Abstract
Objective
This study examined associations between three distinct parent factors (parent personal distress, parenting stress, and spina bifida-specific parenting stress) and youth- and parent proxy-report of youth health-related quality of life (HRQOL) over time.
Method
Participants were recruited as part of a longitudinal study, and data were collected at three time points, spaced two years apart. Parents and youth completed questionnaires, and youth completed neuropsychological assessment tasks to determine youth IQ during home visits.
Results
Analyses revealed that higher levels of maternal SB-specific parenting stress were related to lower levels of youth-reported HRQOL at Time 1. Other parent factors were not associated with youth-report of HRQOL at the earlier time points, though higher levels of maternal SB-specific parenting stress and paternal parenting stress were associated with lower levels of youth HRQOL at Time 3. For mothers and fathers, increased parent personal distress, parenting stress, and SB-specific parenting stress were associated with decreased proxy-report of youth HRQOL. SB-specific parenting stress was consistently the most strongly associated to parent proxy-report of youth HRQOL.
Conclusion
Parenting stress and distress are important targets for interventions, and these interventions may improve youth outcomes, especially as youth age. Future research is needed to identify other factors influencing youth HRQOL over time.
Keywords: parenting stress, psychosocial functioning, quality of life, spina bifida
Spina bifida (SB) is a congenital birth defect that occurs in approximately 3 of every 10,000 live births in the United States.1 SB occurs in the early weeks of gestation, when the neural tube fails to close completely. Though the severity of SB varies, the condition is associated with a number of complications, including paralyzed lower extremities, urinary and bowel dysfunction, and hydrocephalus.1 Given the risk for secondary medical conditions and complications (e.g., skin breakdowns, urinary tract infections, pain, obesity)1, individuals with SB often follow an extensive medical regimen, including medications, catheterization, bowel programs, and skin checks.1 Adherence to these prescribed tasks is critical as these individuals seek to maintain their health in early adulthood.
Research has shown that health-related quality of life (HRQOL) – how individuals with a chronic health condition perceive the impact of their condition on their physical and psychological functioning2– is impaired in individuals with SB. Children and adolescents with SB have been found to have significantly lower HRQOL than both typically developing youth and youth with other chronic health conditions (e.g., asthma, diabetes).2 Lower HRQOL in individuals with SB is associated with higher age, female sex, lower SES, and increased severity of medical issues (e.g., number of operations, mobility impairment, and pain).2–3 While these factors are important to consider, they are also non-modifiable or difficult-to-modify. As HRQOL has been implicated as an important predictor of health outcomes, including adherence to prescribed medical regimen,4 it is necessary to identify modifiable factors that affect HRQOL in this population, such as parent factors.
The clinical symptoms of SB place considerable physical, psychological, and social demands on individuals with SB and their families.5 The majority of youth with SB complete multiple daily medical routines with at least some assistance from a parent or other caretaker.1 Thus, youth with SB are especially reliant on their parents. Increased dependence on parents continues for youth with SB from childhood through adolescence.6 In fact, research has found that these youth experience delayed autonomy development, lagging behind TD peers in this area by approximately two years.7 Therefore, parent factors may have a prolonged impact on these youth. The current study examines the impact of three distinct parent adjustment factors – parent personal distress, parenting stress, and SB-specific parenting stress – on youth HRQOL.
Parent personal distress is operationalized as the degree of overwhelming sadness, anxiety, or pain experienced by an individual (who is also a parent).8–9 Parents of youth with SB have been found to experience clinical levels of personal distress.10 However, it is unclear how the experience of personal distress by a parent affects youth adjustment, specifically HRQOL. One study found that maternal psychological distress predicted lower HRQOL in youth with SB.11 Still, more research is necessary to elucidate this relationship.
Parents are often faced with balancing many responsibilities and may, therefore, experience an increased amount of stress (when compared to non-parents). Parenting stress is operationally defined as the mental or emotional strain or pressure an individual experiences as a direct result of being a parent.8,12 This can include the stress a caretaker experiences due to enforcing bedtimes, preparing meals, or arranging after-school activities. Studies have found that more than one-third of mothers of youth with SB experience clinically significant levels of general parenting stress.13 One study comparing mothers of youth with SB and mothers of typically-developing children found that mothers of youth with SB had lower educational levels, were more likely to be single parents, and were more likely to be unemployed,14 all of which could result in increased levels of general parenting stress. While increased parenting stress has been found to be associated with decreased youth HRQOL in other illness populations (e.g., obesity),15 this relation has been understudied in children with SB.
Parenting a child with SB can have negative effects on parent stress levels. In fact, parents of children with SB appear to experience more stress than parents of TD children.10 One qualitative study found that parents of youth with SB consistently described adhering to daily medical regimen as a major challenge in their everyday lives.16 Additionally, the ambulatory status of youth with SB may impact parents’ experiences of stress, as parents of youth with SB who are able to walk independently report lower parenting stress than parents of youth who use a wheelchair.17 Stress that is a direct result of these condition-related factors can be described as SB-specific parenting stress. Given the negative impact of general parenting stress on youth outcomes,12 it is hypothesized that increased SB-specific parenting stress may also lead to poorer child outcomes, including HRQOL. Still, it is important to distinguish among general parenting stress and this context-specific parenting stress. In other child disability populations, parents have reported high levels of context-specific parenting stress while their experiences of general parenting stress were similar to those of parents of TD children.18 Therefore, more research on associations between SB-specific parenting stress and HRQOL is needed.
Differences may exist between mothers and fathers in their adjustment to and coping with chronic illness. However, a review of the literature revealed that fathers are rarely included in data collections and/or analyses.19 It has been hypothesized that mothers may experience more psychological distress than fathers, as mothers are often more directly involved in youth’s medical care.20 However, more studies including fathers of youth with SB are needed to test this hypothesis.
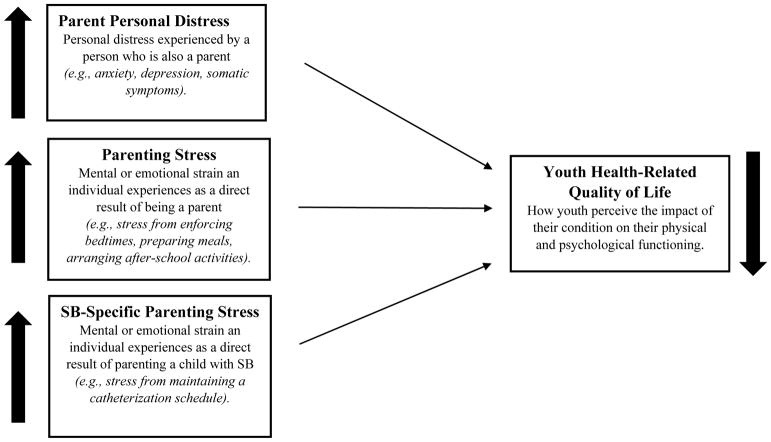
The current study aims to distinguish between the unique contributions of parent personal distress, parenting stress, and SB-specific parenting stress to youth HRQOL. As both demographic (e.g., caregiver and child age, employment status/income)20 and condition-specific (e.g., lesion level, shunt status)21 factors have been found to contribute to the experience of stress and personal distress in the parents of youth with SB, these will be included as covariates in the analyses. It was hypothesized that higher levels of parent personal distress, parenting stress, and SB-specific parenting stress would be related to lower levels of HRQOL (Objective 1, Figure 1). This study also sought to determine which of the three parent factors was most strongly associated with youth HRQOL (Objective 2). It is also possible that the interactions of these three distinct parent factors contribute to youth HRQOL (e.g., associations between parenting stress and HRQOL may be particularly strong when parental personal distress is high). Therefore, this study also sought to identify any significant interactions among the three parent factors (Objective 2). Given the limited research on the relations between these parent factors and HRQOL for youth with SB, these latter analyses were considered exploratory. It is believed that findings from this study will inform future research, as well as the development of evidence-based family interventions aimed at decreasing parental personal distress and parenting stress and improving quality of life in this population.
Figure 1.
Proposed longitudinal associations between parent distress, parenting stress, and SB-specific parenting stress and youth HRQOL in youth with spina bifida. As each of the three parent factors increases, youth HRQOL is expected to decrease.
METHODS
Participants
Participants were recruited for an ongoing longitudinal study examining family and peer relationships, neuropsychological functioning, and psychological adjustment of families of youth with SB.8 The present study’s analyses focus on the first three time points: Time 1(baseline; youth age 8–15 years), Time 2 (2 years after baseline; youth age 10–17 years), and Time 3 (4 years after baseline; youth age 12–19 years). Families of youth with SB were recruited from four Midwestern hospitals, a statewide SB association, in person at regularly scheduled clinic visits, and through recruitment letters. Interested families were screened in person or by phone by a member of the research team. Families were invited to participate if the child met the following criteria at Time 1: (a) diagnosis of SB (including myelomeningocele, lipomeningocele, and myelocystocele); (b) age 8–15 years; (c) ability to speak and read English or Spanish; (d) involvement of at least one primary caregiver; and (e) residence within 300 miles of the lab (to allow for data collection at participants’ homes).
A total of 246 families were approached during recruitment, and 163 families agreed to participate. Of these 163 families, 21 families could not be contacted or declined to participate after their initial consent and two families did not meet inclusion criteria. The final sample of participants included 140 families of children with SB (at Time 1, 53.6% female, Mage = 11.40; Table 1). Youth of families who declined to participate did not differ from participants with respect to type of SB (myelomeningocele or other), χ2 (1) = .0002, p > .05, shunt status, χ2 (1) = .003, p > .05, or occurrence of shunt infections, χ2 (1) = 1.08, p > .05. For all two-parent households, both parents were encouraged to participate, and the final sample included 128 mothers and 102 fathers (Table 1), with both parents participating at Time 1 for 95 families (67.9%).
Table 1.
Youth and Parent Demographic and Condition-Severity Information at Time 1.
| Youth (N=140) M (SD) or N (%) |
Mother (N=128) M (SD) or N (%) |
Father (N=102) M (SD) or N (%) |
|
|---|---|---|---|
| Gender: female | 75(53.6%) | -- | -- |
| Age | 11.43(2.46) | 40.94(6.88) | 42.90(6.94) |
| Race | |||
| Caucasian | 74(52.86%) | 79(61.72%) | 68(65.38%) |
| African-American/Black | 19(13.57%) | 14(10.94%) | 7(6.73%) |
| Hispanic | 39(27.86%) | 29(22.65%) | 26(25.00%) |
| Asian | 2(1.43%) | 1(0.78%) | 1(0.96%) |
| Bi-racial | 6(4.28%) | 1(0.78%) | 0(0.00%) |
| Not Reported | 0(0.00%) | 4(3.13%) | 2(1.92%) |
| Family SES | 39.44(15.90) | -- | -- |
| Two-parent household | 112(69.60%) | ||
| IQ | 85.68(19.68) | -- | -- |
| Condition Severity | 7.86(1.58) | -- | -- |
| SB type | |||
| Myelomeningocele | 123(87.86%) | -- | -- |
| Other | 17(12.14%) | -- | -- |
| Lesion Level | |||
| Thoracic | 29(20.71%) | -- | -- |
| Lumbar | 86(61.42%) | -- | -- |
| Sacral | 18(12.86%) | -- | -- |
| Unknown/not reported | 7(5.00%) | -- | -- |
| Shunt: present | 109(77.86%) | -- | -- |
| Ambulation | |||
| No Assistance | 34(24.28%) | -- | -- |
| K.F.O. or A.F.O. | 16(11.43%) | -- | -- |
| Wheelchair | 83(59.29%) | -- | -- |
| Not reported | 7(5.00%) | -- | -- |
Procedure
The current study was approved by university and hospital Institutional Review Boards and utilized a multi-method, multi-informant longitudinal research design. Data were collected by trained research assistants during home visits that lasted approximately three hours. At Time 1, two 3-hour home visits were conducted, and at Time 2 and Time 3 only one 3-hour home visit was conducted. For home visits with families who primarily spoke Spanish in the home, at least one research assistant was bilingual. Informed consent from parents and assent from youth were obtained at each data collection time point. Youth completed questionnaires and neuropsychological assessments independently from their parents. Research assistants were available to assist youth with the completion of questionnaires (e.g., reading questions aloud, recording responses) as needed. Mothers and fathers completed identical questionnaires separately. Questionnaires that were only available in English were adapted for Spanish speakers using forward and back translation by a trained translation team. The current study used youth- and parent-reported questionnaire data. At Time 3, a sub-sample of participants (roughly 25%) was 18 years old or older. For these participants, only the target young adult (and not parents) completed questionnaires. Because parents were not assessed at Time 3 when youth were >18 an all independent variables were reported by parents, these >18 participants were not included in Time 3 analyses. Hard copies of all questionnaires were de-identified, labeled with an alpha-numeric participant identification code, and stored in a locked cabinet in a locked office.
Measures
Demographics
At Time 1, parents reported on youth and family demographic information. Parents reported on child age, sex, and race/ethnicity. Parents also reported on their own sex, ethnicity, education, employment, income, and relationship to child. The Hollingshead Index of socioeconomic status (SES) was computed using parents’ education and occupation, with higher scores indicating higher SES.22
Youth Illness Severity
At Time 1, parents completed the Medical History Questionnaire (MHQ).5 This survey contains questions about youth’s disease-specific medical information, including bowel and bladder functioning, ambulation, medications, providers and frequency of medical care, and surgery history. In addition to the MHQ, data were collected from participants’ medical charts to assess the following information: type of SB (i.e., lipomeningocele, meningocele, or myelomeningocele), shunt status, lesion level (i.e., sacral, lumbar, or thoracic), and ambulation method (i.e., ankle-foot orthoses [AFOs], knee-ankle-foot orthoses [KAFOs], or hip-knee-ankle-foot orthoses [HKAFOs], wheelchair, or no assistance). These variables were used to compute an illness severity index based on inclusion in a specific group: shunt status (no = 1, yes = 2), myelomeningocele (no = 1, yes = 2), lesion level (sacral = 1, lumbar = 2, thoracic = 3), and ambulation status (no assistance/AFOs = 1, KAFOs/HKAFOs = 2, wheelchair = 3). Scores ranged from four to ten, with higher scores indicating higher levels of severity.
Youth IQ
Youth were administered the Vocabulary and Matrix Reasoning subtests of the Wechsler Abbreviated Scale of Intelligence (WASI) which can be administered to individuals aged 6 to 90 years.23 These subtests have demonstrated high levels of internal consistency for individuals aged 6 to 16 years (α = .89 for Vocabulary, α = .92 for Matrix Reasoning).23 Scores on these two subtests can be used to compute an estimated Full Scale IQ (FSIQ), which functions as a proxy for general intellectual functioning. IQ data from Time 1 only were used in this study.
Parent Personal Distress
Mothers and fathers separately completed the Symptom Checklist-Revised (SCL-90-R)24 at each time point. This measure assesses psychological symptoms experienced by parents in the last week. The SCL-90-R is made up of nine symptom subscales and three larger indices, but only the Global Severity Index (GSI) was used in this study. The GSI is the average of all items from all subscales, with higher scores indicating higher global distress. Previous studies using the GSI with this sample have demonstrated high internal consistency (α = .95 - .98).
Parenting Stress
An abbreviated version of the Parenting Stress Index (PSI) was used to assess parenting stress (e.g., stress an individual experiences as a direct result of being a parent) at each time point.25 This abbreviated measure included three subscales (Sense of Competence, Role Restriction, and Social Isolation) from the larger instrument, as these subscales capture the functioning of parents in their roles as parents. A parenting stress total score was computed by converting raw scores to z-scores so that 4- and 5-point scale items could be totaled together. Higher scores on this measure indicate higher reported parenting stress. In this study, the PSI demonstrated high internal consistency for both mother- and father-report (α = .85 – .88). It should be noted that the PSI has only been validated for parents of children ages 2–12 years.25 However, this measure has frequently been used in studies of parents of children with disabilities or chronic illnesses of any age.26
SB-specific Parenting Stress
Parents completed the Family Stress Scale (FSS), a 19-item scale assessing common stressors in families of a child with SB.27 This scale assesses the stress an individual experiences as a direct result of parenting a child with SB. Of the 19 items, 13 are non-disease specific (e.g., “mealtimes and bedtimes”) and 6 are disease-specific (e.g., “medical care/appointments”). Items are rated on a 5-point Likert scale (1= “not at all stressful” and 5 = “extremely stressful”). The current study used a total score comprised of the 6 disease-specific items with higher total scores indicating higher levels of SB-specific parenting stress. In the current study, internal consistency for the FSS was high for both mother- and father-report (α = .87 – .92).
Youth HRQOL
Discrepancies have been found between youth and parent proxy report of HRQOL within families of youth with SB. Specifically, parents have been found to report lower HRQOL than do youth.2 Given these discrepancies, youth’s HRQOL was assessed using both youth and parent proxy-report on the Pediatric Quality of Life Scale (PedsQL™ 4.0 Generic Core Scales)28 at each time point. The PedsQL assesses both physical and psychosocial aspects of quality of life. Due to the physical limitations associated with SB, the 8-item physical scale of the PedsQL was not used in this study. The 15-item psychosocial scale is comprised of three subscales: emotional (5 items), social (5 items), and school/work functioning (5 items). Each item asks how much of a problem each aspect of quality of life has been over the last month (for example, “I hurt or ache”), with higher scores indicating better HRQOL. The three psychosocial subscales were found to be highly correlated for each reporter at each time point, so only the composite psychosocial scale was used in this study. In the current study, internal consistency for all three reporters was adequate (α’s = 0.83 – 0.90).
Statistical Analysis
Objective 1
To evaluate the degree to which the parent factors were associated with HRQOL at each time point, a series of hierarchical multiple regression analyses were conducted.i All analyses included youth IQ, SB condition severity, child age, and family SES as covariates, as all four of these sociodemographic factors may contribute to parent personal distress, parenting stress, SB-specific parenting stress, and youth HRQOL. Cross-sectional regression analyses were run to determine associations between parent factors at Time 1, Time 2, and Time 3 with HRQOL at each time point, respectively. When running these analyses, independent variables were entered in the following order: (Step 1) covariates – IQ, illness, severity, SES; (Step 2) individual independent variables (parent personal distress, parenting stress, or SB-specific parenting stress). Separate regressions were run for each independent variable, and separate sets of regression analyses were run for self-, mother proxy-, and father proxy-reports of HRQOL and for the maternal and paternal independent variables. Tests of this objective were conducted both with and without using Bonferroni adjusted alpha levels of .0014 (.05/36).
Objective 2
To determine which parent variable (parent personal distress, parenting stress, or SB-specific parenting stress) was most strongly associated with youth HRQOL (self- and parent proxy-reported) and to test the significance of interactions among the three parent variables, cross-sectional hierarchical multiple regression analyses were performed at each time point. Independent variables were entered in the following order: (Step 1): covariates – IQ, illness severity, child age, SES at Time 1; (Step 2): parent distress, parenting stress, and SB-specific parenting stress; (Step 3): interaction terms (parent distress*parenting stress, parent distress*SB-specific parenting stress, parenting stress*SB-specific parenting stress). The Step 2 and Step 3 variables were each entered in a forward selection fashion, such that the variable that significantly improved the model most was entered first; this process was repeated until none of the independent variables significantly improved the model. Separate regressions were run for each study time point, and separate sets of regressions were run for the maternal and paternal variables.
RESULTS
Preliminary Analyses
An a priori power analysis was conducted using G*Power 3.1,29 indicating that the current study using Time 1 data was adequately powered to detect medium-large effect sizes, while analyses at subsequent Time points were powered to detect large effect sizes (due to attrition and the resulting lower sample sizes). All variables were examined for outliers, but none were identified. Additionally, all independent and dependent variables were tested for skewness. Variables were considered skewed if skewness values were greater than 1.0. The results indicated that four variables were positively skewed: mother-report on the SCL-90-R (skewness value = 2.90), father-report on the SCL-90-R (skewness value = 1.39), mother report on the FSS (skewness value = 1.05), and father report on the FSS (skewness value = 1.44). Skewed variables were transformed using log transformations prior to being used in analyses; such transformations corrected the significant levels of skewness for these variables.
Attrition Analyses
As anticipated, though a majority of families participated at all three time points (N = 94; 67%), not all families who participated at Time 1 participated at each of the subsequent time points (NTime 1 only = 18, 12.9%; NTime 1& Time 2 = 18, 12.9%; NTime 1 & Time3 = 10, 7.1%). Univariate analyses of variance (ANOVAs) were performed to compare these four groups at Time 1 on SES, youth IQ, youth age, youth condition severity, and youth-reported HRQOL. No significant differences were found on these factors between those who participated at all three time points, those who participated only at Time 1, those who participated only at Time 1 and Time 2 and those who participated only at Time 1 and Time 3 (SES: F(3, 128) = 1.37, p = .26; IQ: F(3, 128) = 1.50, p = .22; age: F(3, 126) = 1.87, p = .14; condition severity: F(3, 105) = .60, p = .62; HRQOL: F(3, 120) = 1.98, p = .12).
Objective 1
The first objective of this study was to evaluate the degree to which the three parent factors were associated with youth- and parent proxy-report of HRQOL at each time point. The covariate variables (youth IQ, SB condition severity, child age, and family SES) were entered into regressions in a simultaneous block. However, at all three time points, none of these variables were significantly associated with HRQOL.
At all three time points, associations between the three parent factors – parent personal distress, parenting stress, and SB-specific parenting stress – for both mothers and fathers and youth-reported HRQOL were explored. At Time 1, higher levels of maternal SB-specific parenting stress were associated with lower levels of youth self-reported HRQOL. At Time 2, parent factors were not associated with youth-report of HRQOL. At Time 3, higher levels of maternal SB-specific parenting stress and paternal parenting stress were associated with lower levels of youth self-reported HRQOL (Table 2).
Table 2.
Summary of Regression Analyses Examining Associations between Parent Variables and Youth and Parent Proxy-Reported Health-Related Quality of Life.
| Parent Factor | Time 1 Youth Age 8–15 Years |
Time 2 Youth Age 10–17 Years |
Time 3 Youth Age 12–19 Years |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Youth-Report | Mother-Report | Father-Report | Youth-Report | Mother-Report | Father-Report | Youth-Report | Mother-Report | Father-Report | ||||||||||
| β | ΔR2 | β | ΔR2 | β | ΔR2 | β | ΔR2 | β | ΔR2 | β | ΔR2 | β | ΔR2 | β | ΔR2 | β | ΔR2 | |
| Mothers | ||||||||||||||||||
|
| ||||||||||||||||||
| Parent Personal Distress | −.21 | .04 | −.21* | .04 | −− | −− | −.02 | .00 | −.39** a | .14 | −− | -- | −.08 | .01 | −.25 | .06 | -- | -- |
| Parenting Stress | −.11 | .01 | −.19 | .04 | -- | -- | −.01 | .00 | −.04 | .00 | -- | -- | −.21 | .03 | −.47** | .19 | -- | -- |
| SB-Specific Parenting Stress | −.25* | .06 | −.41**a | .17 | -- | -- | −.16 | .02 | −.37** | .12 | -- | -- | −.68** a | .40 | −.50** a | .22 | -- | -- |
|
| ||||||||||||||||||
| Fathers | ||||||||||||||||||
|
| ||||||||||||||||||
| Parent Personal Distress | −.07 | .01 | -- | -- | −.26* | .06 | −.23 | .05 | -- | -- | −.13 | .02 | −.14 | .02 | -- | -- | −.24 | .04 |
| Parenting Stress | −.02 | .00 | -- | -- | −.22 | .04 | .01 | .00 | -- | -- | −.01 | .00 | −.61** | .25 | -- | -- | −.42* | .13 |
| SB-Specific Parenting Stress | −.16 | .03 | -- | -- | −.43** a | .17 | −.16 | .02 | -- | -- | −.35* | .10 | .06 | .00 | -- | -- | −.10 | .01 |
Note: Separate regressions were run for each independent variable for each parent. For all analyses, the covariates of age, IQ, SES, and illness severity were entered at Step 1. These covariates were not found to be significantly associated with youth HRQOL across all analyses.
p < .05,
p < .01,
p < .0014 (Bonferroni adjusted alpha levels of .0014 [.05/36])
The analyses were repeated with parent-proxy report of HRQOL as the dependent variable. At all three time points, parent factors were associated with parent proxy-report of HRQOL. For mothers, higher levels of personal distress, parenting stress, and SB-specific parenting stress were significantly associated with lower levels of maternal-reported youth HRQOL at one or more time points (Table 2). For fathers, higher levels of personal distress, parenting stress, and SB-specific parenting stress were significantly associated with lower levels of paternal-reported youth HRQOL at one or more time points (Table 2). All of these effects were in the expected direction, with increased distress and stress being related to decreased parent proxy-report of HRQOL.
Given concerns about Type I error (due to the high number of analyses performed), these analyses were also considered using a Bonferroni adjusted alpha level (p = .0014). When this adjustment was applied, maternal SB-specific parenting stress at Time 3 was the only parent factor that remained significantly associated with youth-report of HRQOL (Table 2). For mothers, SB-specific parenting stress (Time 1, Time 3) and maternal personal distress (Time 2) were significantly associated with mother proxy-report of youth HRQOL (Table 2). For fathers, only SB-specific parenting stress (Time 1) remained significantly associated with father proxy-report of HRQOL (Table 2).
Objective 2
The second objective of the study was to determine which of the three parent factors was the most strongly associated with youth- and parent proxy-report of HRQOL and to identify any test the significance of interactions among the parent factors. As maternal SB-specific parenting stress and paternal parenting stress were the only significant independent variables identified in the analyses using youth self-report of HRQOL as the dependent variable, these analyses were only performed for mother and father proxy-reports of youth of HRQOL.
At Time 1 and Time 3, maternal SB-specific parenting stress was the only independent variable found to be significantly associated with mother proxy-report of youth HRQOL (T1: β=−.40, p<.001; T3: β=−.50, p<.01). At Time 2, maternal personal distress was the only independent variable significantly associated with mother proxy-report of youth HRQOL (β=−.39, p<.01). There were no significant interaction effects for maternal variables at any time point (all p’s>.05). Parallel analyses were run for father variables. At Time 1 and Time 2, paternal SB-specific parenting stress was the only independent variable found to be significantly associated with father proxy-report of youth HRQOL (T1: β=−.43, p<.001; T2: β=−.35, p<.05). At Time 3, paternal parenting stress was the only independent variable significantly associated with father proxy-report of HRQOL (β=−.43, p<.05). Interaction effects for paternal variables were non-significant across time points (all p’s>.05). Overall, for both mothers and fathers, SB-specific parenting stress was most often the independent variable most strongly associated with parent proxy-report of youth HRQOL.
DISCUSSION
The current study examined the impact of three distinct parent-related factors – parent personal distress, parenting stress, and SB-specific parenting stress – on HRQOL in youth with SB. Despite previous research indicating that parent factors may influence HRQOL in youth with SB regardless of age, the current study found that parent personal distress, parenting stress, and SB-specific parenting stress was not consistently significantly associated with youth-reported HRQOL in childhood or early adolescence. Given the considerable influence of the family on psychosocial adjustment in youth with chronic illnesses, the finding that none of the parent variables were associated with youth-reported HRQOL at Time 2 was surprising. Previous studies have found associations between parent variables (e.g., parental hope, parental overprotection, maternal psychological distress) and youth HRQOL in this population.11 However, the results of this study suggest that parent-specific factors may not significantly impact youth-reported HRQOL consistently across developmental periods.
On the other hand, some associations between the parent-related variables and youth-reported HRQOL were found, which are noteworthy since these associations cannot be attributed to common-method variance. Specifically, youth of mothers reporting greater SB-specific parenting stress were found to self-report lower HRQOL. It is likely that younger children with SB are more dependent on their parents for assistance in completing daily medical tasks (e.g., clean intermittent catheterization).1 Further, research has shown that mothers of youth with SB are more likely than fathers to take on the role of managing their child’s medical regimen.30 Therefore, younger children may be impacted more significantly by maternal SB-specific parenting stress.
The results of the current study also suggest that as youth become older (at Time 3), their reports of HRQOL may be more affected by parents’ levels of stress (either SB-specific [mothers] or general parenting stress [fathers]). Adolescence is a developmental period when youth seek opportunities for independence and autonomy. However, youth with SB have been found to display more dependent behavior than their typically developing peers consistently from childhood through late adolescence.6 As youth mature, they may become more aware of the mental states of those around them. Adolescents with SB likely spend a good deal of time with their parents; and, thus, may be more greatly impacted by their parents psychosocial functioning (e.g., stress level) than is the case for typically developing youth. Additionally, as youth with SB mature, they are more likely to take responsibility for their own medical care.31 The transfer of this responsibility from parent to adolescent may be a stress-inducing process for parents and may also lead to poorer medical adherence in youth with SB.31 Therefore, the relationship between parenting stress and youth-reported HRQOL found in this study at Time 3 could be related to the transfer of medical responsibility from parent to child, which is more likely to occur for more youth in the Time 3 age range (i.e., 12–19 years) than for those in the Times 1 or 2 age ranges (8–15 years and 10–17 years, respectively). The association between maternal SB-specific parenting stress and youth-reported HRQOL remained significant after applying the Bonferroni correction, further supporting the hypothesis that adolescents with SB are impacted by medically-related parental stress. Still, further research is needed to better understand this process and the relationship between parenting stress (both general and condition-specific) and youth-reported HRQOL.
Given that there were relatively few associations between the independent variables and youth-reported HRQOL and the fact that none of the previously established associations between this study’s covariates (age, SES, IQ, and illness severity) and youth-reported HRQOL were found to be significant, the validity of the youth HRQOL measure is called into question. It is possible that this generic measure of HRQOL may not be the “best” assessment of HRQOL for youth with SB. Though many chronic illnesses share common features (such as family conflict, fatigue, pain, stigmatization by peers, and financial burden), specific illnesses also have unique characteristics that may not be adequately assessed by a generic HRQOL measure. SB is one such condition that has effects that may not be captured by a general assessment of HRQOL.3 SB is a congenital disorder with a chronic course. Youth with SB experience a chronic type of stress due to the daily struggles of a complex medical regimen involving multiple domains, including managing limited mobility and bowel and bladder routines.1 HRQOL instruments developed for healthy children or children with other chronic illnesses (e.g., diabetes) may not capture the small but clinically important variability in this population because they are not designed to measure the impact of SB on HRQOL. The need for a SB-specific HRQOL questionnaire has been recognized,3 and recently, two new assessments of HRQOL in this population were developed, validated, and published.32–33 The use of these instruments will likely improve the assessment of HRQOL in this population.
The analyses using parent-proxy reports of HRQOL highlight the importance of assessing SB-specific factors. For both mothers and fathers, higher levels of SB-specific parenting stress were consistently the most significantly associated with proxy-report of youth HRQOL, and these associations remained significant after applying the Bonferroni correction. Though questions on the psychosocial subscale of the PedsQL did not specifically mention SB-specific medical issues (such as bowel/bladder management), it is possible that parents considered these daily struggles when responding to these questions. Given the impact that decreased mobility and bowel and bladder management have on parenting stress,13 it is possible that assessments of HRQOL that include these specific condition-related domains (such as the Spina Bifida Pediatric Questionnaire33 or Quality of Life Assessment in Spina Bifida for Children [which has both adolescent and adult versions]32) may better allow for the detection of a relationship between parenting constructs and youth HRQOL. It is also possible that parents may have been better able to understand the impact that SB has on their child’s overall functioning and, therefore, more successfully translated the daily stressors these youth experience into their report of HRQOL. It was surprising that illness- severity was not significantly related to youth or parent-proxy reports of youth HRQOL. However, this study’s assessment of illness-severity did not include questions concerning bowel and bladder functioning. It is possible that bowel and bladder dysfunction is the illness-related factor most impactful on HRQOL. The significant association between SB-specific parenting stress (but not illness-severity) and parent proxy-report of youth HRQOL highlights the importance of including the bowel/bladder domain when assessing HRQOL in youth with SB.
Strengths, Limitations, and Future Research
This study had several strengths. First, the current study sought to expand the limited knowledge of modifiable factors affecting HRQOL in youth with SB. Second, longitudinal data were used to examine relationships at multiple time points, which allows for consideration of developmental changes in childhood and adolescence as well as providing initial support for causal conclusions. Third, the study used data from multiple reporters, including fathers. It cannot be assumed that all caregivers (mothers and fathers) experience their role as caretakers identically, and it is important to include fathers in research studies so that these potential differences can be better understood.
However, there are several limitations of the current study that should be addressed in future work. First, the current study used the PedsQL to assess HRQOL in youth with SB. This measure has not been normed in this population specifically. Due to the limited mobility of many youth with SB, the physical subscale of this measure was not used as the items were deemed inappropriate for these youth. It is possible that a SB-specific measure of youth HRQOL would be more appropriate for the assessment of this construct in this population. An additional measurement limitation is the use of the PSI with parents of older children (as it is only validated for use with parents of children ages 2–12 years).25 Use of a measure of parenting stress specific to parents of adolescents could yield different results. Further, many of the significant findings of this study emerged when parent-report was used for both the independent and dependent variables. Therefore, common-method variance cannot be ruled-out as an explanation for these significant associations. On the other hand, common method variance is not the only explanation for these findings since the parent-related factors were differentially associated with HRQOL. Fourth, the number of analyses run introduce Type 1 error as a potential explanation for the significant findings. However, the results are presented both with and without the application of a Bonferroni correction, and many of the associations remain significant when this correction was applied. Fifth, though the majority of the sample had myelomeningocele (~88%), youth with less severe types of SB were also included in the study. The inclusion of families of youth with both myelomeningocele and other types of SB may have influenced the findings. Lastly, it is possible that families that chose not to participate at later data collection time points were experiencing greater levels of stress and distress. This also may indicate that an especially at-risk group exists (but is not represented in the study). Therefore, the results and clinical implications of the current study should be interpreted with caution.
Clinical Implications
The results of the current study have important implications for work with families of youth with SB. Given the consistently lower HRQOL of youth with SB and the potentially important role that HRQOL plays in adherence and disease management, it is critical that factors that affect HRQOL during this developmental period in this population be identified. Previous research has found that youth with SB have significant social difficulties;5 social difficulties may also significantly impact HRQOL for these youth. Thus, in future work, it will be important to expand the types of variables used as predictors of HRQOL. In this study, parent factors were found to be associated with youth perceptions of HRQOL primarily for older participants and with parents’ perceptions of youth HRQOL at all ages. These perceptions may influence the way parents treat their child with SB. Therefore, interventions targeting parenting stress and distress in this population could have clinically significant effects not only for parents, but for youth with SB as well. Golfenshtein et al.’s (2016) review highlights potential parenting stress reduction interventions for parents of youth with pediatric health conditions, though it should be noted that most current interventions have failed to demonstrate long-term reductions in parenting stress.34 Further, health providers for youth with SB could play a critical role in identifying at-risk parents by incorporating the use of brief screening instruments (e.g., the Patient Health Questionnaire −2 [PHQ-2]) and clinical interviewing into regular clinic visits.35 Finally, special consideration should be given when choosing an instrument to assess HRQOL in this population, as SB-specific instruments may have more construct validity with such youth.32–33
Acknowledgments
Funding
This research was supported in part by grants from the National Institute of Nursing Research and the Office of Behavioral and Social Sciences Research (R01 NR016235), National Institute of Child Health and Human Development (R01 HD048629), and the March of Dimes Birth Defects Foundation (12-FY13-271). This study is part of an ongoing, longitudinal study.
The authors would like to thank the Illinois Spina Bifida Association as well as staff of the spina bifida clinics at Ann & Robert H. Lurie Children’s Hospital of Chicago, Shriners Hospital for Children-Chicago, and Loyola University Medical Center. We also thank the many undergraduate and graduate research assistants who helped with data collection and entry. Finally, and most importantly, we would like to thank the parents and children who participated in this study.
Footnotes
Longitudinal analyses were also completed using Time 1 parent factors to predict self- and parent proxy-reported youth HRQOL at Times 2 and 3. When running these longitudinal regression analyses, independent variables were entered in the following order: (Step 1) HRQOL at Time 1 (for Time 2 outcome) or HRQOL at Time 2 (for Time 3 outcome); (Step 2) covariates – age, IQ, SES, illness severity; (Step 3) independent variable(s). Analyses paralleled those reported in this manuscript, such that regressions were run using each individual parent factor (Objective 1) and with all parent factors entered in a single model (Objective 2). However, these analyses were non-significant, and, thus, are not reported in this manuscript. Such non-significance was due, in part, to the high level of stability for HRQOL (i.e., the high correlation between Time 1, Time 2, and Time 3 HRQOL).
References
- 1.Copp AJ, Adzick NS, Chitty LS, et al. Spina bifida. Nat Rev Dis Primers. 2015;1:15007. doi: 10.1038/nrdp.2015.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murray CB, Holmbeck GN, Ros AM, et al. A longitudinal examination of health-related quality of life in children and adolescents with spina bifida. J Pediatr Psychol. 2015;40:419–430. doi: 10.1093/jpepsy/jsu098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sawin KJ, Bellin MH. Quality of life in individuals with spina bifida: a research update. Dev Disabil Res Rev. 2010;16:47–59. doi: 10.1002/ddrr.96. [DOI] [PubMed] [Google Scholar]
- 4.Loon SC, Jin J, Jin GM. The relationship between quality of life and adherence to medication in glaucoma patients in Singapore. J Glaucoma. 2015;24:e36–e42. doi: 10.1097/IJG.0000000000000007. [DOI] [PubMed] [Google Scholar]
- 5.Holmbeck GN, Westhoven VC, Phillips WS, et al. A multimethod, multi-informant, and multidimensional perspective on psychosocial adjustment in preadolescents with spina bifida. J Consult Clin Psychol. 2003;71:782–796. doi: 10.1037/0022-006x.71.4.782. [DOI] [PubMed] [Google Scholar]
- 6.Lennon JM, Murray CB, Bechtel CF, et al. Resilience and disruption in observed family interactions in youth with and without spina bifida: An eight-year, five-wave longitudinal study. J Pediatr Psychol. 2015;40:943–955. doi: 10.1093/jpepsy/jsv033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Devine KA, Wasserman RM, Gershenson LS, et al. Mother-adolescent agreement regarding decision-making autonomy: A longitudinal comparison of families of adolescents with and without spina bifida. J Pediatr Psychol. 2011;36:277–288. doi: 10.1093/jpepsy/jsq093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Driscoll CFB, Stern A, Ohanian D, et al. Parental perceptions of child vulnerability in families of youth with spina bifida: The role of parental distress and parenting stress. J Pediatr Psychol. 2017 doi: 10.1093/jpepsy/jsx133. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silver EJ, Westbrook LE, Stein RE. Relationship of parental psychological distress to consequences of chronic health conditions in children. J Pediatr Psychol. 1998;23:5–15. doi: 10.1093/jpepsy/23.1.5. [DOI] [PubMed] [Google Scholar]
- 10.Holmbeck GN, Gorey-Ferguson L, Hudson T, et al. Maternal, paternal, and marital functioning in families of preadolescents with spina bifida. J Pediatr Psychol. 1997;22:167–181. doi: 10.1093/jpepsy/22.2.167. [DOI] [PubMed] [Google Scholar]
- 11.Abad MC. Unpublished doctoral dissertation. Loyola University Chicago; Chicago, IL: 2007. Predictors of quality of life in youth with spina bifida. [Google Scholar]
- 12.Deater-Deckard K, Chen N, El Mallah S. Oxford Bibliographies. May, 2013. Parenting Stress. Online [serial online] [Google Scholar]
- 13.Kanaheswari Y, Razak N, Chandra V, et al. Predictors of parenting stress in mothers of children with spina bifida. Spinal Cord. 2011;49:376–380. doi: 10.1038/sc.2010.125. [DOI] [PubMed] [Google Scholar]
- 14.Ong LC, Norshireen NA, Chandran V. A comparison of parenting stress between mothers of children with spina bifida and able bodied controls. Dev Neurorehabil. 2011;14:22–28. doi: 10.3109/17518423.2010.523057. [DOI] [PubMed] [Google Scholar]
- 15.Frontini R, Moreira H, Canavarro MC. Parenting stress and quality of life in pediatric obesity: the mediating role of parenting styles. J Child Fam Stud. 2016;25:1011–1023. [Google Scholar]
- 16.Sawin KJ, Bellin MH, Roux G, et al. The experience of parenting an adolescent with spina bifida. Rehabil Nurs. 2003;28:173–185. doi: 10.1002/j.2048-7940.2003.tb02057.x. [DOI] [PubMed] [Google Scholar]
- 17.Antiel RM, Adzick NS, Thom EA, et al. Impact on family and parental stress of prenatal vs postnatal repare of myelomeningocele. Am J Obstet Gynecol. 2016;215:522.e1–522e6. doi: 10.1016/j.ajog.2016.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quittner AL, Barker DH, Cruz I, et al. Parenting stress among parents of deaf and hearing children: Associations with language delays and behavior problems. Parent Sci Pract. 2010;10:136–155. doi: 10.1080/15295190903212851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cassano M, Adrian M, Veits G, et al. The inclusion of fathers in the empirical investigation of child psychopathology: an update. J Clin Child Adolesc Psychol. 2006;35:583–589. doi: 10.1207/s15374424jccp3504_10. [DOI] [PubMed] [Google Scholar]
- 20.Vermaes IP, Janssens JM, Bosman AM, et al. Parents’ psychological adjustment in families of children with spina bifida: a meta-analysis. BMC Pediatr. 2005;5:32. doi: 10.1186/1471-2431-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malm-Buatsi E, Aston CE, Ryan J, et al. Mental health and parenting characteristics of caregivers of children with spina bifida. J Pediatr Urol. 2015;11:65.e1–65.e7. doi: 10.1016/j.jpurol.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 22.Hollingshead AB. Four Factor Index of Social Status. New Haven, CT: Yale University; 1975. [Google Scholar]
- 23.Wechsler D. WASI: Wechsler Abbreviated Scale of Intelligence Manual. San Antonio, TX: Harcourt Assessment, Inc; 1999. [Google Scholar]
- 24.Derogatis LR, Rickels K, Rock AF. The SCL-90 and the MMPI: a step in the validation of a new self-report scale. Br J Psychiatry. 1976;128:280–289. doi: 10.1192/bjp.128.3.280. [DOI] [PubMed] [Google Scholar]
- 25.Abidin RR. Parenting Stress Index Short Form. Lutz, FL: Psychological Assessment Resources, Inc; 1990. [Google Scholar]
- 26.Hayes SS, Watson SL. The impact of parenting stress: A meta-analysis of studies comparing the experience of parenting stress in parents of children with and without Autism Spectrum Disorder. J Autism Dev Disord. 2013;43:629–642. doi: 10.1007/s10803-012-1604-y. [DOI] [PubMed] [Google Scholar]
- 27.Quittner AL, Glueckauf RL, Jackson DN. Chronic parenting stress: moderating versus mediating effects of social support. J Pers Soc Psychol. 1990;59:1266–1278. doi: 10.1037//0022-3514.59.6.1266. [DOI] [PubMed] [Google Scholar]
- 28.Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care. 2001;39:800–812. doi: 10.1097/00005650-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Faul F, Erdfelder E, Buchner A, et al. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Beh Res Methods. 2009;41:1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- 30.Brekke I, Fruh EA, Kvarme LG, et al. Long-time sickness absence among parents of pre-school children with cerebral palsy, spina bifida, and down syndrome: a longitudinal study. BMC Pediatr. 2017;17:26. doi: 10.1186/s12887-016-0774-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Psihogios AM, Kolbuck V, Holmbeck GN. Condition self-management in pediatric spina bifida: a longitudinal investigation of medical adherence, responsibility-sharing, and independence skills. J Pediatr Psychol. 2015;40:790–803. doi: 10.1093/jpepsy/jsv044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Szymanski KM, Misseri R, Whittam B, et al. Quality of Life Assessment in Spina Bifida for Children (QUALAS-C): development and validation of a novel health-related quality of life instrument. Urology. 2016;87:178–184. doi: 10.1016/j.urology.2015.09.027. [DOI] [PubMed] [Google Scholar]
- 33.Velde VS, Laridaen J, Van Hoecke E, et al. Development and validation of a spina bifida-specific pediatric quality of life questionnaire: the Spina Bifida Pediatric Questionnaire, SBPQ. Childs Nerv Syst. 2016;32:105–110. doi: 10.1007/s00381-015-2903-3. [DOI] [PubMed] [Google Scholar]
- 34.Gofrenshtein N, Srulovici E, Deatrick JA. Interventions for reducing parenting stress in families with pediatric conditions: An integrative review. J Fam Nurs. 2016;4:460–492. doi: 10.1177/1074840716676083. [DOI] [PubMed] [Google Scholar]
- 35.National Research Council and Institute of Medicine Committee on Depression, Parenting Practices, and the Healthy Development of Children. Screening for depression in parents. In: England MJ, Sim LJ, editors. Depression in Parents, Parenting, and Children: Opportunities to Improve Identification, Treatment, and Prevention. Washington, D.C: 2009. [PubMed] [Google Scholar]