Abstract
A wealth of evidence has led to the conclusion that virtually all cases of cervical cancer are attributable to persistent infection by a sub-set of HPV types, especially HPV16 and HPV18. These HPVs also cause a proportion of other cancers, including vulvar, vaginal, anal, penile, and oropharyngeal cancers. Although cervical cancer screening, primarily via the Pap smear, has reduced the incidence of this cancer in industrialized countries, cervical cancer remains the second most common cause of death from cancer in women worldwide, as the developing world has lacked the resources for widespread high quality screening. In addition to advances in Pap smear technology, identification of HPV as the etiologic agent has produced two recent advances that may have a major impact on approaches to reduce the incidence of this disease. The first is development of a preventive vaccine whose current versions appear to prevent close to 100% of persistent genital infection and disease caused by HPV16 and HPV18; future second generation vaccines may be able to protect against oncogenic infections by a broader array of HPV types. The second is the incorporation of HPV testing into screening programs. In women over 30, HPV testing can identify high grade dysplasias earlier than Pap smears, with acceptable rates of specificity. These results, together with the high sensitivity of HPV testing, imply that such testing could permit increased intervals for screening. An inexpensive HPV test in development might, if successful, be incorporated as part of an economically viable “screen-and-treat” approach in the developing world. The manner in which vaccination and screening programs are integrated will need to be carefully considered, so they can efficiently reduce the overall incidence of cervical cancer.
Introduction
The past six decades have witnessed a dramatic change in the approach to cervical cancer and the understanding of its pathogenesis. The emphasis has switched to prevention of invasive cancer before it occurs. The introduction of the Pap smear in the 1940s and its validation permitted the identification of easily-treated cervical intraepithelial neoplasia (CIN) and early cervical cancer, initiating a transition towards progressively greater emphasis being given to the prevention of this cancer. Starting in the 1950’s, widespread Pap smear implementation led to reductions in the incidence of cervical cancer in communities with the resources for high quality screening programs. However, the incidence of this cancer has remained high in the developing world, which lacks the resources for widespread screening programs as practiced in nations with high resources. A second achievement was the recognition, from observations initiated in the 1970s, which reached their fruition in the 1980s and early 1990s, that human papillomaviruses (HPV) were etiologically linked to cervical cancer. This major advance in our understanding of the etiology and pathogenesis of cervical cancer also led to two important clinical advances: a preventive HPV vaccine for the primary prevention of cervical cancer and HPV assays to improve secondary prevention (screening programs). The availability of these etiology-based interventions for the primary and secondary prevention of cervical cancer provides an opportunity for even greater and more efficient reductions in the incidence of this cancer in settings with established secondary screening programs and may offer the possibility of bringing cost-effective cervical cancer prevention strategies to the developing world. There is an urgent need for such interventions, as cervical cancer remains the second most common cause of death from cancer among women worldwide, accounting for more than 250,000 deaths each year.1
This review is divided into three parts. It first summarizes the current understanding of cervical cancer pathogenesis, then considers the use of vaccination and other approaches aimed at the primary prevention of cervical cancer, and finally discusses strategies for secondary prevention in vaccinated and unvaccinated populations.
HPV infection and cervical cancer pathogenesis
HPV infection and disease
HPVs are a group of more than 150 related DNA viruses that infect cutaneous and mucosal epithelia, where acute infection causes benign cutaneous lesions such as non-genital and genital warts, flat cervical condylomas, or low-grade CINs.2 HPV genomes are genetically quite stable, in contrast to the relatively high mutation rate of many RNA viruses.3 A subset of approximately 15 HPVs that infect the genital tract have the potential to cause malignant tumors, most commonly in the cervix.4,5 The cancer-associated HPV types are designated high-risk (or oncogenic) types, while those not associated with cervical cancer are designated low-risk types. Two closely-related low-risk types (HPV6 and HPV11) cause most cases of genital warts (condyloma acuminatum), but many other low-risk types may cause virtually no pathology. The high-risk types are phylogenetically related,5 and HPV-associated carcinomas arise as the result of long-term persistent infection with high-risk types. HPV DNA is almost universally found in primary cervical tumors (regardless of histology) and their metastases. The HPV types associated with cervical cancer are similar throughout the world, although minor regional differences in frequency have been noted.6 HPV16 is the type most frequently identified in all regions, accounting for about 50% of all cervical cancers. In most regions, HPV18 is the next most common type, typically being found in 15–20% of squamous cell cancers and a larger proportion of adenocarcinomas. HPVs are also implicated in the development of a variable proportion of vulvar, vaginal, anal, penile, and oropharyngeal cancers, with HPV16 accounting for the vast majority of the HPV-associated tumors at these sites.1 Worldwide, cervical cancer represents about 80% of the cancers attributable to genital-mucosal HPV infection. However, in countries with effective cervical cancer screening programs, the non-cervical cancers, which tend not to be subject to widespread screening, represent a higher proportion of these cancers.
Genital HPV infection is believed to be the most common sexually transmitted infection.7 Young women are at particularly high risk of acquiring HPV soon after initiating sexual activity. Those in their early 20s have point prevalence rates on cross-sectional screening of 20–40%, with a roughly equal distribution between high-risk and low-risk HPVs.8 The cumulative incidence depends on the frequency of sampling. One longitudinal study with semi-annual visits showed that sexually active women aged 15–19 had a 3-year cumulative incidence of more than 40%.9 Cumulative lifetime risk of infection cannot be accurately estimated, but is probably 75% or more for one or more genital HPV infection. The great majority of these infections are self-limited or controlled by the immune system, and the prevalence of HPV infection among women over 30 years of age is substantially lower than among women soon after the average age of first sexual intercourse,10 although age-specific prevalence at older ages varies for unclear reasons. Clearance of infection is believed to be immune-mediated and largely type-specific, as evidenced by the association between degree of immunosuppression and rates of infection among immune compromised individuals, altered immune profiles of women with persistent infections, and the independent clearance of specific HPV types among women with two or more HPV infections.11,12 Neutralizing antibodies that develop in response to infection are type-restricted, with limited evidence of cross-reaction observed for some closely related HPV types.13,14
Importance of persistent infection and viral oncogenes
Women who, instead of clearing their infection, become persistently infected with a high-risk HPV type, are at increased risk of developing cervical cancer.15 The risk of progression to high-grade CIN and invasive cancer is greater for women infected with HPV16 and HPV18 than those infected with other high-risk types (Figure 1).16 Certain variants of HPV16 and HPV18 may be associated with a different risk of progression to high-grade CIN or cancer.17 Among HPV-infected women, some etiologic co-factors may be associated with an increased risk of persistent infection and/or progression to high-grade CIN or invasive cancer. The most likely co-factors include cigarette smoking, multi-parity, long-term hormonal contraceptive use, and HIV infection or other causes of long-term immunosuppression.11,18–20
Figure 1.
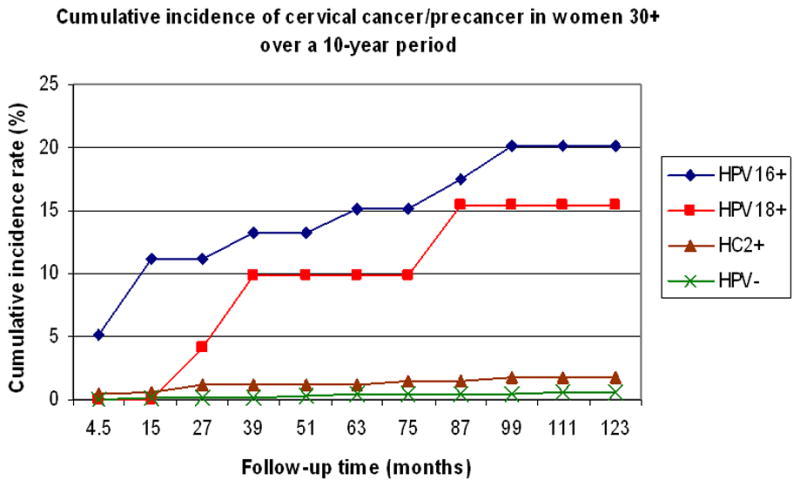
Cumulative incidence of cervical cancer/precancer in women over 30 during a 10-year period. Women with normal cytology were tested once, at enrollment, for HPV16, HPV18, and Hybrid Capture II (HC2, a cocktail of multiple high-risk HPV types, including HPV16 and HPV18). Each woman was classified as being positive for HPV16 (HPV16+), HPV18 (HPV18+), HC2 positive but negative for HPV16 or HPV18 (HC2+), or negative for HPV (HPV-), and followed prospectively for 10 years. From reference 16.
Characteristic features of most cervical cancers that contain HPV16 and almost all with HPV18 DNA are that the viral DNA is integrated in the host genome and that only the viral E6 and E7 genes are usually expressed, with the other viral genes being deleted or mutated.21,22 (Tumors with unintegrated viral DNA are discussed below.) These findings suggested that E6 and E7 are important viral oncogenes, and a variety of experimental findings have validated this possibility. For example, HPV16 E6 and E7 together can induce cervical cancer in transgenic mice, and their tumorigenic activity is substantially greater when both genes are expressed together compared with expressing only one of them.23 The E6 and E7 genes of high-risk HPVs also possess in vitro biological properties and associated biochemical activities that are lacking, or less prominent, in the E6 and E7 genes of low-risk HPVs. When expressed together, high-risk E6 and E7 cooperate to immortalize primary human keratinocytes. E6 and E7 expression also appears to be necessary for maintenance of the transformed phenotype, as suppression of their expression in cervical cancer cell lines leads to growth arrest or apoptosis.21,22 High-risk E6 and E7 proteins each encode multiple biochemical activities; key features include the ability of E6 to inactivate the p53 tumor suppressor protein and E7 to inactivate the pRB tumor suppressor protein.24 A characteristic cellular response to pRb inactivation is expression of the p16 tumor suppressor gene, which is therefore usually detected in benign and malignant lesions.25 The biological importance of p53 and pRb inactivation is underscored by the observations that E6 and E7 mutants lacking these activities are deficient in their ability to contribute to keratinocyte immortalization, as are low-risk E6 and E7. Thus, E6 and E7 are important determinants of the oncogenicity of high-risk HPVs. Whether differences in E6/E7 properties among high-risk HPV types exist and explain the predominance of HPV-16/18 in cancers is currently not well understood.
Cancer only develops after many years of persistent infection with a high-risk type.26 Low-grade cytopathic changes (ASC-US or LSIL cytology, CIN1 histology) often develop shortly after infection.27 Of these minor abnormalities, LSIL cytology is the most reproducible sign of HPV;28 both ASC-US cytology and CIN1 histology are poorly reproduced in inter-pathologist studies.29 Whether or not minor lesions occur, acute HPV infection has a high likelihood of regressing without intervention.12 High-grade CIN (most clearly represented as CIN3 because CIN2 may represent a mixture of acute infection and proto-CIN3) is typically detected 5–15 years after infection depending on the intensity and sensitivity of screening.15 Progression of CIN3 to invasive cancer generally takes many years or even decades. The interval from first infection to high-grade CIN is usually less than the time from high-grade CIN to cancer.26 Overall, the long interval between first infection and cancer implies that while the virus may initiate the chain of events that lead to cancer, a series of cellular alterations, in the target cell and/or stroma, that collaborate with the virus occur during the process of carcinogenesis. Consistent with this hypothesis, many genetic and epigenetic changes have been observed in tumors.22,30–32 These changes in the tumors, which are a consequence of long-term infection and/or exposure to exogenous co-factors, include activation of oncogenes and anti-apoptotic genes and inactivation of tumor suppressors, pro-apoptotic genes, and genes implicated in antigen processing and presentation.
Co-expression of HPV16 E6 and E7 induces genomic instability, which is believed to contribute to aneuploidy and viral DNA integration.31 For HPV16-associated lesions, which have been examined in the greatest detail, aneuploidy is very uncommonly detected in low-grade CIN, but may be found in about one-third of high-grade CIN and the vast majority of invasive cancers. The frequency of viral DNA integration follows a similar pattern; however, in high grade CIN, integration in one study was only about one-half as frequent as aneuploidy, while this difference in frequency was not seen in cancers.33 It seems likely that aneuploidy usually precedes viral DNA integration, as almost all lesions with integrated viral DNA are aneuploid, while a proportion of aneuploid lesions lack detectable integrated viral DNA.
Although integration of viral DNA is detected in the majority of tumors associated with HPV16, HPV18, and HPV45 (which is very closely related phylogenetically to HPV18), integration is not an obligatory step for the development of invasive cancer.34 This point has recently been emphasized by showing, with a sensitive molecular assay, that the majority of tumors associated with HPV31 and HPV33 did not contain detectable integrated viral DNA.35 The tumors associated with the latter HPV types also appeared to develop more slowly. Integrated viral DNA is reported to be associated with increased expression of E6 and E7, which might further enhance the degree of genomic instability and foster a faster rate of cellular alterations.36
It is unclear what viral properties may account for differences in oncogenicity observed between subtypes and variants of a given high-risk HPV type or between the different high-risk HPV types, including the degree to which the differences may be attributable to biological activities of the virus or to host response. Obtaining insight into these issues would be highly worthwhile, given the medical importance of HPV as a carcinogen and the opportunity to correlate experimental analyses with ongoing prospective natural history studies of genital infection by various HPVs. As sequence divergence between HPV types is distributed throughout their genomes, it seems likely that the particular oncogenic properties of a given virus could be attributable to quantitative differences in several genes.
Primary Prevention
Introduction
Until very recently, cervical cancer prevention has involved mainly secondary prevention, specifically screening based on the Pap test. However, the recognition that virtually all cases of cervical cancer are attributable to HPV infection implies that primary prevention of a high proportion of the infections that lead to cervical cancer could represent a powerful complementary approach to reducing the incidence of this cancer and other cancers attributable to HPV infection.
The current high incidence of genital HPV infection suggests that traditional approaches to reduce infection rates have had limited efficacy. One reason is that condoms, as used by most people, afford limited protection against HPV, in contrast to their relatively high protection against pregnancy and efficacy against several other sexually transmitted infections.37–39 There is evidence that male circumcision may reduce the incidence of infection among sexual partners.40, 41 Nevertheless, the incidence of HPV infection among circumcised males and their partners remains high. Reducing the number of sexual partners could also reduce incidence, although the high frequency of HPV exposure among sexually experienced individuals and the apparent high transmissibility of infection suggests that other approaches to primary prevention are needed.
HPV vaccination: theoretical considerations
In this regard, approaches more specifically directed toward preventing HPV infection could have a more substantial impact on infection rates and subsequent cancer. Theoretically, the long interval between infection and cancer development implies that a therapeutic vaccine could have high utility. However, it has proven easier to develop effective preventive vaccines, as indicated by the fact that most approved vaccines against other infections are preventive rather than therapeutic. Furthermore, preventive vaccination has historically been found to be a cost-effective approach that can dramatically reduce the incidence of many infections.42
Preventive vaccines may be composed of virions (virus particles) that have been chemically inactivated to render them non-infectious, as with the Salk poliovirus vaccine; attenuated live viruses, as with the Sabin poliovirus vaccine; or a sub-unit vaccine that is not infectious because it lacks some components required for infection, as with the hepatitis B virus (HBV) vaccine.43, 44 Such subunit vaccines can be delivered directly or using live vectors, with direct delivery perhaps posing fewer theoretical regulatory issues. At the present time, the sub-unit approach is the most appropriate for an HPV vaccine, as the presence of HPV oncogenes, the difficulty of making preparative amounts of authentic HPV, and the lack of an animal model for testing the pathogenic activity of an HPV, because of its species specificity, make other approaches less suitable.
Preventive HPV vaccine
Neutralizing antibodies, the class of antibodies that inhibit infection by binding to viral proteins in infectious virions, are thought to be the main protective activity induced by preventive vaccines.45,46 Such antibodies are typically induced by virion proteins, of which papillomaviruses have two, L1 and L2, designated the major and minor structural proteins, respectively, because of their relative abundance in the virion. Both L1 and L2 can induce neutralizing antibodies, with levels of antibodies induced by L1 being substantially higher; the current commercial sub-unit HPV vaccines are composed of L1.47 These non-infectious, protein-based vaccines are based on the preclinical observations that multiple copies of the L1 protein will self-assemble into virus-like particles (VLPs) that induce high levels of neutralizing antibodies and are highly protective in animal papillomavirus models.48 Passive transfer of immune IgG can confer protection to naïve animals, implying that neutralizing antibodies are probably the main protective activity induced by the vaccine. The particulate nature of the immunogen appears to be important, as the neutralizing antibodies are directed against L1 epitopes that are conformationally dependent; denaturation of VLPs leads to an L1 immunogen that does not induce neutralizing antibodies and is ineffective as a vaccine in a preclinical model.48 The repetitive structure of the VLPs probably contributes to their high immunogenicity.49 Because the neutralizing antibodies induced by VLPs are predominantly type-specific,13,50 commercial versions of the vaccine contain VLPs from more than one HPV type.
There are two commercial versions of the vaccine. One, produced by GlaxoSmithKline (GSK; Cervarix), is a bivalent vaccine composed of L1 VLPs from HPV16 and HPV18.51 These two types are responsible for about 70% of cervical cancer. The other vaccine, produced by Merck (Gardasil), is a quadrivalent vaccine. It contains HPV16 and HPV18 VLPs, but also has VLPs from HPV6 and HPV11, which together cause about 90% of genital warts.52,53 Both vaccines are administered in three intramuscular doses given over a 6 month period. The Merck vaccine is produced in yeast and uses a simple aluminum salt as an adjuvant. The GSK vaccine is produced in insect cells and uses a proprietary adjuvant, AS04, which contains an aluminum salt and monophosphoryl lipid A (MPL). Both vaccines induce seroconversion in more than 99.5% of vaccinees.
Vaccine efficacy
Published clinical efficacy trials of the two vaccines have been carried out in women 15–26 (Table 1). In controlled trials, both vaccines have shown a high level of protection against incident persistent infection and disease caused by the HPV types in the respective vaccine (vaccine types), and a good safety record. In fully vaccinated women (according to protocol [ATP] analysis), in which women who were positive for a given HPV type at enrollment or during the vaccination period were excluded from analysis for that type, protection has been close to 100% against incident end-points involving vaccine types. For the Merck vaccine, such protection has been shown, in their interim analysis of phase III trials, for HPV16- and HPV18-associated cases of moderate- and high-grade CIN and moderate- and high-grade vulvar and vaginal dysplasia, as well as for external genital warts associated with any of the four vaccine types.53,54 As expected, most of the genital warts were associated HPV6 and HPV11. A similar degree of protection against incident persistent infection or high-grade CIN attributable to HPV16 or HPV18 infection has been shown for the GSK vaccine for fully vaccinated women.55 The interim analysis of the GSK phase III trials was by a modified intention to treat (MITT) protocol, where women who were positive for a given HPV type at enrollment were excluded from analysis for that type, but they were not excluded if they became positive during the vaccination period.51 The MITT analysis gave somewhat lower protection rates, which may reflect decreased protection during the vaccination period, rather than an actual reduction in vaccine potency. Prophylactic protection from the vaccines has been shown to last at least 5 years in phase II trials.55,56 The women in the phase III trials, which of necessity started after the phase II trials, have thus far been followed for a shorter time period.
Table 1.
Prophylactic Efficacy of VLP Vaccines Against Vaccine Targeted HPV Types
| OUTCOME ATP or MITT | Test vaccine (reference No.) | Controls Events/group | Vaccinees Events/group | Efficacy (95% CI) |
|---|---|---|---|---|
| CIN2+ | ||||
| MITT | GSK (ref. 55) | 5/470 | 0/481 | 100 (−7–100) |
| MITT | GSK (ref. 51) | 21/7838 | 2/7788 | 90 (53–99) |
| ATP | Merck (ref. 52) | 44/2258 | 0/2241 | |
| ATP | Merck (ref. 58) | 42/5260 | 1/5305 | 98 (86–100) |
| CIN1+ | ||||
| MITT | GSK (ref. 55) | 8/470 | 0/481 | 100 (42–100) |
| MITT | GSK (ref. 51) | 28/7838 | 3/7788 | 89 (59–99) |
| ATP | Merck (ref. 52) | 65/2258 | 0/2241 | 100 (94–100) |
| Persistent HPV DNA | ||||
| ATP (12 mo.) | GSK (ref. 55) | 9/385 | 0/414 | 100 (61–100) |
| MITT (12 mo.) | GSK (ref. 55) | 16/470 | 1/481 | 94 (78–99) |
| MITT (12 mo.) | GSK (ref. 51) | 46/3437 | 11/3386 | 76 (48–90) |
| ATP (4 mo.) | Merck (ref, 56) | 45/233 | 2/235 | 96 (83–100) |
| MITT (4 mo.) | Merck (ref. 56) | 48/254 | 4/256 | 94 (83–98) |
| External genital warts | ||||
| ATP | Merck (ref. 52) | 48/2279 | 0/2261 | 100 (92–100) |
GSK vaccine: bivalent HPV16/18
Merck vaccine: quadrivalent HPV6/11/16/18
ATP (According to Protocol: fully vaccinated women who were negative for a given vaccine type at enrollment and throughout the vaccination period)
MITT (Modified Intention to Treat: women who were negative for a given vaccine type at enrollment)
Persistent HPV DNA (the number in parenthesis indicates the minimum number of months between positive HPV tests required for the infection to be defined as persistent)
Despite their efficacy in preventing incident infection and disease, the vaccines do not influence the rate of clearance of prevalent HPV16 or HPV18 infections and/or CIN.57,58 This observation is consistent with preclinical animal papillomavirus studies in which the VLP vaccine did not induce regression of established lesions.59 It is, therefore, not surprising that when the Merck vaccine was been analyzed by an intention to treat (ITT) protocol, which includes a mixture of prevalent and incident infections, as women who were positive for a given HPV type at enrollment were not excluded from analysis for that type, its level of protection was much lower than when only incident infections were counted.52,58
In addition to protecting against incident infection and disease caused by the vaccine types, limited cross-protection against other HPVs has been observed, as published in the GSK phase II and phase III trials51,55 and reported at meetings for the Merck vaccine. In the published studies, cross-protection has been limited to those HPV types that are most closely related to HPV16 and HPV18. These results indicate that protection is type-restricted, rather than being strictly type-specific. The cross-protection apparently increases the overall level of protection against high-grade CIN associated with any HPV type, but approximately one-quarter of such lesions may not be prevented by the vaccine. In vitro assays indicate that neutralization titers against a given vaccine type is at least 10-fold higher than against even a closely related HPV type, raising the possibility that the duration of protection against non-vaccine HPV types might wane sooner than that against vaccine types. It is therefore noteworthy that the published phase II trial data indicate that the cross-protection lasts several years.55 This observation raises the possibility that protection against the vaccine types could last substantially longer, if it is assumed that the in vitro neutralization results are clinically relevant.
HPV vaccine: regulatory aspects and recommendations
The Merck vaccine was approved in the US and the European Union in 2006, and in many other countries. The GSK vaccine was approved in the European Union in 2007, and in several other countries. GSK has applied for licensure in the US as well, and a decision is anticipated in 2008. In the US and many other countries, approval (of the Merck vaccine) has been limited to young women, 9–26. As noted above, published efficacy studies have been limited to women 16–26. Immunological bridging studies to adolescent girls 9–15 years of age, which showed an even more robust immune response than among the young women in the efficacy trials, were accepted by the FDA as implying they would also be protected. Two relevant considerations were the widespread recognition that it would be extremely difficult to carry out efficacy trials in young adolescents and data from the US, and many other countries, indicating that young adolescents should be the main target group for the vaccine. That is because the vaccine should be most cost-effective (i.e., prevent the most cases of high-grade CIN and cancer for a given public expenditure) if it is given before women become sexually active, and US behavioral surveys indicate that about one-quarter of 15 year olds have been sexually active, with this proportion increasing to about 70% of 18-year olds.
The European Union approved the Merck vaccine for 9–15 year old males, in addition to 9–26 year old females, based on their acceptance of immunological bridging studies of 9–15 year old boys showing results that were virtually identical to those of the 9–15 year old girls. The FDA will presumably consider approval for vaccination of males only after efficacy has been demonstrated in them, perhaps because an experimental herpes simplex virus subunit vaccine was shown to confer partial protection in women, but none in men.60 This gender-specific response raised the possibility that prophylactic vaccination against mucosal genital infections might have reduced efficacy among men. Efficacy trials of the HPV vaccine in men have been initiated.
In the US, the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) makes national recommendations for approved vaccines, although implementation is at the state and local level. The ACIP recommended routine vaccination of 11–12 year old girls as the main target group for the vaccine, with catch-up vaccination for girls and women 13–26, and vaccination of 9–10 year old girls at the discretion of the medical personnel involved. However, the recommendation for catch-up vaccination of women aged 19 and older has not been embraced universally.61
The ACIP also recommended that the federal government purchase vaccine through its vaccine for children’s (VFC) program, which can now provide the HPV vaccine for girls 18 years old or younger who come from poor families. This aspect of vaccine implementation could be particularly important for public health, as women from this socio-economic background tend to have less access to screening when they are older and are, therefore, at greater risk of developing cervical cancer.62 Traditional vaccines that are part of the VCF program have achieved broad coverage for eligible children, especially when the vaccines are required.63
Because the vaccine may not prevent at least 25% of infections that may lead to cervical cancer, it is recommended that vaccinated women should, at least for now, continue to follow the same cervical cancer screening guidelines as non-vaccinated women (see the section below on integration of screening and vaccination). The vaccine is in the process of being made widely available in the industrialized world. However, its current high cost ($120 per dose) probably means that it will not be widely implemented in the developing world in the near future, even with tiered pricing.
Implementation may be divided into vaccination of the target group and catch-up vaccination for girls and young women up to 26 years of age. The number of cases of cervical cancer prevented will be greater if the vaccine is administered before becoming sexually active, given the high risk for infection soon after initiating sexual activity and the lack of vaccine efficacy against established infection. An instructive theoretical model has been developed for females in Finland,64 although a somewhat lower percentage of Finnish girls 18 and younger are sexually active (~10% of 15 year olds in Finland vs. 25% in the US and 65% of 18 year olds in Finland vs. 69% in the US).65 The model predicts the percentage of cervical cancers attributable to HPV16 that would be prevented if 70% of Finnish females were vaccinated when they were 12, 15, 18, or 21, with vaccination postulated to be followed by lifelong protection (Figure 2). The number of cases protected is similar for vaccination at 12 or 15 years of age, because so few Finnish girls are sexually active at these time points. However, if the vaccine were not given until they were 18, it would prevent only about half as many cases, and if it were given when they were 21, it would prevent only about one quarter as many cases. Merck has recently presented results at conferences suggesting that the vaccine may be as efficacious in preventing incident infection and disease in those 27–45 year old women who happen to be naïve to HPV16, HPV18, HPV6 or HPV11 as in similarly unexposed younger women. However, as the number of unexposed women decreases greatly with age, the number of cancers prevented is likely to be inversely proportional to the age at which women are vaccinated. Independent of choices by individual women, this population-wide phenomenom is likely to influence public health oriented implementation recommendations for women older than 26.
Figure 2.
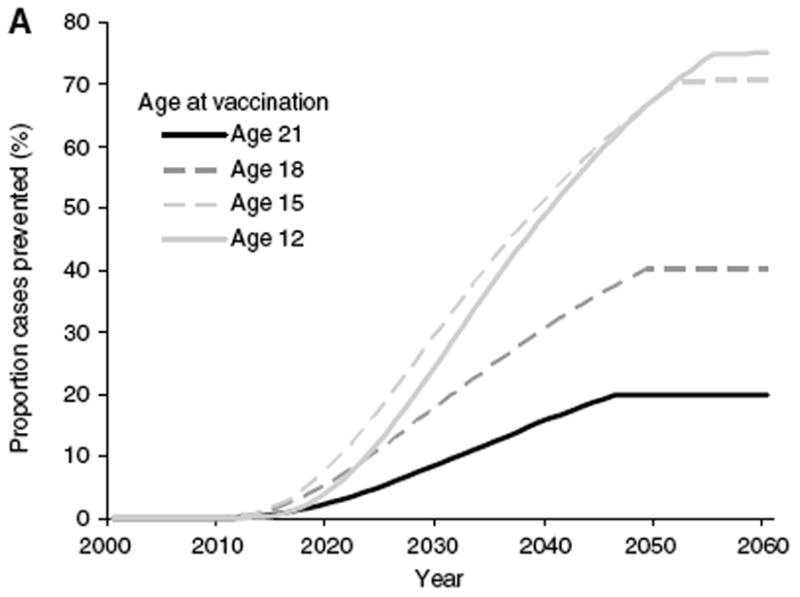
Model, from Finnish women, of the proportion of annual incident HPV16-associated cervical cancer cases prevented with different ages at vaccination, if coverage is 70% of females only and is initiated in 2008. From reference 64.
It will be important to determine if the vaccine will maintain its excellent safety record and to monitor the actual duration of protection afforded by the vaccines. If protection is found to wane, one or more booster vaccinations will probably be needed during a woman’s lifetime to maintain a high level of protection. The administration of boosters would, of course, increase the overall cost and logistical complexity of vaccination.
Another important issue is the public health benefits of vaccinating males, if the vaccine is protective in males. Vaccination of women is the top priority for public health, since worldwide 90% of HPV-associated cancers occur in females, and modeling has suggested that if there is high vaccine coverage of women the benefit from vaccinating males would be rather limited.64 However, if a country has sufficient resources and only a minority of the female target population is actually being vaccinated, male vaccination would probably contribute to reductions in infection of both males and females, although less efficiently than if those vaccinations were given to females.
Potential second generation vaccines
A number of second generation prophylactic vaccines are under consideration and/or active development.66 However, with the exception of upper respiratory delivery of purified VLPs, these approaches have not been tested clinically.67 Second generation vaccines seek to address one or more of the inherent limitations of the current vaccines, such as high cost of production and implementation and type-restricted efficacy. In a simple extension of the current approach, both companies are considering increasing the number of VLP types in their vaccines. Increasing VLP valency would likely increase the percentage of cervical cancers that would be prevented by the vaccines. However, after HPV16 and HPV18, no single type accounts for more than a few percent of cancers. From a broad public health perspective, therefore, this approach would be cost-effective primarily if it did not increase further increase the cost of vaccination, or if could lead to decreased costs of cervical cancer screening (see next section). Although such a multivalent vaccine would probably be of value in the industrialized world, the increased manufacturing complexity could further delay the time when the vaccine might be affordable in the developing world.
Live bacterial vectors expressing L1 might be inexpensive to produce and deliver, if given orally. Live L1-recombinants of the widely employed Salmonella typhi vaccine strain Ty21a induce high titers of neutralizing antibodies in mice,68 and a clinical trial of this vaccine is under consideration. Decreased production and delivery costs might also be achieved with VLPs produced in plants69 or L1 capsomeres in bacteria.70,71
Most approaches for second generation vaccines are based on L1, but neutralizing antibodies can also be generated against L2, the minor capsid protein. Unlike the case for L1, relatively short L2 polypeptides can induce neutralizing antibodies.72 These antibodies have been found to be broadly cross-neutralizing against divergent HPV types and can induce protection against heterologous virus challenge in animal papillomavirus models,73,74 raising the possibility of a simple monovalent vaccine that would prevent a broad spectrum of HPV infections. However, the titers of L2 neutralizing antibodies achieved to date are considerably lower than the neutralizing titers induced by L1 VLPs, and current efforts are focused on increasing the immunogenicity of L2 vaccines.
Secondary Prevention
Introduction
Despite the high efficacy of the vaccine against the high-risk HPV types that cause the greatest morbidity and mortality, cervical cancer screening will continue to play a critical role in the control of cervical cancer.75 It will take at least two decades for a prophylactic HPV vaccine program targeting a cohort of adolescent girls to reduce substantially their incidence of invasive cervical cancer in middle-age and beyond (Figure 2).76 In addition, even if worldwide vaccine coverage were achievable today, secondary prevention would be required because the vaccine does not appear to alter the natural history of prevalent infections, and some cancer causing infections will not be prevented by the vaccine.57, 58
Nonetheless, it is important to start thinking now about HPV vaccination and cervical screening programs together, in part because successful vaccination programs will affect screening performance much sooner than they reduce cancer rates. The vaccines decrease the high-grade cancer precursors that screening is designed to detect and treat. As vaccine coverage increases, and more polyvalent vaccines are presumably introduced, screening programs will have to change to remain cost-effective (see below). In the interim, the use of improved cervical screening methods that recognize the central etiologic role of persistent HPV infection could save hundreds of thousands of lives, especially in low-resource regions.77
Current cytology- and colposcopy-based programs
Current, conventional cervical cancer prevention programs include: repeated rounds of screening of women in the general population; triage of equivocal screening results by additional testing or heightened surveillance; histologic diagnosis of abnormal screening results by colposcopically-directed biopsy; post-colposcopic follow-up if no immediate treatment is performed; and assessment of cure if treatment is performed.78 At present, these programs rely mainly on the microscopic (cytologic and histologic) and visual (colposcopic) correlates of HPV infection to predict the cervical cancer risk of women with different screening or diagnostic results.
New technologies for high-resource settings
New technologies provide several options for improvement of cervical cancer secondary prevention. Improvements in cytology slide preparation and interpretation have increased screening efficiency and might increase sensitivity.79 However, to date, no computer-assisted technology has been proven to dramatically increase accuracy.80, 81
In a few countries, testing for HPV directly by molecular assays is already being used with cytology to provide better risk prediction and the possibility of fewer cycles of screening.82–84 HPV DNA testing is more reproducible than cytologic screening and colposcopy for the detection of existent and incipient cervical precancerous conditions and cancer.85–87 As a corollary of the high sensitivity of HPV testing, a negative test for carcinogenic HPV types provides a degree and duration of reassurance not achievable by any other diagnostic method. However, especially among young women, a single positive HPV test has low specificity and poor positive predictive value.16 On the other hand, HPV persistence of approximately 2 years or more predicts a substantial risk of diagnosis of high-grade CIN within the subsequent 5–10 years.15,26 Few women or physicians will wish to wait to know whether an HPV infection clears. It would be very useful to have biomarkers that can predict carcinogenic HPV persistence and risk of progression to high-grade CIN, obviating the need for follow-up. Validation of such a biomarker with high sensitivity and high specificity might even permit the use of molecular testing (with or without HPV assays) at younger ages at which HPV testing alone is non-specific. For example, it has been proposed that p16INK4 assays88 or E6/E7 expresson89 might serve this role, but sufficient validation trials have not yet been conducted.
With the data already at hand, the use of HPV testing to complement or even replace cervical cytology for screening is likely to be accelerated by the results of a series of randomized controlled trials reported in the last year, which all showed that testing for high-risk HPV types is more sensitive than cytology in the early detection of high-grade CIN.90–93 It is important to note that HPV testing detected the same number of cases as cytology in these trials, but earlier, suggesting that the molecular tests are detecting true cancer precursors. In addition, these data also suggested that the choice of HPV testing at longer intervals, versus repeated cytology at shorter intervals, is largely a matter of programmatic efficiency and cost, at least in high-resource regions where patients are infrequently lost to follow-up.
Similarly, HPV testing has also been shown, in comparative trials, to be more efficient than repeat cytology in triage of equivocal cytologic interpretations.94 Because persistent infections with high-risk HPV genotypes cause cervical cancer and all true precursor lesions, HPV testing identifies possible cervical cancer precursors and avoids unnecessary follow-up or treatment of the great number of “look-alike” lesions.95 Finally, HPV testing is being proposed, based on recent studies, for use in following women post-colposcopy and post-treatment.87,96
Applicability of HPV-based methods in low-resource settings
New technologies tend to be expensive and difficult to implement but, because >80% of all cervical cancer and its related-mortality occur in low-resource settings,1 it is desirable for new technologies to be adapted for these underserved populations. Cost, infrastructure, and acceptability must be addressed to achieve widespread use. In low-resource regions, screening is most effective if women are reached at the ages of peak risk of treatable precancerous conditions attributable to persistent infection (10–15 years after the population median age of sexual debut) and before the average age at which most frankly invasive cancers occur.77, 97 Simple visual inspection with acetic acid has been shown to reduce cervical cancer incidence in low-resource settings.98 Although this technique saves lives, it does not appear to be sensitive or specific enough to be considered the long-term optimal approach.
It is intuitively appealing, if feasible, to use secondary prevention methods for cervical cancer based directly on HPV detection. Candidate HPV-based tests are now being developed into rapid, robust, easy-to-use, and inexpensive formats.99 In low-resource regions, screening might target women a total of 1–2 times in the age range of peak risk of treatable high-grade CIN and earliest cancer.97 One-visit “screen-and-treat” strategies would minimize loss-to-follow-up that frequently reduces the effectiveness of screening programs. Two limitations of screen-and-treat strategies still remain. It will be difficult to fully rule out lesions that require advanced care.100 Also, full success of this innovative strategy awaits development of improved safe, inexpensive, and effective outpatient treatment for HPV-positive women.101,102
Effect of HPV vaccination on all forms of cervical screening
In any setting, the amount of public funding to be used for HPV vaccination will be balanced against the need to continue screening. This balancing act will be made more difficult because screening will gradually become less cost-effective, following the widespread use of HPV vaccines, as described below.103 While the elimination of CIN3 and cancers attributable to types 16 and 18 will be welcome, vaccination will leave behind more equivocal and less predictive abnormalities, as the most evident and risky abnormal results by cytology, HPV tests, and colposcopy are caused by HPV16. For vaccinated women, therefore, the positive predictive value of a positive result for CIN3 and cancer will decrease for cytology, HPV testing, and colposcopy.103
For cytology, vaccination will reduce the number of high-grade cytologic findings disproportionately to the reduction in equivocal and low grade abnormalities.104 The effect of vaccination on testing protocols relying on HPV tests that pool all carcinogenic types will parallel the issues raised for cytology, as the power of HPV testing derives in large part from detection of HPV16 and HPV18.16 As with cytology, the number of HPV infections found that predict cancer or high-grade CIN will diminish relative to the number of HPV-positive women destined not to get cervical cancer or even high-grade CIN. Successful vaccination will also affect colposcopy (and presumably its lower-cost replacements such as visual assessment with acetic acid) as strongly if not more than it will affect cytology.105 The visual appearance of cervical HPV infections, as evaluated by colposcopy or derivative techniques, is even more highly variable and difficult to classify than cytopathic effects. In fact, the lack of reproducibility and accuracy of colposcopy represents a considerable, but underappreciated, clinical challenge.106,107
HPV16 is associated with the highest probability of clearly recognizable lesions, including those lesions that lead to a histologic diagnosis of high-grade CIN.108 Therefore, the removal of HPV16 by vaccination will leave an even greater challenge for colposcopists approaching the already difficult task of targeting lesions for biopsy diagnosis.
The above considerations imply that vaccination duplicates important parts of screening, doing a large part of the same job of raising safety and removing the most dangerous cervical abnormalities. As described above, vaccination will reduce some of the underlying value and efficiency of screening, but it will not entirely eliminate the need for screening. However, if the long-term intensity of screening were to remain unchanged indefinitely for vaccinated cohorts, it would mean that the cost of vaccination had simply been added to the current cost of screening, except for the relatively modest savings resulting from the decrease in the number of positive screening tests.
One potentially cost-effective long-term strategy to consider would be to raise the age of initiation of screening for vaccinated women. Emerging data suggest that the risk of early cancers in young women might be preferentially linked to HPV16 and HPV18.35 If true, and if data demonstrate further reductions in early cancer risk for vaccinated women, it might be medically justifiable not to begin screening until the mid twenties, or even later in some regions of the world. In addition to initiating screening later, we may want to stretch out screening intervals, if vaccine durability proves truly long-term, if it is demonstrated that boosters are cost-effective, and especially if HPV testing is added routinely to cervical cytology.
Conclusions
The justifiable excitement over primary prevention by vaccination should be coordinated with screening efforts. Further understanding of HPV as a uniquely powerful human carcinogen also remains an important research goal. In the post-vaccination era, screening must continue, but will need to be changed to preserve cost-effectiveness of the total program. The challenge will be to screen women in high-resource regions appropriately, while applying the latest advances rationally and equitably to low-resource regions.
References
- 1.Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006;118(12):3030–44. doi: 10.1002/ijc.21731. [DOI] [PubMed] [Google Scholar]
- 2.de Villiers EM, Fauquet C, Broker TR, Bernard HU, zur Hausen H. Classification of papillomaviruses. Virology. 2004;324(1):17–27. doi: 10.1016/j.virol.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 3.Chan S-Y, Ho L, Ong C-K, Chow V, Drecher B, Durst M, et al. Molecular variants of human papillomaviru-16 from four continents suggest pandemic spread of the virus and its coevolution with humankind. Journal of Virology. 1992;66:2057–66. doi: 10.1128/jvi.66.4.2057-2066.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Munoz N, Bosch FX, Castellsague X, Diaz M, de Sanjose S, Hammouda D, et al. Against which human papillomavirus types shall we vaccinate and screen? The international perspective. Int J Cancer. 2004;111(2):278–85. doi: 10.1002/ijc.20244. [DOI] [PubMed] [Google Scholar]
- 5.Schiffman M, Herrero R, Desalle R, Hildesheim A, Wacholder S, Rodriguez AC, et al. The carcinogenicity of human papillomavirus types reflects viral evolution. Virology. 2005;337(1):76–84. doi: 10.1016/j.virol.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Smith JS, Lindsay L, Hoots B, Keys J, Franceschi S, Winer R, et al. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. Int J Cancer. 2007;121(3):621–32. doi: 10.1002/ijc.22527. [DOI] [PubMed] [Google Scholar]
- 7.Baseman JG, Koutsky LA. The epidemiology of human papillomavirus infections. J Clin Virol. 2005;32 (Suppl 1):S16–24. doi: 10.1016/j.jcv.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 8.Herrero R, Castle PE, Schiffman M, Bratti MC, Hildesheim A, Morales J, et al. Epidemiologic profile of type-specific human papillomavirus infection and cervical neoplasia in Guanacaste, Costa Rica. J Infect Dis. 2005;191(11):1796–807. doi: 10.1086/428850. [DOI] [PubMed] [Google Scholar]
- 9.Woodman CB, Collins S, Winter H, Bailey A, Ellis J, Prior P, et al. Natural history of cervical human papillomavirus infection in young women: a longitudinal cohort study. Lancet. 2001;357(9271):1831–6. doi: 10.1016/S0140-6736(00)04956-4. [DOI] [PubMed] [Google Scholar]
- 10.Franceschi S, Herrero R, Clifford GM, Snijders PJ, Arslan A, Anh PT, et al. Variations in the age-specific curves of human papillomavirus prevalence in women worldwide. Int J Cancer. 2006;119(11):2677–84. doi: 10.1002/ijc.22241. [DOI] [PubMed] [Google Scholar]
- 11.Palefsky JM, Holly EA. Chapter 6: Immunosuppression and co-infection with HIV. J Natl Cancer Inst Monogr. 2003;(31):41–6. doi: 10.1093/oxfordjournals.jncimonographs.a003481. [DOI] [PubMed] [Google Scholar]
- 12.Plummer M, Schiffman M, Castle PE, Maucort-Boulch D, Wheeler CM. A 2-year prospective study of human papillomavirus persistence among women with a cytological diagnosis of atypical squamous cells of undetermined significance or low-grade squamous intraepithelial lesion. J Infect Dis. 2007;195(11):1582–9. doi: 10.1086/516784. [DOI] [PubMed] [Google Scholar]
- 13.Pastrana DV, Buck CB, Pang YY, Thompson CD, Castle PE, FitzGerald PC, et al. Reactivity of human sera in a sensitive, high-throughput pseudovirus-based papillomavirus neutralization assay for HPV16 and HPV18. Virology. 2004;321(2):205–16. doi: 10.1016/j.virol.2003.12.027. [DOI] [PubMed] [Google Scholar]
- 14.Bishop B, Dasgupta J, Klein M, Garcea RL, Christensen ND, Zhao R, et al. Crystal structures of four types of human papillomavirus L1 capsid proteins: understanding the specificity of neutralizing monoclonal antibodies. J Biol Chem. 2007;282(43):31803–11. doi: 10.1074/jbc.M706380200. [DOI] [PubMed] [Google Scholar]
- 15.Rodriguez AC, Schiffman M, Herrero R, Wacholder S, Burk RD, et al. Rapid clearance of HPV should lead to clinical focus on persistent infections. J Natl Cancer Inst. doi: 10.1093/jnci/djn044. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khan MJ, Castle PE, Lorincz AT, Wacholder S, Sherman M, Scott DR, et al. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J Natl Cancer Inst. 2005;97(14):1072–9. doi: 10.1093/jnci/dji187. [DOI] [PubMed] [Google Scholar]
- 17.Xi LF, Koutsky LA, Hildesheim A, Galloway DA, Wheeler CM, Winer RL, et al. Risk for high-grade cervical intraepithelial neoplasia associated with variants of human papillomavirus types 16 and 18. Cancer Epidemiol Biomarkers Prev. 2007;16(1):4–10. doi: 10.1158/1055-9965.EPI-06-0670. [DOI] [PubMed] [Google Scholar]
- 18.Cervical carcinoma and reproductive factors: collaborative reanalysis of individual data on 16,563 women with cervical carcinoma and 33, 542 women without cervical carcinoma from 25 epidemiological studies. Int J Cancer. 2006;119(5):1108–24. doi: 10.1002/ijc.21953. [DOI] [PubMed] [Google Scholar]
- 19.Appleby P, Beral V, Berrington de Gonzalez A, Colin D, Franceschi S, Goodill A, et al. Carcinoma of the cervix and tobacco smoking: collaborative reanalysis of individual data on 13,541 women with carcinoma of the cervix and 23,017 women without carcinoma of the cervix from 23 epidemiological studies. Int J Cancer. 2006;118(6):1481–95. doi: 10.1002/ijc.21493. [DOI] [PubMed] [Google Scholar]
- 20.Comparison of risk factors for invasive squamous cell carcinoma and adenocarcinoma of the cervix: collaborative reanalysis of individual data on 8,097 women with squamous cell carcinoma and 1,374 women with adenocarcinoma from 12 epidemiological studies. Int J Cancer. 2007;120(4):885–91. doi: 10.1002/ijc.22357. [DOI] [PubMed] [Google Scholar]
- 21.zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002;2(5):342–50. doi: 10.1038/nrc798. [DOI] [PubMed] [Google Scholar]
- 22.Howley PM, Lowy DR. Papillomaviruses. In: Knipe DM, Howley PH, editors. Fields Virology. 5. Vol. 2. Philadelphia: Lippincott Williams & Wilkins; 2007. pp. 2299–354. [Google Scholar]
- 23.Song S, Liem A, Miller JA, Lambert PF. Human papillomavirus types 16 E6 and E7 contribute differently to carcinogenesis. Virology. 2000;267(2):141–50. doi: 10.1006/viro.1999.0106. [DOI] [PubMed] [Google Scholar]
- 24.Wise-Draper TM, Wells SI. Papillomavirus E6 and E7 proteins and their cellular targets. Front Biosci. 2008;13:1003–17. doi: 10.2741/2739. [DOI] [PubMed] [Google Scholar]
- 25.Hampl M, Wentzensen N, Vinokurova S, von Knebel-Doeberitz M, Poremba C, Bender HG, et al. Comprehensive analysis of 130 multicentric intraepithelial female lower genital tract lesions by HPV typing and p16 expression profile. J Cancer Res Clin Oncol. 2007;133(4):235–45. doi: 10.1007/s00432-006-0162-0. [DOI] [PubMed] [Google Scholar]
- 26.Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007;370(9590):890–907. doi: 10.1016/S0140-6736(07)61416-0. [DOI] [PubMed] [Google Scholar]
- 27.Castle PE, Wacholder S, Lorincz AT, Scott DR, Sherman ME, Glass AG, et al. A prospective study of high-grade cervical neoplasia risk among human papillomavirus-infected women. J Natl Cancer Inst. 2002;94(18):1406–14. doi: 10.1093/jnci/94.18.1406. [DOI] [PubMed] [Google Scholar]
- 28.Zuna RE, Wang SS, Rosenthal DL, Jeronimo J, Schiffman M, Solomon D. Determinants of human papillomavirus-negative, low-grade squamous intraepithelial lesions in the atypical squamous cells of undetermined significance/low-grade squamous intraepithelial lesions triage study (ALTS) Cancer. 2005;105(5):253–62. doi: 10.1002/cncr.21232. [DOI] [PubMed] [Google Scholar]
- 29.Stoler MH, Schiffman M. Interobserver reproducibility of cervical cytologic and histologic interpretations: realistic estimates from the ASCUS-LSIL Triage Study. JAMA. 2001;285(11):1500–5. doi: 10.1001/jama.285.11.1500. [DOI] [PubMed] [Google Scholar]
- 30.Lazo PA. The molecular genetics of cervical carcinoma. Brit J Cancer. 1999;80:2008–18. doi: 10.1038/sj.bjc.6690635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Munger K, Baldwin A, Edwards KM, Hayakawa H, Nguyen CL, Owens M, et al. Mechanisms of human papillomavirus-induced oncogenesis. J Virol. 2004;78(21):11451–60. doi: 10.1128/JVI.78.21.11451-11460.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Snijders PJ, Steenbergen RD, Heideman DA, Meijer CJ. HPV-mediated cervical carcinogenesis: concepts and clinical implications. J Pathol. 2006;208(2):152–64. doi: 10.1002/path.1866. [DOI] [PubMed] [Google Scholar]
- 33.Melsheimer P, Vinokurova S, Wentzensen N, Bastert G, von Knebel Doeberitz M. DNA aneuploidy and integration of human papillomavirus type 16 e6/e7 oncogenes in intraepithelial neoplasia and invasive squamous cell carcinoma of the cervix uteri. Clin Cancer Res. 2004;10(9):3059–63. doi: 10.1158/1078-0432.ccr-03-0565. [DOI] [PubMed] [Google Scholar]
- 34.Cullen AP, Reid R, Campion M, Lörincz AT. Analysis of the physical state of different human papillomavirus DNAs in intraepithelial and invsasive cervical neoplasm. J Virol. 1991;65:606–12. doi: 10.1128/jvi.65.2.606-612.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vinokurova S, Wentzensen N, Kraus I, Klaes R, Driesch C, Melsheimer P, et al. Type-dependent integration frequency of human papillomavirus genomes in cervical lesions. Cancer Res. 2008;68(1):307–13. doi: 10.1158/0008-5472.CAN-07-2754. [DOI] [PubMed] [Google Scholar]
- 36.Jeon S, Lambert PF. Integration of human papillomavirus type 16 DNA into the human genome leads to increased stability of E6 and E7 mRNAs: implications for cervical carcinogenesis. Proc Natl Acad Sci U S A. 1995;92(5):1654–8. doi: 10.1073/pnas.92.5.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vaccarella S, Franceschi S, Herrero R, Munoz N, Snijders PJ, Clifford GM, et al. Sexual behavior, condom use, and human papillomavirus: pooled analysis of the IARC human papillomavirus prevalence surveys. Cancer Epidemiol Biomarkers Prev. 2006;15(2):326–33. doi: 10.1158/1055-9965.EPI-05-0577. [DOI] [PubMed] [Google Scholar]
- 38.Winer RL, Hughes JP, Feng Q, O’Reilly S, Kiviat NB, Holmes KK, et al. Condom use and the risk of genital human papillomavirus infection in young women. N Engl J Med. 2006;354(25):2645–54. doi: 10.1056/NEJMoa053284. [DOI] [PubMed] [Google Scholar]
- 39.Nielson CM, Harris RB, Dunne EF, Abrahamsen M, Papenfuss MR, Flores R, et al. Risk factors for anogenital human papillomavirus infection in men. J Infect Dis. 2007;196(8):1137–45. doi: 10.1086/521632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Castellsague X, Bosch FX, Munoz N, Meijer CJ, Shah KV, de Sanjose S, et al. Male circumcision, penile human papillomavirus infection, and cervical cancer in female partners. N Engl J Med. 2002;346(15):1105–12. doi: 10.1056/NEJMoa011688. [DOI] [PubMed] [Google Scholar]
- 41.Baldwin SB, Wallace DR, Papenfuss MR, Abrahamsen M, Vaught LC, Giuliano AR. Condom use and other factors affecting penile human papillomavirus detection in men attending a sexually transmitted disease clinic. Sex Transm Dis. 2004;31(10):601–7. doi: 10.1097/01.olq.0000140012.02703.10. [DOI] [PubMed] [Google Scholar]
- 42.Plotkin SA, Orenstein WA, Offit PA. Vaccines. 5. Saunders; 2008. p. 1725. [Google Scholar]
- 43.Kao JH, Chen DS. Global control of hepatitis B virus infection. Lancet Infect Dis. 2002;2(7):395–403. doi: 10.1016/s1473-3099(02)00315-8. [DOI] [PubMed] [Google Scholar]
- 44.Mast EE, Ward JW. Hepatitis B vaccines. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. 5. Saunders; 2008. pp. 205–41. [Google Scholar]
- 45.Robbins JB, Schneerson R, Szu SC. Hypothesis: serum IgG antibody is sufficient to confer protection against infectious diseases by inactivating the inoculum. J Infect Dis. 1995;171:1387–98. doi: 10.1093/infdis/171.6.1387. [DOI] [PubMed] [Google Scholar]
- 46.Zinkernagel RM. On natural and artificial vaccinations. Annu Rev Immunol. 2003;21:515–46. doi: 10.1146/annurev.immunol.21.120601.141045. [DOI] [PubMed] [Google Scholar]
- 47.Roden RB, Yutzy WHt, Fallon R, Inglis S, Lowy DR, Schiller JT. Minor capsid protein of human genital papillomaviruses contains subdominant, cross- neutralizing epitopes. Virology. 2000;270(2):254–7. doi: 10.1006/viro.2000.0272. [DOI] [PubMed] [Google Scholar]
- 48.Lowy DR, Schiller JT. Prophylactic human papillomavirus vaccines. J Clin Invest. 2006;116(5):1167–73. doi: 10.1172/JCI28607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bachmann MF, Zinkernagel RM. Neutralizing antiviral B cell responses. Annu Rev Immunol. 1997;15:235–70. doi: 10.1146/annurev.immunol.15.1.235. [DOI] [PubMed] [Google Scholar]
- 50.Roden RBS, Hubbert NL, Kirnbauer R, Christensen ND, Lowy DR, Schiller JT. Assessment of the serological relatedness of genital human papillomaviruses by hemagglutination inhibition. J Virol. 1996;70:3298–301. doi: 10.1128/jvi.70.5.3298-3301.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paavonen J, Jenkins D, Bosch FX, Naud P, Salmeron J, Wheeler CM, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet. 2007;369(9580):2161–70. doi: 10.1016/S0140-6736(07)60946-5. [DOI] [PubMed] [Google Scholar]
- 52.Garland SM, Hernandez-Avila M, Wheeler CM, Perez G, Harper DM, Leodolter S, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356(19):1928–43. doi: 10.1056/NEJMoa061760. [DOI] [PubMed] [Google Scholar]
- 53.Joura EA, Leodolter S, Hernandez-Avila M, Wheeler CM, Perez G, Koutsky LA, et al. Efficacy of a quadrivalent prophylactic human papillomavirus (types 6, 11, 16, and 18) L1 virus-like-particle vaccine against high-grade vulval and vaginal lesions: a combined analysis of three randomised clinical trials. Lancet. 2007;369(9574):1693–702. doi: 10.1016/S0140-6736(07)60777-6. [DOI] [PubMed] [Google Scholar]
- 54.Ault KA. Effect of prophylactic human papillomavirus L1 virus-like-particle vaccine on risk of cervical intraepithelial neoplasia grade 2, grade 3, and adenocarcinoma in situ: a combined analysis of four randomised clinical trials. Lancet. 2007;369(9576):1861–8. doi: 10.1016/S0140-6736(07)60852-6. [DOI] [PubMed] [Google Scholar]
- 55.Harper DM, Franco EL, Wheeler C, Moscicki A-B, Romanowski B, Roteli-Martins CM, et al. Sustained efficacy up to 4·5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet. 2006;367(9518):1247–55. doi: 10.1016/S0140-6736(06)68439-0. [DOI] [PubMed] [Google Scholar]
- 56.Villa LL, Costa RL, Petta CA, Andrade RP, Paavonen J, Iversen OE, et al. High sustained efficacy of a prophylactic quadrivalent human papillomavirus types 6/11/16/18 L1 virus-like particle vaccine through 5 years of follow-up. Br J Cancer. 2006;95(11):1459–66. doi: 10.1038/sj.bjc.6603469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hildesheim A, Herrero R, Wacholder S, Rodriguez AC, Solomon D, Bratti MC, et al. Effect of human papillomavirus 16/18 L1 viruslike particle vaccine among young women with preexisting infection: a randomized trial. JAMA. 2007;298(7):743–53. doi: 10.1001/jama.298.7.743. [DOI] [PubMed] [Google Scholar]
- 58.Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356(19):1915–27. doi: 10.1056/NEJMoa061741. [DOI] [PubMed] [Google Scholar]
- 59.Kirnbauer R, Chandrachud L, O’Neil B, Wagner E, Grindlay G, Armstrong A, et al. Virus-like particles of Bovine Papillomavirus type 4 in prophylactic and therapeutic immunization. Virology. 1996;219:37–44. doi: 10.1006/viro.1996.0220. [DOI] [PubMed] [Google Scholar]
- 60.Stanberry LR, Spruance SL, Cunningham AL, Bernstein DI, Mindel A, Sacks S, et al. Glycoprotein-D-adjuvant vaccine to prevent genital herpes. N Engl J Med. 2002;347(21):1652–61. doi: 10.1056/NEJMoa011915. [DOI] [PubMed] [Google Scholar]
- 61.Saslow D, Castle PE, Cox JT, Davey DD, Einstein MH, Ferris DG, et al. American Cancer Society Guideline for human papillomavirus (HPV) vaccine use to prevent cervical cancer and its precursors. CA Cancer J Clin. 2007;57(1):7–28. doi: 10.3322/canjclin.57.1.7. [DOI] [PubMed] [Google Scholar]
- 62.Freeman H, Wingrove B. Excess Cervical Cancer Mortality: A Marker for Low Access to health Care in Poor Communities. Rockville, MD: National Cancer Institute, Center to Reduce Cancer Health Disparities; 2005. NIH Pub. No. 05–5282. [Google Scholar]
- 63.Orenstein WA, Rodewald LE, Hinman AR, Schuchat A. Immunization in the United States. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. 5. Saunders; 2008. pp. 1479–510. [Google Scholar]
- 64.French KM, Barnabas RV, Lehtinen M, Kontula O, Pukkala E, Dillner J, et al. Strategies for the introduction of human papillomavirus vaccination: modelling the optimum age- and sex-specific pattern of vaccination in Finland. Br J Cancer. 2007;96(3):514–8. doi: 10.1038/sj.bjc.6603575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mosher WD, Chandra A, Jones J. Sexual behavior and selected health measures: men and women 15–44 years of age, United States, 2002. Adv Data. 2005;(362):1–55. [PubMed] [Google Scholar]
- 66.Schiller JT, Nardelli-Haefliger D. Chapter 17: Second generation HPV vaccines to prevent cervical cancer. Vaccine. 2006;24 (Suppl 3):S147–53. doi: 10.1016/j.vaccine.2006.05.123. [DOI] [PubMed] [Google Scholar]
- 67.Nardelli-Haefliger D, Lurati F, Wirthner D, Spertini F, Schiller JT, Lowy DR, et al. Immune responses induced by lower airway mucosal immunisation with a human papillomavirus type 16 virus-like particle vaccine. Vaccine. 2005;23(28):3634–41. doi: 10.1016/j.vaccine.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 68.Fraillery D, Baud D, Pang SY, Schiller J, Bobst M, Zosso N, et al. Salmonella enterica serovar Typhi Ty21a expressing human papillomavirus type 16 L1 as a potential live vaccine against cervical cancer and typhoid fever. Clin Vaccine Immunol. 2007;14(10):1285–95. doi: 10.1128/CVI.00164-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maclean J, Koekemoer M, Olivier AJ, Stewart D, Hitzeroth, Rademacher T, et al. Optimization of human papillomavirus type 16 (HPV-16) L1 expression in plants: comparison of the suitability of different HPV-16 L1 gene variants and different cell-compartment localization. J Gen Virol. 2007;88(Pt 5):1460–9. doi: 10.1099/vir.0.82718-0. [DOI] [PubMed] [Google Scholar]
- 70.Rose RC, White WI, Li M, Suzich JA, Lane C, Garcea RL. Human papillomavirus type 11 recombinant L1 capsomeres induce virus-neutralizing antibodies. J Virol. 1998;72:6151–54. doi: 10.1128/jvi.72.7.6151-6154.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yuan H, Estes PA, Chen Y, Newsome J, Olcese VA, Garcea RL, et al. Immunization with a pentameric L1 fusion protein protects against papillomavirus infection. J Virol. 2001;75(17):7848–53. doi: 10.1128/JVI.75.17.7848-7853.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chandrachud LM, Grindlay GJ, McGarvie GM, O’Neil BW, Wagner ER, Jarrett WF, et al. Vaccination of cattle with the N-terminus of L2 is necessary and sufficient for preventing infection by bovine papillomavirus-4. Virology. 1995;211(1):204–8. doi: 10.1006/viro.1995.1392. [DOI] [PubMed] [Google Scholar]
- 73.Pastrana DV, Gambhira R, Buck CB, Pang YY, Thompson CD, Culp TD, et al. Cross-neutralization of cutaneous and mucosal Papillomavirus types with anti-sera to the amino terminus of L2. Virology. 2005;337(2):365–72. doi: 10.1016/j.virol.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 74.Gambhira R, Jagu S, Karanam B, Gravitt PE, Culp TD, Christensen ND, et al. Protection of rabbits against challenge with rabbit papillomaviruses by immunization with the N terminus of human papillomavirus type 16 minor capsid antigen L2. J Virol. 2007;81(21):11585–92. doi: 10.1128/JVI.01577-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Franco EL, Cuzick J, Hildesheim A, de Sanjose S. Chapter 20: Issues in planning cervical cancer screening in the era of HPV vaccination. Vaccine. 2006;24 (Suppl 3):S171–7. doi: 10.1016/j.vaccine.2006.05.061. [DOI] [PubMed] [Google Scholar]
- 76.Bosch FX, Castellsague X, de Sanjose S. HPV and cervical cancer: screening or vaccination? Br J Cancer. 2008;98(1):15–21. doi: 10.1038/sj.bjc.6604146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schiffman M, Castle PE. The promise of global cervical-cancer prevention. N Engl J Med. 2005;353(20):2101–4. doi: 10.1056/NEJMp058171. [DOI] [PubMed] [Google Scholar]
- 78.Solomon D. Chapter 14: Role of triage testing in cervical cancer screening. J Natl Cancer Inst Monogr. 2003;(31):97–101. doi: 10.1093/oxfordjournals.jncimonographs.a003489. [DOI] [PubMed] [Google Scholar]
- 79.Biscotti CV, Dawson AE, Dziura B, Galup L, Darragh T, Rahemtulla A, et al. Assisted primary screening using the automated ThinPrep Imaging System. Am J Clin Pathol. 2005;123(2):281–7. [PubMed] [Google Scholar]
- 80.Davey E, Barratt A, Irwig L, Chan SF, Macaskill P, Mannes P, et al. Effect of study design and quality on unsatisfactory rates, cytology classifications, and accuracy in liquid-based versus conventional cervical cytology: a systematic review. Lancet. 2006;367(9505):122–32. doi: 10.1016/S0140-6736(06)67961-0. [DOI] [PubMed] [Google Scholar]
- 81.Arbyn M, Bergeron C, Klinkhamer P, Martin-Hirsch P, Siebers AG, Bulten J. Liquid compared with conventional cervical cytology: a systematic review and meta-analysis. Obstet Gynecol. 2008;111(1):167–77. doi: 10.1097/01.AOG.0000296488.85807.b3. [DOI] [PubMed] [Google Scholar]
- 82.Cuzick J, Szarewski A, Cubie H, Hulman G, Kitchener H, Luesley D, et al. Management of women who test positive for high-risk types of human papillomavirus: the HART study. Lancet. 2003;362(9399):1871–6. doi: 10.1016/S0140-6736(03)14955-0. [DOI] [PubMed] [Google Scholar]
- 83.Ronco G, Giorgi-Rossi P, Carozzi F, Dalla Palma P, Del Mistro A, De Marco L, et al. Human papillomavirus testing and liquid-based cytology in primary screening of women younger than 35 years: results at recruitment for a randomised controlled trial. Lancet Oncol. 2006;7(7):547–55. doi: 10.1016/S1470-2045(06)70731-8. [DOI] [PubMed] [Google Scholar]
- 84.Ronco G, Segnan N, Giorgi-Rossi P, Zappa M, Casadei GP, Carozzi F, et al. Human papillomavirus testing and liquid-based cytology: results at recruitment from the new technologies for cervical cancer randomized controlled trial. J Natl Cancer Inst. 2006;98(11):765–74. doi: 10.1093/jnci/djj209. [DOI] [PubMed] [Google Scholar]
- 85.Castle PE, Sideri M, Jeronimo J, Solomon D, Schiffman M. Risk assessment to guide the prevention of cervical cancer. Am J Obstet Gynecol. 2007;197(4):356, e1–6. doi: 10.1016/j.ajog.2007.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wright TC, Jr, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D. 2006 consensus guidelines for the management of women with cervical intraepithelial neoplasia or adenocarcinoma in situ. Am J Obstet Gynecol. 2007;197(4):340–5. doi: 10.1016/j.ajog.2007.07.050. [DOI] [PubMed] [Google Scholar]
- 87.Wright TC, Jr, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D. 2006 consensus guidelines for the management of women with abnormal cervical cancer screening tests. Am J Obstet Gynecol. 2007;197(4):346–55. doi: 10.1016/j.ajog.2007.07.047. [DOI] [PubMed] [Google Scholar]
- 88.Wentzensen N, von Knebel Doeberitz M. Biomarkers in cervical cancer screening. Dis Markers. 2007;23(4):315–30. doi: 10.1155/2007/678793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Castle PE, Dockter J, Giachetti C, Garcia FA, McCormick MK, Mitchell AL, et al. A cross-sectional study of a prototype carcinogenic human papillomavirus E6/E7 messenger RNA assay for detection of cervical precancer and cancer. Clin Cancer Res. 2007;13(9):2599–605. doi: 10.1158/1078-0432.CCR-06-2881. [DOI] [PubMed] [Google Scholar]
- 90.Bulkmans NW, Berkhof J, Rozendaal L, van Kemenade FJ, Boeke AJ, Bulk S, et al. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year follow-up of a randomised controlled implementation trial. Lancet. 2007;370(9601):1764–72. doi: 10.1016/S0140-6736(07)61450-0. [DOI] [PubMed] [Google Scholar]
- 91.Mayrand MH, Duarte-Franco E, Rodrigues I, Walter SD, Hanley J, Ferenczy A, et al. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med. 2007;357(16):1579–88. doi: 10.1056/NEJMoa071430. [DOI] [PubMed] [Google Scholar]
- 92.Naucler P, Ryd W, Tornberg S, Strand A, Wadell G, Elfgren K, et al. Human papillomavirus and Papanicolaou tests to screen for cervical cancer. N Engl J Med. 2007;357(16):1589–97. doi: 10.1056/NEJMoa073204. [DOI] [PubMed] [Google Scholar]
- 93.Ronco G, Segnan N. HPV testing for primary cervical cancer screening. Lancet. 2007;370(9601):1740–2. doi: 10.1016/S0140-6736(07)61480-9. [DOI] [PubMed] [Google Scholar]
- 94.Arbyn M, Sasieni P, Meijer CJ, Clavel C, Koliopoulos G, Dillner J. Chapter 9: Clinical applications of HPV testing: A summary of meta-analyses. Vaccine. 2006;24 (Suppl 3):S78–89. doi: 10.1016/j.vaccine.2006.05.117. [DOI] [PubMed] [Google Scholar]
- 95.Results of a randomized trial on the management of cytology interpretations of atypical squamous cells of undetermined significance. Am J Obstet Gynecol. 2003;188(6):1383–92. doi: 10.1067/mob.2003.457. [DOI] [PubMed] [Google Scholar]
- 96.Arbyn M, Paraskevaidis E, Martin-Hirsch P, Prendiville W, Dillner J. Clinical utility of HPV-DNA detection: triage of minor cervical lesions, follow-up of women treated for high-grade CIN: an update of pooled evidence. Gynecol Oncol. 2005;99(3 Suppl 1):S7–11. doi: 10.1016/j.ygyno.2005.07.033. [DOI] [PubMed] [Google Scholar]
- 97.Goldie SJ, Gaffikin L, Goldhaber-Fiebert JD, Gordillo-Tobar A, Levin C, Mahe C, et al. Cost-effectiveness of cervical-cancer screening in five developing countries. N Engl J Med. 2005;353(20):2158–68. doi: 10.1056/NEJMsa044278. [DOI] [PubMed] [Google Scholar]
- 98.Sankaranarayanan R, Esmy PO, Rajkumar R, Muwonge R, Swaminathan R, Shanthakumari S, et al. Effect of visual screening on cervical cancer incidence and mortality in Tamil Nadu, India: a cluster-randomised trial. Lancet. 2007;370(9585):398–406. doi: 10.1016/S0140-6736(07)61195-7. [DOI] [PubMed] [Google Scholar]
- 99.Study Finds New Version of QIAGEN’s HPV Test for Developing Countries Could Reduce Risk of Cervical Cancer by More than Half When Combined With Appropriate Treatment.
- 100.Jeronimo J, Castle PE, Herrero R, Burk RD, Schiffman M. HPV testing and visual inspection for cervical cancer screening in resource-poor regions. Int J Gynaecol Obstet. 2003;83(3):311–3. doi: 10.1016/s0020-7292(03)00299-6. [DOI] [PubMed] [Google Scholar]
- 101.Mariategui J, Santos C, Taxa L, Jeronimo J, Castle PE. Comparison of depth of necrosis achieved by CO(2)- and N(2)O-cryotherapy. Int J Gynaecol Obstet. 2008;100(1):24–6. doi: 10.1016/j.ijgo.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 102.Seamans Y, Loesel C, Jeronimo J, Sellors J, Castle PE. Effect of cough technique and cryogen gas on temperatures achieved during simulated cryotherapy. BMC Womens Health. 2007;7:16. doi: 10.1186/1472-6874-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Schiffman M. Integration of human papillomavirus vaccination, cytology, and human papillomavirus testing. Cancer. 2007;111(3):145–53. doi: 10.1002/cncr.22751. [DOI] [PubMed] [Google Scholar]
- 104.Kovacic MB, Castle PE, Herrero R, Schiffman M, Sherman ME, Wacholder S, et al. Relationships of human papillomavirus type, qualitative viral load, and age with cytologic abnormality. Cancer Res. 2006;66(20):10112–9. doi: 10.1158/0008-5472.CAN-06-1812. [DOI] [PubMed] [Google Scholar]
- 105.Jeronimo J, Schiffman M. Colposcopy at a crossroads. Am J Obstet Gynecol. 2006;195(2):349–53. doi: 10.1016/j.ajog.2006.01.091. [DOI] [PubMed] [Google Scholar]
- 106.Gage JC, Hanson VW, Abbey K, Dippery S, Gardner S, Kubota J, et al. Number of cervical biopsies and sensitivity of colposcopy. Obstet Gynecol. 2006;108(2):264–72. doi: 10.1097/01.AOG.0000220505.18525.85. [DOI] [PubMed] [Google Scholar]
- 107.Jeronimo J, Massad LS, Castle PE, Wacholder S, Schiffman M. Interobserver agreement in the evaluation of digitized cervical images. Obstet Gynecol. 2007;110(4):833–40. doi: 10.1097/01.AOG.0000281665.63550.8f. [DOI] [PubMed] [Google Scholar]
- 108.Jeronimo J, Massad LS, Schiffman M. Visual appearance of the uterine cervix: correlation with human papillomavirus detection and type. Am J Obstet Gynecol. 2007;197(1):47, e1–8. doi: 10.1016/j.ajog.2007.02.047. [DOI] [PubMed] [Google Scholar]


