Abstract
A new rosane diterpenoid, 3α-hydroxy-5,15-rosadien-11-one (3), was isolated, together with a known rosane diterpenoid, 5,15-rosadiene-3,11-dione (4), and an aromadendrane sesquiterpenoid, ent-cyclocolorenone (5), from the Et2O extract of an unidentified Argentine liverwort Anastrophyllum species. Moreover, four known sesquiterpene lactones 6–9 and two known bibenzyls 10, 11 were isolated from the Et2O extracts of Argentine Frullania brasiliensis and Radula voluta, respectively. The structures of compounds 3–11 were determined by the use of NMR techniques.
Keywords: Jungermanniales, liverwort, Radula voluta, Frullania brasiliensis, Anastrophyllum species, bibenzyl, rosane, chemosystematics
1. Introduction
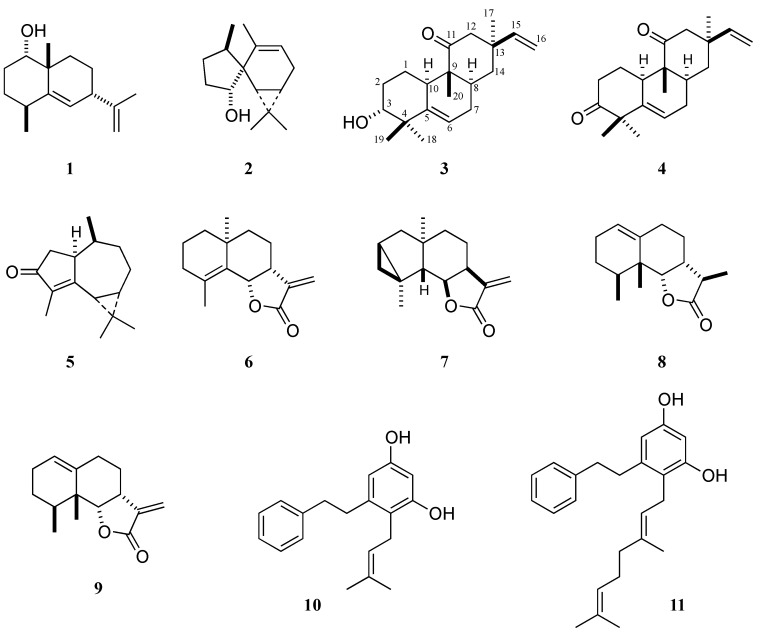
Due to their small morphology, liverworts (Hepaticae) are difficult to classify and identify. However, they are a rich source of terpenoids and aromatic compounds, which can be used to evaluate their chemosystematics [1,2]. In our search for new biologically active substances, we continue to study the chemical constituents of liverworts. Many liverworts are endemic to the southern hemisphere, including Oceania and South America. Recently, we have reported the structures of new sesqui- and diterpenoids from New Zealand liverworts [3]. We also reported the isolation of new sesquiterpenoids 1 and 2 from an unidentified Gackstroemia species from New Zealand [4]. During the course of our investigation of the chemical constituents of three Argentine Jungermanniales species (unidentified Anastrophyllum species, Radula voluta and Frullania brasiliensis), we isolated a new rosane diterpenoid 3 and six previously known compounds: Rosane 4, aromadendrane 5, two eudesmanes 6 and 7, two eremophilanes 8 and 9, and two bibenzyls 10 and 11 (Figure 1) and characterized their chemical structures. The chemosystematics of these three species from Argentina are discussed.
Figure 1.
Terpenoids and bibenzyls isolated from Argentine liverworts.
2. Results and Discussion
The new rosane diterpenoid 3 was isolated from the ether extract of an unidentified Anastrophyllum species by chromatographic separation (silica gel and Sephadex LH-20), together with ent-cyclocolorenone (5) [5] and 5,15-rosadiene-3,11-dione (4) [6], whose spectral and physical data were identical with those of authentic samples.
The mass spectrum of 3 showed m/z 302 [M]+ and its molecular formula, C20H30O2 (calcd. 302.2245), was confirmed by HR-EIMS. The IR spectrum demonstrated the presence of hydroxy and carbonyl groups. The 1H-NMR spectrum (Table 1) of 3 showed the signals of terminal vinyl protons (δ 4.94 dd, 4.96 dd, 5.83 dd), an olefinic proton (δ 5.56 d), a proton (δ 3.23 dd) on a carbon bearing a hydroxy group and four tertiary methyls. The 13C-NMR spectrum (Table 2) exhibited 20 carbons, and its DEPT spectrum indicated the presence of trisubstituted olefinic carbons (δ 117.0 d, 145.5 s), terminal vinyl carbons (δ 110.2 t, 147.9 d), a carbonyl carbon (δ 214.7) and a methine (δ 77.2) with a hydroxy group, together with four methyls, four methylenes, two methines and three quaternary carbons. Since the 13C-NMR (Table 2) data was similar to those of the 5,15-rosadiene-3,11-dione (4) [6], compound 3 was suggested to be a rosane diterpenoid.
Table 1.
1H-NMR data of 3 (600 MHz, CDCl3) a.
| H | H | ||
|---|---|---|---|
| 1 | 1.01 m | 14 | 1.36 dd (10.2, 2.5) α |
| 1.97 m | 1.76 m β | ||
| 2 | 1.69 m | 15 | 5.83 dd (17.3, 10.7) |
| 1.78 m | |||
| 3 | 3.23 dd (11.5, 4.7)b | 16 | 4.94 dd (10.7, 0.8) |
| 4.96 dd (17.3, 0.8) | |||
| 6 | 5.56 d (6.3) | 17 | 0.97 s |
| 7 | 1.87 m | 18 | 0.99 s |
| 1.98 m | |||
| 8 | 1.77 m | 19 | 1.15 s |
| 10 | 2.72 m | 20 | 0.98 s |
| 12 | 1.95 dd (12.6, 2.5) α | ||
| 2.73 d (12.6) β |
a Chemical shift values are in δ (ppm); b Coupling constants are in Hz.
Table 2.
13C-NMR of 3 and 4 (100 MHz, CDCl3) a.
| C | 3 | 4 [6] | C | 3 | 4 [6] |
|---|---|---|---|---|---|
| 1 | 25.3 | 25.2 | 11 | 214.7 | 214.4 |
| 2 | 30.3 | 37.8 | 12 | 48.4 | 48.5 |
| 3 | 77.2 | 214.6 | 13 | 41.6 | 41.6 |
| 4 | 42.1 | 51.0 | 14 | 38.4 | 38.4 |
| 5 | 145.5 | 144.2 | 15 | 147.9 | 147.7 |
| 6 | 117.0 | 118.2 | 16 | 110.2 | 110.4 |
| 7 | 28.9 | 29.0 | 17 | 23.5 | 23.5 |
| 8 | 38.3 | 38.2 | 18 | 21.4 | 22.7 |
| 9 | 48.9 | 49.4 | 19 | 24.4 | 29.5 |
| 10 | 37.9 | 38.6 | 20 | 12.7 | 12.0 |
a Chemical shift values are in δ (ppm).
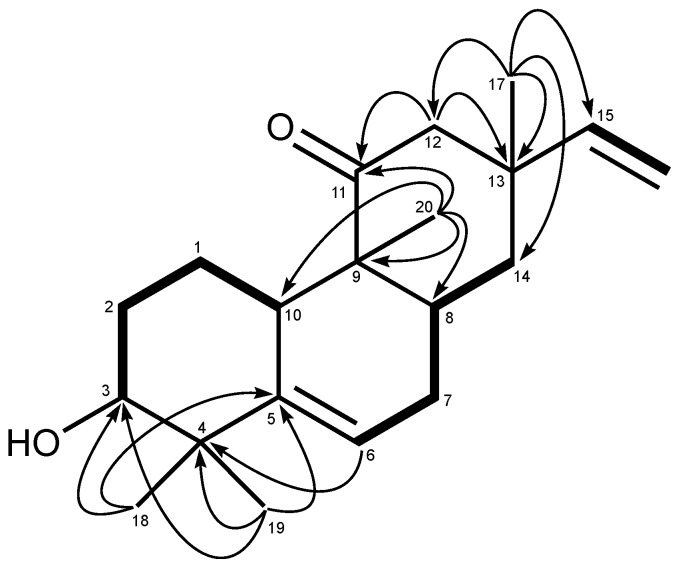
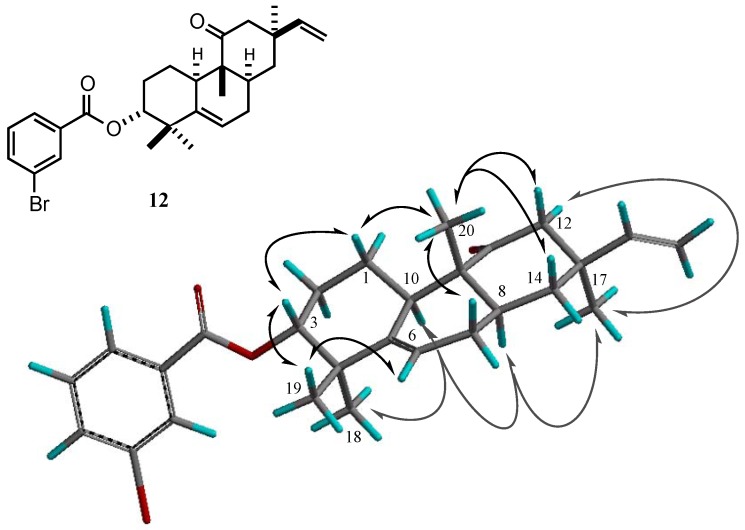
The 1H-1H COSY of 3 confirmed three partial segments: (A) -CH(OH)-CH2-CH2-CH-, (B) -CH2-CH-CH2-CH=C-, and (C) –CH=CH2. As seen in the HMBC spectrum (Figure 2), the tertiary methyl at Η−17 correlated with the methylene carbon at C-4 in segment B, the terminal vinyl carbon at C-15, the quaternary carbon at C-13 and the isolated methylene carbon at C-12, methylene protons at H-12 of which correlated with the carbonyl carbon at C-11. The other tertiary methyl at H-20 correlated with the methine at C-10 in segment A, the carbonyl carbon, the quaternary carbon at C-9 and the aliphatic methine in segment B. The methyl groups at H-18 and 19 correlated with the methine at C-3 bearing hydroxy group in segment A, an aliphatic quaternary carbon and a trisubstituted olefinic quaternary carbon. On the basis of the above results, the structure of 3 was elucidated to be 3-hydroxy-5,15-rosadien-11-one. The stereochemistry of 3 was clarified by the NOESY spectrum of the m-bromobenzoate derivative 12 of 3. NOE correlations (Figure 3) of 12 were observed between: (i) H-3 and H-1β, H-18; (ii) H-1β and H-20; (iii) H-20 and H-7β, H-12β, H-14β; (iv) H-19 and H-10; (v) H-10 and H-8; (vi) H-8 and H-17. Moreover the CD spectrum of 3 showed a positive Cotton effect (λmax 297) as the same Cotton effect (λmax 298) as 4 [6]. Thus, the structure of 3 was shown to be 3α-hydroxy-5,15-rosadien-11-one. However, the absolute configuration of 3 has not yet clarified because the use of only the Cotton effect of the CD spectrum was not able to establish it unequivocally.
Figure 2.
1H-1H (bold line) and long range 1H-13C (arrows) correlations of 3.
Figure 3.
NOE correlations of 12.
Frullania brasiliensis contains sesquiterpene lactones [7]. We reexamined the same species for the purpose of potentially isolating new compounds. A chromatographic separation of the ether extract of Frullania brasiliensis resulted in the isolation of two eudesmanes, (+)-frullanolide (6), which causes potent allergenic contact dermatitis [8], and nepalensolide A (7) [9], and two eremophilanes, 5-epi-dilatanolide A (8) [7] and 5-epi-dilatanolide B (9) [7].
Two bibenzyl compounds, 3,5-dihydroxy-2-(3-methyl-2-butenyl)bibenzyl (10) [10,11] and 2-geranyl-3,5-dihydroxybibenzyl (11) [12], were chromatographically isolated from the ether extract of Radula voluta.
Fusicoccane and sphenolobane diterpenoids are common chemical components isolated from Anastrophyllum species, e.g., from A. minutum [13], A. aurztum [14] and A. donnianum [15]. These diterpenoids are very characteristic for Anastrophyllum genus belonging to the Jungermanniaceae. However, neither sphenolobane nor fusicoccane were isolated from the present unidentified Anastrophyllum species. Those isolates included naturally rare rosane diterpenoid and aromadendrane sesquiterpenoid. Therefore, the unidentified Anastrophyllum species may be from a chemically different taxon from the other Anastrophyllum species.
Eudesmane and eremophilane sesquiterpene lactones have been isolated from F. brasiliensis (Frullaniaceae) [7] and are considered the most important chemical markers of the Frullaniaceae [1,2,16]. The present F. brasiliensis also contained the same eudesmanolides and eremophilanolides as those reported.
A number of bibenzyls and prenyl bibenzyls were isolated from European, New Zealand, Ecuador and Japanese Radula species (Radulaceae) [1,2,17]. R. voluta also produced bibenzyls 10 and 11, which are ubiquitous components in the Radula species. The Radula including R. voluta is chemically very isolated from the other liverworts examined so far, since the presence of terpenoids is very rare.
3. Experimental
3.1. General
1H and 13C-NMR: 200, 400 and 600 MHz (1-NMR) and 100, 150 MHz (13C-NMR). Chemical shift values were expressed in δ (ppm) downfield from tetramethylsilane as an internal standard (1H-NMR) and δ 77.03 (ppm) from CDCl3 as a standard (13C-NMR). TLC: Visualized under UV (254 nm) light and by spraying with 10% H2SO4 or Godin reagent [18] followed by heating at 120–130 °C. MeOH-CH2Cl2 (1:1) was used for Sephadex LH-20. [α]D: CHCl3. Radula voluta Tayl. ex Gott., Lindenb. & Nees, Frullania brasiliensis Raddi and unidentified Anastrophyllum species were collected in Argentina in 2005 and identified by Prof. Dr. S. R. Gradstein (University of Göttingen, Germany). The voucher specimen was deposited at the Institute of Pharmacognosy, Tokushima Bunri University.
3.2. Extraction and Isolation
The dry material (14.7 g) of the unidentified Anastrophyllum species was ground and extracted with Et2O. The crude extract (471.4 mg) was divided into 10 fractions by column chromatography on silica gel (n-hexane-EtOAc gradient). Fraction 4 gave ent-cyclocolorenone (5) ([α]D +376.9° c 1.45; 15.6 mg) and 5,15-rosadiene-3,11-dione (4) (6 mg) by rechromatography on silica gel (n-hexane-EtOAc 17:3, CH2Cl2-Et2O 49:1). 3α-Hydroxy-5,15-rosadien-11-one (3) (4.5 mg) was purified from Fraction 6 by Sephadex LH-20 and silica gel (n-hexane-EtOAc 19:1).
The crude Et2O extract (1.9 g) of F. brasiliensis (90.8 g) was chromatographed on silica gel (n-hexane-EtOAc gradient) to give six fractions. Fraction 3 was rechromatographed on Sephadex LH-20, silica gel (n-hexane-EtOAc or n-hexane-Et2O), MPLC (Si-60, toluene) and prep. HPLC (Cosmosil 5SL-II, n-hexane-Et2O 98:2) to give (+)-frullanolide (6) (59.3 mg) and nepalensolide A (7) (15.1 mg). 5-Epi-dilatanolide A (8) (13.9 mg) and 5-epi-dilatanolide B (9) (5.4 mg) were isolated from Fraction 5 by a combination of reverse phase silica gel (CH3CN), MPLC (Si-60, n-hexane-Et2O 4:1) and prep. HPLC (UK-silica, n-hexane-EtOAc 9:1).
The dry material (690 mg) of R. voluta was ground and extracted with Et2O. The crude extract (79 mg) was chromatographed on Sephadex LH-20 and silica gel to give 3,5-dihydroxy-2-(3-methyl-2-butenyl)bibenzyl (10) (17.6 mg) and 2-geranyl-3,5-dihydroxybibenzyl (11) (3.1 mg).
3α-Hydroxy-5,15-rosadien-11-one (3): [α]D +71.3° (c 1.31); CD (EtOH): Δε297nm +1.70, Δε211nm −0.51 (c = 9.77 × 10−4); FTIR νmax cm−1: 3438, 1704; 1H-NMR see Table 1; and 13C-NMR see Table 2; HR-EIMS: calcd for C20H30O2: 302.2245. Found: 302.2253; EIMS m/z (rel. int.): 302[M]+(10), 284(100), 269(47), 241(76), 226(11), 211(11), 201(11), 187(47), 173(47), 171(46), 159(23), 151(34), 145(21), 134(27), 119(26), 105(29), 91(25), 79(113), 67(11), 55(13), 41(15).
m-Bromobenzoate 12:To compound 3 (3.5 mg) in dry CH2Cl2 (2 mL) was added m-bromobenzoic acid (5 mg), DCC (4 mg) and DMAP (2 mg) and the solution was stirred at r.t. overnight. The reaction mixture was filtered and chromatographed on silica gel (n-hexane-EtOAc 19:1) to yield m-bromobenzoate 12 (4.2 mg). 1H-NMR (600 MHz, CDCl3): δ 8.17 (1H, t, J = 1.9 Hz), 7.98 (1H, dd, J = 8.0, 1.1 Hz), 7.69 (1H, ddd, J = 8.0, 1.9, 1.1 Hz), 7.33 (1H, t, J = 8.0 Hz), 2.05 (1H, m, H-1α), 1.16 (1H, dd, J = 12.9, 4.1 Hz, H-1β), 1.82-1.95 (3H, m, H-2, H-2, H-7α), 4.73 (1H, dd, J = 11.3, 4.7 Hz, H-3), 5.62 (1H, d, J = 6.3 Hz, H-6), 1.99 (1H, m, H-7β), 1.80 (1H, m, H-8), 2.81 (1H, brd, J = 12.4 Hz, H-10), 1.97 (1H, dd, J = 12.6, 2.2 Hz, H-12α), 2.74 (1H, d, J = 12.6 Hz, H-12β), 1.38 (1H, dd, J = 9.9, 2.5 Hz, H-8α), 1.77 (1H, d, J = 12.4 Hz, H-14β), 5.84 (1H, dd, J = 17.3, 10.7 Hz, H-15), 4.95 (1H, dd, J = 10.7, 0.8 Hz, H-16), 4.97 (1H, dd, J = 17.3, 0.8 Hz, H-16), 0.99 (3H, s, H-17), 1.22 (3H, s, H-18), 1.10 (3H, s, H-19), 1.02 (3H, s, H-20); 13C-NMR (100 MHz, CDCl3): δ 214.6, 164.8, 147.9, 144.5, 135.8, 132.8, 132.6, 130.0, 128.2, 122.5, 117.9, 110.2, 80.0, 49.0, 48.4, 41.6, 41.2, 38.4, 38.3, 37.9, 28.9, 26.7, 25.0, 24.6, 23.5, 23.1, 12.6.
4. Conclusions
A new rosane diterpenoid 3 was isolated from the unidentified Argentine liverwort Anastrophyllum species, together with a known rosanediterpenoid 4 and an aromadendranesesquiterpenoid 5. The known bibenzyls 10 and 11 and sesquiterpene lactones 6–9 were isolated from Argentine Radula voluta and Frullania brasiliensis.
Acknowledgements
We thank A. Bardón (Tucumán National University, Argentina) for the collection of the liverworts and S.R. Gradstein (University of Göttingen, Germany) for the identification of their species. Thanks are also due to M. Tanaka (TBU) and Miss Y. Okamoto (TBU) for measurements of 600 MHz NMR spectra and mass spectra, and N. Minakami and Miss H. Morinaga for their technical assistance.
Footnotes
Sample Availability: Samples of the compounds 5–10 are available from the authors.
References and Notes
- 1.Asakawa Y. Chemical constituents of the Hepaticae. In: Herz W., Grisebach H., Kirby G.W., editors. Progress in the Chemistry of Organic Natural Products. Vol. 42. Springer-Verlag; Wien, Austria: 1982. pp. 1–269. [Google Scholar]
- 2.Asakawa Y. Chemical constituents of the Bryophytes. In: Herz W., Kirby G.W., Moore R.E., Steglich W., Tamm Ch., editors. Progress in the Chemistry of Organic Natural Products. Vol. 65. Springer-Verlag; Wien, Austria: 1995. pp. 1–618. [Google Scholar]
- 3.Asakawa Y., Toyota M., Nagashima F., Hashimoto T. Chemical constituents of selected Japanese and New Zealand liverworts. Nat. Prod. Commun. 2008;3:289–300. [Google Scholar]
- 4.Nagashima F., Kuba Y., Ogata A., Asakawa Y. Sesqui- and diterpenoids from three New Zealand liverworts Bazzania novae-zelandiae, Gackstroemia sp. and Dendromastigophora sp. Nat. Prod. Res. 2010;24:68–75. doi: 10.1080/14786410902800749. [DOI] [PubMed] [Google Scholar]
- 5.Matsuo A., Nakayama M., Sato S., Nakamoto T., Uto S., Hayashi S. (−)-Maalioxide and (+)-cyclocolorenone, enantiomeric sesquiterpenoids from the liverwort, Plagiochila acanthophylla subsp. japonica. Experientia. 1974;30:321–322. [Google Scholar]
- 6.Feld H., Zapp J., Becker H. Secondary metabolites from the liverwort Tylimanthus renifolius. Phytochemistry. 2003;64:1335–1340. doi: 10.1016/j.phytochem.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 7.Bardón A., Mitre G.B., Kamiya N., Toyota M., Asakawa Y. Eremophilanolides and other constituents from the Argentine liverwort Frullania brasiliensis. Phytochemistry. 2002;59:205–213. doi: 10.1016/s0031-9422(01)00452-6. [DOI] [PubMed] [Google Scholar]
- 8.Asakawa Y., Muller J.-C., Ourisson G., Foussereau J., Ducombs G. Nouvelles lactones sesquiterpéniques de Frullania (Hépaticae). Isolement, structures, propriétés allergisantes. Bull. Soc. Chim. France. 1976:1465–1466. [Google Scholar]
- 9.Tori M., Miyazaki N., Kondo K., Taira Z., Asakawa Y. Nepalensolide A, Novel sesquiterpene lactone from the liverwort Frullania nepalensis. Compound breaking the Samek rule. A study by NOE and X-ray. Chem. Lett. 1990:2115–2116. [Google Scholar]
- 10.Asakawa Y., Toyota M., Takemoto T. Seven bibenzyls and a dihydrochalcone from Radula variabilis. Phytochemistry. 1978;17:2005–2010. doi: 10.1016/S0031-9422(00)88752-X. [DOI] [Google Scholar]
- 11.Crombie L.W., Crombie W.M.L., Firth D.F. Synthesis of bibenzyl cannabinoids, hybrids of two biogenetic series found in Cannabis sativa. J. Chem. Soc. Perkin Trans. 1. 1988:1263–1270. [Google Scholar]
- 12.Asakawa Y., Kondo K., Tori M., Hashimoto T., Ogawa S. Prenyl bibenzyls from the liverwort Radula kojana. Phytochemistry. 1991;30:219–234. doi: 10.1016/0031-9422(91)84129-G. [DOI] [Google Scholar]
- 13.Beyer J., Becker H., Toyota M., Asakawa Y. Diterpenoids with a novel skeleton from the liverwort Anastrophyllum minutum. Phytochemistry. 1987;26:1085–1089. doi: 10.1016/S0031-9422(00)82355-9. [DOI] [Google Scholar]
- 14.Zapp J., Burkhardt G., Becker H. Sphenolobane and fusicoccane diterpenoids from the liverwort Anastrophyllum aurztum. Phytochemistry. 1994;37:787–793. doi: 10.1016/S0031-9422(00)90359-5. [DOI] [Google Scholar]
- 15.Buchanan M.S., Connolly J.D., Rycroft D.S. Sphenolobane diterpenoids from the liverwort Anastrophyllum donnianum. Phytochemistry. 1996;43:1297–1301. doi: 10.1016/S0031-9422(96)00466-9. [DOI] [Google Scholar]
- 16.Asakawa Y. Chemosystematics of Hepaticae. Phytochemistry. 2004;65:623–669. doi: 10.1016/j.phytochem.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Kraut L., Mues R., Zinsmeister H.D. Prenylated bibenzyl derivatives from Lethocolea glossophylla and Radula voluta. Phytochemistry. 1997;45:1249–1255. [Google Scholar]
- 18.Godin P. A new spray reagent for paper chromatography of polyols and cetoses. Nature (London) 1954;174:134. [Google Scholar]