Abstract
BACKGROUND:
Children with acute lymphoblastic leukemia (ALL) have increased risk for obesity and short stature. Data on patients treated on contemporary protocols without cranial irradiation are limited.
METHODS:
Changes in body mass index (BMI), height, and weight Z-scores from diagnosis to 5 years off therapy were evaluated with multivariable analysis in 372 children with ALL (aged 2–18 years at diagnosis) enrolled on the St. Jude Total XV protocol in 2000–2007.
RESULTS:
The percentage of overweight/obese patients increased from 25.5% at diagnosis to approximately 50% during the off-therapy period. Median BMI Z-scores increased significantly during glucocorticoid therapy (induction [Δ0.56; P<0.001, 95% confidence interval [CI]: 0.29–0.64] and re-induction II [Δ0.22; P=0.001, 95% CI: 0.13–0.49]) and during the first year post-therapy (Δ0.18; P=0.006, 95% CI: 0.08–0.46). Among patients who were of healthy weight/underweight at diagnosis, those aged 2 to <10 years at diagnosis had a significantly higher risk of becoming overweight/obese during or after therapy than did those aged ≥10 years (P=0.001). Height Z-scores declined during treatment and improved post-therapy. Age 2 to <10 years at diagnosis, low-risk status, white blood cell count (WBC) < 50×109/L at diagnosis, and negative central nervous system (CNS) disease were associated with significantly better improvement in height Z-scores during the off-therapy period than were age ≥10 years, standard/high-risk status, WBC ≥ 50×109/L, and positive CNS disease, respectively.
CONCLUSIONS:
Obesity was prevalent, and height growth, especially in patients with identified risk factors, was compromised. Multidisciplinary intervention should begin during induction therapy and continue during the off-therapy period.
Keywords: acute lymphoblastic leukemia, children, body mass index, height, weight
PRECIS
Body mass index Z-scores increased during induction therapy and remained elevated even after therapy completion; therefore, multidisciplinary intervention for obesity should begin during induction therapy and continue during the off-therapy period.
Height Z-scores declined during therapy and improved after therapy completion; however, they remained low in patients aged ≥10 years at diagnosis, in those with standard/high-risk status, in those with a white blood cell count of ≥50×109/L at diagnosis, and in those with positive central nervous system disease.
INTRODUCTION
As a result of improved risk-directed treatment and supportive care, the survival rate of children with acute lymphoblastic leukemia (ALL) has improved dramatically to approximately 90%1. Childhood obesity is prevalent in the United States (17.0% of US children were obese in 2011–2014)2; however, children with ALL are at increased risk of becoming overweight or obese3–6, which can result in substantial physical and psychosocial morbidity and further complicate other therapy-associated adverse effects, such as infections, osteopenia, and osteonecrosis3. Reductions in linear growth and final adult height are well-known complications of ALL treatment, especially that involving cranial irradiation, which can cause hypothalamic-pituitary dysfunction7–9. Data on obesity and linear growth in children with ALL treated uniformly on a single contemporary protocol without cranial irradiation are limited.
It has been suggested that interventions to promote healthy body composition should be initiated during therapy10–12. However, guidelines regarding the timing and nature of such efforts are lacking and we have not conducted intervention trials at our institution. To evaluate the appropriate timing and duration of intervention, we analyzed longitudinal changes in body mass index (BMI), height, and weight, along with clinical factors associated with these changes, in pediatric patients with ALL during and after treatment without prophylactic cranial irradiation on the St. Jude Children’s Research Hospital (St. Jude) Total XV protocol13.
MATERIALS AND METHODS
Patients
Children and adolescents with ALL (aged 1–18 years at diagnosis) were enrolled on the Total XV protocol at St. Jude between 2000 and 200713. Patients who died, experienced relapse, received re-intensification therapy because of refractory disease, or underwent a hematopoietic stem cell transplant were censored at the time of event. The study was approved by the St. Jude Institutional Review Board.
Patient characteristics were recorded, including sex, age at diagnosis (2 to <10 vs. ≥10 years), risk stratification (low risk [LR] vs. standard/high risk [SR/HR]), white blood cell count (WBC) at diagnosis (<50×109/L vs. ≥50×109/L), central nervous system (CNS) disease status (CNS-1 vs. CNS-2, CNS-3, or traumatic lumbar puncture with blast cells), and immunophenotype (B-ALL vs. T-ALL). Race was recorded based on genetic ancestry (black, white, Native American, or other) as determined by DNA single-nucleotide polymorphisms14. Because of the small number of patients in the Native American and “other” groups, the analysis of race was expressed as comparisons between black and white races only, although all races were incorporated in the mixed model.
Treatment
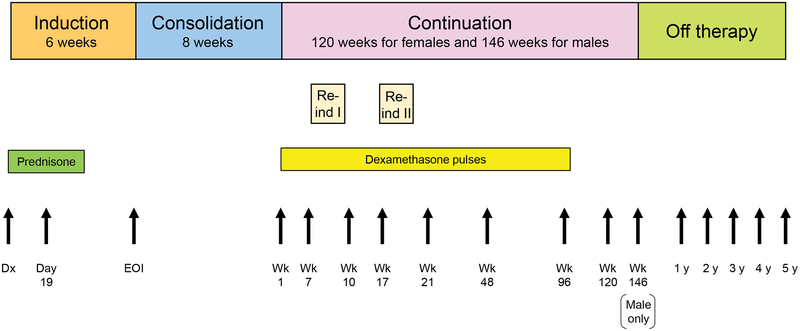
Total XV therapy lasted approximately 2.5 years for females and 3 years for males and consisted of 3 treatment phases: induction (6 weeks), consolidation (8 weeks), and continuation (120 weeks for females, 146 weeks for males) (Figure 1)13. All patients received prednisone at 40 mg/m2/day for 28 days during induction and dexamethasone at 8 mg/m2/day on days 1–8 and 15–21 during re-induction I (continuation weeks 7–9) and re-induction II (weeks 17–19). Five-day dexamethasone pulses (every 3–4 weeks) were given during continuation therapy at 8 mg/m2/day for LR patients and 12 mg/m2/day for SR/HR patients from continuation week 1 to week 100, except in the weeks of re-induction I and II. Depending on their risk and CNS status, patients received 13 to 25 intrathecal treatments without cranial irradiation. After completing therapy, patients were followed up at least yearly.
Figure 1.
Treatment schema of the Total XV protocol and time-points for height and weight measurements. Abbreviations: Re-ind, reinduction therapy; Dx, diagnosis; EOI, end of induction; Wk, week.
BMI, Weight, and Height
Height and weight data were obtained from routine clinic visits at St. Jude at 16 time-points for females and 17 time-points for males: the start of induction (± 3 days); day 19 of induction (± 3 days); the end of induction (± 7 days); continuation weeks 1, 7 (the start of re-induction I), 10, 17 (the start of re-induction II), and 21 (± 7 days), as well as weeks 48, 96, 120 (the end of therapy for females), and 146 (the end of therapy for males) (± 14 days); and every year thereafter (± 180 days) until the patients were 5 years off therapy (Figure 1). A broader time range (± 180 days) was allowed for data collection during the off-therapy period, as patient scheduling preferences and appointment availability did not always align with the off-therapy anniversary, especially after 3 years off therapy, when patients transition to our survivorship clinic, which has different schedule availability. Each individual’s height and weight data were checked for outlier values by first fitting a quadratic local regression polynomial to the data, using the “smooth” function with the “loess” method in MATLAB (MathWorks, version R2017a). An outlier value was defined as a height with an absolute residual greater than 5 cm or 15% or a weight with an absolute residual greater than 10 kg or 25%. Outlier values were replaced with interpolated values, again using quadratic local regression polynomials and the remaining non-outlier data. BMI was calculated as the weight in kilograms divided by the square of the height in meters. BMI, height, and weight data for patients aged 2–20 years were converted to age- and sex-adjusted Z-scores by using the SAS program for the Centers for Disease Control and Prevention (CDC) growth charts15. For patients older than 20 years and for final height analysis, Z-scores were calculated based on reference data for individuals aged 20 years10,16. For BMI category (underweight, healthy weight, overweight, or obese), percentile criteria were used for patients aged 2–20 years and the actual BMIs were used for those older than 20 years. The final height was defined when the growth velocity was less than 2 cm over a 12-month period after completing therapy17. Permanent loss of height was calculated as the difference between the predicted final height based on the height Z-score at diagnosis and the actual final height. Short stature was defined as a height Z-score < −2.
We also calculated Z-scores for National Health and Nutrition Examination Survey (NHANES) 2005–2006 participants (aged 2–19 years) by using the CDC 2000 growth charts, and we used survey sampling methodology to calculate the means by age and sex15,18. We then applied indirect standardization based on the distribution of age and sex in our cohort.
Statistical Analysis
Z-score changes in BMI, height, and weight between time-points were evaluated using repeated measures, adjusting for the effects of baseline Z-scores, age, sex, ancestry, treatment risk, CNS status, WBC count at diagnosis, and time. The correlation of Z-scores at each time-point with those at diagnosis was examined via the Pearson correlation coefficient. The associations of Z-scores with clinical features at each time-point were analyzed by the Wilcoxon rank-sum test (for 2 groups) or Kruskal-Wallis test (for >2 groups) in univariate analysis, then longitudinal data of Z-scores were evaluated using a mixed model, with factors with P-values of <0.1 at 2 or more time-points in univariate analysis, as well as treatment risk, sex, race, baseline Z-scores, and time-points. Risk factors for patients who were underweight or of healthy weight at diagnosis becoming overweight or obese were evaluated with Chi-square tests by using clinical characteristics, and a multivariable logistic regression model was used for characteristics with P-values of <0.1. Missing data and data for censored patients were not imputed in our statistical analyses. P-values were not adjusted for multiple comparisons.
RESULTS
Patient Characteristics
Of 408 children with ALL enrolled on the St. Jude Total XV protocol, 10 with Down syndrome and 26 aged between 1 and 2 years at diagnosis were excluded, leaving 372 for study (Table 1). The median age at diagnosis was 5.9 years (range, 2.0–18.8 years), and the median follow-up was 10.7 years (range, 3.2–14.8 years) at the time of analysis.
TABLE 1.
Patient Characteristics
| Clinical Characteristics | Total (n=372) |
|---|---|
| Age at diagnosis; N (%) | |
| 2 to <10 years | 266 (71.5) |
| ≥10 years | 106 (28.5) |
| Sex; N (%) | |
| Female | 159 (42.7) |
| Male | 213 (57.3) |
| Ancestry; N (%) | |
| White | 253 (68.0) |
| Black | 63 (16.9) |
| Native American | 26 (7.0) |
| Other | 22 (5.9) |
| No dataa | 8 (2.2) |
| Lineage; N (%) | |
| B cell | 309 (83.1) |
| T cell | 63 (16.9) |
| WBC at diagnosis; N (%) | |
| <50 × 109/L | 272 (73.1) |
| ≥50 × 109/L | 100 (26.9) |
| CNS status; N (%) | |
| CNS-1 | 282 (75.8) |
| Other | 90 (24.2) |
| Risk; N (%) | |
| Low | 180 (48.4) |
| Standard/high | 192 (51.6) |
Abbreviations: WBC, white blood cell count CNS, central nervous system
Germline samples for ancestry analysis were not available.
Longitudinal Changes in BMI, Height, and Weight Z-Scores During and After Therapy
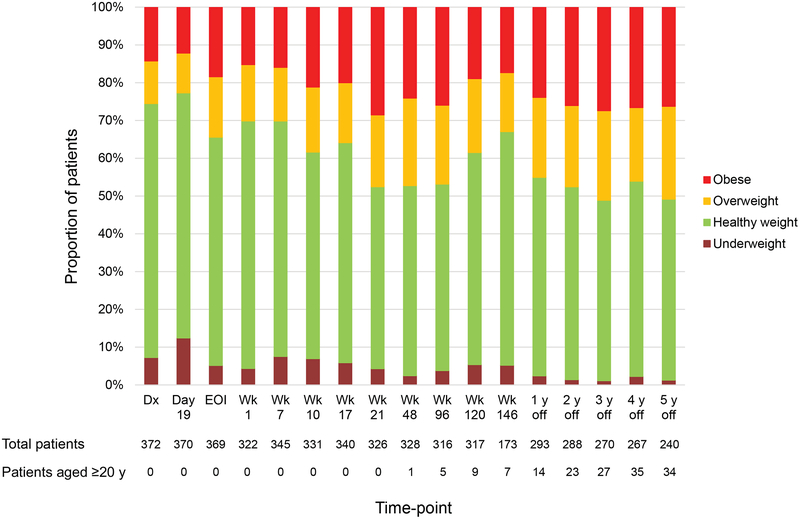
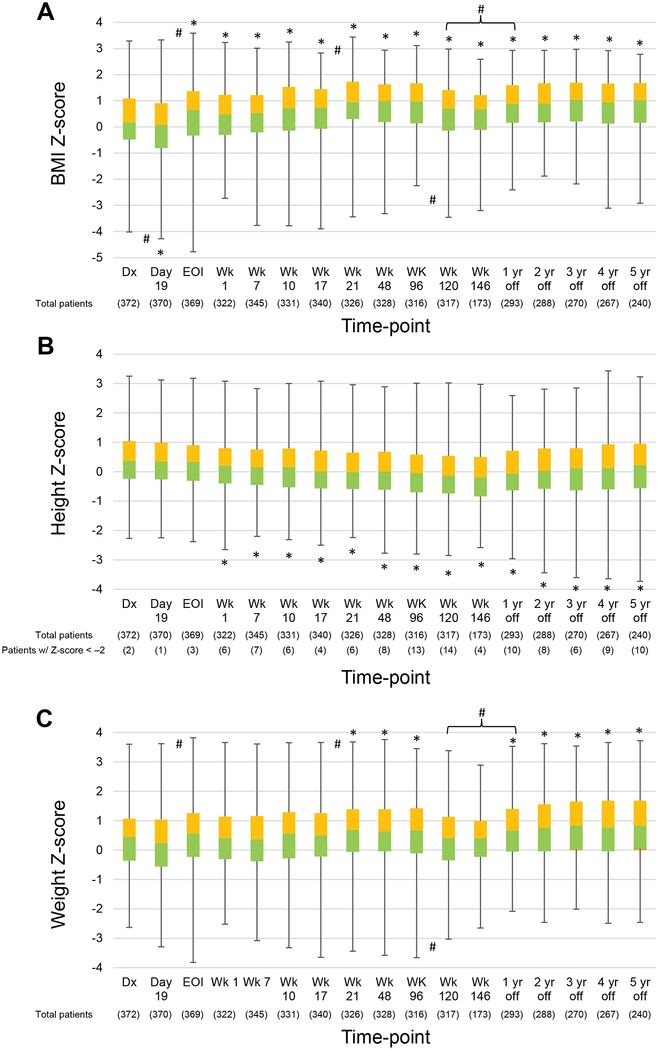
Longitudinal changes in the percentage of patients in each BMI category showed the percentage of overweight/obese patients increasing and that of underweight patients decreasing over time (Figure 2). Although a broader time range (± 180 days) was allowed for data collection during the off-therapy period, the percentages of patients beyond the 90-day point were as follows: 1 year off therapy, 3.4%; 2 years off therapy, 5.2%; 3 years off therapy, 13.3%; 4 years off therapy, 21.0%; and 5 years off therapy, 24.6%. There were very few outliers requiring interpolation (representing less than 0.7% of the data for height and less than 0.1% for weight). Figure 3A and Supporting Table 1 show the longitudinal changes in the median BMI Z-score. The median BMI Z-score at all on- and off-therapy time-points, except day 19 of induction, was significantly higher than at diagnosis (P≤0.015 for all). Median BMI Z-scores increased significantly between induction day 19 and the end of induction (Δ0.56; P<0.001, 95% confidence interval [CI]: 0.29–0.64), during re-induction II (weeks 17–21, Δ0.22; P=0.001, 95% CI: 0.13–0.49), and between week 120 and 1 year off therapy (Δ0.18; P=0.006, 95% CI: 0.08–0.46) (Figure 3A). In contrast, median BMI Z-scores decreased significantly between diagnosis and induction day 19 (Δ−0.09; P=0.034, 95% CI: ‒0.36 to ‒0.01) and between weeks 96 and 120 (Δ−0.27; P=0.004, 95% CI: ‒0.46 to ‒0.09), corresponding to the discontinuation of dexamethasone pulses after week 100.
Figure 2.
Longitudinal changes in the percentage of patients in each body mass index category. Abbreviations: Dx, diagnosis; EOI, end of induction; Wk, week.
Figure 3.
Longitudinal changes in the median body mass index (BMI) (A), height (B), and weight (C) Z-scores of patients during therapy and in the off-therapy period.
Boxes show scores from the first quartile to the third quartile. The border between the 2 boxes shows the median Z-score. Error bars show minimum and maximum values. Significant differences from baseline values and intervals with significant changes in Z-scores are indicated by the symbols * and #, respectively. As only males received week 146 therapy, interval changes between week 120 and 1 year off therapy are evaluated. Symbols above the line represent increases in Z-scores; those below the line show decreases between 2 time-points. Abbreviations: BMI, body mass index; Dx, diagnosis; EOI, end of induction; Wk, week.
Figure 3B and Supporting Table 2 show the longitudinal changes in the median height Z-scores. From diagnosis (median Z-score = 0.37), median height Z-scores decreased during ALL therapy (to −0.13 at week 120) then improved during the off-therapy period (to 0.22 at 5 years off therapy), although they never improved to the levels at diagnosis. After week 1, all median height Z-scores were significantly lower than the score at diagnosis (P≤0.008 for all). The incidence of short stature, defined as height Z-score < ‒2, increased from 2 of 372 patients (0.5%) at diagnosis to 14 of 317 patients (4.4%) at week 120 (Figure 3B). Twenty-six patients had height Z-scores < −2 during or after therapy, and 24 patients were evaluable for follow-up. Seven were seen by the endocrinology department because of significant concerns regarding their growth velocity: 3 of the 7 underwent dynamic testing that ruled out growth hormone deficiency, 2 had normal-screening plasma insulin-like growth factor-1 (IGF-1) levels, and 2 were observed without testing because of familial short stature (both reached final heights consistent with the mid-parental heights). Seventeen patients were not seen by endocrinology, and 12 experienced spontaneous improvement in their growth velocity (among these, 6 had normal IGF-1 levels and 6 were not tested), so that referral was not deemed necessary. For the remaining 5 patients, there was no information regarding their familial height or growth potential. Three of these patients had normal IGF-1 levels and 2 were not tested. None of the patients were treated with growth hormone.
The median weight Z-scores in weeks 21, 48, and 96 and at 1 year off therapy and after were significantly higher than the median score at diagnosis (0.45) (P≤0.041 for all) and increased during the off-therapy period (Figure 3C and Supporting Table 3). The median weight Z-scores increased significantly between induction day 19 and the end of induction (Δ0.33; P=0.001, 95% CI: 0.11–0.45), during re-induction II (weeks 17–21, Δ0.19; P=0.032, 95% CI: 0.02–0.37), and between week 120 and 1 year off therapy (Δ0.26; P=0.001, 95% CI: 0.13–0.50) (Figure 3C). The median weight Z-scores decreased significantly between weeks 96 and 120 (Δ−0.26, P=0.016, 95% CI: ‒0.41 to ‒0.04).
Supporting Table 4 shows the significant correlations of the BMI, height, and weight Z-scores at diagnosis with subsequent values (P<0.001 for all), although the correlations became less significant at the later time-points.
As obesity has become more of a problem in the general pediatric population since the growth curves were constructed in 20002,19, we estimated the BMI, height, and weight Z-scores in NHANES 2005–2006 participants (aged 2–19 years) who matched to our patient population by age and sex. The mean Z-scores ± standard error for BMI, height, and weight were 0.33 ± 0.13, 0.23 ± 0.12, and 0.37 ± 0.13, respectively.
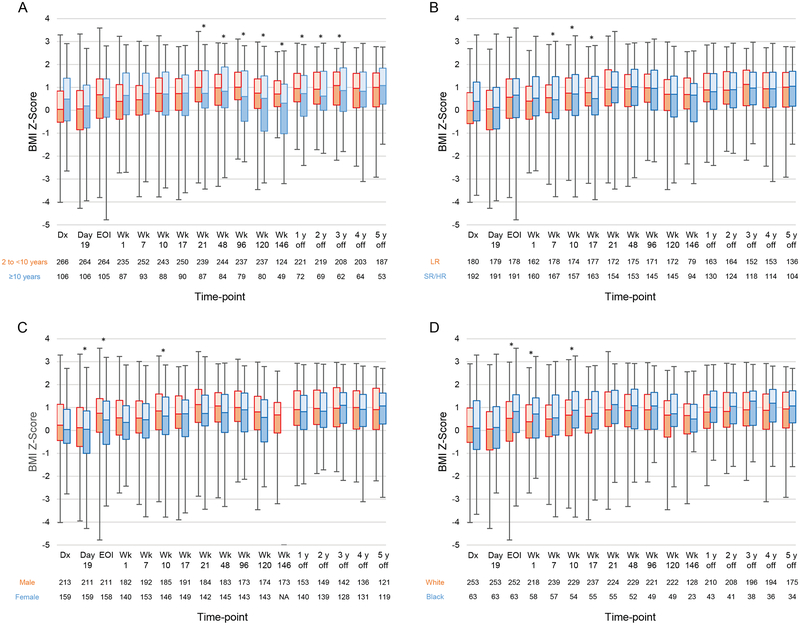
Clinical Characteristics Affecting BMI Z-Scores During and After Therapy
Supporting Table 5 shows the significant differences in median BMI Z-scores for clinical characteristics at each time-point, as determined by univariate analysis. In multivariable analysis (Figure 4 and Supporting Table 6), patients aged 2 to <10 years at diagnosis had median BMI Z-scores significantly higher than those of patients aged ≥10 years at diagnosis between week 21 and 3 years off therapy (P≤0.033 for all) (Figure 4A). Median Z-scores in older patients declined from week 48 to the end of therapy but increased during the off-therapy period. Median BMI Z-scores were higher for LR patients than for SR/HR patients in weeks 7, 10, and 17 (P≤0.005 for all) (Figure 4B); higher for males than for females on day 19, at the end of induction, and in week 10 (P≤0.025 for all) (Figure 4C); and higher for black patients than for white patients at the end of induction and in weeks 1 and 10 (P≤0.043 for all) (Figure 4D). However, these differences disappeared later in the off-therapy period.
Figure 4.
Longitudinal changes in the median body mass index (BMI) Z-scores according to (A) age (2 to <10 years vs. ≥10 years); (B) treatment risk (low risk [LR] vs. standard/high risk [SR/HR]); (C) sex (male vs. female); and (D) race (white vs. black). Boxes show scores from the first quartile to the third quartile. The border between the 2 boxes shows the median Z-score. Error bars show minimum and maximum values. Time-points with significant differences between the 2 groups in the mixed model are indicated by asterisks. Abbreviations: BMI, body mass index; Dx, diagnosis; EOI, end of induction; Wk, week.
We also evaluated risk factors in patients who were of healthy weight or underweight at diagnosis but became overweight or obese during or after therapy (Table 2). Multivariable analysis showed that patients aged 2 to <10 years at diagnosis had a significantly higher risk of becoming overweight/obese than those aged ≥10 years (P=0.001).
TABLE 2.
Characteristics of Patients Who Were of Healthy Weight or Underweight at Diagnosis and Became Overweight/Obese During or After Therapy
| Variables | Total (n=277) | Overweight/Obese | Univariate Analysis | Multivariable Analysisa | |
|---|---|---|---|---|---|
| No (n=115) | Yes (n=162) | P-Value | P-Value | ||
| Age at diagnosis; N (%) | |||||
| 2 to <10 years | 211 | 72 (34.1) | 139 (65.9) | <0.001 | 0.001 |
| ≥10 years | 66 | 43 (65.2) | 23 (34.8) | ||
| Sex; N (%) | |||||
| Female | 122 | 48 (39.3) | 74 (60.7) | 0.515 | |
| Male | 155 | 67 (43.2) | 88 (56.8) | ||
| Ancestry; N (%) | |||||
| White | 193 | 80 (41.5) | 113 (58.5) | 0.216 | |
| Black | 43 | 21 (48.8) | 22 (51.2) | ||
| Native American | 19 | 4 (21.1) | 15 (78.9) | ||
| Other | 17 | 6 (35.3) | 11 (64.7) | ||
| No datab | 5 | 4 (80.0) | 1 (20.0) | ||
| Lineage; N (%) | |||||
| B cell | 237 | 92 (38.8) | 145 (61.2) | 0.027 | 0.548 |
| T cell | 40 | 23 (57.5) | 17 (42.5) | ||
| WBC at diagnosis; N (%) | |||||
| <50 × 109/L | 212 | 84 (39.6) | 128 (60.4) | 0.248 | |
| ≥50 × 109/L | 65 | 31 (47.7) | 34 (52.3) | ||
| CNS status; N (%) | |||||
| CNS-1 | 216 | 90 (41.7) | 126 (58.3) | 0.924 | |
| Other | 61 | 25 (41.0) | 36 (59.0) | ||
| Risk; N (%) | |||||
| Low | 144 | 44 (30.6) | 100 (69.4) | <0.001 | 0.092 |
| Standard/high | 133 | 71 (53.4) | 62 (46.6) | ||
Multivariable analysis was performed to determine the independent effect of each factor that had a P-value of less than 0.10 in univariate analysis.
Germline samples for ancestry analysis were not available.
Clinical Characteristics Affecting Height Z-Scores During and After Therapy
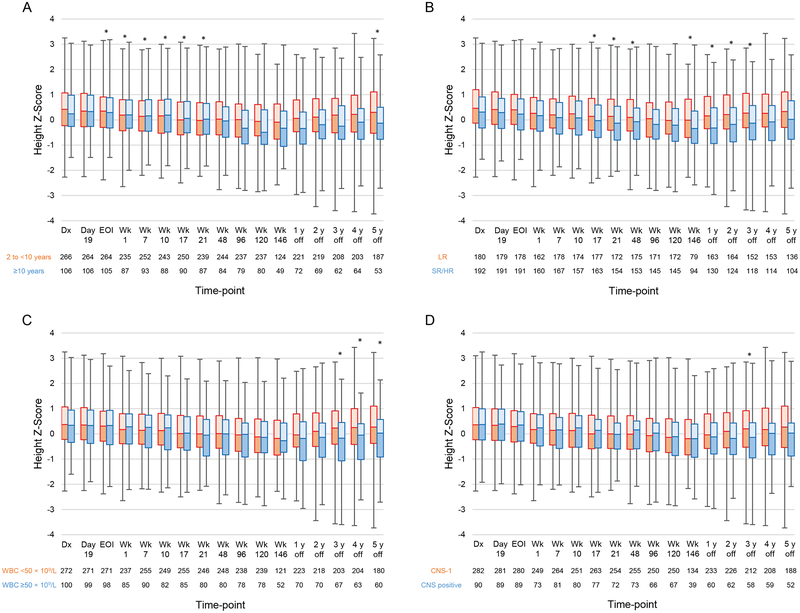
The results of univariate analysis of median height Z-scores with respect to clinical characteristics are shown in Supporting Table 7. The results of multivariable analysis are shown in Figure 5 and Supporting Table 8. Patients aged ≥10 years at diagnosis had median height Z-scores significantly higher than those of patients aged 2 to <10 years at diagnosis at the end of induction and in continuation weeks 1–21 (P≤0.038 for all); however, the median height Z-scores at 5 years off therapy were significantly higher for patients aged 2 to <10 years at diagnosis than for patients aged ≥10 years at diagnosis (P=0.011) (Figure 5A). Median height Z-scores were higher for LR patients than for SR/HR patients in weeks 17, 21, 48, and 146 and at 1–3 years off therapy (P≤0.024 for all) (Figure 5B). At 3–5 years off therapy, the median height Z-scores of patients with WBCs < 50×109/L at diagnosis were higher than those of patients with WBCs ≥ 50×109/L at diagnosis (P≤0.018 for all) (Figure 5C). Compared with patients with CNS disease (CNS-2, CNS-3, or a traumatic tap with blasts), those with CNS-1 status had significantly higher median height Z-scores at 3 years off therapy (P=0.029) (Figure 5D). Median height Z-scores were higher for males than for females in weeks 96 and 120 (P≤0.009 for all) and higher for white patients than for black patients at 2–4 years off therapy (P≤0.027 for all).
Figure 5.
Longitudinal changes in the median height Z-scores according to (A) age (2 to <10 years vs. ≥10 years); (B) treatment risk (low risk [LR] vs. standard/high risk [SR/HR]; (C) white blood cell count (WBC) at diagnosis (<50×109/L vs. ≥50×109/L); and (D) central nervous system (CNS) disease status at diagnosis (CNS-1 vs. CNS positive). Boxes show scores from the first quartile to the third quartile. The border between the 2 boxes shows the median Z-score. Error bars show minimum and maximum values. Time-points at which there were significant differences between the 2 groups in the mixed model are indicated by asterisks. Abbreviations: Dx, diagnosis; EOI, end of induction; Wk, week.
Of 106 patients aged ≥10 years at diagnosis, 68 had reached their final height after completing therapy; the median Z-scores at final height (−0.31) were significantly lower than those at diagnosis (0.28) (P=0.003) (Supporting Figure 1). The median permanent loss of height and height Z-score in female patients (N=30) were 1.90 cm (range, ‒3.00 cm to 12.05 cm) and 0.29 (range, ‒0.46 to 1.89), respectively, and the corresponding values in male patients (N=38) were 2.13 cm (range, ‒8.00 cm to 25.50 cm) and 0.30 (range, ‒1.13 to 3.56). When analysis was limited to patients aged 10–15 years at diagnosis, the median permanent loss of height and its Z-score in female patients (N=19) were 4.60 cm (range, ‒1.80 cm to 12.05 cm) and 0.71 (range, ‒0.28 to 1.89), respectively, and the corresponding values in male patients (N=25) were 5.75 cm (range, ‒8.00 cm to 25.50 cm) and 0.81 (range, ‒1.13 to 3.56).
Clinical Characteristics Affecting Weight Z-Scores During and After Therapy
The results of univariate analysis are shown in Supporting Table 9. In multivariable analysis (Supporting Figure 2 and Supporting Table 10), patients aged 2 to <10 years at diagnosis had median weight Z-scores significantly lower than those of patients aged ≥10 years at diagnosis in week 7 (P=0.025); however, from week 96 to 1 year off therapy, patients aged 2 to <10 years at diagnosis had median weight Z-scores higher than those of patients aged ≥10 years at diagnosis (P≤0.007 for all) (Supporting Figure 2A). Median weight Z-scores were higher for LR patients than for SR/HR patients in weeks 7, 10, 17, and 146 and at 4 years off therapy (P≤0.048 for all) (Supporting Figure 2B). Median weight Z-scores were higher for males than for females at the end of induction and in weeks 10, 21, and 120 (P≤0.047 for all) (Supporting Figure 2C) and higher for black patients than for white patients at the end of induction and in week 1 (P≤0.038 for all) (Supporting Figure 2D).
DISCUSSION
By analyzing data acquired at multiple time-points, we studied the longitudinal changes in BMI, height, and weight, and their associations with other characteristics, of pediatric patients with ALL treated with a single contemporary regimen without cranial irradiation. Median BMI Z-scores started to increase during induction therapy and remained elevated after the completion of chemotherapy. Median height Z-scores decreased during therapy then improved during the off-therapy period; however, they remained low in patients aged ≥10 years at diagnosis, in those with standard/high-risk status, and in those with positive CNS disease. Median weight Z-scores were relatively stable during therapy, except for increases during intensive glucocorticoid treatment; however, they increased in the off-therapy period. The median BMI Z-scores increased during therapy because of significant decreases in the median height Z-scores and moderate increases in the median weight Z-scores, whereas the increases in off-therapy median BMI Z-scores were primarily due to increases in the median weight Z-scores. When we estimated the BMI, height, and weight Z-scores in the recent population, our patients still showed comparatively higher BMI and weight Z-scores, especially between continuation weeks 21 and 96 and during the off-therapy period.
Previous studies of children and adolescents with ALL showed increases in BMI during and after treatment, regardless of whether cranial irradiation was used, without recovery to the baseline value4,5. Although our patients’ BMI Z-scores declined in the first 19 days of induction therapy, possibly because of leukemia-related illness, they increased significantly in the latter 4 weeks of induction therapy, which is when BMI started to increase. Another significant increase in BMI occurred during re-induction therapy II. Patients received intensive glucocorticoid therapy in these periods. There were significant decreases in BMI between continuation weeks 96 and 120 after the discontinuation of glucocorticoid pulses. Glucocorticoid treatment increases energy intake, stimulates cellular lipid accumulation by inducing differentiation of pre-adipocytes to adipocytes, and increases insulin resistance20–22. Many patients with ALL also exhibit an imbalance between their caloric intake from an unhealthy diet and their energy expenditure that reflects a sedentary lifestyle due to chemotherapy-related fatigue and parental permission23,24, leading to increased BMI and weight Z-scores. Further significant increases in BMI Z-scores due to weight gain were seen after patients completed therapy. It is likely that unhealthy dietary and behavioral habits established over 2.5 years of treatment persist after therapy completion25.
In the multivariable analysis of BMI Z-scores, scores for LR, male, and black patients during the early phase of therapy were higher than for SR/HR, female, and white patients, respectively. Patients aged 2 to <10 years at diagnosis had higher BMI-Z-scores than did patients aged ≥10 years at diagnosis from the continuation therapy phase to the early off-therapy period. Healthy-weight/underweight patients in this age group had a higher risk of becoming overweight/obese during or after therapy than did older patients. Younger age was associated with obesity in several studies26,27. Low-risk patients receive less-intensive therapy than do SR/HR patients. Some studies have suggested that female patients are at a higher risk than male patients of becoming overweight/obese, whereas other studies found no such difference between the sexes4. A meta-analysis showed that the BMI of female survivors increased during the off-therapy period5, which may explain why off-therapy BMI Z-scores were similar for male and female survivors in our study. The higher BMI Z-scores for black patients than for white patients have been reported previously2. However, these observed differences disappeared later in the off-therapy period, suggesting that BMI gain after therapy completion is prevalent in survivors of ALL regardless of their clinical characteristics.
We evaluated the longitudinal changes in height Z-scores, which decreased during therapy and improved after its completion. Previous studies showed that patients receiving chemotherapy without cranial irradiation can catch up with respect to their height Z-scores after completing therapy9,28. Importantly, we found that the median height Z-scores of patients aged ≥10 years at diagnosis were higher than those of patients aged 2 to <10 years at diagnosis until the early phase of continuation therapy but showed no improvement after therapy, and at 5 years off therapy they had become lower than those of patients aged 2 to <10 years at diagnosis. Older patients would have been in the midst of their growth spurt at diagnosis and early in their treatment, but chemotherapy could have slowed this process until they attained their final height, which might have led to weight and BMI gain during the off-therapy period. The changes in median height Z-score from diagnosis to week 120 and 146 were −0.50 and −0.56, respectively. Although the changes at other time-points were smaller than these, the permanent loss of height in patients aged 10‒15 years at diagnosis was approximately 5 cm. We consider these findings to be clinically significant.
Height Z-scores were also lower in SR/HR patients and in those with initial WBCs ≥ 50×109/L. Intensive therapy given to SR/HR patients, such as high doses of methotrexate, doxorubicin, cyclophosphamide, or cytarabine, would have affected their linear growth. Previously obtained data indicates that children in whom ALL was diagnosed before the age of 4 years and whose treatment included cranial irradiation had significant linear growth deficits27. However, younger patients, especially LR patients, experienced a growth spurt during the off-therapy period, and their height Z-scores then became comparable with those at diagnosis if they were treated without cranial irradiation. We confirmed that the height Z-scores of patients who were older at diagnosis were lower than at diagnosis when these patients attained their final height, but it is important to confirm that younger patients have Z-scores at their final heights that are comparable to those at diagnosis. Although it was significant at 3 years off therapy only, CNS involvement at diagnosis was associated with lower height Z-scores. This is possibly due to the frequent intrathecal chemotherapy received by patients with CNS disease or to the direct effect of leukemia involvement in the CNS on linear growth. Further study is needed to confirm this finding. Previous studies on height included patients treated with multiple protocols and some who received cranial irradiation. Our data on patients treated with a single frontline ALL regimen without cranial irradiation are, therefore, useful, as more patients with ALL are being treated without irradiation.
We recommend that interventions to prevent obesity be introduced for all patients during induction therapy, because excessive weight gain early in treatment is unlikely to be reversed. Evidence-based obesity-prevention guidelines recommend using motivational interviewing to work with the patient and family to establish goals for behavioral change such as decreasing sugar-sweetened beverage consumption and decreasing screen time29. Initial interactions during induction would focus on introducing concepts and delivering education to set the stage for future lifestyle changes. Great care would be taken to meet the patient/family “where they are” in terms of readiness for change29,30. In a survey of parents of children undergoing ALL therapy, nearly one-third of them recommended that these conversations begin during induction (E. Browne, unpublished data). Because of the significant association between values at diagnosis and those at subsequent follow-up, patients exhibiting obesity and/or short stature at diagnosis require particular attention. Such interventions should involve a multidisciplinary team of oncologists, nurses, dietitians, physical therapists, psychologists, and endocrinologists31, and they must continue during the off-therapy period. Such efforts can focus on modifiable behaviors, using a family-centered approach to support healthy nutritional intake and reduce sedentary behavior32. For patients with a linear growth deficit, especially those with identified risk factors (older age, SR/HR, higher WBCs, and/or CNS involvement), endocrine evaluation for growth hormone deficiency should be considered. It is also important to examine whether reducing the cumulative doses of glucocorticoids or other agents and using emerging therapeutic approaches, such as molecularly targeted agents or immune therapy, can reduce the dosing of conventional chemotherapeutic agents and their toxicities without compromising their anti-leukemia effects1.
This study is strengthened by involving only patients treated with a single contemporary regimen without cranial irradiation. Multiple time-points corresponding to different phases of therapy were evaluated to identify interval changes in the Z-scores of 3 parameters. BMI is not the best indicator of body composition but is still frequently used as a cost-effective, easily attainable, and clinically feasible anthropometric measurement. We did not perform routine dual-energy x-ray absorptiometry scans or waist circumference measurements, which can show obesity missed by BMI measurements.
In conclusion, in children with ALL, BMI Z-scores increase significantly because of decreases in height Z-scores and increases in weight Z-scores during therapy and mainly because of increases in weight Z-scores after therapy completion. Neither BMI nor weight Z-scores recover to baseline. Although height Z-scores improve after chemotherapy completion, patients aged ≥10 years at diagnosis, at SR/HR, with an initial WBC ≥ 50×109/L, and/or with positive CNS disease show less improvement than do other patients. Accordingly, all patients should be monitored for excess weight gain and linear growth and be exposed to multidisciplinary healthy lifestyle promotions, beginning in induction therapy. These efforts should continue even in the off-therapy period.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Keith A. Laycock, PhD, ELS, for editing the manuscript and Mary Egan Clark for data collection.
Funding sources: This study was supported in part by Cancer Center Core Grant CA21765 and P50 GM115279 from the National Institutes of Health and by ALSAC.
Footnotes
Conflict of interest disclosures:
The authors have no potential conflicts of interest, including specific financial interests, relationships, or affiliations relevant to the subject of this manuscript.
REFERENCES
- 1.Inaba H, Greaves M, Mullighan CG. Acute lymphoblastic leukaemia. Lancet. 2013;381:1943–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Lawman HG, et al. Trends in obesity prevalence among children and adolescents in the United States, 1988‒1994 through 2013‒2014. JAMA. 2016;315:2292–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diller L, Chow EJ, Gurney JG, et al. Chronic disease in the Childhood Cancer Survivor Study cohort: a review of published findings. J Clin Oncol. 2009;27:2339–2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang FF, Kelly MJ, Saltzman E, Must A, Roberts SB, Parsons SK. Obesity in pediatric ALL survivors: a meta-analysis. Pediatrics. 2014;133:e704–e715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang FF, Liu S, Chung M, Kelly MJ. Growth patterns during and after treatment in patients with pediatric ALL: a meta-analysis. Pediatr Blood Cancer. 2015;62:1452–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chow EJ, Pihoker C, Hunt K, Wilkinson K, Friedman DL. Obesity and hypertension among children after treatment for acute lymphoblastic leukemia. Cancer. 2007;110:2313–2320. [DOI] [PubMed] [Google Scholar]
- 7.Clayton PE, Shalet SM, Morris-Jones PH, Price DA. Growth in children treated for acute lymphoblastic leukaemia. Lancet. 1988;1:460–462. [DOI] [PubMed] [Google Scholar]
- 8.Sklar C, Mertens A, Walter A, et al. Final height after treatment for childhood acute lymphoblastic leukemia: comparison of no cranial irradiation with 1800 and 2400 centigrays of cranial irradiation. J Pediatr. 1993;123:59–64. [DOI] [PubMed] [Google Scholar]
- 9.Vandecruys E, Dhooge C, Craen M, Benoit Y, De Schepper J. Longitudinal linear growth and final height is impaired in childhood acute lymphoblastic leukemia survivors after treatment without cranial irradiation. J Pediatr. 2013;163:268–273. [DOI] [PubMed] [Google Scholar]
- 10.Esbenshade AJ, Simmons JH, Koyama T, Koehler E, Whitlock JA, Friedman DL. Body mass index and blood pressure changes over the course of treatment of pediatric acute lymphoblastic leukemia. Pediatr Blood Cancer. 2011;56:372–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.White J, Flohr JA, Winter SS, Vener J, Feinauer LR, Randsdell LB. Potential benefits of physical activity for chilren with acute lymphoblastic leukaemia. Pediatr Rehabil. 2005;8:53–58. [DOI] [PubMed] [Google Scholar]
- 12.Withycombe JS, Post-White JE, Meza JL, et al. Weight patterns in children with higher risk ALL: a report from the Children’s Oncology Group (COG) for CCG 1961. Pediatr Blood Cancer. 2009;53:1249–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pui CH, Campana D, Pei D, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med. 2009;360:2730–2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang JJ, Cheng C, Devidas M, et al. Ancestry and pharmacogenomics of relapse in acute lymphoblastic leukemia. Nat Genet. 2011;43:237–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. A SAS Program for the 2000 CDC Growth Charts (ages 0 to <20 years). Available from URL: http://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm (accessed 06/22/2018).
- 16.Must A, Anderson SE. Body mass index in children and adolescents: considerations for population-based applications. Int J Obes (Lond). 2006;30:590–594. [DOI] [PubMed] [Google Scholar]
- 17.Chemaitilly W, Boulad F, Heller G, et al. Final height in pediatric patients after hyperfractionated total body irradiation and stem cell transplantation. Bone Marrow Transplant. 2007;40:29–35. [DOI] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey: 2005–2006 raw data. NHANES archive home page: https://www.cdc.gov/nchs/nhanes/archive_new_nhanes.htm (accessed 06/22/2018).
- 19.Lindemulder SJ, Stork LC, Bostrom B, et al. Survivors of standard risk acute lymphoblastic leukemia do not have increased risk for overweight and obesity compared to non-cancer peers: a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2015;62:1035–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reilly JJ, Brougham M, Montgomery C, Richardson F, Kelly A, Gibson BE. Effect of glucocorticoid therapy on energy intake in children treated for acute lymphoblastic leukemia. J Clin Endocrinol Metab. 2001;86:3742–3745. [DOI] [PubMed] [Google Scholar]
- 21.Oeffinger KC, Adams-Huet B, Victor RG, et al. Insulin resistance and risk factors for cardiovascular disease in young adult survivors of childhood acute lymphoblastic leukemia. J Clin Oncol. 2009;27:3698–3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galitzky J, Bouloumie A. Human visceral-fat‒specific glucocorticoid tuning of adipogenesis. Cell Metab. 2013;18:3–5. [DOI] [PubMed] [Google Scholar]
- 23.Cohen J, Wakefield CE, Tapsell LC, Walton K, Fleming CA, Cohn RJ. Exploring the views of parents regarding dietary habits of their young cancer-surviving children. Support Care Cancer. 2015;23:463–471. [DOI] [PubMed] [Google Scholar]
- 24.Winter C, Müller C, Hoffmann C, Boos J, Rosenbaum D. Physical activity and childhood cancer. Pediatr Blood Cancer. 2010;54:501–510. [DOI] [PubMed] [Google Scholar]
- 25.Ness KK, Leisenring WM, Huang S, et al. Predictors of inactive lifestyle among adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Cancer. 2009;115:1984–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baillargeon J, Langevin AM, Lewis M, et al. Demographic correlates of body size changes in children undergoing treatment for acute lymphoblastic leukemia. Pediatr Blood Cancer. 2007;49:793–796. [DOI] [PubMed] [Google Scholar]
- 27.Dalton VK, Rue M, Silverman LB, et al. Height and weight in children treated for acute lymphoblastic leukemia: relationship to CNS treatment. J Clin Oncol. 2003;21:2953–2960. [DOI] [PubMed] [Google Scholar]
- 28.Viana MB, Vilela MI. Height deficit during and many years after treatment for acute lymphoblastic leukemia in children: a review. Pediatr Blood Cancer. 2008;50:509–516; discussion 517. [DOI] [PubMed] [Google Scholar]
- 29.Davis MM, Gance-Cleveland B, Hassink S, Johnson R, Paradis G, Resnicow K. Recommendations for prevention of childhood obesity. Pediatrics. 2007;120 Suppl 4:S229–S253. [DOI] [PubMed] [Google Scholar]
- 30.Spahn JM, Reeves RS, Keim KS, et al. State of the evidence regarding behavior change theories and strategies in nutrition counseling to facilitate health and food behavior change. J Am Diet Assoc. 2010;110:879–891. [DOI] [PubMed] [Google Scholar]
- 31.Ligibel JA, Alfano CM, Courneya KS, et al. American Society of Clinical Oncology position statement on obesity and cancer. J Clin Oncol. 2014;32:3568–3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spear BA, Barlow SE, Ervin C, et al. Recommendations for treatment of child and adolescent overweight and obesity. Pediatrics. 2007;120 Suppl 4 S254–S288. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.