Abstract
Neoadjuvant chemotherapy is used to allow more limited breast surgery without compromising local control. We sought to evaluate nationwide surgical trends in patients with operable breast cancer treated with neoadjuvant chemotherapy and factors associated with surgical type. We used the National Cancer Database to identify 235,339 women with unilateral T1–3N0–3M0 breast cancer diagnosed between 2010 and 2014, and treated with surgery and chemotherapy. Of these, 59,568 patients (25.3%) were treated with neoadjuvant chemotherapy. Rates of pathologic complete response to neoadjuvant chemotherapy increased from 33.3% at the start of the study period in 2010 to 46.3% at the end of the period in 2014 (p=0.02). Rates of breast- conserving surgery changed little, from 37.0% to 40.8% (p=0.22). While rates of unilateral mastectomy decreased from 43.3% to 34.7% (p=0.02) and rates of bilateral mastectomy without immediate reconstruction remained similar (11.7% to 11.5%, p=0.82), rates of bilateral mastectomy with immediate reconstruction rose from 8.0% to 13.1% (p=0.02). Patients who were younger, with private/managed care insurance, and diagnosed in more recent years were more likely to achieve pathologic complete response; however, these same characteristics were associated with receipt of bilateral mastectomy (versus breast-conserving surgery). Additionally, non-Hispanic white race and higher area education attainment were both associated with bilateral mastectomy. These findings did not differ by age or molecular subtype. Further study of non-clinical factors that influence selection of more extensive surgery despite excellent response to neoadjuvant chemotherapy is warranted.
Keywords: contralateral prophylactic mastectomy, neoadjuvant chemotherapy, pathologic complete response, reconstruction, breast cancer
Introduction
Breast-conserving surgery (BCS) with radiotherapy provides survival rates equivalent to those of mastectomy while allowing for a less extensive surgical procedure and preservation of the breast, and was endorsed by a National Institutes of Health Consensus Conference in 1990 as a standard of excellence in breast cancer care for women with early-stage breast cancer1.
Neoadjuvant chemotherapy (NAC) was initially used for locally advanced breast cancers but has recently been increasingly used in earlier stage settings. Potential benefits of this strategy include providing prognostic information and allowing tailoring of adjuvant therapy, allowing time for surgical decision-making without delaying initiation of therapy, and serving as a research platform to test novel therapies and biomarkers2–5. Furthermore, neoadjuvant chemotherapy (NAC) has been shown to allow more limited surgery in the breast without compromising local control, and is often used to try to achieve BCS in patients who would have otherwise required a mastectomy6–9. However, recent clinical trials have not shown an increase in BCS despite rising rates of pathological complete response (pCR) with the neoadjuvant use of newer systemic therapies10–13. There are few data on the extent to which this is true outside of clinical trials. For patients with unilateral breast cancer who do not have elevated genetic risk, bilateral mastectomy (or contralateral prophylactic mastectomy) has not been shown to improve survival 14,15, and may lead to larger, more costly operations requiring inpatient hospitalization and a prolonged recovery period compared to BCS16,17.
While previous studies have found that white women of higher socioeconomic status tend to seek more aggressive breast surgery14,18,19, the majority of patients on these studies did not receive chemotherapy prior to surgery. Using a large, nationwide cohort treated in an era of molecular subtyping and modern systemic therapies, we sought to evaluate trends in mastectomy in the growing population of patients with operable breast cancer treated with NAC, and to quantify the contribution of demographic characteristics to changing trends.
Methods
Study overview
We performed a retrospective cohort study using the National Cancer Database (NCDB) 2014 Participant User File with the approval of our institutional review board.
Database and cohort selection
The NCDB is a joint program of the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society. It is a hospital-based registry that collects data from more than 1,500 CoC-accredited cancer hospitals and contains detailed information including demographics, disease stage, comorbidity, and first course of treatment delivered for 70% of all incident cancer cases in the United States20 (20). The American College of Surgeons and the CoC have not verified and are not responsible for the analytic or statistical methodology used or for the conclusions drawn from these data.
We identified adult women with unilateral invasive breast cancer [International Classification of Diseases for Oncology-3 (ICD-O-3) site C50.0–50.9; ICD-O-3 histology 800x-803x, 805x, 814x-852x, 854x-857x] who underwent neoadjuvant chemotherapy followed by surgery. We included patients diagnosed between 2010 and 2014 because site-specific factors such as HER2+ status and pathological complete response (pCR) were available beginning in 2010. We excluded patients with T4 cancers, metastatic disease, or incomplete staging or molecular subtype information. We excluded patients who had prior cancer diagnoses and whose sequence of chemotherapy and surgery was not known.
Covariates
We included relevant patient, tumor and treatment characteristics available in the NCDB in our analysis. We classified histology codes 8500–8508 and 8521–8523 as ductal cancer, and 8520 and 8524–8525 as lobular cancer14. Molecular subtype was categorized into the following categories: 1) ER/PR+, HER2- (ER or PR positive and HER2 negative), 2) ER/PR+, HER2+ (ER or PR positive and HER2 positive), 3) ER/PR-, HER2+ (ER and PR negative and HER2 positive), and 4) ER/PR-, HER2- (ER, PR, and HER2 negative). The NCDB variable for HER2 was based on both information from immunohistochemistry and fluorescent in situ hybridization.
Patient comorbidities were categorized as 0, 1, or 2 according to the Charlson- Deyo comorbidity score. Patient distance from facility was based on the “great circle” distance in miles between the patient’s residence and the hospital that reported the case21 (21). Patient residence was coded according to published files by the U.S. Department of Agriculture Economic Research Service. Median educational attainment (percent of adults who did not graduate from high school) for each patient’s zip code of residence was derived from the 2012 American Community Survey data. Reporting facilities were assigned a category classification by the COC program.
We determined chemotherapy sequencing using both the NCDB variables for sequencing of systemic therapy and for date of surgery and chemotherapy initiation. We considered women who initiated chemotherapy within the period of 84 to 270 days before surgery as receiving neoadjuvant chemotherapy, and women who initiated chemotherapy within 90 days after surgery as receiving adjuvant chemotherapy, as previous studies have done22,23 (22,23). BCS and unilateral and bilateral mastectomy with or without reconstruction were classified based on Surgery of the Primary Site codes24. We used the response to neoadjuvant therapy variable in the NCDB to identify patients who had pathologic complete response (pCR) and those who did not after neoadjuvant chemotherapy (NAC). This variable is based on review of the medical record for a specific statement by a physician about the response to neoadjuvant therapy, and not based on interpretation of response based on pathology report or other elements of the medical record.
Statistical analysis
Pearson chi-square tests were used to assess associations between surgery type and patient, tumor and treatment characteristics. Trends over time were evaluated using Mann-Kendall non-parametric test. Multivariable logistic models for bilateral mastectomy versus BCS and unilateral mastectomy versus BCS were created. We also created polytomous logistic regression to model surgery use, but because results were not materially different (data not shown), we present only the results of our binomial logistic models for ease of interpretation. Covariates in the model were selected a priori and included the following variables available in the database: age, race/ethnicity, year of diagnosis, Charlson-Deyo comorbidity score, metropolitan vs urban/rural residence, patient distance from treating facility, area education attainment, insurance type, clinical stage, histology, and molecular subtype. We also separately looked at the groups most likely to be ideal candidates for BCS (patients with initial clinical T1/2 disease and patients who achieved pCR). We did not include region of the country and reporting facility type in any of our main models because this data was missing for patients under 40 years of age. We performed sensitivity analyses and constructed separate models adjusting for region of the country and facility type, and adjusting for reporting facility. All tests were 2-sided, and P < 0.05 was considered statistically significant. Statistical analyses were performed using SAS Enterprise Guide (version 7.12, SAS Institute, Cary, North Carolina).
Results
Patient and treatment characteristics
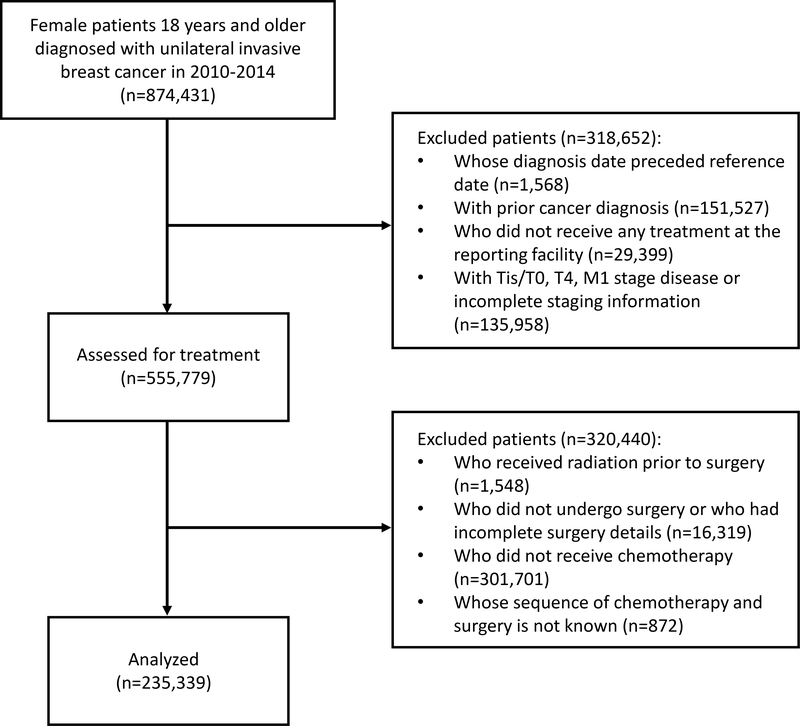
We identified 235,339 patients who fulfilled our inclusion criteria (Figure 1). Of these, 59,568 patients (25.3%) were treated with neoadjuvant chemotherapy (NAC). Table 1 shows their baseline clinical and demographic characteristics.
Figure 1:
Cohort selection flow diagram.
Table 1.
Baseline characteristics.
Abbreviations: pathological complete response (pCR).
| Patients who underwent unilateral mastectomy |
Patients who underwent bilateral mastectomy |
Patients who underwent breast conserving surgery |
|||||
|---|---|---|---|---|---|---|---|
| No. | (%) | No. | (%) | No. | % | P | |
| Age | <0.0001 | ||||||
| <40 years old | 3,691 | (39.6%) | 3,427 | (36.8%) | 2,193 | (23.6%) | |
| 40–49 years old | 6,330 | (37.8%) | 4,631 | (27.6%) | 5,793 | (34.6%) | |
| 50–64 years old | 9,496 | (37.9%) | 4,635 | (18.5%) | 10,905 | (43.6%) | |
| 65 years old and older | 3,600 | (42.5%) | 980 | (11.6%) | 3,887 | (45.9%) | |
| Race | <0.0001 | ||||||
| Non-Hisoanic white | 14,947 | (37.0%) | 10,344 | (25.6%) | 15,160 | (37.5%) | |
| Hispanic | 2,365 | (46.3%) | 942 | (18.4%) | 1,805 | (35.3%) | |
| Non-Hispanic black | 4,124 | (40.1%) | 1,687 | (16.4%) | 4,462 | (43.4%) | |
| Asian/Other | 1,477 | (45.5%) | 600 | (18.5%) | 1,170 | (36.0%) | |
| Unknown | 204 | (42.1%) | 100 | (20.6%) | 181 | (37.3%) | |
| Year of Diagnosis | <0.0001 | ||||||
| 2010 | 4,006 | (43.3%) | 1,826 | (19.7%) | 3,422 | (37.0%) | |
| 2011 | 4,300 | (40.6%) | 2,262 | (21.4%) | 4,027 | (38.0%) | |
| 2012 | 4,509 | (39.9%) | 2,590 | (22.9%) | 4,195 | (37.1%) | |
| 2013 | 4,964 | (38.1%) | 3,213 | (24.7%) | 4,854 | (37.2%) | |
| 2014 | 5,338 | (34.7%) | 3,782 | (24.6%) | 6,280 | (40.8%) | |
| Charlson-Deyo comorbidity score | <0.0001 | ||||||
| 0 | 20,191 | (38.5%) | 12,296 | (23.4%) | 20,012 | (38.1%) | |
| 1 | 2,473 | (40.9%) | 1,200 | (19.8%) | 2,375 | (39.3%) | |
| 2 or higher | 453 | (44.4%) | 177 | (17.3%) | 391 | (38.3%) | |
| Locationa | <0.0001 | ||||||
| Northeast | 3,383 | (39.1%) | 1,536 | (17.7%) | 3,742 | (43.2%) | |
| South | 7,940 | (39.0%) | 4,264 | (21.0%) | 8,149 | (40.0%) | |
| Central | 4,697 | (37.7%) | 2,605 | (20.9%) | 5,154 | (41.4%) | |
| West | 3,406 | (38.8%) | 1,841 | (21.0%) | 3,540 | (40.3%) | |
| Unknown | 3,691 | (39.6%) | 3,427 | (36.8%) | 2,193 | (23.6%) | |
| Facility typea | <0.0001 | ||||||
| Community cancer program | 1,649 | (39.0%) | 759 | (17.9%) | 1,821 | (43.1%) | |
| Comprehensive community cancer program | 8,091 | (37.5%) | 4,711 | (21.9%) | 8,746 | (40.6%) | |
| Academic/Research Program | 7,248 | (40.9%) | 3,281 | (18.5%) | 7,201 | (40.6%) | |
| Integrated Network Cancer Program | 2,438 | (36.1%) | 1,495 | (22.1%) | 2,817 | (41.7%) | |
| Other/Unknown | 3,691 | (39.6%) | 3,427 | (36.8%) | 2,193 | (23.6%) | |
| Facility type | <0.0001 | ||||||
| Non-academic program | 12,178 | (37.4%) | 6,965 | (21.4%) | 13,384 | (41.1%) | |
| Academic/Research Programb | 7,248 | (40.9%) | 3,281 | (18.5%) | 7,201 | (40.6%) | |
| Other/Unknown | 3,691 | (39.6%) | 3,427 | (36.8%) | 2,193 | (23.6%) | |
| Residence | 0.002 | ||||||
| Metropolitan | 19,852 | (38.9%) | 11,619 | (22.8%) | 19,573 | (38.3%) | |
| Urban/Rural | 2,695 | (38.8%) | 1,695 | (24.4%) | 2,557 | (36.8%) | |
| Unknown | 570 | (36.1%) | 359 | (22.8%) | 648 | (41.1%) | |
| Distance from reporting facility | <0.0001 | ||||||
| Within 50 miles of facility | 21,106 | (38.7%) | 12,360 | (22.6%) | 21,104 | (38.7%) | |
| >50 miles of facility | 1,941 | (40.2%) | 1,276 | (26.4%) | 1,611 | (33.4%) | |
| Unknown | 70 | (41.2%) | 37 | (21.8%) | 63 | (37.1%) | |
| Education less than high school | <0.0001 | ||||||
| 21% or more | 4,411 | (43.8%) | 1,777 | (17.6%) | 3,886 | (38.6%) | |
| 13–20.9 °%o | 5,850 | (40.4%) | 3,107 | (21.4%) | 5,541 | (38.2%) | |
| 7–12.9 °%o | 7,027 | (37.2%) | 4,680 | (24.8%) | 7,202 | (38.1%) | |
| <7°%o | 5,764 | (36.2%) | 4,080 | (25.6%) | 6,088 | (38.2%) | |
| Unknown | 65 | (41.9%) | 29 | (18.7%) | 61 | (39.4%) | |
| Insurance | <0.0001 | ||||||
| Medicaid/Not Insuredc | 4,677 | (44.5%) | 1,978 | (18.8%) | 3,846 | (36.6%) | |
| Private/Managed care | 14,345 | (36.4%) | 10,370 | (26.3%) | 14,653 | (37.2%) | |
| Medicare | 3,759 | (42.1%) | 1,178 | (13.2%) | 3,995 | (44.7%) | |
| Unknown | 336 | (43.8%) | 147 | (19.2%) | 284 | (37.0%) | |
| Clinical T category | <0.0001 | ||||||
| 1 | 3,445 | (31.7%) | 2,713 | (24.9%) | 4,725 | (43.4%) | |
| 2 | 11,671 | (34.4%) | 7,309 | (21.5%) | 14,965 | (44.1%) | |
| 3 | 8,001 | (54.3%) | 3,651 | (24.8%) | 3,088 | (20.9%) | |
| Clinical N category | <0.0001 | ||||||
| 0 | 7,741 | (30.7%) | 5,875 | (23.3%) | 11,620 | (46.0%) | |
| 1 | 11,861 | (43.5%) | 6,140 | (22.5%) | 9,240 | (33.9%) | |
| 2 | 2,218 | (49.0%) | 1,004 | (22.2%) | 1,303 | (28.8%) | |
| 3 | 1,297 | (50.5%) | 654 | (25.5%) | 615 | (24.0%) | |
| Histology | <0.0001 | ||||||
| Ductal | 20,331 | (37.7%) | 12,299 | (22.8%) | 21,283 | (39.5%) | |
| Lobular or lobular component | 1,875 | (54.6%) | 885 | (25.7%) | 677 | (19.7%) | |
| Other | 911 | (41.1%) | 489 | (22.0%) | 818 | (36.9%) | |
| Tumor Grade | <0.0001 | ||||||
| Well differentiated | 1,358 | (45.0%) | 671 | (22.2%) | 988 | (32.7%) | |
| Moderately differentiated | 7,747 | (41.5%) | 4,236 | (22.7%) | 6,685 | (35.8%) | |
| Poorly differentiated/undifferentiated | 12,055 | (36.5%) | 7,620 | (23.1%) | 13,343 | (40.4%) | |
| Unknown | 1,957 | (40.2%) | 1,146 | (23.6%) | 1,762 | (36.2%) | |
| Molecular subtype | <0.0001 | ||||||
| ER/PR+, HER2- | 10,082 | (43.6%) | 5,258 | (22.8%) | 7,762 | (33.6%) | |
| ER/PR+, HER2+ | 4,216 | (36.0%) | 2,709 | (23.2%) | 4,773 | (40.8%) | |
| ER/PR-, HER2+ | 2,367 | (37.5%) | 1,405 | (22.3%) | 2,534 | (40.2%) | |
| ER/PR-, HER2- | 5,202 | (33.4%) | 3,666 | (23.5%) | 6,712 | (43.1%) | |
| Unknown | 1,250 | (43.4%) | 635 | (22.0%) | 997 | (34.6%) | |
| pCR | <0.0001 | ||||||
| Yes | 4,058 | (30.2%) | 3,386 | (25.2%) | 6,000 | (44.6%) | |
| No | 8,843 | (43.2%) | 4,463 | (21.8%) | 7,151 | (35.0%) | |
| Unknown | 10,216 | (39.8%) | 5,824 | (22.7%) | 9,627 | (37.5%) | |
Not available for patients<40 years old
Includes NCI designated comprehensive cancer centers
Includes Medicaid, uninsured and other government insurance
Temporal trends
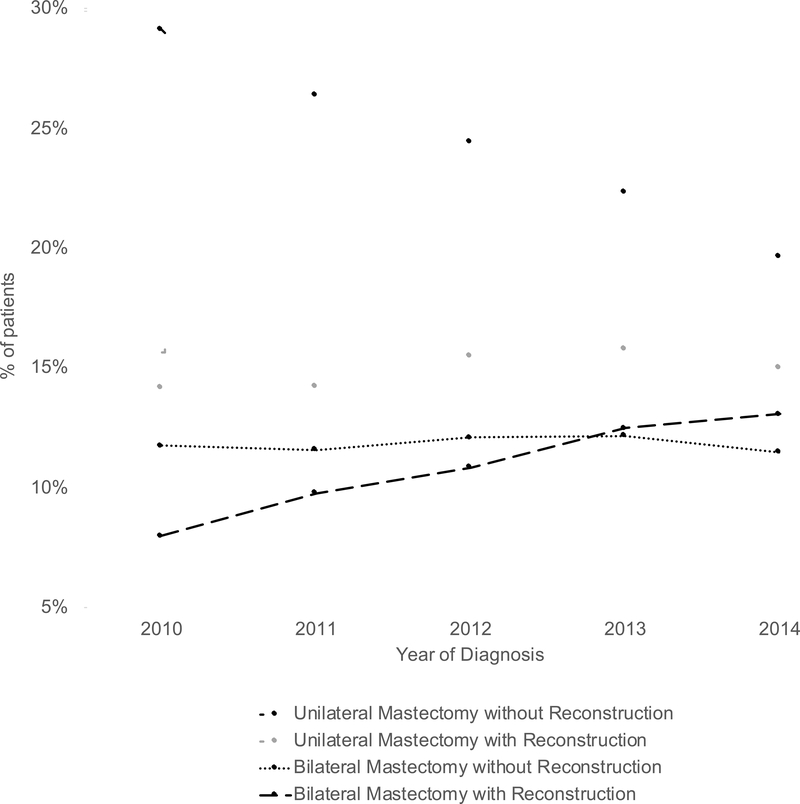
Rates of neoadjuvant chemotherapy use increased from 20.6% at the start of the study period in 2010 to 32.0% at the end of the period in 2014 (p=0.02). Rates of pathologic complete response (pCR) to NAC increased from 33.3% to 46.3% over this period (p=0.02). Figure 2 shows surgical trends over the study time period. Rates of breast-conserving surgery (BCS) changed little, from 37.0% to 40.8% (p=0.22). Rates of unilateral mastectomy decreased from 43.3% to 34.7% (p=0.02). Rates of bilateral mastectomy without immediate reconstruction remained similar (11.7% to 11.5%, p=0.82). However, rates of bilateral mastectomy with immediate reconstruction rose from 8.0% to 13.1% (p=0.02). These trends did not differ by age (<40 years old vesus 40 years or older) or by molecular subtype (Supplemental Material).
Figure 2: Mastectomy trends over time among patients who received neoadjuvant chemotherapy.
Mann-Kendall test for trend: unilateral mastectomy without reconstruction (p=0.23), unilateral mastectomy with reconstruction (p=0.02), bilateral mastectomy without reconstruction (p=0.82), bilateral mastectomy with reconstruction (p=0.02).
Factors associated with pCR
Table 2 shows patient and tumor characteristics associated with attainment of pCR. Patients who were younger, diagnosed in more recent years, with fewer comorbidities, with earlier stage disease, and with private/managed care insurance were more likely to achieve pCR. Patients with lobular and ER/PR+, HER2- subtype cancers were less likely to achieve pCR.
Table 2.
Logistic regression adjusted odds ratios for associations with attainment of pathological complete response (pCR) among all patients who received neoadjuvant chemotherapy. Significant associations are bolded.
Abbreviations : odds ratio (OR), confidence interval (CI).
| Adjusted OR for achieving pCR | |
|---|---|
| Age | |
| <40 years old | Reference |
| 40–49 years old | 0.94 (0.87–1.01) |
| 50–64 years old | 0.80 (0.75–0.86) |
| 65 years old and older | 0.65 (0.57–0.73) |
| Race | |
| Non-Hispanic white | Reference |
| Hispanic | 0.99 (0.90–1.08) |
| Non-Hispanic black | 0.96 (0.90–1.03) |
| Asian/Other | 0.88 (0.79–0.98) |
| Year of Diagnosis | |
| 2010 | Reference |
| 2011 | 1.08 (1.00–1.17) |
| 2012 | 1.22 (1.13–1.33) |
| 2013 | 1.32 (1.22–1.44) |
| 2014 | 1.50 (1.39–1.62) |
| Charlson-Deyo comorbidity score | |
| 0 | Reference |
| 1 | 0.92 (0.85–1.00) |
| 2 or higher | 0.77 (0.63–0.94) |
| Residence | |
| Metropolitan | Reference |
| Urban/Rural | 1.10(1.01–1.20) |
| Distance from reporting facility | |
| Within 50 miles of facility | Reference |
| >50 miles of facility | 1.00 (0.91–1.10) |
| Education less than high school | |
| 21% or more | Reference |
| 13–20.9% | 1.03 (0.95–1.12) |
| 7–12.9% | 1.05 (0.97–1.13) |
| <7% | 1.04 (0.96–1.14) |
| Insurance | |
| Medicaid/Not Insureda | Reference |
| Private/Managed care | 1.17 (1.10–1.26) |
| Medicare | 1.11 (0.99–1.25) |
| Clinical T stage | |
| 1–2 | Reference |
| 3 | 0.64 (0.60–0.68) |
| Clinical N stage | |
| 0 | Reference |
| 1 | 0.83 (0.80–0.88) |
| 2 | 0.85 (0.78–0.94) |
| 3 | 0.83 (0.73–0.94) |
| Histology | |
| Ductal | Reference |
| Lobular or lobular component | 0.53 (0.46–0.61) |
| Other | 1.00 (0.88–1.14) |
| Molecular subtype | |
| ER/PR+, HER2- | Reference |
| ER/PR+, HER2+ | 3.15(2.94–3.37) |
| ER/PR-, HER2+ | 6.55 (6.03–7.11) |
| ER/PR-, HER2- | 3.39 (3.18–3.61) |
Includes Medicaid, uninsured and other government insurance
Factors associated with unilateral and bilateral mastectomy
Table 3 shows the results of our adjusted logistic models for the entire cohort. The unadjusted temporal trends toward more bilateral and less unilateral mastectomy persisted in the adjusted analysis. Furthermore, we found no significant interaction between year of diagnosis and age or between year of diagnosis and molecular subtype for surgery type. Younger patients, those at greater distance from their treating facility and those with more advanced stage were more likely to receive unilateral or bilateral mastectomy (versus BCS). Non-Hispanic white race and higher area education attainment were both associated with bilateral mastectomy. Private or managed care insurance (versus public insurance or no insurance) was directly associated with bilateral mastectomy, but inversely associated with unilateral mastectomy.
Table 3.
Logistic regression adjusted odds ratios for associations with receipt of unilateral or bilateral mastectomy (versus lumpectomy) among all patients who receive neoadjuvant chemotherapy. Significant associations are bolded.
Abbreviations: odds ratio (O R), confidence interval (CI).
| All patients who received neoadjuvant chemotherapy |
||
|---|---|---|
| Adjusted OR (95% CI) for Unilateral Mastectomy | Adjusted OR (95% CI) for Bilateral Mastectomy | |
| Age | ||
| <40 years old | Reference | Reference |
| 40–49 years old | 0.62 (0.58–0.67) | 0.48 (0.44–0.51) |
| 50–64 years old | 0.50 (0.46–0.53) | 0.24 (0.23–0.26) |
| 65 years old and older | 0.52 (0.47–0.57) | 0.15(0.13–0.17) |
| Race | ||
| Non-Hispanic white | Reference | Reference |
| Hispanic | 1.07 (0.99–1.16) | 0.71 (0.64–0.78) |
| Non-Hispanic black | 0.84 (0.79–0.89) | 0.53 (0.50–0.57) |
| Asian/Other | 1.21 (1.10–1.32) | 0.67 (0.60–0.76) |
| Year of Diagnosisa | ||
| 2010 | Reference | Reference |
| 2011 | 0.92 (0.86–0.99) | 1.10 (1.00–1.20) |
| 2012 | 0.96 (0.90–1.03) | 1.28 (1.18–1.39) |
| 2013 | 0.95 (0.88–1.01) | 1.42 (1.30–1.54) |
| 2014 | 0.82 (0.77–0.88) | 1.37 (1.26–1.48) |
| Charlson-Deyo comorbidity score | ||
| 0 | Reference | Reference |
| 1 | 1.10 (1.03–1.18) | 1.07 (0.98–1.16) |
| 2 or higher | 1.34 (1.15–1.56) | 1.22 (1.00–1.49) |
| Residence | ||
| Metropolitan | Reference | Reference |
| Urban/Rural | 0.95 (0.89–1.02) | 1.02 (0.94–1.11) |
| Distance from reporting facility | ||
| Within 50 miles of facility | Reference | Reference |
| >50 miles of facility | 1.24 (1.14–1.35) | 1.33 (1.21–1.46) |
| Education less than high school | ||
| 21% or more | Reference | Reference |
| 13–20.9% | 0.96 (0.90–1.03) | 1.14 (1.05–1.24) |
| 7–12.9% | 0.90 (0.85–0.96) | 1.26 (1.16–1.36) |
| <7% | 0.88 (0.83–0.95) | 1.24 (1.14–1.35) |
| Insurance | ||
| Medicaid/Not Insuredb | Reference | Reference |
| Private/Managed care | 0.91 (0.86–0.96) | 1.37 (1.28–1.47) |
| Medicare | 0.95 (0.87–1.05) | 1.11 (0.98–1.25) |
| Clinical T stage | ||
| 1–2 | Reference | Reference |
| 3 | 2.92 (2.78–3.08) | 2.17 (2.04–2.31) |
| Clinical N stage | ||
| 0 | Reference | Reference |
| 1 | 1.78 (1.70–1.85) | 1.31 (1.25–1.38) |
| 2 | 2.31 (2.12–2.50) | 1.57 (1.42–1.73) |
| 3 | 2.82 (2.52–3.15) | 2.09 (1.83–2.37) |
| Histology | ||
| Ductal | Reference | Reference |
| Lobular or lobular component | 2.32 (2.10–2.56) | 2.24 (1.99–2.52) |
| Other | 1.22 (1.09–1.36) | 1.08 (0.95–1.23) |
| Molecular subtype | ||
| ER/P R+, HE R2- | Reference | Reference |
| ER/P R+, HE R2+ | 0.79 (0.75–0.83) | 0.85 (0.79–0.90) |
| ER/P R -, HE R2+ | 0.79 (0.74–0.85) | 0.91 (0.84–0.99) |
| ER/P R -, HE R2- | 0.69 (0.66–0.73) | 0.91 (0.86–0.97) |
When year of diagnosis is included as a continuous variable in our models, the adjusted odds ratio for bilateral and unilateral mastectomy (versus lumpectomy) were 1.085 (95% CI 1.067–1.104, p<0.001) and 0.961 (95% CI 0.948–0.976, p<0.001) per increase in year, respectively.
Includes Medicaid, uninsured and other government insurance
In sensitivity analyses limiting our patient cohort to those who initiating presented with clinical T1–2 disease as well as those who achieved pCR at surgery (Table 4, Supplemental Material), and after further controlling for facility, facility type, and region of the country (Supplemental Material), our results were materially unchanged. We found the likelihood of undergoing bilateral mastectomy (versus BCS) increased over time, and that sociodemographic factors such as age, race, area education attainment, and insurance type were associated with surgery type. Additionally, we found that patients treated at academic facilities were more likely to undergo unilateral mastectomies, and less likely to undergo bilateral mastectomies, as compared to breast-conserving surgery.
Table 4.
Logistic regression adjusted odds ratios for associations with receipt of unilateral or bilateral mastectomy (versus lumpectomy) among patients who achieved pathological complete response after neoadjuvant chemotherapy and patients with clinical T1–2 tumors prior to neoadjuvant chemotherapy. Significant associations are bolded.
Abbreviations: odds ratio (OR), confidence interval (CI), number (n).
| Patients who achieved a pathological complete response (n=13,444) |
Patients with initial clinical T1/T2 tumors (n=44,828) |
|||
|---|---|---|---|---|
| Adjusted OR (95% CI) for Unilateral Mastectomy | Adjusted OR (95% CI) for Bilateral Mastectomy | Adjusted OR (95% CI) for Unilateral Mastectomy | Adjusted OR (95% CI) for Bilateral Mastectomy | |
| Age | ||||
| <40 years old | Reference | Reference | Reference | Reference |
| 40–49 years old | 0.55 (0.48–0.63) | 0.48 (0.42–0.55) | 0.61 (0.57–0.66) | 0.48 (0.44–0.52) |
| 50–64 years old | 0.47 (0.41–0.53) | 0.24(0.21–0.27) | 0.48 (0.44–0.51) | 0.24 (0.22–0.26) |
| 65 years old and older | 0.48 (0.39–0.60) | 0.12(0.10–0.16) | 0.47 (0.42–0.53) | 0.14(0.12–0.16) |
| Race | ||||
| Non-Hispanic white | Reference | Reference | Reference | Reference |
| Hispanic | 0.96 (0.81–1.14) | 0.72 (0.59–0.87) | 1.07 (0.98–1.17) | 0.74 (0.66–0.83) |
| Non-Hispanic black | 0.78 (0.69–0.88) | 0.54 (0.47–0.62) | 0.87 (0.81–0.92) | 0.55 (0.51–0.60) |
| Asian/Other | 1.43 (1.19–1.73) | 0.70 (0.55–0.88) | 1.20 (1.08–1.32) | 0.68 (0.60–0.78) |
| Year of Diagnosis | ||||
| 2010 | Reference | Reference | Reference | Reference |
| 2011 | 0.90 (0.77–1.04) | 1.15 (0.97–1.36) | 0.94 (0.87–1.02) | 1.15 (1.04–1.28) |
| 2012 | 0.92 (0.79–1.06) | 1.33 (1.13–1.58) | 0.99 (0.92–1.07) | 1.33 (1.21–1.47) |
| 2013 | 0.97 (0.83–1.12) | 1.52 (1.29–1.79) | 0.95 (0.88–1.03) | 1.51 (1.37–1.66) |
| 2014 | 0.84 (0.73–0.96) | 1.50 (1.29–1.75) | 0.85 (0.79–0.92) | 1.47 (1.34–1.61) |
| Charlson-Deyo comorbidity score | ||||
| 0 | Reference | Reference | Reference | Reference |
| 1 | 1.00 (0.86–1.16) | 0.98 (0.83–1.15) | 1.11 (1.03–1.19) | 1.06 (0.97–1.16) |
| 2 or higher | 1.44 (1.01–2.07) | 1.39 (0.91–2.11) | 1.27 (1.07–1.50) | 1.21 (0.97–1.51) |
| Residence | ||||
| Metropolitan | Reference | Reference | Reference | Reference |
| Urban/Rural | 0.96 (0.83–1.12) | 1.20 (1.02–1.41) | 0.95 (0.88–1.03) | 0.98 (0.89–1.08) |
| Distance from reporting facility | ||||
| Within 50 miles of facility | Reference | Reference | Reference | Reference |
| >50 miles of facility | 1.36 (1.15–1.62) | 1.20(1.02–1.44) | 1.25 (1.14–1.37) | 1.36 (1.22–1.51) |
| Education less than high school | ||||
| 21% or more | Reference | Reference | Reference | Reference |
| 13–20.9% | 1.02 (0.89–1.18) | 1.08 (0.92–1.28) | 0.98 (0.91–1.06) | 1.14 (1.04–1.24) |
| 7–12.9% | 0.94 (0.82–1.09) | 1.18 (1.01–1.38) | 0.90 (0.84–0.97) | 1.21 (1.10–1.32) |
| <7% | 0.95 (0.82–1.10) | 1.21 (1.02–1.42) | 0.89 (0.82–0.96) | 1.19 (1.08–1.31) |
| Insurance | ||||
| Medicaid/Not Insureda | Reference | Reference | Reference | Reference |
| Private/Managed care | 0.93 (0.83–1.06) | 1.41 (1.22–1.62) | 0.92 (0.86–0.98) | 1.40 (1.29–1.52) |
| Medicare | 0.86 (0.70–1.06) | 1.24 (0.96–1.59) | 1.00 (0.90–1.11) | 1.15 (1.00–1.32) |
| Clinical T stage | ||||
| 1–2 | Reference | Reference | ||
| 3 | 2.57 (2.30–2.88) | 1.90 (1.67–2.15) | * | * |
| Clinical N stage | ||||
| 0 | Reference | Reference | Reference | Reference |
| 1 | 1.73 (1.58–1.91) | 1.25 (1.13–1.38) | 1.72 (1.64–1.80) | 1.22 (1.15–1.29) |
| 2 | 2.37 (1.99–2.82) | 1.50 (1.23–1.84) | 2.35 (2.14–2.58) | 1.51 (1.35–1.70) |
| 3 | 2.94 (2.35–3.68) | 1.79 (1.40–2.32) | 2.63 (2.31–2.99) | 1.98 (1.70–2.30) |
| Histology | ||||
| Ductal | Reference | Reference | Reference | Reference |
| Lobular or lobular component | 1.90 (1.38–2.62) | 2.00 (1.42–2.83) | 2.27 (2.01–2.56) | 2.05 (1.77–2.38) |
| Other | 1.19 (0.94–1.50) | 1.22 (0.96–1.56) | 1.21 (1.06–1.37) | 1.09 (0.94–1.27) |
| Molecular subtype | ||||
| ER/PR+, HER2- | Reference | Reference | Reference | Reference |
| ER/PR+, HER2+ | 1.03 (0.90–1.17) | 0.84 (0.73–0.97) | 0.80 (0.75–0.85) | 0.84 (0.78–0.90) |
| ER/PR-, HER2+ | 1.09 (0.95–1.25) | 0.96 (0.82–1.11) | 0.80 (0.74–0.86) | 0.90 (0.82–0.99) |
| ER/PR-, HER2- | 0.85 (0.75–0.97) | 0.98 (0.86–1.12) | 0.70 (0.66–0.74) | 0.97 (0.91–1.04) |
Includes Medicaid, uninsured and other government insurance
Discussion
Using the National Cancer Database, we found that the use of neoadjuvant chemotherapy and rates of pathologic complete response (pCR) have increased over time among patients with operable unilateral breast cancers in the United States. However, while unilateral mastectomy rates have declined, the rate of bilateral mastectomy with reconstruction has increased.
The use of bilateral mastectomy has been previously reported to be increasing among patients who are candidates for primary BCS14,25,26, raising questions as to what drives decision-making in this population. We found similar nationwide surgical trends among the population of patients receiving NAC. As in the non-NAC setting, the decision to pursue bilateral mastectomy for unilateral breast cancers after NAC is multifactorial and likely influenced by patient, physician and treatment factors, such as increased use of magnetic resonance imaging27,28, increased genetic testing and identification of high risk patients who benefit from bilateral mastectomy29, surgeon’s preference30, and patient preference due to fear of additional therapies, recurrence or anticipated regret31–34 or desire for cosmetic symmetry and increased access to reconstruction35. In our study, we observed that receipt of bilateral mastectomy is more common among younger patients from a more advantaged sociodemographic background even though these characteristics were associated with attainment of pCR. Being non-Hispanic white, living in areas with higher educational attainment, and having private insurance were factors independently associated with receipt of bilateral mastectomy. Others have also found similar associations for bilateral mastectomy in the early stage breast cancer setting14,18,19,36. It may be that these are the patients who have the capacity to participate more in their breast cancer surgical decisions, which has been linked to receipt of more aggressive treatment31,37. Additionally, younger women may be driven more by fear of recurrence and concern for more aggressive disease38–41, as well as have a higher probability of carrying genetic mutations. However, more extensive surgery may result from this decision-making process if patients are misinformed about their risk of recurrence or risk of complications from surgery19. It may also be that these patients have the resources to access reconstruction and new bilateral reconstruction approaches that achieve cosmetic symmetry, which have been shown to influence surgical decision-making35,42. Additional cosmetic considerations such as breast size to tumor size ratio, which are not available in the database, may also have influenced surgical choice.
NAC is increasingly used to tailor locoregional therapy for patients with breast cancer. As pCR rates increase with the use of newer systemic agents, more patients should be eligible for consideration of BCS and even omission of breast surgery (clinical trial: NCT02945579). We acknowledge that pCR is not available at the time of surgical decision-making or necessary for BCS, and that some definitions of pCR include in situ disease which can sometimes preclude BCS. However, good-responding patients who achieve pCR should be the most likely candidates for BCS after NAC. Among this group, we nonetheless found similar sociodemographic associations with type of surgery received. We similarly looked at patients who should be candidates for BCS at presentation (those with clinical T1/2 disease), and found similar results.
Notable strengths of the study include the use of a large, nationwide cohort treated in an era of molecular subtyping and modern systemic therapies, and the availability of pCR data, which is not included in other similar SEER-based analyses36. Limitations of the study include the lack of patient and tumor details that may have influenced treatment decisions, and thus we can only speculate about the reasons patients had for pursuing bilateral mastectomy, and whether that decision was clinically appropriate. For example, the NCDB does not contain data on genetic testing or family history, imaging findings, or important pathological details such as extent of microcalcifications. pCR was determined based on physician statement in the medical record rather than pathology reports. There is similarly a lack of data on patient preferences and physician recommendations, so we cannot distinguish whether surgical decisions are being driven by one or the other.
We found that many non-clinical factors may influence patients to choose more extensive surgery after neoadjuvant chemotherapy, a phenomenon that may be unique to North America. A recent study found that patients with triple negative breast cancer without germline BRCA mutations in Europe and Asia were more likely to choose BCS than patients in North America (55 vs. 80%) after neoadjuvant chemotherapy43. Improved understanding of characteristics associated with selecting mastectomy over BCS when BCS is appropriate would help clarify whether these decisions are toward more personalized medicine or overtreatment.
Supplementary Material
Footnotes
There are no financial disclosures or conflicts of interest.
Novelty/Impact: We used of a large, nationwide cohort treated in an era of molecular subtyping and modern systemic therapies to evaluate surgical trends in patients with operable breast cancer treated with neoadjuvant chemotherapy.
References
- 1.Treatment of Early-Stage Breast Cancer. JAMA 1991;265:391–5. [PubMed] [Google Scholar]
- 2.Masuda N, Lee S-J, Ohtani S, Im Y-H, Lee E-S, Yokota I, Kuroi K, Im S-A, Park B-W, Kim S-B, Yanagita Y, Ohno S, et al. Adjuvant Capecitabine for Breast Cancer after Preoperative Chemotherapy. N Engl J Med 2017;376:2147–59. [DOI] [PubMed] [Google Scholar]
- 3.Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, Bonnefoi H, Cameron D, Gianni L, Valagussa P, Swain SM, Prowell T, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. The Lancet 2014;384:164–72. [DOI] [PubMed] [Google Scholar]
- 4.Park JW, Liu MC, Yee D, Yau C, van ‘t Veer LJ, Symmans WF, Paoloni M, Perlmutter J, Hylton NM, Hogarth M, DeMichele A, Buxton MB, et al. Adaptive Randomization of Neratinib in Early Breast Cancer. N Engl J Med 2016;375:11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rugo HS, Olopade OI, DeMichele A, Yau C, van ‘t Veer LJ, Buxton MB, Hogarth M, Hylton NM, Paoloni M, Perlmutter J, Symmans WF, Yee D, et al. Adaptive Randomization of Veliparib– Carboplatin Treatment in Breast Cancer. N Engl J Med 2016;375:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mauri D, Pavlidis N, Ioannidis JPA. Neoadjuvant versus adjuvant systemic treatment in breast cancer: a meta-analysis. J Natl Cancer Inst 2005;97:188–94. [DOI] [PubMed] [Google Scholar]
- 7.Mieog JSD, van der Hage JA, van de Velde CJH. Neoadjuvant chemotherapy for operable breast cancer. Br J Surg 2007;94:1189–200. [DOI] [PubMed] [Google Scholar]
- 8.van der Hage JA, van de Velde CJ, Julien JP, Tubiana-Hulin M, Vandervelden C, Duchateau L. Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer trial 10902. J Clin Oncol Off J Am Soc Clin Oncol 2001;19:4224–37. [DOI] [PubMed] [Google Scholar]
- 9.Fisher B, Brown A, Mamounas E, Wieand S, Robidoux A, Margolese RG, Cruz AB, Fisher ER, Wickerham DL, Wolmark N, DeCillis A, Hoehn JL, et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol Off J Am Soc Clin Oncol 1997;15:2483–93. [DOI] [PubMed] [Google Scholar]
- 10.Guarneri V, Frassoldati A, Bottini A, Cagossi K, Bisagni G, Sarti S, Ravaioli A, Cavanna L, Giardina G, Musolino A, Untch M, Orlando L, et al. Preoperative Chemotherapy Plus Trastuzumab, Lapatinib, or Both in Human Epidermal Growth Factor Receptor 2–Positive Operable Breast Cancer: Results of the Randomized Phase II CHER-LOB Study. J Clin Oncol 2012;30:1989–95. [DOI] [PubMed] [Google Scholar]
- 11.Untch M, Loibl S, Bischoff J, Eidtmann H, Kaufmann M, Blohmer J-U, Hilfrich J, Strumberg D, Fasching PA, Kreienberg R, Tesch H, Hanusch C, et al. Lapatinib versus trastuzumab in combination with neoadjuvant anthracycline-taxane-based chemotherapy (GeparQuinto, GBG 44): a randomised phase 3 trial. Lancet Oncol 2012;13:135–44. [DOI] [PubMed] [Google Scholar]
- 12.Baselga J, Bradbury I, Eidtmann H, Di Cosimo S, de Azambuja E, Aura C, Gómez H, Dinh P, Fauria K, Van Dooren V, Aktan G, Goldhirsch A, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet Lond Engl 2012;379:633–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bear HD, Anderson S, Brown A, Smith R, Mamounas EP, Fisher B, Margolese R, Theoret H, Soran A, Wickerham DL, Wolmark N, National Surgical Adjuvant Breast and Bowel Project Protocol B-27. The effect on tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: preliminary results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol Off J Am Soc Clin Oncol 2003;21:4165–74. [DOI] [PubMed] [Google Scholar]
- 14.Kurian AW, Lichtensztajn DY, Keegan THM, Nelson DO, Clarke CA, Gomez SL. Use of and mortality after bilateral mastectomy compared with other surgical treatments for breast cancer in California, 1998–2011. JAMA 2014;312:902–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lostumbo L, Carbine NE, Wallace J. Prophylactic mastectomy for the prevention of breast cancer. Cochrane Database Syst Rev 2010;CD002748. [DOI] [PubMed] [Google Scholar]
- 16.Roberts A, Habibi M, Frick KD. Cost-effectiveness of contralateral prophylactic mastectomy for prevention of contralateral breast cancer. Ann Surg Oncol 2014;21:2209–17. [DOI] [PubMed] [Google Scholar]
- 17.Barton MB, West CN, Liu I-LA, Harris EL, Rolnick SJ, Elmore JG, Herrinton LJ, Greene SM, Nekhlyudov L, Fletcher SW, Geiger AM. Complications following bilateral prophylactic mastectomy. J Natl Cancer Inst Monogr 2005;61–6. [DOI] [PubMed] [Google Scholar]
- 18.Hawley ST, Jagsi R, Morrow M, Janz NK, Hamilton A, Graff JJ, Katz SJ. Social and Clinical Determinants of Contralateral Prophylactic Mastectomy. JAMA Surg 2014;149:582–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jagsi R, Hawley ST, Griffith KA, Janz NK, Kurian AW, Ward KC, Hamilton AS, Morrow M, Katz SJ. Contralateral Prophylactic Mastectomy Decisions in a Population-Based Sample of Patients With Early-Stage Breast Cancer. JAMA Surg 2017;152:274–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boffa DJ, Rosen JE, Mallin K, Loomis A, Gay G, Palis B, Thoburn K, Gress D, McKellar DP, Shulman LN, Facktor MA, Winchester DP. Using the National Cancer Database for Outcomes Research: A Review. JAMA Oncol 2017;3:1722–8. [DOI] [PubMed] [Google Scholar]
- 21.Onega T, Duell EJ, Shi X, Wang D, Demidenko E, Goodman D. Geographic access to cancer care in the U.S. Cancer 2008;112:909–18. [DOI] [PubMed] [Google Scholar]
- 22.Mougalian SS, Soulos PR, Killelea BK, Lannin DR, Abu-Khalaf MM, DiGiovanna MP, Sanft TB, Pusztai L, Gross CP, Chagpar AB. Use of neoadjuvant chemotherapy for patients with stage I to III breast cancer in the United States. Cancer 2015;121:2544–52. [DOI] [PubMed] [Google Scholar]
- 23.Rusthoven CG, Rabinovitch RA, Jones BL, Koshy M, Amini A, Yeh N, Jackson MW, Fisher CM. The impact of postmastectomy and regional nodal radiation after neoadjuvant chemotherapy for clinically lymph node-positive breast cancer: a National Cancer Database (NCDB) analysis. Ann Oncol Off J Eur Soc Med Oncol 2016;27:818–27. [DOI] [PubMed] [Google Scholar]
- 24.Kummerow KL, Du L, Penson DF, Shyr Y, Hooks MA. Nationwide Trends in Mastectomy for EarlyStage Breast Cancer. JAMA Surg 2015;150:9–16. [DOI] [PubMed] [Google Scholar]
- 25.Tuttle TM, Habermann EB, Grund EH, Morris TJ, Virnig BA. Increasing use of contralateral prophylactic mastectomy for breast cancer patients: a trend toward more aggressive surgical treatment. J Clin Oncol Off J Am Soc Clin Oncol 2007;25:5203–9. [DOI] [PubMed] [Google Scholar]
- 26.Arrington AK, Jarosek SL, Virnig BA, Habermann EB, Tuttle TM. Patient and surgeon characteristics associated with increased use of contralateral prophylactic mastectomy in patients with breast cancer. Ann Surg Oncol 2009;16:2697–704. [DOI] [PubMed] [Google Scholar]
- 27.Katipamula R, Degnim AC, Hoskin T, Boughey JC, Loprinzi C, Grant CS, Brandt KR, Pruthi S, Chute CG, Olson JE, Couch FJ, Ingle JN, et al. Trends in mastectomy rates at the Mayo Clinic Rochester: effect of surgical year and preoperative magnetic resonance imaging. J Clin Oncol Off J Am Soc Clin Oncol 2009;27:4082–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Onega T, Weiss JE, Goodrich ME, Zhu W, DeMartini WB, Kerlikowske K, Ozanne E, Tosteson ANA, Henderson LM, Buist DSM, Wernli KJ, Herschorn SD, et al. Relationship between preoperative breast MRI and surgical treatment of non-metastatic breast cancer. J Surg Oncol 2017;116:1008–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heemskerk-Gerritsen B a. M, Menke-Pluijmers MBE, Jager A, Tilanus-Linthorst MMA, Koppert LB, Obdeijn IMA, van Deurzen CHM, Collée JM, Seynaeve C, Hooning MJ Substantial breast cancer risk reduction and potential survival benefit after bilateral mastectomy when compared with surveillance in healthy BRCA1 and BRCA2 mutation carriers: a prospective analysis. Ann Oncol Off J Eur Soc Med Oncol 2013;24:2029–35. [DOI] [PubMed] [Google Scholar]
- 30.Morrow M, Jagsi R, Alderman AK, Griggs JJ, Hawley ST, Hamilton AS, Graff JJ, Katz SJ. Surgeon recommendations and receipt of mastectomy for treatment of breast cancer. JAMA 2009;302:1551–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katz SJ, Lantz PM, Janz NK, Fagerlin A, Schwartz K, Liu L, Deapen D, Salem B, Lakhani I, Morrow M. Patient Involvement in Surgery Treatment Decisions for Breast Cancer. J Clin Oncol 2005;23:5526–33. [DOI] [PubMed] [Google Scholar]
- 32.Collins ED, Moore CP, Clay KF, Kearing SA, O’Connor AM, Llewellyn-Thomas HA, Barth RJ, Sepucha KR. Can women with early-stage breast cancer make an informed decision for mastectomy? J Clin Oncol Off J Am Soc Clin Oncol 2009;27:519–25. [DOI] [PubMed] [Google Scholar]
- 33.Fisher CS, Martin-Dunlap T, Ruppel MB, Gao F, Atkins J, Margenthaler JA. Fear of recurrence and perceived survival benefit are primary motivators for choosing mastectomy over breast-conservation therapy regardless of age. Ann Surg Oncol 2012;19:3246–50. [DOI] [PubMed] [Google Scholar]
- 34.Katz SJ, Morrow M. Addressing overtreatment in breast cancer: The doctors’ dilemma. Cancer 2013;119:3584–8. [DOI] [PubMed] [Google Scholar]
- 35.King TA, Sakr R, Patil S, Gurevich I, Stempel M, Sampson M, Morrow M. Clinical management factors contribute to the decision for contralateral prophylactic mastectomy. J Clin Oncol Off J Am Soc Clin Oncol 2011;29:2158–64. [DOI] [PubMed] [Google Scholar]
- 36.Wapnir IL, Kurian AW, Lichtensztajn DY, Clarke CA, Gomez SL. Rising Bilateral Mastectomy Rates Among Neoadjuvant Chemotherapy Recipients in California From 1998 to 2012. Ann Surg 2016; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hawley ST, Griggs JJ, Hamilton AS, Graff JJ, Janz NK, Morrow M, Jagsi R, Salem B, Katz SJ. Decision involvement and receipt of mastectomy among racially and ethnically diverse breast cancer patients. J Natl Cancer Inst 2009;101:1337–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Balch CM, Jacobs LK. Mastectomies on the rise for breast cancer: “the tide is changing.” Ann Surg Oncol 2009;16:2669–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McGuire KP, Santillan AA, Kaur P, Meade T, Parbhoo J, Mathias M, Shamehdi C, Davis M, Ramos D, Cox CE. Are mastectomies on the rise? A 13-year trend analysis of the selection of mastectomy versus breast conservation therapy in 5865 patients. Ann Surg Oncol 2009;16:2682–90. [DOI] [PubMed] [Google Scholar]
- 40.Adkisson CD, Vallow LA, Kowalchik K, McNeil R, Hines S, Deperi E, Moreno A, Roy V, Perez EA, McLaughlin SA. Patient age and preoperative breast MRI in women with breast cancer: biopsy and surgical implications. Ann Surg Oncol 2011;18:1678–83. [DOI] [PubMed] [Google Scholar]
- 41.Nixon AJ, Neuberg D, Hayes DF, Gelman R, Connolly JL, Schnitt S, Abner A, Recht A, Vicini F, Harris JR. Relationship of patient age to pathologic features of the tumor and prognosis for patients with stage I or II breast cancer. J Clin Oncol Off J Am Soc Clin Oncol 1994;12:888–94. [DOI] [PubMed] [Google Scholar]
- 42.Alderman AK, Hawley ST, Waljee J, Mujahid M, Morrow M, Katz SJ. Understanding the impact of breast reconstruction on the surgical decision-making process for breast cancer. Cancer 2008;112:489–94 [DOI] [PubMed] [Google Scholar]
- 43.Golshan M, Loibl S, Huober J, Joyce O’Shaughnessy. Breast conservation after neoadjuvant chemotherapy for triple-negative breast cancer: Surgical results from an international randomized trial (BrighTNess). J Clin Oncol 35, 2017. (suppl; abstr 514). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.