Abstract
This study examined whether selective neonatal hippocampal lesions in monkeys (Macaca mulatta), which left the surrounding cortical areas (parahippocampal cortex) intact, affect contextual learning and memory compared to controls. Monkeys were tested with an automated touch-screen apparatus so that stimuli and contextual cues could be manipulated independently of one another. The data suggest that animals with neonatal hippocampal lesions have sparing of function in regards to contextual learning and memory when (1) contextual information is irrelevant or (2) relevant for good discrimination performance, and (3) when transferring a contextual rule to new discriminations. These findings are at odds with studies examining contextual learning and memory in monkeys with selective adult-onset hippocampal lesions, and those with non-selective neonatal hippocampal lesions, which have demonstrated impairment in contextual learning and memory. Therefore, the sparing of function seen in this study may be due to the early nature of the damage and the plastic nature of the infant brain, as well as the intact medial temporal lobe cortical areas as a result of the lesion methodology. Specifically, by removing the hippocampus early in life, before it has begun to function, the parahippocampal (TH/TF) and perirhinal cortices and its interactions with the lateral prefrontal cortex may be able to support context processing throughout life.
Keywords: facilitation, development, medial temporal lobe, memory, context
INTRODUCTION
An extensive body of literature has provided evidence that the hippocampus plays a critical role in contextual discrimination learning in monkeys. For instance, Ridley and colleagues (Ridley et al. 1995, 2001) demonstrated that surgical removal of the CA fields, subiculum and presubiculum or just the CA1 field in adult marmosets impaired performance on discrimination problems when information about the background context was required to indicate which object was rewarded. Thus, on one background (A), one object (a) was “correct” and on the other background (B), the other object (b) was “correct”. Furthermore, Dore and colleagues (Dore et al. 1998) showed that monkeys with selective damage to the hippocampus did not use background contextual cues to enhance performance on a discrimination task. Specifically, monkeys were trained on a discrimination paradigm in which they were required to learn which of five objects presented in an array would deliver a reward when chosen. Monkeys with excitotoxic hippocampal lesions were impaired relative to controls when learning these discrimination problems, indicating that the hippocampus is required for using contextual cues provided by the existence of other objects in an array. Finally, using an incidental recognition task (visual paired-comparison or object/context-VPC), Bachevalier and colleagues (Bachevalier et al. 2015) demonstrated a lack of novelty preference in monkeys with selective hippocampal lesions sustained in adulthood when a familiar object was presented over a new background. Similar findings have also been reported in humans, rabbits and rodents (Burgess et al. 2001; Freeman et al. 1997; Kennedy & Shapiro 2004; Kim & Fanselow 1992; Kim et al. 2012; Mayes et al. 1992; Smith & Mizumori 2006; Penick & Solomon 1991; Phillips & LeDoux 1992; Rudy et al. 2002; Sill & Smith 2012; Pascalis et al. 2009; Spiers et al. 2001).
The effects of early damage to the hippocampus on contextual discrimination learning remain to be fully explored. Neonatal damage to the hippocampal formation (i.e. < 21 days) disrupts the emergence of hippocampal-dependent functions, when the use of contextual information was critical for good performance on the task (van Praag, Qu et al. 1998). Exploratory behavior of adult rats that had received unilateral electrolytic hippocampal ablations at one day of age were assessed in a Morris water maze. Adult rats with neonatal hippocampal damage did not respond to novelty, spending an equivalent amount of time exploring a quadrant with a new stimulus than when no stimulus was present. In contrast, rats with adult-onset hippocampal lesions and control animals spent an increased amount of time in the quadrant with a new stimulus. These results indicate that rats with neonatal hippocampal lesions may be unable to process contextual cues and suggest that early hippocampal damage can yield impairment not present when the damage is sustained in adulthood. Similar contextual memory impairment was demonstrated following neonatal hippocampal lesions in monkeys. Thus, hippocampectomized infant monkeys were able to learn discrimination problems, but were unable to retrieve the contextual information in which these problems occurred (Killiany et al. 2005; Rehbein et al. 2005). In addition, neonatal hippocampal lesions in monkeys (Pascalis et al. 2009) altered recognition memory when the background onto which the objects were presented changed from study to test. Nevertheless, these few developmental lesion studies in primates are inconclusive in regards to the role of the hippocampus in contextual memory given that in all developmental studies so far, the damage to the hippocampus was extended to include cortical areas adjacent to the hippocampus, which in turn could have been responsible, either alone or in combination with the hippocampal lesions, for the impairment noted (Diana et al. 2007; 2012).
More recently, we investigated the role of the hippocampus in the development of memory processes using more selective hippocampal lesions made via injections of neurotoxins performed when animals were 8-12 days of age. Like adult-onset hippocampal lesions, these neonatal lesions yielded a delay-dependent incidental object recognition memory impairment that emerged when the monkeys were 18 months of age (Zeamer et al. 2010) and was still present when the animals were re-tested in adulthood (Zeamer & Bachevalier 2013). In addition, the same monkeys with neonatal hippocampal lesions also had impaired spatial relational memory (Blue et al. 2013) and memory for food/place associations (Glavis-Bloom et al. 2013). The goal of the current study was to assess whether these same animals with selective neonatal hippocampal lesions would also be impaired in contextual discrimination learning. We designed a series of experiments using a touch screen computer that allowed us to manipulate stimuli (images of objects) and the contextual cues (background screens) within each discrimination problem using a 24-hr concurrent discrimination task. We first carried out two experiments (Experiments 1 and 2) to rule out any effects of the use of different backgrounds onto which stimuli are displayed on performance of animals with neonatal hippocampal lesions on 24-hr concurrent discrimination. In these two experiments, the use of contextual cues was irrelevant for good performance on the task. Three additional experiments (Experiments 3-5) used three different versions of the 24-hr discrimination task in which monkeys had to use contextual cues to be correct. This type of association between an object and the background on which it was presented, has been specifically termed “contextual binding” (Chalfonte & Johnson 1996, Mitchell et al. 2000). We predicted that animals with neonatal hippocampal lesions would show normal discrimination learning when the context onto which the discrimination problems were presented was made irrelevant for good performance on the task (Experiments 1 and 2), but would show robust impairment in learning discrimination problems when the context onto which these problems was relevant for good performance. Finally, to compare the results of neonatal hippocampal lesions on object/context association with those obtained in monkeys with adult-onset hippocampal lesions, we also tested the animals in the incidental recognition object/context VPC task, used in our earlier study in adult monkeys (Bachevalier et al. 2015). Preliminary reports of the findings were published in abstract form (Glavis-Bloom et al. 2010).
METHOD
Subjects
Twelve adult rhesus macaques (Macaca mulatta) of both sexes, weighing between 5-10 kg and ranging between 7 and 9 years of age at the beginning of testing, were divided into two groups. Between eight and twelve days of age, 6 animals received MRI-guided neurotoxic lesions of the hippocampus (Group Neo-Hibo; 4 males, 2 females) and 6 others received sham-operations (Group Neo-C; 3 males, 3 females).
All animals were acquired from the MD Anderson Cancer Center Science Park breeding facility (Bastrop, TX), and brought to the primate nursery at MD Anderson Cancer Center (Houston, TX) between one and four days of age. They were hand fed a diet of infant Similac formula, and were surrogate-peer reared according to procedures developed by Sackett and colleagues (Sackett et al. 2002). These procedures included daily social interactions with peers and humans, along with cognitive testing (for more details, see Goursaud & Bachevalier, 2007). At 1.5 years of age, animals were transferred to the Yerkes National Primate Research Center where they were housed in pairs for three years and then housed individually. They were given water ad libitum, and fed fresh fruit, vegetables, and monkey chow (Lab Diet #5037, PMI Nutrition International Inc., Brentwood, MO) daily. Animal housing rooms were maintained on a 12:12 hour light-dark cycle, and all testing occurred during the light phase.
The MRI-guided surgical procedures were carried out while the animals were at the University of Texas Health Science Center in Houston and were approved by the Internal Animal Care and Use Committee of the University of Texas-Houston. Behavioral testing for the current experiment occurred at the Yerkes National Primate Research Center and was approved by the Internal Animal Care and Use Committee of Emory University.
Neuroimaging and Surgical Procedures
Neuroimaging Procedures
Neuroimaging and surgical procedures have been described in detail elsewhere (Goursaud & Bachevalier, 2007, Zeamer et al., 2010). Briefly, before surgery, each infant monkey in Group Neo-Hibo underwent an MRI of the brain to acquire both structural high-resolution T1 FSPGR images, which were used to calculate the coordinates of injection sites within the hippocampus as well as Fluid Attenuated Inversion Recovery (FLAIR) images with CSF suppression, which were used to evaluate areas of hypersignal (high water density) one week after the neurotoxin injections.
The day of surgery, animals were maintained under gas anesthesia (Isoflurane 1.0 – 3.0% to effect), their head was shaved, and an intravenous catheter was placed in the saphenous vein to deliver a solution of dextrose and 0.45% sodium chloride to maintain hydration throughout the procedures. EMLA cream (lidocaine 2.5% and prilocaine 2.5%) was applied to the ear canals and to the skin just below the eye orbits to reduce pain caused by the pressure of the ear bars and eye pieces of the stereotaxic apparatus, respectively, and ophthalmic ointment was applied to the eyes to prevent ocular dryness during the procedure. The animals were then secured in a sterotaxic apparatus (Crist Instruments Co., Inc., Damascus, MD), centered in the GE Sigma 1.5 Tesla Echo Speed scanner (GE Medical Systems, Milwaukee, WI), and MR images were acquired with a 5-cm surface coil. The entire scanning session lasted 45 to 60 minutes during which the animal’s heart rate, body temperature, and SPO 2 were continuously monitored. Five to eight days after surgery, the same sets of MR images were acquired and used to insure the ibotenic acid had targeted the hippocampus and estimate the lesion extent.
Surgical procedures
After completion of the pre-surgical scans, animals remained anesthetized and secured in the stereotaxic apparatus, and immediately transported to the surgical suite where they were prepared for the surgical procedures. The scalp was disinfected with Nolvasan Solution and a local anesthetic (Marcaine 25%, 1.5m., s.c.) was injected along the incision line at the midline to reduce pain. The skin was cut at the midline from the mid-supra-orbital ridges to the occipital notch and the skin and galea were gently retracted. A small bone opening was made just above the injection sites in each hemisphere and the exposed dura was slit open to allow passage of the Hamilton syringes held onto the Kopf electrode manipulators (David Kopf Instruments, Tujunga, CA). Injections of the neurotoxin, ibotenic acid (Biosearch Technologies, Novato, CA) were made in each hemisphere simultaneously for each of the 7-8 sites selected at 2-mm intervals along the anterior-posterior axis of the hippocampus. A total of 3.2-5.4 μl (10mg/ml in PBS, pH 7.4) was injected at a rate of 0.2 μl/30sec. After each injection, the needles were kept in place for an additional three minutes to maximize diffusion of the neurotoxin into the hippocampal tissue and minimize its spread along the needle path when the needle was retracted. At the end of the injections, the incision was closed in anatomical layers. The animal was removed from Isoflurane gas anesthesia and recovered in the surgical facility until it regained consciousness.
Sham operations followed the same neuroimaging and surgical procedures outlined above, except that there were no needle penetrations and no injections performed.
Pre- and Post-surgical Treatment
Beginning 12 hours before surgery, and continuing for seven days post-surgery, all animals received dexamethazone sodium phosphate (0.4 mg/kg, s.c.) and oral doses of Cephazolin (25 mg/kg) to control swelling and minimize risk of infection, respectively. A topical antibiotic ointment was also applied to the incision daily. Additionally, acetaminophen (10mg/kg, p.o.) was given four times a day for three days after surgery to reduce pain.
Lesion Verification
At the conclusion of behavioral testing, the monkeys were given a lethal dose of pentobarbital sodium and were perfused intracardially with normal saline followed by aldehyde fixatives. The brains were then removed, frozen at −80°C, and then cut at 50 μm in the coronal plane. A series of sections from each brain was stained with thionin and galea. Extent of cell loss identified on each thionin-stained section through the hippocampus is illustrated in Figures 1 and 2 for each case. Table 1 provides the percentage of hippocampal cell loss in each hemisphere in all six cases.
Figure 1:
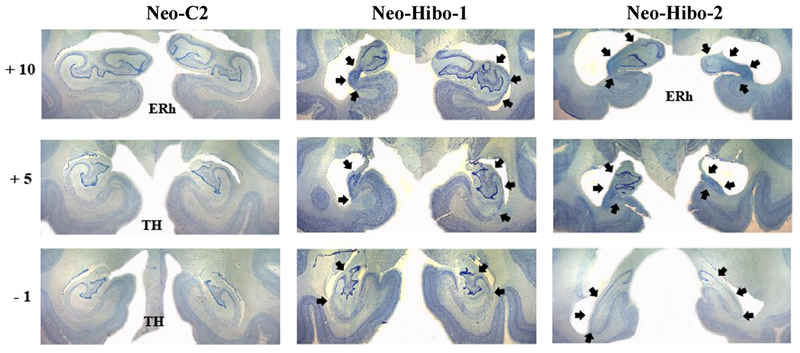
Coronal histological sections (thionin stain) at four levels through the hippocampus of a control animal (Neo-C-2) and through the extent of hippocampal damage of two cases with neonatal hippocampal lesions (Neo-Hibo1 and -2). The numerals on the left of each coronal section indicate the distance in millimeters from the interaural plane. Arrows point to cell loss within the hippocampus. Abbreviations: ERh: entorhinal cortex; TH: cytoarchitectonic fields described by von Bonin and Bailey (1947). More detailed description of the lesions is provided in the text.
Figure 2:
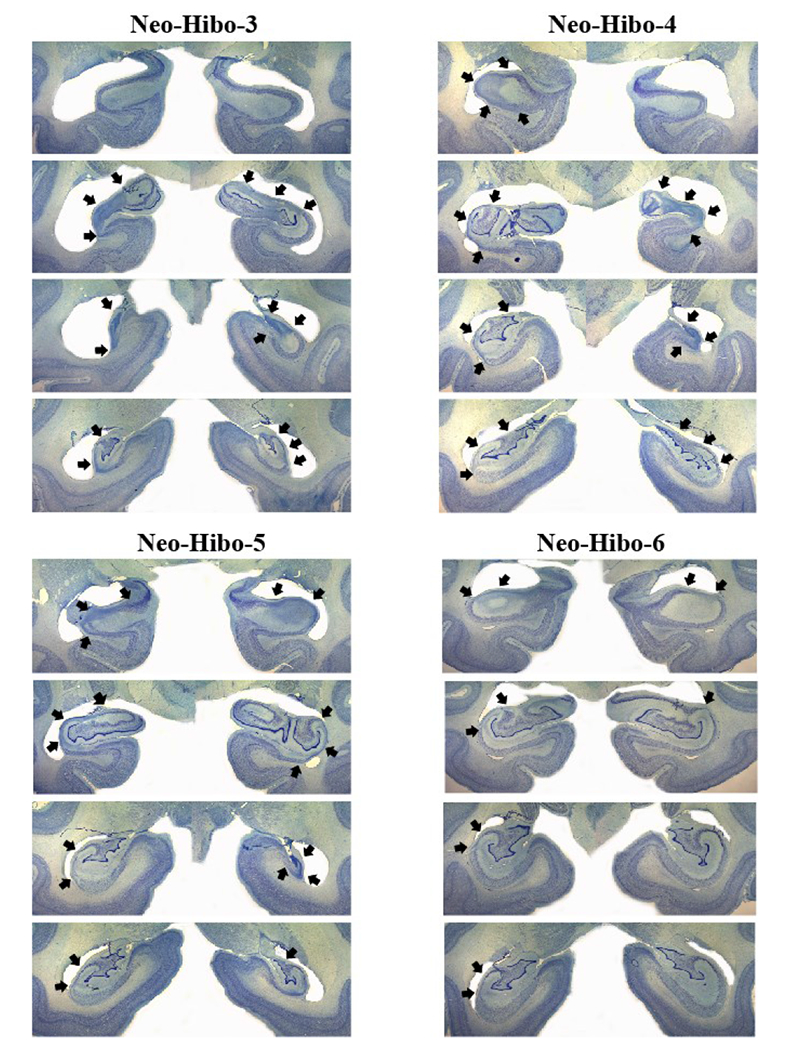
Coronal histological sections (thionin stain) at four levels through the extent of damage of four cases with neonatal hippocampal lesions (Neo-Hibo3, -4, -5, and -6). Conventions as in Figure 1.
Table 1:
Volume of hippocampal Cell Loss
| Subject | L% | R% | X% | W |
|---|---|---|---|---|
| Neo-Hibo1 | 65.63 | 23.77 | 44.69 | 15.59 |
| Neo-Hibo2 | 53.62 | 79.00 | 66.31 | 42.36 |
| Neo-Hibo3 | 63.41 | 42.02 | 52.72 | 26.65 |
| Neo-Hibo4 | 33.89 | 69.95 | 51.92 | 23.71 |
| Neo-Hibo5 | 37.59 | 64.95 | 51.27 | 24.41 |
| Neo-Hibo6 | 23.82 | 7.48 | 15.65 | 1.78 |
| X | 46.32 | 47.86 | 47.09 | 22.42 |
Data are the percentage of cell loss as assessed from post-mortem histological investigation. L: percent cell loss in left hemisphere; R: percent cell loss in right hemisphere; X%: average of L and R; W = (L × R)/100 [weighted index as defined by Hodos and Bobko (1984)]; X: group mean
Neo-Hibo-1 had an almost complete lesion through the left hippocampus, including all CA fields and the dentate gyrus, whereas the lesion on the right side was restricted to CA1, CA2 and CA3 through the entire length of the hippocampus. The lesion in Neo-Hibo-2 included the entire hippocampus bilaterally with little sparing of hippocampus fields. Neo-Hibo-3 had an extensive lesion of the entire length of the hippocampus on the left, sparing only the anterior-most portion, whereas the lesion on the right was mostly restricted to CA1, CA2, and CA3 fields. The reverse was true for Neo-Hibo-4 that had a more extensive lesion on the right than on the left. The lesion in Neo-Hibo-5 was less extended and restricted to the CA fields bilaterally, whereas Neo-Hibo-6 had the smallest lesion of the hippocampus, including the CA2 and CA3 fields through the entire length of the left hippocampus, but only a small amount of damage in the CA2 field in the anterior-most portion of the right hippocampus. As shown in the figures, cell loss was absent in all adjacent medial temporal areas (entorhinal cortex, parahippocampal areas and area TE).
General Behavioral Procedures
Animals went through a series of six experiments in the order they are labeled in the text (i.e., Experiment 1 first and Experiment 6 last) and no other tasks were given in between these six experiments. Experiments 1 through 5 took place within a sound-attenuated testing box equipped with a touch-screen computer and an automatic mini M&M dispenser (Med Associates, Inc., St. Albans, VT) mounted on a shelf secured to the outside of the testing box. Each day, the animal was brought to the testing room in a transport cage that was positioned approximately 15 cm in front of the touch screen. The software “Presentation” (Neurobehavioral Systems Inc., Albany, CA) was used to present stimuli to the monkey on the touch screen computer. The stimuli were digitized pictures of objects, approximately 10 cm × 10 cm, displayed over either a black background or a wall paper background (see Figures 3A-6A for examples of stimuli). The program controlled the number and order of trials, locations of the stimuli on the screen, delivery of the food rewards (mini M&Ms), and length of the inter-trial interval (ITI). The computer program also recorded several parameters about the animal’s performance on each experiment, including test session and trial number, correct or incorrect choice, the location on the screen of the correct choice and the chosen location, and the latency from stimulus onset to choice. In all cases, trials remained displayed on the screen until the monkey made a choice. When a correct choice was made, a “ding” coincided with the dispensing of a mini M&M into a food cup located directly underneath and in the center of the touch screen, and the offset of the stimuli. When an incorrect choice was made, a “door closing” sound coincided with the offset of the stimuli, but no reward was dispensed. The details of stimuli presentations will be described below for each experiment.
Figure 3:
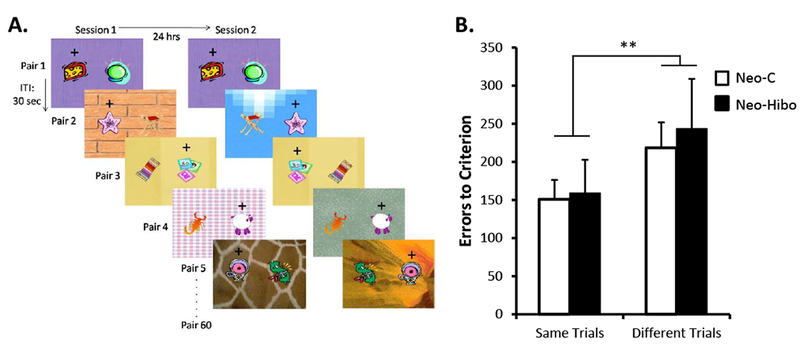
A- Example of stimuli used in Experiment 2. Within each daily session, thirty pairs were always presented on the same background (“Same” trials, see Pairs 1 and 3) and thirty pairs were presented on different background (“Different” trials, see Pairs 2, 4, and 5). The location of the objects on the background varied pseudo randomly across trials within a daily session. B- Mean number of errors (±SEM) to criterion for the two types of trials. Black bars illustrate performance of animals with neonatal hippocampal lesions and white bars illustrate performance of control animals. ** indicates p < .01.
Figure 6:
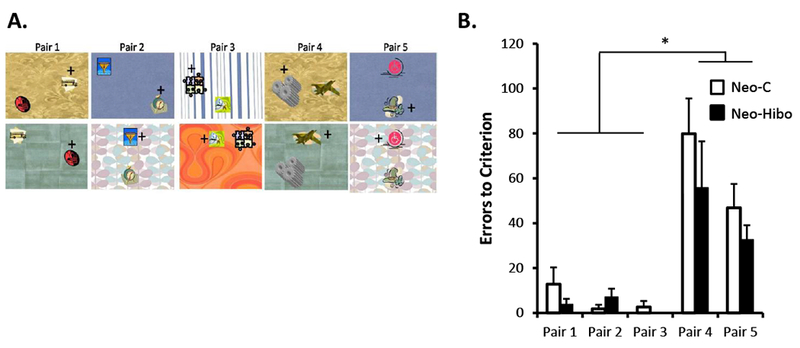
A- Example of stimuli used in Experiment 5. Animals were presented with three biconditional discrimination problems they learned in Experiment 4 (Trials 1-3) and two new problems in which the pairs of objects were presented onto backgrounds already occurring in Trials 1 and 2 (trials 4 and 5). All five Pairs were presented concurrently as in Experiment 4. For all experiments, location of the objects on the background varied across trials within a daily session. B- Mean number of errors (±SEM) to criterion for the each of the five Pairs. Black bars illustrate performance of animals with neonatal hippocampal lesions and white bars illustrate performance of control animals.
Statistical Procedures
All statistical analyses were performed using the SPSS 12.0 statistical analyses package. Data were analyzed using ANOVAs, with group as the between-subjects factor and repeated measures were used for the within-subjects factor when needed. A Huynh-Feldt correction for degrees of freedom was used for the repeated measures. Post-hoc pairwise comparisons were made using univariate analysis of variance and/or Bonferroni corrected t-tests, as appropriate. Nonparametric analyses (Mann-Whitney or Kruskal-Wallis) were used when data were not normally distributed. For all ANOVAs, when p value failed just short of significance, effect sizes were reported using partial eta squared (ηp2) and for all t-tests, effect sizes were reported using Cohen’s d (dCohen). Finally, correlations between performance on the tasks and extent of hippocampal cell loss were performed using Pearson correlations.
Experiment 1:
Although an earlier study reported normal performance of the Neo-Hibo animals on a 60-pair three-dimensional object concurrent discrimination task with 24-hrs ITI administered in a Wisconsin General Testing Apparatus (WGTA) (Glavis-Bloom et al. 2008), this first experiment tested whether this normal performance of the Neo-Hibo animals would be maintained when the task was delivered via a computerized testing apparatus, which subsequently permitted easy manipulation of the stimuli and the backgrounds onto which they were presented.
Behavioral Procedures
One hundred and twenty colored pictures of different objects were paired to form 60 discrimination problems. These pictures of objects were presented on a uniform black background. On each trial, a pair of stimuli appeared 10 cm apart on the left and right sides of the screen. For each pair, one of the objects was designated as positive (delivery of a reward followed if selected) and the other was designated as negative (no delivery of reward followed if selected). After the animal selected one of the stimuli, the monitor screen went black for an inter-trial interval of 30 sec, after which the second pair of objects was presented for choice and so on until the 60 pairs of stimuli were presented once each. The 60 discrimination problems were presented in the same order each day, but the left/right position of the positive object of the pair was counterbalanced each day. Daily testing was continued until the animal reached a criterion of 85% correct responses over three consecutive days of testing.
Results
Monkeys with neonatal hippocampal lesions acquired the task at the same rate (average ± SEM: 12 ± 2.9 sessions, 730 ± 179.6 trials, 244 ± 48.3.1 errors) as sham-operated animals (average ± SEM: 13 ± 3 sessions, 790 ± 180.2 trials, 282 ± 68.9 errors). The two groups did not differ in any of the three measures (Mann-Whitney for Sessions: U = 17.5, NS; Trials: U = 17.5, NS; Errors: U = 16.0, NS). In addition, both groups had similar latencies to select the correct or incorrect stimuli (Group: F(1, 10) = 1.34, NS; Latency: F (1, 10) = 0.49, NS; Interaction: F(1, 10) = 0.12, NS). Because the neonatal hippocampal lesions did not affect the response latency to stimuli, this parameter was not analyzed in subsequent experiments. Finally, a comparison of performance on the computerized version of the task using 2D stimuli with performance of the same animals when tested previously in the manual version using 3D objects (Glavis-Bloom et al. 2008) indicated that for both groups the number of sessions with the 2D objects was comparable to that obtained with the 3D objects (average ± SEM with 3D objects: 7 ± 1.2 sessions for Neo-Hibo and 12 ± 0.9 sessions for sham-operated controls).
Experiment 2:
This second experiment introduced a background under each pair of stimuli to ensure that the use of backgrounds would not affect performance on the concurrent discrimination task, and more so for animals with neonatal hippocampal damage than for sham-operated controls. The discrimination task was modified such that a background was added to each discrimination problem of the concurrent discrimination task. Thus, on 30 discrimination problems, the background under each pair of stimuli was kept the same from day to day, and for the other 30 problems, the background changed on each day. However, the information provided by the backgrounds was irrelevant for good performance on the task and the task could be solved using the rule learned in Experiment 1. Thus, we predicted that animals with neonatal hippocampal lesions would continue to perform normally on both types of discrimination problems (i.e., same or different backgrounds from day to day).
Behavioral Procedures
The basic concurrent discrimination task was used and 60 new pairs of stimuli were selected. However, instead of a uniform black background, both stimuli of a pair were presented on a colored/patterned background. As shown in Figure 3A, the backgrounds differed for each pair and thirty of the pairs were presented on the same background each day (“same” trials), whereas the other thirty pairs were presented on a different new background each day (“different” trials). These two types of discrimination problems were pseudo randomly intermixed within the daily session. As for Experiment 1, each pair of stimuli was presented one at a time with the objects in the pair presented on the left and right sides of the screen, counterbalanced across trials. Pairs were each presented once per day, in the same order, until a learning criterion (85% average over three consecutive days) was achieved for each type of trials separately as well as for both types of trials overall.
Results
Analyses of the two types of discrimination problems (Figure 3B) revealed that all animals, regardless of the group, acquired the discriminations presented over the same background (“same” trials) faster than discriminations presented over different backgrounds (“different” trials). Analyses of the sessions revealed a significant main effect of trial type (F(1,10) = 18.11, p = .002), but no effect of group (F(1,10) = .000, NS), and no interaction (F(1,10) = .003, NS). Similar findings were obtained with analyses of trials [Group: F(1, 10) = .000, NS; Trial type: F(1, 10) = 18.11, p = .002; interaction: F(1, 10) = .003, NS] and of errors [Group: F(1,10) = .08, NS; Trial type: F(1, 10) = 15.16, p = .003; interaction: F(1, 10) = 0.19, NS]. In addition, although overall performance across the 60 discrimination problems was similar for both groups [Neo-Hibo: 21 sessions, 1260 trials, 436 errors and Neo-C: 22 sessions, 1340 trials, 419 errors; Sessions: t(10) = .22, NS; Trials: t(10) = .22, NS; Errors: t(10) = −0.131, NS], the use of backgrounds and possibly the intermixing of “same trials” and “different trials” within the same daily session slightly reduced the speed of learning in both groups. Thus, when comparing overall performance in Experiments 1 and 2, animals in both groups made twice more errors in Experiment 2 than in Experiment 1 [Neo-Hibo: 436 versus 244 errors; Neo-C: 419 versus 282 errors, respectively; [Experiment effect: F(1,10) = 20.82, p = .001; Group effect: F(1,10) = .01, NS; interaction: F(1,10) = .56, NS].
Experiment 3:
Given the critical contribution of the hippocampus in processing object-context associations (Bachevalier et al. 2015; Burgess et al. 2001; Kim & Fanselow 1992; Pascalis et al. 2009; Phillips & LeDoux 1992; Smith & Mizumori 2006; Spiers et al. 2001; Eichenbaum et al. 2007; Diana et al. 2007), the concurrent discrimination task was modified in a third experiment to assess whether neonatal hippocampal damage would affect learning of the discriminations when the background was made relevant for correct performance on the task. To this end, four new pairs of stimuli and eight new backgrounds were selected for the task. Each pair of stimuli was presented over two different backgrounds, such that on one background one stimulus was correct, and on the other background the other stimulus was correct (see Figure 4A). Each pair of objects and their backgrounds were introduced serially, and by the end of training all four pairs/backgrounds were intermixed in a daily session.
Figure 4:
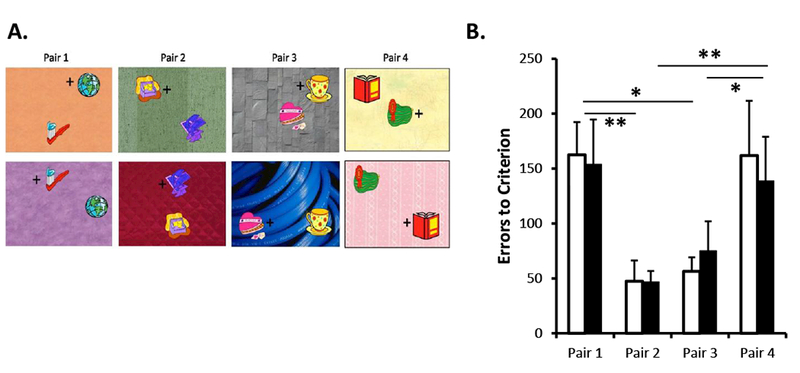
A- Example of stimuli used in Experiment 3. Animals learned four biconditional discrimination problems and in each problem one of the stimuli was rewarded when the pair was presented on top of background X and the other stimulus was rewarded when the pair was presented on top of background Y. Animals first learned Pair 1, then Pair 2 was introduced, then Pair 3 and finally Pair 4; each new pair was introduced as animals reached 70% correct. The location of the objects on the background varied pseudo randomly across trials within a daily session. B- Mean number of errors (±SEM) to criterion for the each of the four Pairs. Black bars illustrate performance of animals with neonatal hippocampal lesions and white bars illustrate performance of control animals. ** p < .001; * p < .05
Behavioral Procedures
Training began with presentation of the first pair of stimuli over two different backgrounds. Animals received 60 trials in which for half of the trials the pair of stimuli was presented over one background and for the other half the pair of stimuli was presented over the other background for that pair. These two types of trials were randomized across the 60-trial session. Animals were trained on this first discrimination problem until they demonstrated initial learning. (i.e., at least 70% correct on one daily session). Afterward, the second pair of discrimination problems over two different backgrounds was added to the daily session and intermixed with trials from the first pair. Animals then received a total of 60 trials per session (30 trials of Pair 1 with its two backgrounds and 30 trials of Pair 2 with its two backgrounds). The order of pair presentation was pseudorandomized across the 60-trial session so that each pair was presented 15 times onto one of its backgrounds and 15 times onto the other background. Again, initial learning criterion was set for at least 70% correct on Pair 2 over two daily sessions of 60 trials. When Pairs 1 and 2 were intermixed, initial learning criterions were taken across two days of testing (i.e., 60 trials for each pair in a “block” of trials). The third pair of discriminations (Pair 3) was then added to the training and intermixed with presentations of Pairs 1 and 2. The 60 trials per daily session comprised of 20 trials for Pairs 1, 2, and 3, and for each pair, 10 trials were presented over one background and 10 trials over the other background. Again, initial learning criterion was set for at least 70% correct on Pair 3 over three daily sessions, in order to again keep 60 trials of each pair in a “block” of trials across sessions. Then, the final pair (Pair 4) was added to the training sessions, animals received 15 trials for each Pair (1, 2, 3, and 4). Due to uneven number of trials for each pair in a daily session, trials were counterbalanced across daily sessions to maintain an equal number of presentations of each pair on each of its backgrounds within a four-session block of trials. Animals were tested in this last phase until they reached a learning criterion of 85% correct for each pair.
Results
Neo-Hibo animals acquired these contextual discrimination problems as rapidly as sham-operated controls (Group Neo-Hibo: 18 ± 3.14 sessions, 1040 ± 188.26 trials, and 581 ± 140.16 errors; Group Neo-C: 18 ± 2.90 sessions, 1070 ± 174.18 trials and 611 ± 88.0 errors). The group difference did not reach significance on any of the three learning parameters (Sessions: t(10) = .12, NS; Trials: t(10) = .12, NS; Errors: t(10) = .18, NS). To investigate whether animals in the two groups learned each contextual discrimination problem similarly, data for each pair were analyzed separately (see Fig. 4B for the errors to criterion). Although no reliable group differences emerged for any of the four pairs [Errors: F(1, 10) = .01, NS; interaction F(2.2, 21.79 Huynh-Feldt corrected) = .22, NS], the main effect of pair was significant (F(2.2, 21.79 Huynh-Feldt corrected) = 9.5, p = .001). Thus, both groups made significantly more errors to learn Pairs 1 and 4 as compared to Pair 2 (p = .000 and p = .011) and Pair 3 (p = .000 and p = .02). Performance on Pair 2 did not differ from performance on Pair 3.
Experiment 4:
Although animals in both groups performed equally well on Experiment 3, the lack of impairment following neonatal hippocampal lesions could be associated with the procedure used for the task. Specifically, it is possible that the small number of pairs to be learned and the fact that the pairs were presented one at a time until initial learning was demonstrated may have aided animals with neonatal hippocampal damage to chunk information on each trial and to learn each of the 8 discriminations separately without having to use a contextual rule. To test this possibility in Experiment 4, we added one additional discrimination problem to increase the set size from 4 to 5 and presented these five new pairs of objects associated with 10 new backgrounds all intermixed within each daily session (see Fig. 5A). We predicted that if animals with neonatal hippocampal lesions had learned a configural rule in Experiment 3, they should be able to transfer this rule to the new pairs of stimuli and rapidly reach criterion, whereas control animals may require more trials to learn each discrimination problem using a more relational rule.
Figure 5:
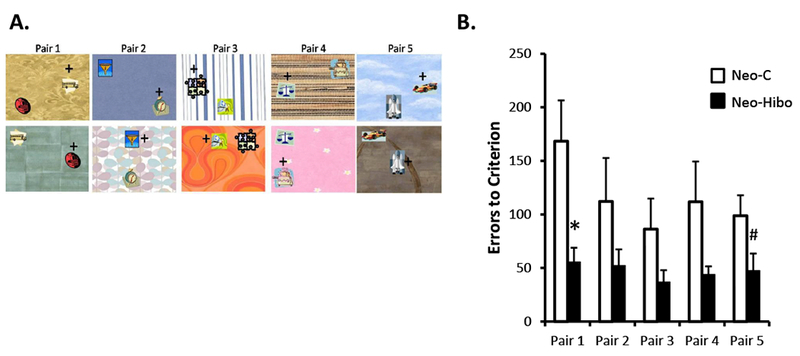
A- Example of stimuli used in Experiment 4. Animals learned five new biconditional discrimination problems presented concurrently within daily sessions, following the same rules as Experiment 3. The location of the objects on the background varied pseudo randomly across trials within a daily session. B- Mean number of errors (±SEM) to criterion for the each of the five Pairs. Black bars illustrate performance of animals with neonatal hippocampal lesions and white bars illustrate performance of control animals. * p < .05; # p < .06
Behavioral Procedures
Animals received 100 trials per daily session, consisting of 20 trials of each pair (10 trials presented over one background and 10 trials presented over the other background). The ITI was reduced from 30 seconds to 15 seconds to maintain daily training to 35-45 minutes. The order of presentation of the pairs and the spatial location of the stimuli within the background were randomized. Animals were tested until they reached a learning criterion of 85% correct for each pair.
Results
The mean number of sessions, trials, and errors to the 85% criterion for each pair as well as across the five pairs are presented for each animal of both groups in Table 2. Animals in Group Neo-Hibo acquired the five contextual discrimination problems rapidly and faster than animals in Group Neo-C. Their overall performance across the five pairs differed significantly from performance of controls for Errors [t(6.18) = 2.32, p = .058, dCohen=1.34], sessions [t(7.162) = 2.35, p = .05] and trials [t(7.162) = 2.35, p = .05]. As illustrated in Figure 5B for the errors, the group effect failed short of significance but the effect size was large [F(1, 10) = 4.23, p = .067; ηp2=0.297], and the Group × Pair interaction was significant [F(4, 40) = 2.68, p = .045]. Post-hoc comparisons indicated that group Neo-Hibo differed significantly from Group Neo-C on Pair 1 (56 vs 168 errors; t = 2.79, p = .019) and approached significance for Pair 5 (48 vs 99 errors; t = 2.07, p = .06). The two groups did not differ for Pair 2 (52 vs 112 errors, t = 1.39, NS), Pair 3 (37 vs 86 errors; t = 1.62, NS) and Pair 4 (44 vs. 112 errors; t = 1.77, NS).
Table 2:
Experiment 4 – Sessions, Trials, Errors to criterion
| Subject | Sessions |
Trials |
Errors |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pair 1 | Pair 2 | Pair 3 | Pair 4 | Pair 5 | Total | Pair 1 | Pair 2 | Pair 3 | Pair 4 | Pair 5 | Total | Pair 1 | Pair 2 | Pair 3 | Pair 4 | Pair 5 | Total | |
| Neo-C1 | 12 | 5 | 6 | 5 | 6 | 13 | 720 | 300 | 360 | 300 | 360 | 3900 | 250 | 105 | 122 | 126 | 147 | 929 |
| Neo-C2 | 5 | 4 | 1 | 3 | 3 | 6 | 300 | 240 | 60 | 180 | 180 | 1800 | 98 | 64 | 16 | 54 | 83 | 385 |
| Neo-C3 | 3 | 3 | 2 | 3 | 2 | 4 | 180 | 180 | 120 | 180 | 120 | 1200 | 58 | 48 | 36 | 62 | 48 | 303 |
| Neo-C4 | 11 | 5 | 5 | 5 | 5 | 12 | 660 | 300 | 300 | 300 | 300 | 3600 | 197 | 73 | 90 | 91 | 77 | 730 |
| Neo-C5 | 13 | 14 | 9 | 12 | 8 | 15 | 780 | 840 | 540 | 720 | 480 | 4500 | 294 | 311 | 205 | 290 | 166 | 1378 |
| Neo-C6 | 5 | 4 | 3 | 2 | 3 | 6 | 300 | 240 | 180 | 120 | 180 | 1800 | 113 | 72 | 49 | 48 | 72 | 425 |
| Average | 8.17 | 5.83 | 4.33 | 5 | 4.5 | 9.33 | 490 | 350 | 260 | 300 | 270 | 2800 | 168 | 112 | 86.3 | 112 | 98.8 | 692 |
| SEM | 1.76 | 1.66 | 1.2 | 1.48 | 0.92 | 1.86 | 106 | 99.7 | 72.1 | 89 | 55.3 | 557 | 38.1 | 40.5 | 28.4 | 37.5 | 19 | 168 |
| Neo-Hibo1 | 3 | 2 | 2 | 2 | 1 | 4 | 180 | 120 | 120 | 120 | 60 | 1200 | 61 | 26 | 43 | 38 | 29 | 286 |
| Neo-Hibo2 | 3 | 3 | 2 | 2 | 2 | 4 | 180 | 180 | 120 | 120 | 120 | 1200 | 46 | 51 | 37 | 54 | 38 | 259 |
| Neo-Hibo3 | 1 | 1 | 0 | 1 | 1 | 2 | 60 | 60 | 0 | 60 | 60 | 600 | 24 | 19 | 0 | 20 | 14 | 103 |
| Neo-Hibo4 | 3 | 4 | 3 | 4 | 4 | 6 | 180 | 240 | 180 | 300 | 240 | 1800 | 47 | 106 | 77 | 67 | 70 | 452 |
| Neo-Hibo5 | 6 | 4 | 3 | 4 | 7 | 8 | 360 | 240 | 180 | 240 | 420 | 2400 | 117 | 88 | 50 | 58 | 116 | 429 |
| Neo-Hibo6 | 2 | 1 | 1 | 1 | 1 | 3 | 120 | 60 | 60 | 60 | 60 | 900 | 39 | 24 | 14 | 26 | 18 | 148 |
| Average | 3 | 2.5 | 1.83 | 2.33 | 2.67 | 4.5 | 180 | 150 | 110 | 150 | 160 | 1350 | 55.7 | 52.3 | 36.8 | 43.8 | 47.5 | 280 |
| SEM | 0.68 | 0.56 | 0.48 | 0.56 | 0.99 | 0.89 | 41 | 33.8 | 28.6 | 40.3 | 59.3 | 266 | 13.2 | 15 | 11.1 | 7.66 | 15.9 | 58 |
Scores are total number of sessions, trials, and errors made before criterion days for learning the 5 biconditional discrimination problems. Neo-C: animals with neonatal sham-operations and Neo-H: animals with neonatal hippocampal lesions.
Experiment 5:
Results from Experiments 3 and 4 demonstrate that animals with neonatal hippocampal damage can learn contextual discrimination problems as well as, or even better than control animals. One possibility that could support such findings includes previous research (Eichenbaum & Bunsey, 1995; Pascalis, Hunkin, Bachevalier, & Mayes, 2009; Eichenbaum et al. 2007) demonstrating that the role of the hippocampus in contextual processing is to process a flexible, relational binding between object and background information such that the two types of information remain separate to be used flexibly and independently of one another. By contrast, the medial temporal cortical areas, perirhinal and parahippocampal cortex, processes object and contextual information respectively. Therefore, without a functioning hippocampus, objects and their contexts could be encoded as a single and inflexible “snapshot” by the cortical areas adjacent to the hippocampus (Eichenbaum et al. 2007; Diana et al. 2007; 2012), so that subsequently the objects and the contexts cannot be retrieved independently of one another. If this were the case, in Experiment 4 animals in Group Neo-Hibo may have learned 10 discrimination problems rather than 5 pairs on two different backgrounds. To test this possibility, the presentation of the discrimination problems in the fifth experiment was manipulated such that the same backgrounds were used for two different discrimination problems. In this manner, we investigated whether monkeys with neonatal hippocampal damage would be impaired when it was necessary to use information from the same background for two different pairs of stimuli.
Behavioral Procedures
As shown in Figure 6A, animals were presented with five pairs of stimuli. Pairs 1, 2, and 3 were identical to those used in Experiment 4, whereas Pair 4 consisted of a new pair of stimuli presented over the background used in Pair 1, and Pair 5 consisted of a new pair of stimuli presented over the background used in Pair 2. To state it differently, two pairs (1 and 4) were presented over the same backgrounds (A and B), two pairs (2 and 5) were presented over the same backgrounds (C and D), and pair 3 was presented over backgrounds E and F. Finally, because Pair 3 was presented on unique backgrounds not used with the other four pairs, it served as a control. The task was delivered in the same manner and with the same contingencies as described for Experiment 4.
Results
Number of sessions, trials, and errors to criterion for each animal of both groups are presented in Table 3 and errors to criterion for each pair are illustrated in Figure 6B. Across all five contextual discrimination problems, animals in Group Neo-Hibo required the same number of sessions and trials, and made slightly fewer errors than Group Neo-C (4.17 ± 1.2 vs 4.83 ± 0.7 sessions, 816.67 ± 207.23 vs 917 ± 203.99 trials, 169 ± 52.7 vs 227 ± 58 errors, respectively), but none of these group differences reached significance [Sessions: t(10) = .48, NS; Trials: t(10) = .34, NS; Errors: t(10) = 0.74, NS]. As illustrated in Figure 6B for the errors, neither the effect of Group nor the interaction Group × Pair reached significance [F(1,8) = .98, NS; F(4,40) = .98, NS, respectively) but the effect of Pairs was significant (Pair: F(4,40) = 26.04, p <.001). The effect of Pair likely arose because Pairs 1, 2, and 3 were already acquired in Experiment 4, whereas Pairs 4 and 5 consisted of new stimuli that the animals had not yet seen and had to learn. Indeed, both groups showed good retention of the three pairs (1, 2, and 3) they had learned in Experiment 4, requiring fewer sessions, trials and errors. However, animals in both groups treated Pairs 4 and 5 as new pairs that had to be learned. For errors to criterion, there were no significant effects of Experiment and no significant Group × Experiment interaction for Pairs 1 (Exp. 4) and 4 (Exp. 5) [F(1,10) = 2.90, NS; F(1,10) = 2.92, NS, respectively] and the same was true for Pairs 2 (Exp. 4) and 5 (Exp. 5) [F(1,10) = 4.24, NS; F(1,10) = 1.22, NS), respectively]. However, the Group effect was significant for Pairs 1-4 (F(1,10) = 9.84, p = .01), but not for Pairs 2-5 (F(1,10) = 2.35, NS). Thus, the results suggested that both groups learned the two novel discrimination problems (Pairs 4 and 5) at the same rate when presented over familiar backgrounds.
Table 3:
Experiment 5 – Sessions, Trials, Errors to criterion
| Subject | Sessions |
Trials |
Errors |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pair 1 | Pair 2 | Pair 3 | Pair 4 | Pair 5 | Total | Pair 1 | Pair 2 | Pair 3 | Pair 4 | Pair 5 | Total | Pair 1 | Pair 2 | Pair 3 | Pair 4 | Pair 5 | Total | |
| Neo-C1 | 0 | 0 | 0 | 2 | 1 | 3 | 0 | 0 | 0 | 120 | 60 | 900 | 0 | 0 | 0 | 52 | 23 | 127 |
| Neo-C2 | 1 | 0 | 0 | 4 | 2 | 5 | 60 | 0 | 0 | 240 | 120 | 1500 | 15 | 0 | 0 | 89 | 36 | 221 |
| Neo-C3 | 3 | 1 | 1 | 7 | 5 | 8 | 180 | 60 | 60 | 420 | 300 | 800 | 47 | 11 | 16 | 152 | 78 | 495 |
| Neo-C4 | 0 | 0 | 0 | 3 | 4 | 5 | 0 | 0 | 0 | 180 | 240 | 500 | 0 | 0 | 0 | 67 | 66 | 257 |
| Neo-C5 | 0 | 0 | 0 | 4 | 3 | 5 | 0 | 0 | 0 | 240 | 180 | 1500 | 0 | 0 | 0 | 73 | 64 | 137 |
| Neo-C6 | 1 | 0 | 0 | 2 | 1 | 3 | 60 | 0 | 0 | 120 | 60 | 300 | 15 | 0 | 0 | 46 | 14 | 127 |
| Average | 0.83 | 0.17 | 0.17 | 3.67 | 2.67 | 4.83 | 50 | 10 | 10 | 220 | 160 | 1450 | 12.8 | 1.83 | 2.67 | 79.8 | 46.8 | 227 |
| SEM | 0.48 | 0.17 | 0.17 | 0.76 | 0.67 | 0.75 | 28.6 | 10 | 10 | 45.6 | 40 | 225 | 7.46 | 1.83 | 2.67 | 15.7 | 10.6 | 58 |
| Neo-Hibo1 | 1 | 1 | 0 | 8 | 2 | 9 | 60 | 60 | 0 | 480 | 120 | 900 | 10 | 11 | 0 | 146 | 45 | 411 |
| Neo-Hibo2 | 0 | 0 | 0 | 1 | 1 | 2 | 0 | 0 | 0 | 60 | 60 | 600 | 0 | 0 | 0 | 17 | 22 | 77 |
| Neo-Hibo3 | 0 | 0 | 0 | 1 | 1 | 2 | 0 | 0 | 0 | 60 | 60 | 600 | 0 | 0 | 0 | 28 | 17 | 100 |
| Neo-Hibo4 | 0 | 2 | 0 | 2 | 3 | 4 | 0 | 120 | 0 | 120 | 180 | 400 | 0 | 22 | 0 | 37 | 54 | 208 |
| Neo-Hibo5 | 1 | 1 | 0 | 5 | 2 | 6 | 60 | 60 | 0 | 300 | 120 | 1800 | 13 | 10 | 0 | 85 | 40 | 148 |
| Neo-Hibo6 | 0 | 0 | 0 | 1 | 1 | 2 | 0 | 0 | 0 | 60 | 60 | 600 | 0 | 0 | 0 | 22 | 18 | 71 |
| Average | 0.33 | 0.67 | 0 | 3 | 1.67 | 4.17 | 20 | 40 | 0 | 180 | 100 | 1250 | 3.83 | 7.17 | 0 | 55.8 | 32.7 | 169 |
| SEM | 0.21 | 0.33 | 0 | 1.18 | 0.33 | 1.17 | 12.6 | 20 | 0 | 71 | 20 | 350 | 2.46 | 3.64 | 0 | 20.6 | 6.42 | 52.7 |
Scores are total number of sessions, trials, and errors made before criterion days for learning the 5 biconditional discrimination problems. Neo-C: animals with neonatal sham-operations and Neo-H: animals with neonatal hippocampal lesions.
Experiment 6:
Results from experiments presented thus far can be explained in a few ways. First, it is possible that these newly designed contextual tasks may be solved by strategies that are not mediated by the hippocampus. In addition, it is possible that the lack of impairment in contextual learning after the neonatal hippocampal lesions may be due to the timing of the hippocampal lesions. The early lesions may have led to sparing of function resulting from functional brain plasticity after early insult to the hippocampus. Although there exist no data on the effects of adult-onset hippocampal lesions on the tasks used in the present studies, we had already demonstrated that adult-onset hippocampal lesions impaired contextual learning when assessed with a visual-paired-comparison task (Context-VPC; Bachevalier et al. 2015). In this task, animals are first familiarized with a stimulus presented over a background (A), and, after a short delay, they are presented with the familiar stimulus and a novel one, both presented over a new background (B) (see Figure 6A-Contex trials). Longer look to the novel objects is used as a measure of recognition memory. As compared to controls, animals with adult-onset hippocampal lesions showed no novelty preference, indicating that they did not recognize the familiar object now presented over a new background (Bachevalier et al. 2015). Thus, to test whether early-onset hippocampal lesions will likewise impaired performance on this incidental contextual task, animals in both groups were then tested on the Context-VPC task.
Behavioral Procedures
Procedures were modeled after Bachevalier and colleagues (Bachevalier et al. 2015) so that results could be compared across studies. Therefore, each trial began with a familiarization phase during which an image was presented on the screen until the monkey accumulated 30 seconds of looking time. Following a 5-sec delay the first test phase ensued, during which two images were presented side-by-side for a total of five seconds. Following another five-second delay, the second test phase ensued, during which the same two images were presented side-by-side for a total of five seconds with their left and right positions on the screen reversed. The short delay of 5 seconds was chosen in order to model the procedures as closely as possible to those described previously (Bachevalier et al. 2015). Additionally, previous research suggests that monkeys with neonatal hippocampal damage from the present study had impaired recognition memory at long delays (Zeamer et al. 2010), but not at short delays. This testing paradigm takes advantage of the monkey’s innate preference for novelty and does not require the learning of a rule. Therefore, the monkey’s eye movements were analyzed frame-by-frame to acquire the percent of time the monkey looked at the novel object on each trial.
Monkeys received a total of 20 trials distributed equally between two types of trials, Control trials and Context trials. For both types of trials, the familiarization phase was similar and consisted of an object presented over a background (see Fig. 7A). In Control Trials, the test phases consisted of the familiar object and a novel object presented over the same background as shown in the familiarization phase. In the Context Trials, the test phase consisted of the familiar object and a novel object presented over a background different from the background used in the familiarization phase. New objects and new backgrounds were used for each trial.
Figure 7:
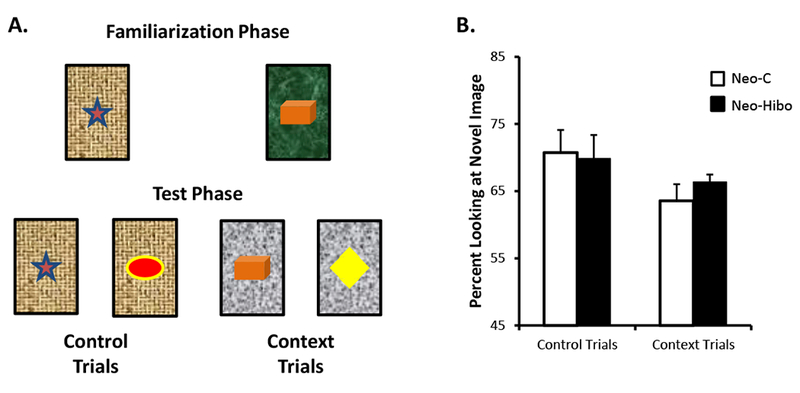
A- Illustration of trial types in the Context-VPC task. For Control trials, in the test phase, the familiar and novel objects were placed on the same background as in the familiarization phase. For Context trials, in the test phase, the familiar and novel objects were placed on a background different than the background in the familiarization phase. B- Mean percent looking (±SEM) to the novel objects in the test phases for both types of trials. Black bars illustrate performance of animals with neonatal hippocampal lesions and white bars illustrate performance of control animals.
Results
Mean percent of time looking at the novel objects for both types of trials and for each group is illustrated in Figure 7B. Animals in both groups demonstrated similar and robust looking preferences for the novel objects on both trial types as revealed by no group effect [F(1, 10) = 0.09, NS] and no interaction [F(1, 10) = 0.59, NS]. Also, both groups looked longer at the novel objects in the Control trials than in the Context Trials as shown by a significant effect of Trial types [F(1, 10) = 4.97, p = .05]. For both groups and both types of trials, preference looking at novel objects was statistically above chance (Group Neo-C: Control Trials: t = 6.17, p = .002, Context Trials: t = 5.50, p = .003; Group Neo-Hibo: Control Trials: t = 5.65, p = .002, Context Trials: t = 14.83, p < .001).
Lesion Correlations
One factor that could be contributing to the lack of impairment seen in the contextual discrimination tasks of Experiments 3, 4, and 5 as well as in Context-VPC task (Experiment 6) relates to the extent of the lesions that each animal in Group Neo-Hibo sustained. It is possible that the unilaterality of the lesions in some animals allowed for normal performance on all contextual tasks described above. To investigate this possibility, we examined whether there were any significant correlations between the unilaterality of the lesions and any performance measures, and found that there were no significant correlations between the extents, location (left vs. right) or symmetry (unilateral or bilateral, i.e. W index) of extent of cell loss for any behavioral measure in any of the experiments (all p values > .05).
DISCUSSION
This series of experiments revealed several significant findings. Selective neonatal hippocampal lesions did not impair the ability of monkeys to learn 60 pair concurrent discrimination problems when the stimuli were presented alone, with black backgrounds (Exp. 1). In addition, like controls, monkeys with Neo-Hibo lesions made fewer errors in acquiring discrimination problems when the background remained the “same” across sessions as opposed to when it “differed” (Exp. 2), even when the context was irrelevant for accurate task performance. Importantly, selective neonatal hippocampal lesions spared monkeys’ ability to learn biconditional discriminations (Exp. 3) and even facilitated the learning of a new set of biconditional discriminations (Exp. 4). Finally, neonatal hippocampal lesions did not affect performance when discriminations were made more ambiguous by using identical backgrounds on different pairs of objects (Exp. 5) or on an incidental recognition memory paradigm requiring the accurate use of context (Exp. 6).
Concurrent discrimination learning
Monkeys with Neo-Hibo lesions were able to learn 60 discrimination problems as rapidly as controls when testing was given via an automated testing apparatus. This normal performance was expected, as previous work in our laboratory has demonstrated intact concurrent discrimination learning in these same animals when tested manually in a Wisconsin General Testing Apparatus (Glavis-Bloom et al. 2008). Additionally, both monkeys with Neo-Hibo lesions and controls performed similarly on trials in which an irrelevant context was added to the discrimination problems, whether or not the context remained the same or changed on subsequent presentations (Exp. 2). In this later experiment, both groups made more errors to attain criterion on discrimination problems presented with an irrelevant background than on discrimination problems presented on a uniform black background. Thus, the presence of the background, even though irrelevant for good task performance, may have served as a distractor, reducing the speed at which animals of both groups reached criterion. These findings suggest that neither Group ignored the background entirely, and indeed are consistent with studies in humans suggesting that there is automatic and obligatory binding of objects and context thought to be supported by the parahippocampal cortex (Hayes et al. 2007; Diana et al. 2012; Memel & Ryan, 2017; 2018). Thus, the findings contrast with those of a previous study showing that monkeys with hippocampal damage did not utilize background contextual cues to enhance performance on a discrimination task (Dore et al. 1998). However, the different outcomes between the two studies may have resulted from the timing of the hippocampal lesions, which occurred early after birth in the present study but in adulthood in the Dore and colleagues study (see below).
Effects of contextual information on biconditional discrimination learning
In Exp. 3 and 4, monkeys were required to utilize background context information to successfully solve biconditional discrimination problems. Monkeys with selective Neo-Hibo lesions solved the task as rapidly as controls. Interestingly, not only were animals with neonatal hippocampal lesions not impaired on biconditional discrimination learning (Exp. 3), but they performed even better than controls when acquiring a second set of novel biconditional discriminations (Exp. 4). One possible explanation is that the two groups may have used different strategies to solve the biconditional task. Control animals may have used information from the background to solve the task encoding a flexible, relational binding between object and background information (Eichenbaum & Bunsey, 1995; Pascalis, Hunkin, Bachevalier, & Mayes, 2009). By contrast, animals with Neo-Hibo lesions may have used an inflexible habit strategy (i.e., encoding fused background-object information for each of the eight discriminations). This habit learning strategy could have facilitated their performance on the new discrimination problems in Exp. 4 as it did when the same animals were tested in a concurrent discrimination task and discrimination reversal task (Glavis-Bloom et al. 2008). Such facilitation in habit learning tasks had already been reported following lesions of the hippocampal system in several species (Eichenbaum et al. 1986; Eichenbaum & Bunsey 1995; Mahut 1972; Schram 1970; Shaw & Aggleton 1993; Staubli et al. 1984; Zola & Mahut 1973). One theory that has been put forth to explain this phenomenon relates to the competitive interactions between the hippocampal dependent and striatal-dependent memory systems (Kapur et al. 1986), such that rendering one system dysfunctional may facilitate the other (Mishkin & Petri 1984).
In order to test whether animals with Neo-Hibo lesions were using a habit-learning strategy when acquiring the biconditional discrimination task, the animals of both groups were trained on a new condition of the task in which they had to learn new pairs of stimuli when presented over backgrounds used in previous problems they had learned. Thus, in Exp. 5, the trials included three biconditional discrimination problems (Trials 1-3) that animals had learned in Exp. 4 as well as two other trials (Trials 4 and 5) in which two new pairs of objects were presented on the same backgrounds already used in Trials 1 and 2 of Exp. 3. We predicted that, in the absence of a functional hippocampus, Neo-Hibo animals may have greater difficulty in learning Trials 4 and 5 than controls if they were relying only on the encoding of an inflexible fused stimulus-background representation. Despite this task manipulation, animals with Neo-Hibo lesions persisted in learning these new discriminations as rapidly as control animals and seemed to treat the new object pairs presented over previously acquired backgrounds as though they were completely new discrimination problems. Thus, to learn normally these new discrimination problems, Neo-Hibo animals had to use either a strategy similar to that used by the controls or, as discussed above, they still could have continued using a “habit” strategy to solve the task.
To assess whether animals with Neo-Hibo lesions were using a habit learning strategy to solve the biconditional discrimination task, in Exp. 6 we trained the animals in an incidental contextual visual paired comparison (VPC) task that did not involve learning discrimination problems since each trial used new backgrounds and new objects. Thus, animals could not encode a unique fused background-object representation to perform well on the task, but had to treat and encode the object and the background separately as two different representations and flexibly use these representations. Still, animals with Neo-Hibo lesions performed as well as controls and both groups performed significantly above chance on both Control and Context Trials. Thus, like controls, monkeys with Neo-Hibo lesions were able to recognize a previously seen object, even if the object was now presented on a new background context in the Context-VPC task. Therefore, it is possible that monkeys with Neo-Hibo lesions were able to encode separate representations of the objects and the backgrounds to solve the discrimination problems in Exp. 3, 4, and 5, as did the control monkeys.
Nevertheless, the normal performance of monkeys with Neo-Hibo lesions could be attributed to the sparing of hippocampal tissue observed in all cases even though correlations between extent of hippocampal lesions and task performance did not reach statistical significance. This explanation seems unlikely given that the same animals were impaired in several memory tasks over the course of their testing history. First, like monkeys with adult-onset hippocampal lesions, the Neo-Hibo monkeys demonstrated a delay-dependent incidental object recognition memory impairment that began at 18 months of age (Zeamer et al. 2010) and persisted into adulthood (Zeamer & Bachevalier, 2013). In addition, the same monkeys also had impaired spatial relational memory (Blue et al. 2013) and impaired memory for specific food/place associations (Glavis-Bloom et al. 2013).
Functional compensation after neonatal hippocampal lesions
The lack of impairment in the biconditional discrimination tasks as well as the Context-VPC task after the Neo-Hibo lesions contrasts with the severe impairment previously reported in monkeys with lesions of the hippocampus performed in infancy or in adulthood. For example, monkeys with neonatal hippocampal lesions were impaired in a conditional object-object association task (Killiany et al. 2005; Rehbein et al. 2005). Two important procedural differences could have resulted in the different outcomes of the neonatal hippocampal lesions. One is the difference in the learning tasks used in the two studies; a conditional object-object association task for the animals in the Killiany and colleagues studies as compared to the biconditional background-object discrimination in the current one. Second, the difference may have resulted from the more extended lesions in the Killiany and colleagues studies, which included not only the hippocampus almost entirely but also a large portion of medial temporal cortical areas (parahippocampal areas TH/TF, visual area TE, perirhinal cortex) as well as the amygdala, as compared to the damage limited to the hippocampus in the present study. Moreover, the spared ability to solve conditional discrimination problems in the present study contrasts with the severe impairment after adult-onset lesions in marmoset reported by Ridley and colleagues (Ridley et al. 1995, 2001). These different outcomes may relate to the use of different species, different timing of the lesions and/or different discrimination tasks.
Although the effects of Neo-Hibo lesions on biconditional discriminations could not be directly compared with the effects of adult-onset hippocampal lesions, the last experiment of the current study allowed for more direct comparisons between the effects of early-onset versus adult-onset restricted hippocampal lesions on contextual tasks using the same species and same task (macaque monkeys and Context-VPC task). The adult-onset hippocampal lesions resulted in a severe impairment in recognizing a familiar object when presented over a new background (Bachevalier et al. 2015), but the Neo-Hibo lesions did not. Thus, the presence of normal ability of monkeys with Neo-Hibo lesions in performing contextual tasks despite their significant impairment in object recognition memory (Zeamer et al. 2010; Zeamer & Bachevalier, 2013), spatial relational memory (Blue et al. 2013) and memory for specific food/place associations (Glavis-Bloom et al. 2013) suggest that the neonatal lesions may have allowed for functional compensation from other neural structures within the neural network supporting contextual learning and memory. Putative candidates include the parahippocampal cortex (TH/TF), which plays an active role in processing contextual information (Arias et al. 2015; Burgess et al. 2001; Diana et al. 2010; Preston & Gabrieli 2008; Gronau et al. 2008; Hayes et al. 2010; Nemanic et al. 2004; Rauch et al. 1997), the retrosplenial cortex, which is involved in the processing of scenes and retrieval of context memory (Kwapis et al. 2015) and may work alongside with TH/TF to process object and context associations (Bar & Aminoff 2003), the perirhinal cortex, which supports both object and contextual memory (Bachevalier et al. 2015; Baxter & Murray 2001; Buckley & Gaffan 1998; Buffalo et al. 1999, 2006; Davachi 2006; Eacott et al. 1994; Gaffan 1994a, b, 1993; Goulet & Murray 2001; Howse et al. 2003; Meunier et al. 1993; Higuchi & Miyashita 1996; Mumby & Pinel 1994; Murray et al. 1993; Murray & Richmond 2001; Tokuyama et al. 2000; Preston & Gabrieli 2008; Staresina & Davachi 2008; Wan et al. 1999; Goh et al 2004), and the prefrontal cortex (Bussey et al., 2001, 2002; Eacott & Gaffan, 1992; Halsband & Passingham, 1982; Lee & Lee, 2013a, b; Mitz et al. 1991; Parker & Gaffan, 1998a, b; Petrides, 1982; Xiang & Brown 2007; Zelikowsky et al. 2013). Thus, it is likely that the sparing of biconditional discrimination learning is the result of the early onset of the hippocampal lesions that allowed for significant functional compensation by other brain structures.
CONCLUSION
In summary, the current data show a significant sparing of non-spatial contextual learning and memory function following early selective lesions to the hippocampus in monkeys. The sparing of function is likely due to both the early nature of the damage and the selectivity of the lesion, which leaves the medial temporal lobe cortical areas relatively intact. Therefore, removing the hippocampus early in life before it has begun to fully function may have facilitated compensatory mechanisms in other brain regions.
Acknowledgments
This work was supported by the National Institute of Mental Health (MH-58846), and the National Center for Research ResourcesP51RR165, currently supported by the Office of Research Infrastructure Programs/ODP51OD11132. We thank the veterinary and animal husbandry staff at the Yerkes National Primate Research Center for expert animal care, the imaging core facility for the care and handling of animals during the MR imaging procedures, and Anthony Gazy and Shala Blue for assistance with the testing of the animals and scoring the videotapes. We also express our gratitude to Patricia Bauer, Elizabeth Buffalo, Robert Hampton, Stuart Zola, and Maria Alvarado for feedback and comments through the execution of this series of experiments, Alyson Weiss for help with the statistical analyses, and Noa Shapiro-Franklin for estimation of the hippocampal cell loss.
Grant sponsor: NIMH, NCRR
Grant number: MH-58846, RR165
References
- Arias N, Mendez M, & Arias J (2015). The recognition of a novel-object in a novel context leads to hippocampal and parahippocampal c-Fos involvement. Behavioral Brain Research, 292,44–9. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Nemanic S, & Alvarado MC (2015). The influence of context on recognition memory in monkeys: Effects of hippocampal, parahippocampal and perirhinal lesions. Behavioral Brain Research, 285, 89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar M, & Aminoff E (2003). Cortical analysis of visual context. Neuron, 38(2), 347–358. [DOI] [PubMed] [Google Scholar]
- Baxter MG, & Murray EA (2001). Opposite relationship of hippocampal and rhinal cortex damage to delayed nonmatching-to-sample deficits in monkeys. Hippocampus, 11(1), 61–71. [DOI] [PubMed] [Google Scholar]
- Blue SN, Kazama AM, & Bachevalier J (2013). Development of memory for spatial locations and object/place associations in infant rhesus macaques with and without neonatal hippocampal lesions. Journal of the International Neuropsychology Society, 19(10), 1053–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley MJ, & Gaffan D (1998). Perirhinal cortex ablation impairs visual object identification. Journal of Neuroscience, 18(6), 2268–2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffalo EA, Bellgowan PS, & Martin A (2006). Distinct roles for medial temporal lobe structures in memory for objects and their locations. Learning & Memory, 13(5), 638–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffalo EA, Ramus SJ, Clark RE,Teng E, Squire LR & Zola SM (1999). Dissociation between the effects of damage to perirhinal cortex and area TE. Learning & Memory, 6(6), 572–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess N, Maguire EA, Spiers HJ,O’Keefe J (2001). A temporoparietal and prefrontal network for retrieving the spatial context of lifelike events. NeuroImage, 14(2,: 439–453. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Wise SP, & Murray EA (2001). The role of ventral and orbital prefrontal cortex in conditional visuomotor learning and strategy use in rhesus monkeys (Macaca mulatta). Behavioral Neuroscience, 115, 971–982. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Wise SP, Murray EA (2002). Interaction of ventral and orbital prefrontal cortex with inferotemporal cortex in conditional visuomotor learning. Behavioral Neuroscience, 116, 703–715. [PubMed] [Google Scholar]
- Chalfonte BL, & Johnson MK (1996). Feature memory and binding in young and older adults. Memory and Cognition, 24(4, 403–416. [DOI] [PubMed] [Google Scholar]
- Davachi L (2006). Item, context and relational episodic encoding in humans. Current Opinion in Neurobiology, 16(6), 693–700. [DOI] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, & Ranganath C (2007). Imaging recollection and familiarity in the medial temporal lobe: a three-component model. Trends in Cognitive Science, 11(9), 379–386. [DOI] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, & Ranganath C (2010). Medial temporal lobe activity during source retrieval reflects information type, not memory strength. Joournal of Cognitive Neuroscience, 22(8), 1808–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, & Ranganath C (2012). Adaptation to cognitive context and item information in the medial temporal lobes. Neuropsychologia, 50(13), 3062–3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dore FY, Thornton JA, White NM, & Murray EA (1998). Selective hippocampal lesions yield nonspatial memory impairments in rhesus monkeys. Hippocampus, 8(4), 323–329. [DOI] [PubMed] [Google Scholar]
- Eacott MJ, & Gaffan D (1992) Inferotemporal-frontal disconnection: the uncinate fascicle and visual associative learning in monkeys. European Journal of Neuroscience, 4,1320–1332. [DOI] [PubMed] [Google Scholar]
- Eacott MJ, Gaffan D, & Murray EA (1994). Preserved recognition memory for small sets, and impaired stimulus identification for large sets, following rhinal cortex ablations in monkeys. European Journal of Neuroscience, 6(9), 1466–1478. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, & Bunsey B (1995). On the binding of associations in memory: clues from studies on the role of the hippocampal region in paired-associate learning. Current Directions in Psychological Science, 4, 19–23. [Google Scholar]
- Eichenbaum H, Fagan A, & Cohen NJ (1986). Normal olfactory discrimination learning set and facilitation of reversal learning after medial-temporal damage in rats: implications for an account of preserved learning abilities in amnesia. Journal of Neuroscience, 6(7), 1876–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AR, & Ranganath C (2007). The medial temporal lobe and recognition memory. Annual Reviews Neuroscience, 30, 123–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman JH Jr., Weible A , Rossi J, & Gabriel M (1997). Lesions of the entorhinal cortex disrupt behavioral and neuronal responses to context change during extinction of discriminative avoidance behavior. Experimental Brain Research, 115(3), 445–457. [DOI] [PubMed] [Google Scholar]
- Gaffan D (1993). Normal forgetting, impaired acquisition in memory for complex naturalistic scenes by fornix-transected monkeys. Neuropsychologia, 31(4), 403–406. [DOI] [PubMed] [Google Scholar]
- Gaffan D (1994a). Dissociated effects of perirhinal cortex ablation, fornix transection and amygdalectomy: evidence for multiple memory systems in the primate temporal lobe. Experimental Brain Research, 99(3), 411–422. [DOI] [PubMed] [Google Scholar]
- Gaffan D (1994b). Scene-specific memory for objects: a model of episodic memory impairment in monkeys with fornix transection. Journal of Cognitive Neuroscience, 6(4,), 305–320. [DOI] [PubMed] [Google Scholar]
- Glavis-Bloom C, Alvarado MC, Bachevalier J (2013). Neonatal hippocampal damage impairs specific food/place associations in adult macaques. Behavioral Neuroscience, 127(1), 9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glavis-Bloom C, Blue SN, & Bachevalier J (2010). Neonatal hippocampal damage spares contextual discrimination learning in adult macaques Society for Neuroscience Abstracts. San Diego, CA. [Google Scholar]
- Glavis-Bloom C, Kazama AM,& Bachevalier J (2008). Paradoxical facilitation of stimulus-reward association learning in rhesus macaques with neonatal hippocampal lesions Society for Neuroscience Abstracts. Washington D.C. [Google Scholar]
- Goh JO, Siong SC, Park D, Gutchess A, Hebrank A, & Chee MW (2004). Cortical areas involved in object, background, and object-background processing revealed with functional magnetic resonance adaptation. Journal of Neuroscience, 24(45), 10223–10228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulet S, & Murray EA (2001). Neural substrates of crossmodal association memory in monkeys: the amygdala versus the anterior rhinal cortex. Behavioral Neuroscience, 115(2), 271–284. [PubMed] [Google Scholar]
- Goursaud AP, & Bachevalier J (2007). Social attachment in juvenile monkeys with neonatal lesion of the hippocampus, amygdala and orbital frontal cortex. Behavioral Brain Research, 176(1), 75–93. [DOI] [PubMed] [Google Scholar]
- Gronau N, Neta M, & Bar M (2008). Integrated contextual representation for objects’ identities and their locations. Journal of Cognitive Neuroscience, 20(3), 371–388. [DOI] [PubMed] [Google Scholar]
- Halsband U, & Passingham RE (1982). The role of premotor and parietal cortex in the direction of action. Brain Research, 240, 368–372. [DOI] [PubMed] [Google Scholar]
- Hayes SM, Baena E, Truong TK, Cabeza R (2010). Neural mechanisms of context effects on face recognition: automatic binding and context shift decrements. Journal of Cognitive Neuroscience, 22(11), 2541–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes SM, Nadel L & Ryan L (2007). The effect of scene context on episodic object recognition: Parahippocampal cortex mediates memory encoding and retrieval success. Hippocampus, 17, 873–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi S, & Miyashita Y (1996). Formation of mnemonic neuronal responses to visual paired associates in inferotemporal cortex is impaired by perirhinal and entorhinal lesions. Proceedings of the National Academy of Science U S A, 93(2), 739–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodos W, & Bobko P (1984). A weighted index of bilateral brain lesions. Journal of Neuroscience Methods, 12, 43–47. [DOI] [PubMed] [Google Scholar]
- Howse DJ, Squires AS, Martin GM, & Skinner DM (2003). Perirhinal cortex lesions impair context aversion learning. Learning & Memory, 10(3), 161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur N, Heath P, Meudell P, Kennedy P (1986). Amnesia can facilitate memory performance: evidence from a patient with dissociated retrograde amnesia. Neuropsychologia, 24(2), 215–221. [DOI] [PubMed] [Google Scholar]
- Kennedy PJ, & Shapiro ML (2004). Retrieving memories via internal context requires the hippocampus. Journal of Neuroscience, 24(31), 6979–6985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killiany R, Rehbein L, & Mahut H (2005). Developmental study of the hippocampal formation in rhesus monkeys (Macaca mulatta): II. Early ablations do not spare the capacity to retrieve conditional object-object associations. Behavioral Neuroscience, 119(3), 651–661. [DOI] [PubMed] [Google Scholar]
- Kim JJ, & Fanselow MS (1992). Modality-specific retrograde amnesia of fear. Science, 256(5057), 675–677. [DOI] [PubMed] [Google Scholar]
- Kim S, Lee J, Lee I (2012). The hippocampus is required for visually cued contextual response selection, but not for visual discrimination of contexts, Frontiers in Behavioral Neuroscience, 6, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwapis JL, Jarome TJ, Lee JL, & Helmstetter FJ (2015). The retrosplenial cortex is involved in the formation of memory for context and trace fear conditioning. Neurobiology of Learning and Memory, 123,110–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I, & Lee CH (2013a). Putting an objcet in context and acting on it: neural mechanisms of goal-directed response to contextual object. Review Neuroscience, 24(1), 27–49. [DOI] [PubMed] [Google Scholar]
- Lee I, Lee SH (2013b). Contextual behavior and neural circuits. Frontiers in Neural Circuits 7:article 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahut H (1972). A selective spatial deficit in monkeys after transection of the fornix. Neuropsychologia, 10(1), 65–74. [DOI] [PubMed] [Google Scholar]
- Mayes AR, MacDonald C, Donlan L, Pears J, & Meudell PR (1992). Amnesics have a disproportionately severe memory deficit for interactive context. Quarterly Journal of Experimental Psychology: A, 45(2), 265–297. [DOI] [PubMed] [Google Scholar]
- Memel M, & Ryan L (2017). Visual integration enhances associative memory equally for young and older adults without reducing hippocampal encoding activation. Neuropsychologia, 100, 195–206. [DOI] [PubMed] [Google Scholar]
- Memel M, & Ryan L (2018). Visual integration of objects and scenes increases recollection-based responding despite differential MTL recruitment in young and older adults. Hippocampus, Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Meunier M, Bachevalier J, Mishkin M, Murray EA (1993). Effects on visual recognition of combined and separate ablations of the entorhinal and perirhinal cortex in rhesus monkeys. Journal of Neuroscience, 13(12), 5418–5432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishkin M, & Petri HL (1984). Memories and habits: Some implications for the analysis of learning and retention In Squire LR & Butters N (Eds). Neuropsychology of Memory (pp. 287–296). New York, NY: Guilford Press. [Google Scholar]
- Mitchell KJ, Johnson MK, Raye CL, Mather M, & D’Esposito M (2000). Aging and reflective processes of working memory: binding and test load deficits. Psychology of Aging, 15(3), 527–541. [DOI] [PubMed] [Google Scholar]
- Mitz AR, Godchalk M, & Wise SP(1991). Learning-dependent neuronal activity in the premotorcortex: activity during the acquisition of conditional motor associations. Journal of Neuroscience, 11,1855–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumby DG, & Pinel JP (1994). Rhinal cortex lesions and object recognition in rats. Behavioral Neuroscience, 108(1), 11–18. [DOI] [PubMed] [Google Scholar]
- Murray EA, Gaffan D, & Mishkin M (1993). Neural substrates of visual stimulus-stimulus association in rhesus monkeys. Journal of Neuroscience, 13(10), 4549–4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EA, & Richmond BJ (2001). Role of perirhinal cortex in object perception, memory, and associations. Current Opinion in Neurobiology, 11(2), 188–193. [DOI] [PubMed] [Google Scholar]
- Nemanic S, Alvarado MC, Bachevalier J (2004). The hippocampal/parahippocampal regions and recognition memory: insights from visual paired comparison versus object-delayed nonmatching in monkeys. Journal of Neuroscience, 24(8), 2013–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker A, & Gaffan D (1998a). Lesions of the primate rhinal cortex cause deficits in flavour-visual associative memory. Behavioral Brain Research, 93(1-2), 99–105. [DOI] [PubMed] [Google Scholar]
- Parker A, & Gaffan D (1998b). Memory after frontal/temporal disconnection in monkeys: conditional and non-conditional tasks, unilateral and bilateral frontal lesions. Neuropsychologia, 36,259–271. [DOI] [PubMed] [Google Scholar]
- Pascalis O, Hunkin NM, Bachevalier J, & Mayes AR (2009). Change in background context disrupts performance on visual paired comparison following hippocampal damage. Neuropsychologia, 47(10), 2107–2113. [DOI] [PubMed] [Google Scholar]
- Penick S, & Solomon PR (1991). Hippocampus, context, and conditioning. Behavioral Neuroscience, 105(5), 611–617. [DOI] [PubMed] [Google Scholar]
- Petrides M (1982). Motor conditional associative learning after selective prefrontal lesions in man. Behavioral Brain Research, 5, 407–413. [DOI] [PubMed] [Google Scholar]
- Phillips RG, & LeDoux JE (1992). Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behavioral Neuroscience, 106(2), 274–285. [DOI] [PubMed] [Google Scholar]
- Preston AR, & Gabrieli JD (2008). Dissociation between explicit memory and configural memory in the human medial temporal lobe. Cerebral Cortex, 18(9), 2192–2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SL, Savage CR, Alpert NM, Dougherty D, Kendrick A, Curran T, Brown HD, Manzo P, Fischman AJ, & Jenike MA (1997). Probing striatal function in obsessive-compulsive disorder: a PET study of implicit sequence learning. Journal of Neuropsychiatry and Clinical Neuroscience, 9(4), 568–573. [DOI] [PubMed] [Google Scholar]
- Rehbein L, Killiany R, & Mahut H (2005). Developmental study of the hippocampal formation in rhesus monkeys (Macaca mulatta): I. Early ablations spare discrimination learning but not recognition memory. Behavioral Neuroscience, 119(3), 635–650. [DOI] [PubMed] [Google Scholar]
- Ridley RM, Hardy A, Maclean CJ, & Baker HF (2001). Non-spatial acquisition and retention deficits following small excitotoxic lesions within the hippocampus in monkeys. Neuroscience, 107(2), 239–248. [DOI] [PubMed] [Google Scholar]
- Ridley RM, Timothy CJ, Maclean CJ, & Baker HF (1995). Conditional learning and memory impairments following neurotoxic lesion of the CA1 field of the hippocampus. Neuroscience, 67(2), 263–275. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Barrientos RM, & O’Reilly RC (2002). Hippocampal formation supports conditioning to memory of a context. Behavioral Neuroscience, 116(4), 530–538. [DOI] [PubMed] [Google Scholar]
- Sackett GP, Ruppenthal GC, Davis AE (2002). Survival, growth, health, and reproduction following nursery rearing compared with mother rearing in pigtailed monkeys (Macaca nemestrina). American Journal of Primatology, 56(3), 165–183. [DOI] [PubMed] [Google Scholar]
- Schram DD (1970). The effect of hippocampal and fornix lesions on the acquisition, transfer, and reversal of a visual discrimination. Ph.D., University of Washington. [Google Scholar]
- Shaw C, & Aggleton JP (1993). The effects of fornix and medial prefrontal lesions on delayed non-matching-to-sample by rats. Behavioral Brain Research, 54(1), 91–102. [DOI] [PubMed] [Google Scholar]
- Sill OC, & Smith DM, (2012). A comparison of the effects of temporary hippocampal lesions on single and dual context versions of the olfactory sequence memory task. Behavioral Neuroscience, 126(4), 588–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DM, & Mizumori SJ (2006). Learning-related development of context-specific neuronal responses to places and events: the hippocampal role in context processing. Journal of Neuroscience, 26(12), 3154–3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiers HJ, Burgess N, Hartley T, Vargha-Khadem F, & O’Keefe J (2001). Bilateral hippocampal pathology impairs topographical and episodic memory but not visual pattern matching. Hippocampus, 11(6), 715–725. [DOI] [PubMed] [Google Scholar]
- Staresina BP, & Davachi L (2008). Selective and shared contributions of the hippocampus and perirhinal cortex to episodic item and associative encoding. Journal of Cognitive Neuroscience, 20(8), 1478–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staubli U, Ivy G, & Lynch G (1984). Hippocampal denervation causes rapid forgetting of olfactory information in rats. Proceedings of the National Academy of Science U S A, 81(18), 5885–5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuyama W, Okuno H, Hashimoto T, Xin Li Y, & Miyashita Y (2000). BDNF upregulation during declarative memory formation in monkey inferior temporal cortex. Nature Neuroscience, 3, 1134–1142. http://dx.doi.org/10.1038/80655 [DOI] [PubMed] [Google Scholar]
- van Praag H, Qu PM, Elliott RC, Wu H, Dreyfus CF, & Black IB (1998). Unilateral hippocampal lesions in newborn and adult rats: effects on spatial memory and BDNF gene expression. Behavioral Brain Research 92(1), 21–30. [DOI] [PubMed] [Google Scholar]
- von Bonin G & Bailey P, The Neocortex of Macaca Mulatta, University of Illinois Press, Urbana, IL, 1947. [Google Scholar]
- Wan H, Aggleton JP, & Brown MW (1999). Different contributions of the hippocampus and perirhinal cortex to recognition memory. Journal of Neuroscience, 19(3), 1142–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang JZ, & Brown MW (2007). Comparison of differential neuronal responsiveness for different regions of the prefrontal cortex in a conditional visual discrimination task. European Journal of Neuroscience, 25,2916–2926. [DOI] [PubMed] [Google Scholar]
- Zeamer A, & Bachevalier J (2013). Long-term effects of neonatal hippocampal lesions on novelty preference in monkeys. Hippocampus, 23(9), 745–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeamer A, Heuer E, & Bachevalier J (2010). Developmental trajectory of object recognition memory in infant rhesus macaques with and without neonatal hippocampal lesions. Journal of Neuroscience, 30(27), 9157–9165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelikowsky M, Bissierec S, Hasta TA, Bennetta RZ, Abdipranotod A, Visseld B, & Fanselow MS (2013). Prefrontal microcircuit underlies contextual learning after hippocampal loss. Proceedings of the National Academy of Science, 110, 9938–9943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zola SM, & Mahut H (1973). Paradoxical facilitation of object reversal learning after transection of the fornix in monkeys. Neuropsychologia, 11(3), 271–284. [DOI] [PubMed] [Google Scholar]


