is that we are quickly approaching an asymptote (diminishing novel returns) in terms of what we can learn from further studies focusing on the activities of native flavonoids. New approaches are now needed to answer the complex questions remaining.
Supplementary Material
HIGHLIGHTS.
Microbial metabolites of flavonoids possess potent activities
Metabolites stimulated glucose-stimulated insulin secretion
Metabolites stimulated beta-cell respiration
Metabolites protected skeletal muscle from oxidative injury
Metabolites did not generally uncouple mitochondrial respiration
5. ACKNOWLEDGEMENT
Funding for this work was provided, in part, by the Virginia Agricultural Experiment Station and the Hatch Program of the National Institute of Food and Agriculture, U.S. Department of Agriculture (APN and DAB), American Diabetes Association (1-JF-05–24 to MWH), the National Institutes of Health-NIDDK (2RO1 DK-078765), R01 HL123647 to DAB), BYU mentoring environment grant (JST), BYU ORCA Grant (BFB), American Diabetes Association (1–17-IBS-101 to JST), and a grant from the Diabetes Action Research and Education Foundation (Grant #461 to JST).
Grants, sponsors, and funding sources: Funding for this work was provided, in part, by the Virginia Agricultural Experiment Station and the Hatch Program of the National Institute of Food and Agriculture, U.S. Department of Agriculture (APN and DAB), American Diabetes Association (1-JF-05–24 to MWH), the National Institutes of Health-NIDDK (2RO1 DK078765), R01 HL123647 to DAB), BYU mentoring environment grant (JST), BYU ORCA Grant (BFB), American Diabetes Association (1–17-IBS-101 to JST), and a grant from the Diabetes Action Research and Education Foundation (Grant #461 to JST).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Guo Y, Bruno RS. Endogenous and exogenous mediators of quercetin bioavailability. J Nutr Biochem 2015;26:201–10. doi:10.1016/j.jnutbio.2014.10.008. [DOI] [PubMed] [Google Scholar]
- [2].Stefanie Wiese, Tuba Esatbeyoglu, Peter Winterhalter, Peter Kruse Hans, Stephanie Winkler, Achim Bub, et al. Comparative biokinetics and metabolism of pure monomeric, dimeric, and polymeric flavan-3-ols: A randomized cross-over study in humans. Mol Nutr Food Res 2015;59:610–21. doi:10.1002/mnfr.201400422. [DOI] [PubMed] [Google Scholar]
- [3].Mulder TPJ, van Platerink CJ, Wijnand Schuyl PJ, van Amelsvoort JMM. Analysis of theaflavins in biological fluids using liquid chromatography–electrospray mass spectrometry. J Chromatogr B Biomed Sci App 2001;760:271–9. doi:10.1016/S03784347(01)00285-7. [DOI] [PubMed] [Google Scholar]
- [4].González-Sarrías A, Espín JC, Tomás-Barberán FA. Non-extractable polyphenols produce gut microbiota metabolites that persist in circulation and show anti-inflammatory and free radical-scavenging effects. Trends Food Sci Technol 2017;69:281–8. doi:10.1016/j.tifs.2017.07.010. [Google Scholar]
- [5].Ulaszewska MM, Trost K, Stanstrup J, Tuohy KM, Franceschi P, Chong MF-F, et al. Urinary metabolomic profiling to identify biomarkers of a flavonoid-rich and flavonoid-poor fruits and vegetables diet in adults: the FLAVURS trial. Metabolomics 2016;12:32. doi:10.1007/s11306-015-0935-z. [Google Scholar]
- [6].Mulder TP, Rietveld AG, van Amelsvoort JM. Consumption of both black tea and green tea results in an increase in the excretion of hippuric acid into urine. Am J Clin Nutr 2005;81:256S–260S. [DOI] [PubMed] [Google Scholar]
- [7].Pereira-Caro G, Moreno-Rojas JM, Brindani N, Del Rio D, Lean MEJ, Hara Y, et al. Bioavailability of Black Tea Theaflavins: Absorption, Metabolism, and Colonic Catabolism. J Agric Food Chem 2017;65:5365–74. doi:10.1021/acs.jafc.7b01707. [DOI] [PubMed] [Google Scholar]
- [8].Chen H, Hayek S, Guzman JR, Gillitt ND, Ibrahim SA, Jobin C, et al. The Microbiota Is Essential for the Generation of Black Tea Theaflavins-Derived Metabolites. PLOS ONE 2012;7:e51001. doi:10.1371/journal.pone.0051001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Goodrich KM, Smithson AT, Ickes AK, Neilson AP. Pan-colonic pharmacokinetics of catechins and procyanidins in male Sprague–Dawley rats. J Nutr Biochem 2015;26:1007–14. [DOI] [PubMed] [Google Scholar]
- [10].Lin W, Wang W, Yang H, Wang D, Ling W. Influence of Intestinal Microbiota on the Catabolism of Flavonoids in Mice. J Food Sci n.d;81:H3026–34. doi:10.1111/17503841.13544. [DOI] [PubMed] [Google Scholar]
- [11].Serra A, Macià A, Romero M-P, Reguant J, Ortega N, Motilva M-J. Metabolic pathways of the colonic metabolism of flavonoids (flavonols, flavones and flavanones) and phenolic acids. Food Chem 2012;130:383–93. doi:10.1016/j.foodchem.2011.07.055. [Google Scholar]
- [12].Castello F, Costabile G, Bresciani L, Tassotti M, Naviglio D, Luongo D, et al. Bioavailability and pharmacokinetic profile of grape pomace phenolic compounds in humans. Arch Biochem Biophys 2018;646:1–9. doi:10.1016/j.abb.2018.03.021. [DOI] [PubMed] [Google Scholar]
- [13].Henning Susanne M., Piwen Wang, Narine Abgaryan, Roberto Vicinanza, de Oliveira Daniela Moura, Yanjun Zhang, et al. Phenolic acid concentrations in plasma and urine from men consuming green or black tea and potential chemopreventive properties for colon cancer. Mol Nutr Food Res 2013;57:483–93. doi:10.1002/mnfr.201200646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Scholl C, Lepper A, Lehr T, Hanke N, Schneider KL, Brockmöller J, et al. Population nutrikinetics of green tea extract. PLOS ONE 2018;13:e0193074. doi:10.1371/journal.pone.0193074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Clifford MN, Hooft VD, Jj J, Crozier A. Human studies on the absorption, distribution, metabolism, and excretion of tea polyphenols. Am J Clin Nutr 2013;98:1619S–1630S. doi:10.3945/ajcn.113.058958. [DOI] [PubMed] [Google Scholar]
- [16].Kawser Hossain M, Abdal Dayem A, Han J, Yin Y, Kim K, Kumar Saha S, et al. Molecular Mechanisms of the Anti-Obesity and Anti-Diabetic Properties of Flavonoids. Int J Mol Sci 2016;17:569. doi:10.3390/ijms17040569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Amiot MJ, Riva C, Vinet A Effects of dietary polyphenols on metabolic syndrome features in humans: a systematic review. Obes Rev 2016;17:573–86. doi:10.1111/obr.12409. [DOI] [PubMed] [Google Scholar]
- [18].Gu Y, Hurst WJ, Stuart DA, Lambert JD. Inhibition of Key Digestive Enzymes by Cocoa Extracts and Procyanidins. J Agric Food Chem 2011;59:5305–11. doi:10.1021/jf200180n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ryan CM, Khoo W, Ye L, Lambert JD, O’Keefe SF, Neilson AP. Loss of Native Flavanols during Fermentation and Roasting Does Not Necessarily Reduce Digestive EnzymeInhibiting Bioactivities of Cocoa. J Agric Food Chem 2016;64:3616–25. doi:10.1021/acs.jafc.6b01725. [DOI] [PubMed] [Google Scholar]
- [20].Bitzer ZT, Glisan SL, Dorenkott MR, Goodrich KM, Ye L, O’Keefe SF, et al. Cocoa procyanidins with different degrees of polymerization possess distinct activities in models of colonic inflammation. J Nutr Biochem 2015. doi:10.1016/j.jnutbio.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Masumoto S, Terao A, Yamamoto Y, Mukai T, Miura T, Shoji T. Non-absorbable apple procyanidins prevent obesity associated with gut microbial and metabolomic changes. Sci Rep 2016;6:31208. doi:10.1038/srep31208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Dorenkott MR, Griffin LE, Goodrich KM, Thompson-Witrick KA, Fundaro G, Ye L, et al. Oligomeric cocoa procyanidins possess enhanced bioactivity compared to monomeric and polymeric cocoa procyanidins for preventing the development of obesity, insulin resistance, and impaired glucose tolerance during high-fat feeding. J Agric Food Chem 2014;62:2216–27. [DOI] [PubMed] [Google Scholar]
- [23].Larrosa M, Luceri C, Vivoli E, Pagliuca C, Lodovici M, Moneti G, et al. Polyphenol metabolites from colonic microbiota exert anti-inflammatory activity on different inflammation models. Mol Nutr Food Res 2009;53:1044–54. doi:10.1002/mnfr.200800446. [DOI] [PubMed] [Google Scholar]
- [24].Qian Y, Babu PVA, Symons JD, Jalili T. Metabolites of flavonoid compounds preserve indices of endothelial cell nitric oxide bioavailability under glucotoxic conditions. Nutr Diabetes 2017;7:e286. doi:10.1038/nutd.2017.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ho GTT, Kase ET, Wangensteen H, Barsett H. Phenolic Elderberry Extracts, Anthocyanins, Procyanidins, and Metabolites Influence Glucose and Fatty Acid Uptake in Human Skeletal Muscle Cells. J Agric Food Chem 2017;65:2677–85. doi:10.1021/acs.jafc.6b05582. [DOI] [PubMed] [Google Scholar]
- [26].Carrasco-Pozo C, Gotteland M, Castillo RL, Chen C. 3,4-dihydroxyphenylacetic acid, a microbiota-derived metabolite of quercetin, protects against pancreatic β-cells dysfunction induced by high cholesterol. Exp Cell Res 2015;334:270–82. doi:10.1016/j.yexcr.2015.03.021. [DOI] [PubMed] [Google Scholar]
- [27].Fernández-Millán E, Ramos S, Alvarez C, Bravo L, Goya L, Martín MÁ. Microbial phenolic metabolites improve glucose-stimulated insulin secretion and protect pancreatic beta cells against tert-butyl hydroperoxide-induced toxicity via ERKs and PKC pathways. Food Chem Toxicol 2014;66:245–53. doi:10.1016/j.fct.2014.01.044. [DOI] [PubMed] [Google Scholar]
- [28].Xue H, Xie W, Jiang Z, Wang M, Wang J, Zhao H, et al. 3,4-Dihydroxyphenylacetic acid, a microbiota-derived metabolite of quercetin, attenuates acetaminophen (APAP)-induced liver injury through activation of Nrf-2. Xenobiotica 2016;46:931–9. doi:10.3109/00498254.2016.1140847. [DOI] [PubMed] [Google Scholar]
- [29].Lee CC, Kim JH, Kim JS, Oh YS, Han SM, Park JHY, et al. 5-(3′,4′-Dihydroxyphenyl-γvalerolactone), a Major Microbial Metabolite of Proanthocyanidin, Attenuates THP-1 Monocyte-Endothelial Adhesion. Int J Mol Sci 2017;18:1363. doi:10.3390/ijms18071363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Wen Liu, Shaoqian Zhao, Jiqiu Wang, Juan Shi, Yingkai Sun, Weiqing Wang, et al. Grape seed proanthocyanidin extract ameliorates inflammation and adiposity by modulating gut microbiota in high‐fat diet mice. Mol Nutr Food Res 2017;61:1601082. doi:10.1002/mnfr.201601082. [DOI] [PubMed] [Google Scholar]
- [31].Esposito D, Damsud T, Wilson M, Grace MH, Strauch R, Li X, et al. Black Currant Anthocyanins Attenuate Weight Gain and Improve Glucose Metabolism in Diet-Induced Obese Mice with Intact, but Not Disrupted, Gut Microbiome. J Agric Food Chem 2015;63:6172–80. doi:10.1021/acs.jafc.5b00963. [DOI] [PubMed] [Google Scholar]
- [32].Brown EM, McDougall GJ, Stewart D, Pereira-Caro G, González-Barrio R, Allsopp P, et al. Persistence of Anticancer Activity in Berry Extracts after Simulated Gastrointestinal Digestion and Colonic Fermentation. PLOS ONE 2012;7:e49740. doi:10.1371/journal.pone.0049740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kim D-H, Jung E-A, Sohng I-S, Han J-A, Kim T-H, Han MJ. Intestinal bacterial metabolism of flavonoids and its relation to some biological activities. Arch Pharm Res 1998;21:17–23. doi:10.1007/BF03216747. [DOI] [PubMed] [Google Scholar]
- [34].van der Pijl PC, Foltz M, Glube ND, Peters S, Duchateau G. Pharmacokinetics of black teaderived phenolic acids in plasma. J Funct Foods 2015;17:667–75. doi:10.1016/j.jff.2015.06.020. [Google Scholar]
- [35].Frisard MI, McMillan RP, Marchand J, Wahlberg KA, Wu Y, Voelker KA, et al. Toll-like receptor 4 modulates skeletal muscle substrate metabolism. Am J Physiol - Endocrinol Metab 2010;298:E988–98. doi:10.1152/ajpendo.00307.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Anderson AS, Roberts PC, Frisard MI, McMillan RP, Brown TJ, Lawless MH, et al. Metabolic changes during ovarian cancer progression as targets for sphingosine treatment. Exp Cell Res 2013;319:1431–42. doi:10.1016/j.yexcr.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Dai W, Cheung E, Alleman RJ, Perry JB, Allen ME, Brown DA, et al. Cardioprotective Effects of Mitochondria-Targeted Peptide SBT-20 in two Different Models of Rat Ischemia/Reperfusion. Cardiovasc Drugs Ther 2016;30:559–66. doi:10.1007/s10557-0166695-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Rowley TJ, Bitner BF, Ray JD, Lathen DR, Smithson AT, Dallon BW, et al. Monomeric cocoa catechins enhance β-cell function by increasing mitochondrial respiration. J Nutr Biochem 2017;49:30–41. doi:10.1016/j.jnutbio.2017.07.015. [DOI] [PubMed] [Google Scholar]
- [39].Kener KB, Munk DJ, Hancock CR, Tessem JS. High-resolution Respirometry to Measure Mitochondrial Function of Intact Beta Cells in the Presence of Natural Compounds. J Vis Exp JoVE 2018. doi:10.3791/57053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Hobson A, Draney C, Stratford A, Becker TC, Lu D, Arlotto M, et al. Aurora Kinase A is critical for the Nkx6.1 mediated β-cell proliferation pathway. Islets 2015;7:e1027854. doi:10.1080/19382014.2015.1027854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Tessem JS, Moss LG, Chao LC, Arlotto M, Lu D, Jensen MV, et al. Nkx6.1 regulates islet βcell proliferation via Nr4a1 and Nr4a3 nuclear receptors. Proc Natl Acad Sci 2014;111:5242–7. doi:10.1073/pnas.1320953111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Draney C, Hobson AE, Grover SG, Jack BO, Tessem JS. Cdk5r1 Overexpression Induces Primary β-Cell Proliferation. J Diabetes Res 2016. doi:10.1155/2016/6375804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Reynolds MS, Hancock CR, Ray JD, Kener KB, Draney C, Garland K, et al. β-Cell deletion of Nr4a1 and Nr4a3 nuclear receptors impedes mitochondrial respiration and insulin secretion. Am J Physiol-Endocrinol Metab 2016;311:E186–201. doi:10.1152/ajpendo.00022.2016. [DOI] [PubMed] [Google Scholar]
- [44].Ray Jason D, Kener Kyle B, Bitner Benjamin F, Wright Brent J, Ballard Matthew S, Barrett Emily J, et al. Nkx6.1‐mediated insulin secretion and β‐cell proliferation is dependent on upregulation of c‐Fos. FEBS Lett 2016;590:1791–803. doi:10.1002/18733468.12208. [DOI] [PubMed] [Google Scholar]
- [45].Anderson EJ, Lustig ME, Boyle KE, Woodlief TL, Kane DA, Lin C-T, et al. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest 2009;119:573–81. doi:10.1172/JCI37048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Owen MR, Doran E, Halestrap AP. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J 2000;348 Pt 3:607–14. [PMC free article] [PubMed] [Google Scholar]
- [47].Childress ES, Alexopoulos SJ, Hoehn KL, Santos WL. Small Molecule Mitochondrial Uncouplers and Their Therapeutic Potential. J Med Chem 2017. doi:10.1021/acs.jmedchem.7b01182. [DOI] [PubMed] [Google Scholar]
- [48].García-Ocaña A, Vasavada RC, Cebrian A, Reddy V, Takane KK, López-Talavera J-C, et al. Transgenic Overexpression of Hepatocyte Growth Factor in the β-Cell Markedly Improves Islet Function and Islet Transplant Outcomes in Mice. Diabetes 2001;50:2752–62. doi:10.2337/diabetes.50.12.2752. [DOI] [PubMed] [Google Scholar]
- [49].Bolca S, Van de Wiele T, Possemiers S. Gut metabotypes govern health effects of dietary polyphenols. Curr Opin Biotechnol 2013;24:220–5. doi:10.1016/j.copbio.2012.09.009. [DOI] [PubMed] [Google Scholar]
- [50].Feliciano RP, Mills CE, Istas G, Heiss C, Rodriguez-Mateos A. Absorption, Metabolism and Excretion of Cranberry (Poly)phenols in Humans: A Dose Response Study and Assessment of Inter-Individual Variability. Nutrients 2017;9. doi:10.3390/nu9030268. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.