Abstract
The screening of several Chinese mangrove plants for insecticidal principles showed that ethanol extract of Ceriops tagal stems and twigs possessed significant feeding deterrent activity against the red flour beetle, Tribolium castaneum (Family: Rhizophoraceae). From the ethanol extract, three feeding deterrent diterpenoids were isolated by bioassay-guided fractionation. The compounds were identified as tagalsin A, B, and H on the basis of their phytochemical and spectral data. Tagalsin A, B, and H exhibited strong feeding deterrent activity against T. castaneum adults with EC50 values of 375.3 ppm, 277.3 ppm, and 285.45 ppm, respectively.
Keywords: feeding deterrents, Ceriops tagal, Tribolium castaneum, tagalsin
1. Introduction
The red flour beetle [Tribolium castaneum (Herbst)] is one of the most widespread and destructive primary insect pests of stored cereals [1]. Infestations not only cause significant losses due to the consumption of grains; they also result in elevated temperature and moisture conditions that lead to an accelerated growth of molds, including toxigenic species [2]. Botanical pesticides have the advantage of providing novel modes of action against insects that can reduce the risk of cross-resistance as well as offering new leads for design of target-specific molecules [3,4]. Control of stored product insects relies heavily on the use of synthetic insecticides and fumigants, which has led to problems such as disturbances of the environment, increasing costs of application, pest resurgence, pest resistance to pesticides and lethal effects on non-target organisms in addition to direct toxicity to users [5]. These problems have highlighted the need for the development of new types of selective stored product pest-control alternatives. During a screening program for new agrochemicals from Chinese medicinal herbs and wild plants, ethanol extract of Chinese mangrove plant, Ceriops tagal (Perr.) C.B. Robinson stems and twigs (Family: Rhizophoraceae) were found to possess significant feeding deterrent activity against T. castaneum. This plant is well distributed in Southern China, Eastern Africa, and Oceania [6]. It is used as a folkloric medicine in China. The bark of C. tagal is a powerful astringent and is used in the treatment of hemorrhage in defecation. The oil of the breed is a kind of antipruritic and used in the treatment of acariasis and chillblain. The leaves, when boiled in water, are used as a substitute for quinine to heal paludism [7]. The bark of this plant has been used for the treatment of infected wounds in Thailand and for obstetric and hemorrhagic conditions in the Philippines [8]. The decoction of its leaves has been used for the treatment of malaria in China [9], whereas that of its bark has been utilized for the treatment of hemorrhage and malignant ulcers in India [10]. The chemical constituents and bioactivities of C. tagal have been extensively studied and the known chemical constituents of this medicinal herb include monoterpenoids, diterpenoids, triterpenoids, flavonoids, alkaloids, polyphenolics, cardiac glycosides, saponins and sterols [7,8,11,12,13,14,15,16,17,18,19,20,21]. However, the bioactive compounds against insects have not been isolated and identified from this plant. In this paper, we report the isolation and identification of three feeding deterrents contained in C. tagal stems and twigs against T. castaneum by bioassay-guided fractionation.
2. Results and Discussion
2.1. Isolated Bioactive Compounds
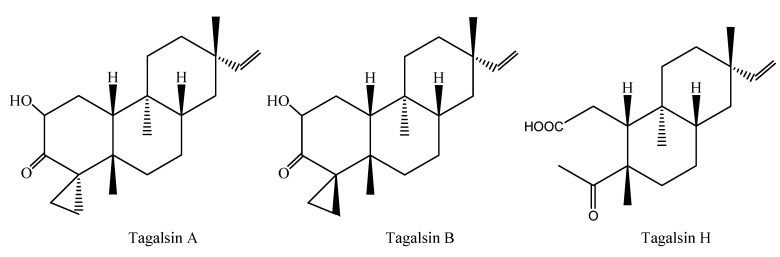
Three bioactive compounds were isolated and based on bioassay-guided fractionation and identified based on their spectroscopic data and comparison with literature vales. Their chemical structures are given in Figure 1.
Figure 1.
Structures of feeding deterrents isolated from Ceriops tagal stems and twigs.
2.2. Feeding Deterrent Activity
The feeding deterrent activity of the three isolated compounds against the red flour beetle is shown in Table 1. The three pure compounds, tagalsin A, B and H exhibited significant feeding deterrent activity against T. castaneum adults at a concentration of 30 ppm and above in a concentration-dependent manner (Table 1). The concentration used in this study (30 ppm) to observe feeding deterrent effects was much higher than for the commercially available products such as margosan-O, active at a 3.75 ppm azadirachtin level [22]. However, it was comparable with another commercially product toosendanin at 20 ppm [23]. The three compounds were evaluated for feeding deterrent activity against stored product insect pests for the first time.
Table 1.
Feeding deterrent activity of the pure compounds isolated from C. tagal stems and twigs against T. castaneum adults.
| Treatment | Concentration (ppm) | Consumption of diet * (% control ± SD) | EC50 (95% FL) | Slope ± SD | Chi square (χ2) |
|---|---|---|---|---|---|
| Control | 100.00 ± 4.83a | - | - | ||
| Tagalsin A | 1000 | 40.05 ± 3.78e | |||
| 300 | 53.15 ± 4.23d | 375.3 | 2.43 ± 0.16 | 27.38 | |
| 100 | 73.43 ± 4.89c | (327.8-428.8) | |||
| 30 | 88.28 ± 3.674b | ||||
| 10 | 97.56 ± 2.79ab | ||||
| Tagalsin B | 1000 | 38.85 ± 4.12e | |||
| 300 | 47.65 ± 4.33d | 277.6 | 2.64 ± 0.15 | 19.36 | |
| 100 | 65.32 ± 5.02c | (245.8-309.3) | |||
| 30 | 84.17 ± 3.43b | ||||
| 10 | 95.23± 3.78a | ||||
| Tagalsin H | 1000 | 35.41 ± 4.57e | |||
| 300 | 49.12 ± 5.03d | 285.4 | 2.56 ± 0.14 | 32.67 | |
| 100 | 63.26 ± 4.09c | (244.9-327.1) | |||
| 30 | 73.72 ± 4.14b | ||||
| 10 | 92.86 ± 3.24a |
* Multiple range test using Tukey’s test (P < 0.05). Within each compound, the same letters denote treatments not significantly different from each other.
Dietary tagalsin A, B and H possessed feeding deterrent activity against T. castaneum adults (EC50 = 375.3, 277.6 and 285.4 ppm, respectively). When compared with the commercial feeding deterrent, toosendanin, the three isolated compounds were 4–5 times less active against T. castaneum adults because toosendanin exhibited feeding deterrent activity against T. castaneum adults with an EC50 value of 66 ppm [23,24]. In the previous report [20], tagalsins Q, R, and U also isolated from C. tagal the stems and twigs showed moderate antifeedant activity against the third-instar larvae of Brontispa longissima at a concentration of 1 mg/mL.
3. Experimental
3.1. Plant Material
Fresh stems and twigs (10 kg) of C. tagal were collected at the mangrove garden in Hainan Island (20.02° N latitude and 110.20° E longitude), China, in September 2010. The stems and twigs were air-dried (7.8 kg) and ground to a powder using a grinding mill (Retsch Muhle , Germany). The species was identified, and the voucher specimens (BNU-HSL-Dushuahan-2010-09-15-003) were deposited at the Herbarium (BNU) of College of Life Sciences, Beijing Normal University.
3.2. Insects
The red flour beetle, T. castaneum were obtained from laboratory cultures maintained for the last 10 years in the dark in incubators at 28–30 °C and 70%–80% relative humidity. T. castaneum was reared on wheat flour mixed with yeast (10:1, w:w). Adults of T. castaneum used in all the experiments were about 2 weeks old.
3.3. Extraction and Isolation of Active Ingredients
The powdered stems and twigs of C. tagal were extracted with 95% ethanol (100 L) at room temperature over a period of three weeks, and the extract was evaporated under reduced pressure using a vacuum rotary evaporator to afford a syrupy gum (324 g). This syrup was partitioned between methanol-water and petroleum ether (3 × 5,000 mL). The petroleum ether extracts were evaporated off to give a residue (35 g). The aqueous layer was re-partitioned with chloroform (3 × 5,000 mL) to provide a residue (187 g) after evaporation of chloroform. Further partitioning with ethyl acetate (3 × 5,000 mL) gave a residue (96 g) after evaporation of ethyl acetate. Based on the previously described bioassays, only the petroleum ether extracts exhibited strong antifeedant action and were chosen for further fractionation. The petroleum ether residue (35 g) was applied to a silica gel column (160–200 mesh, Qingdao Marine Chemical Plant, Shandong Province, China), eluting with petroleum ether containing increasing accounts of ethyl acetate (from 100:1 to 1:2) to give thirteen combined fractions according to TLC detection. Based on the previously described bioassays, fractions 3 and 5 were chosen for further purification. Fraction 3 (0.8 g) was subjected to a Sephadex LH-20 column (18–110 μm, Pharmacia) and eluted with CHCl3-MeOH (1:1) to yield tagalsin H (13.0 mg). Fraction 5 (64 mg) was subjected to silica gel column and eluted with petroleum ether-acetone (8:1) to afford tagalsins A (15.6 mg) and B (17.5 mg). Tagalsin A was recrystallized as needles from acetone. The structures of the compounds were elucidated based on mass spectrometry and nuclear magnetic resonance.
3.4. Feeding Deterrent Activity
A flour disk bioassay was used to direct the isolation of active compounds from C. tagal extracts according to the method of Xie et al. [22] with some modifications [1,24]. Wheat flour (1.0 g) was ultrasonically stirred in distilled water (5 mL) and ethanol (50 µL) containing a fraction or pure compound was added. Pure compounds were first dissolved in ethanol (500 μL) and two drops of Tween-20 (approximately 50 μg) were added to the wheat flour suspension. Aliquots (200 μL) of this stirred suspension were placed on the bottom of a polystyrene Petri dish to form disks. The pipette was fitted with a disposable tip that had an opening enlarged to about 2 mm internal diameter by cutting about 1 cm from the bottom of the tip with a razor blade. The same amounts of ethanol and Tween-20 were applied to produce the control flour disks. The flour disks were left in the fume-hood overnight to air dry. The flour disks were then transferred to an incubator to equilibrate at 28–30 °C and 70%–80% R.H. for 48 h. Each flour disk weighed between 36 and 39 mg. The moisture content of the disk was determined to be 13.5 ± 0.1% using the Kett’s Grain moisture tester (Model PB-1D2, Japan). The disks were placed in glass vials (diameter 2.5 cm, height 5.5 cm) for weighing. Twenty group-weighed, unsexed insects were then added to each vial prior to further weighing. All the insects were starved for 24 h before use. Six replicates were carried out for all treatments and controls. The experimental set-up was left in the incubator for 3 days. Finally, the uneaten parts of the flour disks were weighed. The insect consumption for the different test substances was compared to the control group. Glass vials containing treated flour disks but without insects were prepared to determine any decrease in weights that might have occurred due to evaporation of solvents. Extracts/fractions were tested feeding deterrent activity at a concentration of 1,000 ppm in bioactivity-guided fractionation.
3.5. Apparatus
Melting points were measured on a Buchi 535. 1H- and 13C-NMR spectra were recorded on a Bruker Avance DRX 500 instrument using CDCl3 as solvent with TMS as internal standard. EI-MS were determined on an ThermoQuest Trace 2000 mass spectrometer at 70 eV (probe), ESI-MS were determined on a Finnigan LCQ mass spectrometer.
3.6. Compound Characterization
Tagalsin A. Pale yellow needle crystals. m.p. 68–71 °C [67–69 °C (11)]. EI-MS m/z (%): 316 [M]+ (15), 286 (17), 259 (20), 179 (27), 163 (53), 136 (100), 107 (93), C20H29O3. 1H-NMR δ (ppm): 6.35 (1H, d, J = 6.8 Hz, H-1), 5.80 (1H, dd, J = 10.5, 17.5 Hz, H-15), 4.93 (1H, d, J = 17.5 Hz, H-16), 4.85 (1H, d, J = 10.5 Hz, H-16), 3.43 (1H, d, J = 6.0 Hz, H-18), 2.95 (1H, d, J = 6.0 Hz, H-18), 2.15 (1H, d, J = 6.8 Hz, H-10), 1.52 (1H, ddd, J = 3.0, 4.0, 12.5 Hz, H-11), 1.48 (2H, m, H-7, 12), 1.45 (1H, m, H-6), 1.41 (1H, dd, J = 10.5, 12.5 Hz, H-11), 1.34 (1H, dd, J = 11.5, 12.5 Hz, H-14), 1.21 (1H, dd, J =3.0, 14.0 Hz, H-12), 1.18 (1H, m, H-6), 1.17 (1H, s, H-19), 1.06 (1H, m, H-7), 1.05 (1H, m, H-17), 1.02 (1H, m, H-14), 0.79 (1H, s, H-20). 13C-NMR δ (ppm): 190.8 (C-3), 150.3 (C-15), 146.9 (C-2), 120.8 (C-1), 108.5 (C-16), 60.3 (C-4), 54.5 (C-10), 50.4 (C-18), 40.6 (C-8), 39.8 (C-9), 39.5 (C-14), 36.4 (C-13), 35.9 (C-5), 34.9 (C-11), 33.9 (C-6), 31.8 (C-12), 31.6 (C-19), 27.3 (C-7), 22.8 (C-17), 12.1 (C-20). The 1H and 13C-NMR data were in agreement with the reported data [11].
Tagalsin B. White solid. m.p. 66–69 °C [66–68 °C (11)]. EI-MS m/z (%): 316 [M]+ (17), 283 (20), 255 (18), 175 (49), 136 (63), 107 (100), 81 (69), 67 (43), 55 (50), C20H29O3. 1H-NMR δ (ppm): 6.31 (1H, d, J = 6.5 Hz, H-1), 5.87 (1H, dd, J = 10.5, 17.5Hz, H-15), 4.92 (1H, d, J = 17.5 Hz, H-16), 4.85 (1H, d, J = 10.5 Hz, H-16), 3.13 (1H, d, J = 6.0 Hz, H-18), 3.09 (1H, d, J = 6.0 Hz, H-18), 2.19 (1H, d, J = 6.5 Hz, H-10), 1.60 (1H, m, H-6), 1.56 (1H, m, H-11), 1.46 (2H, m, H-8, 12), 1.43 (1H, m, H-11), 1.34 (1H, dd, J = 13.0, 13.5 Hz, H-14), 1.25 (1H, m, H-12), 1.23 (1H, m, H-7), 1.21 (1H, s, H-19), 1.18 (2H, m, H-6, 7), 1.05 (1H, s, H-17), 0.72 (1H, s, H-20). 13C-NMR δ (ppm): 191.8 (C-3), 150.3 (C-15), 147.3 (C-2), 120.2 (C-1), 108.6 (C-16), 61.3 (C-4), 54.8 (C-10), 55.4 (C-18), 40.1 (C-8), 39.3 (C-14), 39.0 (C-9), 36.9 (C-5), 35.9 (C-13), 34.7 (C-11), 31.9 (C-6), 31.7 (C-12), 29.5 (C-19), 27.0 (C-7), 22.7 (C-17), 13.1 (C-20). The 1H and 13C-NMR data were in agreement with the reported data [11].
Tagalsin H. white powder. m.p. 103–105 °C [101–102 °C (11)]. ESI-MS m/z: 305.18 [M]+, C19H29O3. 1H-NMR δ (ppm): 5.80 (1H, dd, J = 10.5, 17.5 Hz, H-15), 4.88 (1H, d, J = 17.5 Hz, H-16), 4.85 (1H, d, J = 10.5 Hz, H-16), 3.13 (1H, dd, J = 2.0, 17.0 Hz, H-1), 2.66 (1H, dd, J = 6.0, 17.0 Hz, H-1), 2.30 (1H, m, H-6), 2.20 (1H, m, H-18), 1.87 (1H, m, H-10), 1.52 (2H, m, H-8, 12), 1.50 (1H, m, H-11), 1.45 (1H, m, H-7), 1.39 (1H, m, H-14), 1.30 (1H, m, H-6), 1.21 (1H, m, H-12), 1.19 (1H, m, H-7), 1.15 (1H, s, H-19), 1.05 (1H, s, H-17), 1.01 (1H, m, H-14), 0.61 (1H, s, H-20). 13C-NMR δ (ppm): 213.9 (C-4), 180.1 (C-2), 160.3 (C-15), 108.6 (C-16), 54.3 (C-10), 50.8 (C-5), 42.4 (C-8), 39.3 (C-14), 39.0 (C-6), 38.7 (C-9), 35.9 (C-13), 33.7 (C-11), 31.9 (C-12), 31.6 (C-1), 28.5 (C-19), 27.7 (C-18), 27.2 (C-7), 23.0 (C-17), 12.1 (C-20). The 1H and 13C-NMR data were in agreement with the reported data [11,20].
3.7. Data Analyses
Analysis of variance (ANOVA) and Tukey’s test were conducted by using SPSS 10 for Windows 98. Percentage of feeding deterrent index was subjected to an arcsine square-root transformation before ANOVA and Tukey’s tests. The EC50 (the concentration needed to inhibit insect feeding by 50% relative to controls) was determined by linear regression [25].
4. Conclusions
Based on mass screening of medicinal herbs, the ethanol extract of C. tagal stems and twigs was found to possess feeding deterrent activity against the red flour beetles (T. castaneum). Three feeding deterrent compounds were isolated and identified from the ethanol extract of C. tagal by bioactivity-guided fractionation. The concentration used in this study to observe feeding deterrent effects was comparable with that of the commercial product toosendanin. Dietary tagalsin A, B and H possessed feeding deterrent activity against T. castaneum adults, but the three isolated compounds were 4–5 times less active when compared with toosendanin. These findings suggest that the ethanol extract of C. tagal stems and twigs and three isolated compound show potential for development as natural feeding deterrents for the control of stored product insects.
Acknowledgements
This work was funded by National New-drug Innovation Project 2009ZX09501-014 and the Hi-Tech Research and Development of China 2006AA10A209. We thank Liu Q.R. from the College of Life Sciences, Beijing Normal University, Beijing 100875, for the identification of the investigated medicinal herb.
References and Notes
- 1.Liu Z.L., Ho S.H. Bioactivity of the essential oil extracted from Evodia rutaecarpa Hook f. et Thomas against the grain storage insects, Sitophilus zeamais Motsch, and Tribolium castaneum (Herbst) J. Stored Prod. Res. 1999;35:317–328. doi: 10.1016/S0022-474X(99)00015-6. [DOI] [Google Scholar]
- 2.Magan N., Hope R., Cairns V., Aldred D. Postharvest fungal ecology: Impact of fungal growth and mycotoxin accumulation in stored grain. Eur. J. Plant Pathol. 2003;109:723–730. [Google Scholar]
- 3.Isman M.B. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Ann. Rev. Entomol. 2006;51:45–66. doi: 10.1146/annurev.ento.51.110104.151146. [DOI] [PubMed] [Google Scholar]
- 4.Isman M.B. Perspective botanical insecticides: For richer, for poorer. Pest Manag. Sci. 2008;64:8–11. doi: 10.1002/ps.1470. [DOI] [PubMed] [Google Scholar]
- 5.Isman M.B. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Ann. Rev. Entomol. 2006;51:45–66. doi: 10.1146/annurev.ento.51.110104.151146. [DOI] [PubMed] [Google Scholar]
- 6.Flora of China. Vol. 52. Science Press; Beijing, China: 1993. The Editorial Board of Flora China, Chinese Academy of Science; p. 132. [Google Scholar]
- 7.He L., Wang Y.S., Wang Q.J., Lou Z.P. A novel triterpene from Ceriops tagal. Pharmazie. 2005;60:716–718. [PubMed] [Google Scholar]
- 8.Pakhathirathien C., Karalai C., Ponglimanont C., Subhadhirasakul S., Chantrapromma K. Dammarane triterpenes from the hypocotyls and fruits of Ceriops tagal. J. Nat. Prod. 2005;68:1787–1789. doi: 10.1021/np0502793. [DOI] [PubMed] [Google Scholar]
- 9.Lin P., Fu Q. Environmental Ecology and Economic Utilization of Mangroves in China. Higher Education Press; Beijing, China: 1995. pp. 1–95. [Google Scholar]
- 10.Rastogi R.P., Mehrotra B.N. Compendium of Indian Medicinal Plants. Vol. 1 Central Drug Research Institute, Lucknow/Publications and Information Directorate; New Delhi, India: 1991. [Google Scholar]
- 11.Zhang Y., Deng Z.W., Gao T.X., Proksch P., Lin W.H. Tagalsins A–H, dolabrane-type diterpenes from the mangrove plant, Ceriops taga. Phytochemistry. 2005;66:1465–1471. doi: 10.1016/j.phytochem.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y., Lu Y., Mao L., Proksch P., Lin W.H. Tagalsins I and J, two novel tetraterpenoids from the mangrove plant, Ceriops tagal. Org. Lett. 2005;7:3037–3040. doi: 10.1021/ol0509843. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y., Deng Z.W., Gao T.X., Fu H.Z., Lin W.H. Chemical constituents from the mangrove plant Ceriops tagal. Acta Pharm. Sin. 2005;40:935–939. (in Chinese with English abstract). [PubMed] [Google Scholar]
- 14.Ponglimanont C., Thongdeeying P. Lupane-triterpene esters from the leaves of Ceriops tagal (Griff) Ding Hou. Aust. J. Chem. 2005;58:615–618. doi: 10.1071/CH05087. [DOI] [Google Scholar]
- 15.He L., Wang Y.S., Wang Q.J., Xu J.R., Zhang S. Study on chemical constituents of Ceriops tagal. Chin. Pharm. J. 2006;41:341–342. [Google Scholar]
- 16.Chen J.D., Feng D.Q., Yang Z.W., Wang Z.C., Qiu Y., Lin Y.M. Antifouling metabolites from the mangrove plant Ceriops tagal. Molecules. 2008;13:212–219. doi: 10.3390/molecules13020212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen J.D., Qiu Y., Yang Z.W., Lin P., Lin Y.M. Dimeric diterpenes from the roots of the mangrove plant Ceriops tagal. Helv. Chim. Acta. 2008;91:2292–2298. doi: 10.1002/hlca.200890249. [DOI] [Google Scholar]
- 18.Chacha M., Mapitse R., Afolayan A.J., Majinda R.R.T. Antibacterial diterpenes from the roots of Ceriops tagal. Nat. Prod. Commun. 2008;3:17–20. [Google Scholar]
- 19.Wang X.C., Ouyang X.W., Hu L.H. Three new lupane-type triterpenes from Ceriops tagal. J. Asian Nat. Prod. Res. 2010;12:576–581. doi: 10.1080/10286020.2010.485566. [DOI] [PubMed] [Google Scholar]
- 20.Hu W.M., Li M.Y., Li J., Xiao Q., Feng G., Wu J. Dolabranes from the Chinese mangrove, Ceriops Tagal. J. Nat. Prod. 2010;73:1701–1705. doi: 10.1021/np100484w. [DOI] [PubMed] [Google Scholar]
- 21.Ouyang X.W., Wang X.C., Yue Q.X., Hu L.H. A new dolabrane-type diterpene from Ceriops tagal. Nat. Prod. Commun. 2010;5:9–12. [PubMed] [Google Scholar]
- 22.Xie Y.S., Bodnaryk R.P., Fields P.G. A rapid and simple flour-disk bioassay for testing substances active against stored-product insects. Can. Entomol. 1996;128:865–875. [Google Scholar]
- 23.Liu Z.L., Xu Y.J., Wu J., Goh S.H., Ho S.H. Feeding deterrents from Dictamnus dasycarpus Turcz against two stored-product insects. J. Agric. Food Chem. 2002;50:1447–1450. doi: 10.1021/jf010838l. [DOI] [PubMed] [Google Scholar]
- 24.Liu Z.L., Chu S.S., Jiang G.H. Feeding deterrents from Zanthoxylum schinifolium against two stored-product insects. J. Agric. Food Chem. 2009;57:10130–10133. doi: 10.1021/jf9012983. [DOI] [PubMed] [Google Scholar]
- 25.Chen C.C., Chang S.J., Cheng L.L., Hou R.F. Effects of chinaberry fruit extract on feeding, growth and fecundity of the diamondback moth, Plutella xylostella L. (Lep., Yponomeutidae) J. Appl. Entomol. 1996;120:341–345. [Google Scholar]