Abstract
We present an efficient procedure for the synthesis of thirty-six N1,N4-substituted thiosemicarbazones, including twenty-five ones that are reported for the first time, using a microwave-assisted methodology for the reaction of thiosemicarbazide intermediates with aldehydes in the presence of glacial acetic acid in ethanol and under solvent free conditions. Overall reaction times (20–40 min when ethanol as solvent, and 3 min under solvent free conditions) were much shorter than with the traditional procedure (480 min); satisfactory yields and high-purity compounds were obtained. The thiosemicarbazide intermediates were obtained from alkyl or aryl isothiocyanates and hydrazine hydrate or phenyl hydrazine by stirring at room temperature for 60 min or by microwave irradiation for 30 min, with lower yields for the latter. The preliminary in vitro antifungal activity of thiosemicarbazones was evaluated against Aspergillus parasiticus and Candida albicans.
Keywords: thiosemicarbazone, thiosemicarbazide, microwave irradiation, antifungal activity
1. Introduction
Thiosemicarbazones and thiosemicarbazides are now well established as an important class of sulfur/nitrogen donor ligands, particularly for transition metal ions [1,2], because of the remarkably diverse biological activities observed for these compounds. These activities include antiviral [3,4,5], antitumor [6,7], and antimicrobial properties [8], as well as other industrially important activities, including anticorrosion [9] and antifouling [10] effects. Considering all of these properties, it is important to be able to synthesize new series of thiosemicarbazones.
The increasing demand for clean and efficient chemical procedures has been a target for the synthesis of organic compounds. The combined use of microwave irradiation and solvent-free conditions has shown advantages from economic and environmental standpoints [11,12]. Furthermore, microwave-assisted organic reactions generally provide high yields of pure products, minimize the use of organic solvents, and allow for a simplified work-up and shorter reaction times [13].
Our research group has been working on more efficient and cleaner synthetic methods, focusing on microwave irradiation and solvent-free conditions [14,15,16,17]. To extend our investigation and considering the special importance of thiosemicarbazone class, in this paper we report the synthesis of thirty six thiosemicarbazones N1,N4-substituted from thiosemicarbazides with low reaction times and good yields by microwave-assisted reactions. Furthermore, the evaluation of antifungal activity against the Aspergillus parasiticus and Candida albicans was realized.
2. Results and Discussion
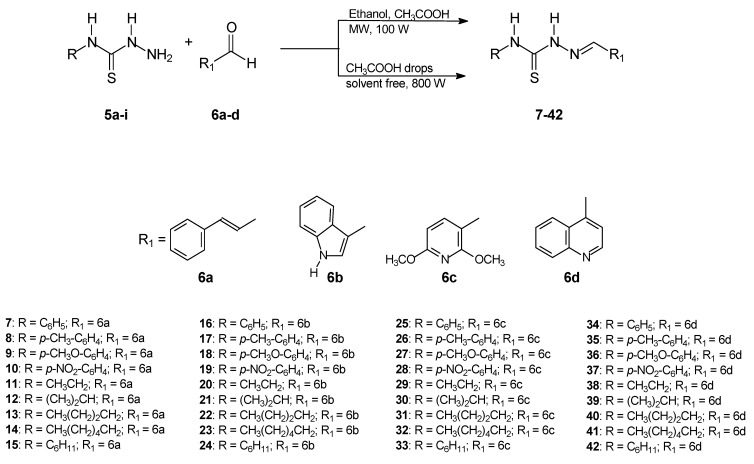
Thirty-six thiosemicarbazones were synthesized using a microwave-assisted methodology; twenty-five are new compounds. The synthetic procedure was performed in two steps starting with aryl thiosemicarbazides 5a–d or alkyl thiosemicarbazides 5e–i, also prepared in this work, and cinnamaldehyde (6a), 3-methyl-indolcarboxaldehyde (6b), 2,6-dimethoxy-pyridincarboxaldehyde (6c) or 4-quinolinecarboxaldehyde (6d) in ethanol as a solvent, and under solvent free conditions, with a few drops of added glacial acetic acid, as outlined in Scheme 1. The reaction mixtures were irradiated in a scientific microwave reactor for 20–40 min at 100 W when ethanol used as solvent, and for 3 min at 800 W in the absence of solvent. The products, a cinnamaldehyde series (compounds 7–15), a 3-methyl-indolcarboxaldehyde series (compounds 16–24), a 2,6-dimethoxypyridincarboxaldehyde series (compounds 25–33) and a 4-quinolinecarboxaldehyde series (compounds 34–42), were obtained with both procedures as fine crystals in high-purity and satisfactory yields after a short time, when compared with the reaction time for the traditional procedure (480 min). The best yields (88–98%) and the short reaction times (3 min) were obtained when the solvent free conditions were used. The thiosemicarbazones 7–10 and 16–19 were also prepared using a reflux method; after 8 h under reflux in ethanol, lower yields were obtained for all the compounds than in the microwave-assisted synthesis. Table 1 shows the reaction times and yields for the target compounds, 7–42.
Scheme 1.
Synthesis of thiosemicarbazones by microwave irradiation.
Table 1.
Thiosemicarbazone yields and reaction times obtained under microwave irradiation using ethanol as solvent and in solvent free conditions (7–42), and under traditional reflux (7–10 and 16–19).
| Compound | Time (min) | Yield (%) | Compound | Time (min) | Yield (%) |
|---|---|---|---|---|---|
| 7 | 3 a /40 b /480 c | 96 a /86 b /83 c | 25 | 3 a /40 b | 93 a /82 b |
| 8 | 3 a /40 b /480 c | 92 a /79 b /74 c | 26 | 3 a /40 b | 86 a /70 b |
| 9 | 3 a /40 b /480 c | 97 a /71 b /70 c | 27 | 3 a /40 b | 91 a /78 b |
| 10 | 3 a /40 b /480 c | 90 a /88 b /68 c | 28 | 3 a /40 b | 90 a /82 b |
| 11 | 3 a /20 b | 89 a /54 b | 29 | 3 a /20 b | 96 a /99 b |
| 12 | 3 a /20 b | 96 a /83 b | 30 | 3 a /20 b | 97 a /99 b |
| 13 | 3 a /20 b | 94 a /71 b | 31 | 3 a /20 b | 92 a /94 b |
| 14 | 3 a /20 b | 92 a /70 b | 32 | 3 a /20 b | 91 a /88 b |
| 15 | 3 a /20 b | 95 a /83 b | 33 | 3 a /20 b | 94 a /97 b |
| 16 | 3 a /40 b /480 c | 91 a /62 b /49 c | 34 | 3 a /40 b | 89 a /77 b |
| 17 | 3 a /40 b /480 c | 97 a /93 b /78 c | 35 | 3 a /40 b | 88 a /84 b |
| 18 | 3 a /40 b /480 c | 88 a /76 b /67 c | 36 | 3 a /40 b | 90 a /57 b |
| 19 | 3 a /40 b /480 c | 96 a /82 b /63 c | 37 | 3 a /40 b | 96 a /79 b |
| 20 | 3 a /20 b | 98 a /73 b | 38 | 3 a /20 b | 95 a /70 b |
| 21 | 3 a /20 b | 93 a /52 b | 39 | 3 a /20 b | 93 a /75 b |
| 22 | 3 a /20 b | 94 a /78 b | 40 | 3 a /20 b | 91 a /81 b |
| 23 | 3 a /20 b | 87 a /52 b | 41 | 3 a /20 b | 89 a /79 b |
| 24 | 3 a /20 b | 95 a /85 b | 42 | 3 a /20 b | 93 a /62 b |
a Solvent free conditions; b using ethanol as solvent; c using traditional reflux.
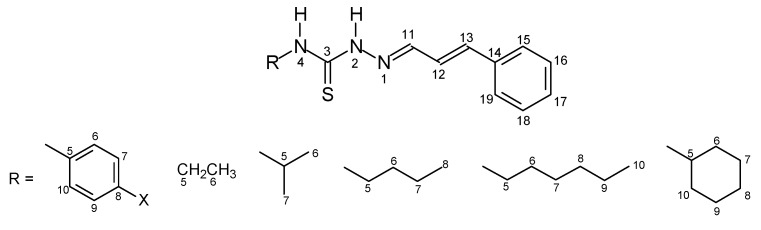
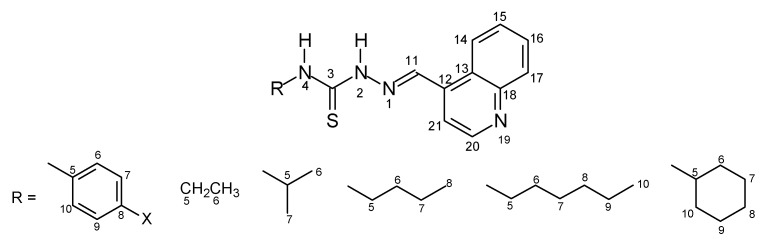
The thiosemicarbazone structures 7–42 were fully characterized by 1H- and 13C-NMR and IR spectroscopy. The 1H- and 13C-NMR shifts (δ) were assigned based on literature data [18,19,20,21,22] and were consistent with the structures proposed. The 1H chemical shifts of H-C=N for the thiosemicarbazone cinnamaldehyde series products 7–15 showed lower values than for the other series. In contrast, the 13C chemical shifts of C=N were assigned with lower and similar values for the 2,6-dimethoxy-3-pyridinecarboxaldehyde and 4-quinolinecarboxaldehyde series products 25–33 and 34–42. The values ranged from δ = 137.02 to 138.62 for the cinnamaldehyde ones 7–15 and from δ = 140.10 to 146.93 for the 3-indolecarboxaldehyde series 16-24. The electronic substituent effects were as expected. The Figure 1–Figure 4 show the numbered structures.
Figure 1.
Cinnamaldehyde series 7–15.
Figure 4.
4-Quinolinecarboxaldehyde series 34-42.
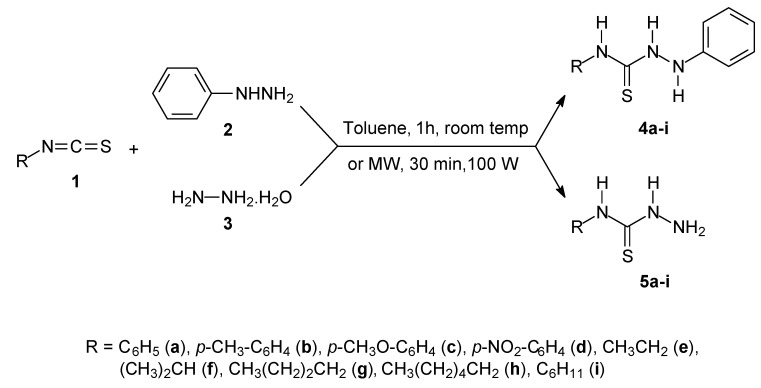
The aryl thiosemicarbazides 5a–d or alkyl thiosemicarbazides 5e–i used as intermediates in thiosemicarbazone preparation were prepared by both traditional and microwave-assisted procedures. Scheme 2 shows the reactions and the conditions for both procedures.
Scheme 2.
Synthesis of thiosemicarbazides.
Different from the thiosemicarbazones, thiosemicarbazide preparation via the traditional methodology with toluene as the solvent under stirring at room temperature was more efficient than the microwave-assisted procedure, affording very good yields in comparison, especially for the alkyl isothiosemicarbazides. This performance may be due to the higher volatility of the alkyl isothiocyanates (1e–i). However, the reaction times using the microwave irradiation method were 30 minutes, whereas stirring at room temperature required 60 minutes. Table 2 lists the yields in the preparation of 5a–i. The thiosemicarbazides were identified by comparison with analytical data found in the literature [23].
Table 2.
Thiosemicarbazide yields and reaction times using microwave irradiation and stirring at room temperature.
| Compound | TraditionalProcedure (%) 60 min | Microwave Irradiation (%) 30 min | Compound | TraditionalProcedure (%) 60 min | Microwave Irradiation (%) 30 min |
|---|---|---|---|---|---|
| 4a | 93 | 83 | 5a | 79 | 65 |
| 4b | 92 | 78 | 5b | 85 | 71 |
| 4c | 92 | 80 | 5c | 80 | 67 |
| 4d | 95 | 73 | 5d | 89 | 77 |
| 4e | 97 | 25 | 5e | 97 | 36 |
| 4f | 99 | 35 | 5f | 99 | 30 |
| 4g | 94 | 22 | 5g | 94 | 28 |
| 4h | 94 | 31 | 5h | 94 | 34 |
| 4i | 93 | 83 | 5i | 98 | 41 |
The preliminary in vitro antimicrobial activity of all thiosemicarbazones synthesized on Aspergillus parasiticus and Candida albicans was evaluated. The thiosemicarbazones showed weak or moderate activity as compared to the standard fungicide itroconazole. However, the observed antimicrobial effects do not exclude from further investigations of these compounds against other fungal strains. Table 3 lists the MIC (μg/mL) values obtained for active thiosemicarbazones.
Table 3.
MIC (μg/mL) values of thiosemicarbazones against C. albicans and A. parasiticus isolates.
| Compound | C. albicans | A. parasiticus | Compound | C. albicans | A. parasiticus |
|---|---|---|---|---|---|
| 11 | 250 | 500 | 20 | NI | 500 |
| 13 | 250 | 500 | 21 | 250 | 500 |
| 14 | 500 | NI | 22 | 500 | 500 |
| 16 | 500 | 500 | 23 | NI | 500 |
| 17 | NI | 500 | 24 | 500 | 500 |
| 18 | 250 | 500 | 41 | NI | 500 |
| 19 | 500 | 500 |
NI: no inhibition up to 500 µg/mL.
3. Experimental
3.1. General
Melting points were determined with a Meltemp II apparatus and were uncorrected. Infrared spectra (KBr pellets) were recorded on a Bruker Vertex 70 spectrophotometer. The 1H- and 13C-NMR spectra were obtained on a Bruker Avance II 400 spectrometer (1H, 400 MHz; 13C, 100 MHz) using tetramethylsilane TMS as the internal standard and acetone-d6 and pyridine-d5 as the solvent. Elemental analyses were performed on a Perkin-Elmer Model 2400 instrument. The microwave-assisted organic reactions were performed in a CEM Discovery System reactor.
3.2. General Procedure for the Preparation of Thiosemicarbazides 5a–i
The thiosemicarbazides were prepared according to methods described elsewhere [23]. Briefly, the alkyl or aryl isothiocyanate (25 mmol) and hydrazine hydrate or phenyl hydrazine (25 mmol) were mixed in the presence of toluene (20 mL). The reaction mixture was kept under stirring for 1 hour at room temperature. The solid obtained was filtered and washed with ice-cold toluene. All thiosemicarbazides were identified by the comparison of analytical data (melting points and NMR) with literature reports.
3.3. General Procedure for the Preparation of Thiosemicarbazides 5a–i Using Microwave Irradiation
The alkyl or aryl isothiocyanate (0.74 mmol) and hydrazine hydrate or phenyl hydrazine (0.74 mmol) were mixed in the presence of toluene (2 mL) and submitted to microwave irradiation for 30 min at 100 W. The solid obtained was filtered and washed with ice-cold toluene. All thiosemicarbazides were identified by comparing their melting points with those previously obtained.
3.4. General Procedure for the Preparation of Thiosemicarbazones 7–42 Using Microwave Irradiation and Ethanol as Solvent
The aldehyde (0.84 mmol) and alkyl or aryl thiosemicarbazide (0.84 mmol) were mixed in the presence of ethanol (5 mL) and few drops of glacial acetic acid and submitted to microwave irradiation for 20–40 min at 100 W. The solid obtained was filtered and washed with ice-cold ethanol several times.
3.5. General Procedure for the Preparation of Thiosemicarbazones 7–42 Using Microwave Irradiation in Solvent-Free Conditions
The aldehyde (0.84 mmols) and alkyl or aryl thiosemicarbazide (0.84 mmols) were mixed in an agate mortar with few drops of glacial acetic acid and submitted to microwave irradiation for 3 min at 800 W. The solid obtained was extracted and recrystallized from ethanol to furnish the pure products.
3.6. General Procedure for the Preparation of Thiosemicarbazones 7–10 and 16–19 Using Traditional Reflux
The aldehyde (1.19 mmol) and aryl thiosemicarbazide (1.19 mmol) were mixed in the presence of ethanol (10 mL) and few drops of glacial acetic acid. The reaction mixture was kept under reflux for 8 hours. The solid obtained was filtered and washed with ice-cold ethanol several times.
Cinnamaldehyde-4-phenyl-thiosemicarbazone (7). Yellow solid; m.p. 174–176 °C (lit. [18] 176 °C); yield 86%; FT-IR (KBr, υ cm−1): 3,271 (N-H), 3,107 (N-H), 1,552 (C=C), 1,517 (C=N), 1,068 (C=S), 991 (C=C); 1H-NMR (acetone-d6) δ 11.80 (s, 1H, H-4), 9.93 (s, 1H, H-2), 7.98 (d, 1H, H-11), 7.60 (d, 2H, H-15 to 19), 7.56 (d, 2H, H-6, H-10), 7.39 (t, 2H, H-7 to 9), 7.30–7.35 (m, 1H, H-17), 7.30–7.35 (m, 2H, H-16, H-18), 7.16 (t, 1H, H-8), 7.06 (d, 1H, H-13), 6.94 (dd, 1H, H-12); 13C-NMR (acetone-d6) δ 177.42 (C-3), 145.81 (C-11), 141.04 (C-17), 140.54 (C-5), 129.62 (C-6, C-10), 128.41 (C-7, C-9), 126.45 (C-8), 126.40 (C-13), 125.24 (C-12), 137.64 (C-14), 130.33 (C-15, C-19), 130.28 (C-16, C-18).
Cinnamaldehyde-4-(p-methyl-phenyl)-thiosemicarbazone (8). Yellow solid; m.p. 178–180 °C (lit. [19] 176 °C); yield 79%; FT-IR (KBr, υ cm−1): 3,340 (N-H), 3,118 (N-H), 2,981 (C-H), 1,556 (C=C), 1,517 (C=N), 1,068 (C=S), 981 (C=C); 1H-NMR (acetone-d6) δ 11.85 (s, 1H, H-4), 9.84 (s, 1H, H-2), 7.96 (d, 1H, H-11), 7.55 (d, 2H, H-15, H-19), 7.44 (d, 2H, H-6, H-10), 7.12 (d, 2H, H-7, H-9), 7.32 (t, 1H, H-17), 7.39 (t, 2H, H-16, H-18), 7.05 (d, 1H, H-13), 6.94 (dd, 1H, H-12), 2.28 (s, 3H, CH3); 13C-NMR (acetone-d6) δ 175.41 (C-3), 144.77 (C-11), 139.14 (C-17), 136.35 (C-8), 135.90 (C-5), 134.18 (C-14), 128.89 (C-15, C-19), 128.55 (C-16, C-18), 126.91 (C-6, C-10), 125.12 (C-13), 124.74 (C-7, C-9), 20.52 (CH3).
Cinnamaldehyde-4-(p-methoxy-phenyl)-thiosemicarbazone (9). Yellow solid; m.p. 152–154 °C (lit. [19] 155 °C); yield: 71%; FT-IR (KBr, υ cm−1): 3,319 (N-H), 3,143 (N-H), 2,985 (C-H), 1,537 (C=C), 1,514 (C=N), 1247 (C-O), 1,201 (O-CH3), 1,026 (C=S), 964 (C=C); 1H-NMR (acetone-d6) δ 11.72 (s, 1H, H-4), 9.80 (s, 1H, H-2), 7.96 (d, 1H, H-11), 7.55 (d, 2H, H-15, H-19), 7.41 (d, 2H, H-6, H-10), 6.88 (d, 2H, H-7, H-9), 7.32 (t, 1H, H-17), 7.39 (t, 2H, H-16, H-18), 7.04 (d, 1H, H-13), 6.93 (dd, 1H, H-12), 3.74 (s, 3H, OCH3); 13C-NMR (acetone-d6) δ 177.95 (C-3), 158.86 (C-8), 145.60 (C-11), 140.81 (C-17), 137.72 (C-14), 133.45 (C-5), 126.57 (C-12), 127.37 (C-6, C-10), 127.26 (C-13), 130.30 (C-15, C-19), 128.41 (C-16, C-18), 114.82 (C-7, C-9), 56.25 (OCH3).
Cinnamaldehyde-4-(p-nitro-phenyl)-thiosemicarbazone (10). Yellow solid; m.p. 198–199 °C; yield 88%; FT-IR (KBr, υ cm−1): 3,263 (N-H), 3,149 (N-H), 1,598 (C=C), 1,562 (C=N), 1,332 (N=O), 1,207 (C=S), 970 (C=C), 850 (C-N); 1H-NMR (acetone-d6) δ 12.12 (s, 1H, H-4), 10.40 (s, 1H, H-2), 8.02 (d, 1H, H-11), 7.58 (d, 2H, H-15, H-19), 8.07 (d, 2H, H-6, H-10), 8.20 (d, 2H, H-7, H-9), 7.34 (t, 1H, H-17), 7.40 (t, 2H, H-16, H-18), 7.11 (d, 1H, H-13), 6.96 (dd, 1H, H-12); 13C-NMR (acetone-d6) δ 176.88 (C-3), 146.93 (C-11), 146.64 (C-8), 145.36 (C-5), 141.98 (C-17), 137.57 (C-14), 130.58 (C-15, C-19), 130.36 (C-16, C-18), 128.53 (C-7, C-9), 126.21 (C-13), 125.35 (C-6, C-10), 124.00 (C-12). Anal. Calcd. for C16H14N4O2S (326.37); C, 58.88; H, 4.32; N, 17.17%. Found: C, 58.84; H, 4.39; N, 17.23%.
Cinnamaldehyde-4-ethyl-thiosemicarbazone (11). White solid; m.p. 166–168 °C; yield 54%; FT-IR (KBr, υ cm−1): 3,315 (N-H), 3,134 (N-H), 3,001 (C-H), 1,554 (C=N), 1,523 (C=C), 1,085 (C=S), 977 (C=C); 1H-NMR (acetone-d6) δ 10.28 (s, 1H, H-2), 7.97 (s, 1H, H-4), 7.94 (d, 1H, H-11), 7.55 (d, 2H, H-15, H-19), 7.38 (t, 2H, H-16, H-18), 7.31 (t, 1H, H-17), 6.99 (d, 1H, H-13), 6.88 (dd, 1H, H-12), 3.69 (q, 2H, H-5), 1.21 (t, 3H, H-6); 13C-NMR (acetone-d6) δ 178.57 (C-3), 144.51 (C-11), 140.31 (C-12), 137.05 (C-14), 130.28 (C-16, C-18), 130.19 (C-17), 129.01 (C-15, C-19), 125.31 (C-13), 39.42 (C-5), 14.20 (C-6). Anal. Calcd. for C12H15N3S (233.33); C, 61.77; H, 6.48; N, 18.01%. Found: C, 61.83; H, 6.44; N, 18.11%.
Cinnamaldehyde-4-isopropyl-thiosemicarbazone (12). White solid; m.p. 195–196 °C; yield 83%; FT-IR (KBr, υ cm−1): 3,296 (N-H), 3,126 (N-H), 2,993 (C-H), 1,556 (C=N), 1,546 (C=C), 1,072 (C=S), 974 (C=C); 1H-NMR (acetone-d6) δ 10.25 (s, 1H, H-2), 7.99 (s, 1H, H-4), 7.94 (d, 1H, H-11), 7.55 (d, 2H, H-15, H-19), 7.38 (t, 2H, H-16, H-18), 7.32 (t, 1H, H-17), 6.99 (d, 1H, H-13), 6.88 (dd, 1H, H-12), 6.64 (s, 1H, H-4), 4.56 (m, 1H, H-5), 1.26 (s, 3H, H-7), 1.25 (s, 3H, H-6); 13C-NMR (acetone-d6) δ 177.62 (C-3), 144.59 (C-11), 139.72 (C-12), 137.11 (C-14), 129.67 (C-18), 129.65 (C-16), 129.59 (C-17), 127.75 (C-15, C-19), 125.97 (C-13), 46.58 (C-5), 22.36 (C-6 to 7). Anal. Calcd. for C13H17N3S (247.36); C, 63.12; H, 6.93; N, 16.99%. Found: C, 63.16; H, 6.89; N, 17.04%.
Cinnamaldehyde-4-butyl-thiosemicarbazone (13). Yellow solid; m.p. 105–107 °C; yield 71%; FT-IR (KBr, υ cm−1): 3,278 (N-H), 3,155 (N-H), 2,997 (C-H), 1,552 (C=N), 1,517 (C=C), 1,091 (C=S), 974 (C=C); 1H-NMR (acetone-d6) δ 10.28 (s, 1H, H-2), 7.98 (s, 1H, H-4), 7.94 (d, 1H, H-11), 7.54 (d, 2H, H-15, H-19), 7.38 (t, 2H, H-16, H-18), 7.31 (t, 1H, H-17), 6.99 (d, 1H, H-13), 6.89 (dd, 1H, H-12), 3.65 (q, 2H, H-5), 1.63 (m, 2H, H-6), 1.37 (m, 2H, H-7), 0.93 (t, 3H, H-8); 13C-NMR (acetone-d6) δ 178.76 (C-3), 144.56 (C-11), 139.63 (C-12), 137.73 (C-19), 137.10 (C-14), 129.59 (C-17), 129.26 (C-16, C-18), 127.73 (C-15), 125.97 (C-13), 44.38 (C-5), 30.04 (C-6), 19.76 (C-7), 14.10 (C-8). Anal. Calcd. for C14H19N3S (261.38); C, 64.33; H, 7.33; N, 16.08%. Found: C, 64.28; H, 7.38; N, 7.39%.
Cinnamaldehyde-4-hexyl-thiosemicarbazone (14). Yellow solid; m.p. 95–97 °C; yield 70%; FT-IR (KBr, υ cm−1): 3,346 (N-H), 3,132 (N-H), 2,927 (C-H), 1,560 (C=N), 1,527 (C=C), 1,099 (C=S), 972 (C=C); 1H-NMR (acetone-d6) δ 10.28 (s, 1H, H-2), 7.97 (s, 1H, H-4), 7.94 (d, 1H, H-11), 7.54 (d, 2H, H-15, H-19), 7.38 (t, 2H, H-16, C-18), 7.31 (t, 1H, H-17), 6.99 (d, 1H, H-13), 6.89 (dd, 1H, H-12), 3.64 (q, 2H, H-5), 1.67 (m, 2H, H-6), 1.33 (m, 6H, H-7, H-9), 0.88 (t, 3H, H-10); 13C-NMR (acetone-d6) δ 178.74 (C-3), 144.55 (C-11), 139.62 (C-12), 137.10 (C-14), 129.67 (C-16, C-18), 129.61 (C-17), 127.72 (C-15, C-19), 125.96 (C-13), 44.68 (C-5), 32.26 (C-8), 29.87 (C-6), 27.22 (C-7), 22.20 (C-9), 17.27 (C-10). Anal. Calcd. for C16H23N3S (289.44); C, 66.40; H, 8.01; N, 14.52%. Found: C, 66.47; H, 7.94; N, 14.59%.
Cinnamaldehyde-4-cyclohexyl-thiosemicarbazone (15). White solid; m.p. 225–226 °C; yield 83%; FT-IR (KBr, υ cm−1): 3,275 (N-H), 3,122 (N-H), 2,925 (C-H), 1,546 (C=N), 1,517 (C=C), 1,068 (C=S), 974 (C=C); 1H-NMR (pyridine-d5) δ 12.58 (s, 1H, H-2), 8.38 (s, 1H, H-4), 8.10 (dd, 1H, H-11), 7.47 (d, 2H, H-15, H-19), 7.35 (t, 2H, H-16, H-18), 7.28 (t, 1H, H-17), 6.99 (d, 1H, H-13), 6.86 (dd, 1H, H-12), 4.75 (m, 1H, H-5), 1.64-1.04 (m, 10H, H-6 to H-10); 13C-NMR (pyridine-d5) δ 177.57 (C-3), 143.60 (C-11), 138.87 (C-12), 136.80 (C-14), 129.26 (C-16, C-18), 129.14 (C-17), 127.36 (C-15, C-19), 125.87 (C-13), 55.39 (C-5), 32.94 (C-6, C-10), 25.81 (C-8), 25.44 (C-7, C-9). Anal. Calcd. for C16H21N3S (287.42); C, 66.86; H, 7.36; N, 14.62%. Found: C, 66.81; H, 7.31; N, 14.71%.
Figure 2.
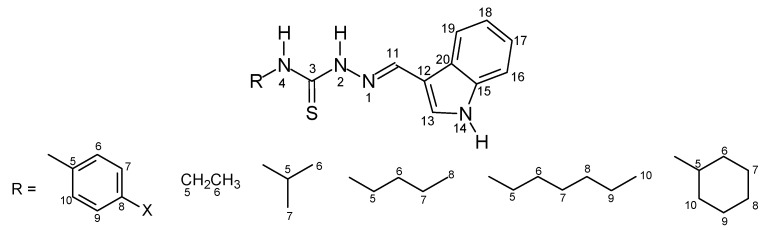
Indole carboxaldehyde series 16–24.
Indole-3-carboxaldehyde-4-phenyl-thiosemicarbazone (16). Beige solid; m.p. 201–203 °C (lit. [20] 197–199 °C); yield: 62%; FT-IR (KBr, υ cm−1): 3,410 (N-H), 3,317 (N-H), 3,314 (N-H), 1,554 (C=N), 1,105 (C=S); 1H-NMR (acetone-d6) δ 11.60 (s, 2H, H-2, H-14), 9.61 (s, 1H, H-4), 8.40 (s, 1H, H-11), 8.23 (d, 1H, H-19), 7.90 (d, 1H, H-13), 7.63 (d, 2H, H-6, H-10), 7.42 (d, 1H, H-16), 7.37 (t, 2H, H-7, H-9), 7.20 (t, 1H, H-18), 7.14 (t, 1H, H-17); 13C-NMR (acetone-d6) δ 176.82 (C-3), 141.99 (C-11), 140.83 (C-15), 138.95 (C-5), 132.05 (C-13), 129.60 (C-6, C-10), 126.17 (C-8), 125.96 (C-20), 125.24 (C-7, C-9), 124.38 (C-17), 123.08 (C-18), 122.42 (C-19), 113.21 (C-12), 113.25 (C-16).
Indole-3-carboxaldehyde-4-(p-methyl-phenyl)-thiosemicarbazone (17). Beige solid; m.p. 198–199 °C (lit. [21] 200 °C); yield 93%; FT-IR (KBr, υ cm−1): 3,404 (N-H), 3,309 (N-H), 3,165 (N-H), 2,920 (C-H), 1,544 (C=N), 1,197 (C=S); 1H-NMR (acetone-d6) δ 11.67 (s, 1H, H-14), 11.54 (s, 1H, H-2), 9.52 (s, 1H, H-4), 8.39 (s, 1H, H-11), 8.20 (d, 1H, H-19), 7.89 (d, 1H, H-13), 7.48 (d, 2H, H-6, H-10), 7.42 (d, 1H, H-16), 7.20 (t, 1H, H-18), 7.15 (d, 2H, H-7, H-9), 7.13 (t, 1H, H-17), 2.30 (s, 3H, CH3); 13C-NMR (acetone-d6) δ 176.88 (C-3), 141.81 (C-11), 138.89 (C-15), 138.21 (C-8), 135.71 (C-5), 131.91 (C-13), 130.05 (C-6, C-10), 126.90 (C-20), 126.38 (C-7 to 9), 124.31 (C-17), 123.00 (C-18), 122.33 (C-19), 113.20 (C-16), 113.05 (C-12), 21.38 (CH3).
Indole-3-carboxaldehyde-4-(p-methoxy-phenyl)-thiosemicarbazone (18). Beige solid; m.p. 202–203 °C (lit. [20] 207–209 °C); yield: 76%; FT-IR (KBr, υ cm−1): 3,435 (N-H), 3,315 (N-H), 3,203 (N-H), 2,972 (C-H), 1,548 (C=N), 1,245 (C-O), 1,110 (C=S), 1,029 (O-CH3); 1H-NMR (acetone-d6) δ 11.67 (s, 1H, H-14), 11.49 (s, 1H, H-2), 9.47 (s, 1H, H-4), 8.39 (s, 1H, H-11), 8.23 (d, 1H, H-19), 7.88 (d, 1H, H-13), 7.44 (d, 2H, H-6, H-10), 7.42 (d, 1H, H-16), 7.19 (t, 1H, H-18), 7.13 (t, 1H, H-17), 6.92 (d, 2H, H-7 to 9), 3.76 (s, 3H, OCH3); 13C-NMR (acetone-d6) δ 177.37 (C-3), 158.77 (C-8), 141.78 (C-11), 138.97 (C-15), 133.80 (C-5), 131.90 (C-13), 127.52 (C-6, C-10), 126.00 (C-20), 124.37 (C-17), 123.16 (C-18), 122.37 (C-19), 114.79 (C-7, C-9), 113.24 (C16), 113.22 (C12), 58.25 (OCH3).
Indole-3-carboxaldehyde-4-(p-nitro-phenyl)-thiosemicarbazone (19). Orange solid; m.p. 210–212 °C; Yield: 82%; FT-IR (KBr, υ cm−1): 3,367 (N-H), 3,296 (N-H), 3,124 (N-H), 1,550 (C=N), 1,334 (N=O), 1,107 (C=S), 844 (C-N); 1H-NMR (acetone-d6) δ 11.97 (s, 1H, H-14), 11.74 (s, 1H, H-2), 10.08 (s, 1H, H-4), 8.44 (s, 1H, H-11), 8.23 (d, 2H, H-6, H-10), 8.18 (d, 1H, H-19), 8.09 (d, 2H, H-7, H-9), 7.95 (d, 1H, H-13), 7.43 (d, 1H, H-16), 7.21 (t, 1H, H-18), 7.15 (t, 1H, H-17); 13C-NMR (acetone-d6) δ 175.97 (C-3), 146.97 (C-8), 145.16 (C-5), 143.17 (C-11), 139.03 (C-15), 132.67 (C-13), 126.99 (C-20), 125.38 (C-7, C-9), 124.55 (C-17), 123.82 (C-6, C-10), 123.16 (C-18), 122.62 (C-19), 113.38 (C-6), 113.04 (C-12). Anal. Calcd. for C16H13N5O2S (339.37); C, 56.63; H, 3.86; N, 20.64%. Found: C, 56.68; H, 3.91; N, 20.69%.
Indole-3-carboxaldehyde-4-ethyl-thiosemicarbazone (20). Beige solid; m.p. 226–228 °C [22]; yield: 73%; FT-IR (KBr, υ cm−1): 3,352 (N-H), 3,238 (N-H), 3,184 (N-H), 2,972 (C-H), 1,546 (C=N), 1,109 (C=S); 1H-NMR (pyridine-d5) δ 12.83 (s, 1H, H-14), 12.29 (s, 1H, H-2), 8.76 (s, 1H, H-11), 8.62 (d, 1H, H-19), 8.51 (s, 1H, H-4),7.89 (d, 1H, H-13), 7.57 (d, 1H, H-16), 7.32 (t, 1H, H-18), 7.23 (t, 1H, H-17), 3.99 (m, 2H, H-5), 1.26 (t, 3H, H-6); 13C-NMR (pyridine-d5) δ 178.10 (C-3), 140.32 (C-11), 138.37 (C-15), 130.85 (C-13), 125.35 (C-20), 123.36 (C-17), 122.44 (C-18), 121.20 (C-19), 112.63 (C-12), 112.45 (C-16), 39.32 (C-5), 15.15 (C-6).
Indole-3-carboxaldehyde-4-isopropyl-thiosemicarbazone (21). Beige solid; m.p. 156–158 °C (lit. [21] 160 °C); yield: 52%; FT-IR (KBr, υ cm−1): 3,346 (N-H), 3,307 (N-H), 3,157 (N-H), 2,922 (C-H), 1,548 (C=N), 1,056 (C=S); 1H-NMR (pyridine-d5) δ 12.86 (s, 1H, H-14), 12.34 (s, 1H, H-2), 8.75 (s, 1H, H-11), 8.55 (d, 1H, H-19), 7.95 (s, 1H, H-4), 7.91 (d, 1H, H-13), 7.59 (d, 1H, H-16), 7.39 (t, 1H, H-18), 7.35 (t, 1H, H-17), 5.00 (m, 1H, H-5), 1.33 (s, 3H, H-6), 1.32 (s, 3H, H-7); 13C-NMR (pyridine-d5) δ 177.21 (C-3), 140.19 (C-11), 130.89 (C-13), 138.46 (C-15), 125.46 (C-20), 123.47 (C-17), 122.03 (C-18), 121.45 (C-19), 112.68 (C-16), 112.62 (C-12), 42.35 (C-5), 22.70 (C-6, C-7).
Indole-3-carboxaldehyde-4-butyl-thiosemicarbazone (22). Beige solid; m.p. 172–178 °C (lit. [21] 170 °C); yield: 78%; FT-IR (KBr, υ cm−1): 3,410 (N-H), 3,365 (N-H), 3,138 (N-H), 2,954 (C-H), 1,537 (C=N), 1,105 (C=S); 1H-NMR (pyridine-d5) δ 12.84 (s, 1H, H-14), 12.34 (s, 1H, H-2), 8.77 (s, 1H, H-11), 8.64 (d, 1H, H-19), 8.45 (s, 1H, H-4), 7.90 (d, 1H, H-13), 7.34 (t, 1H, H-18), 7.58 (d, 1H, H-16), 7.28 (t, 1H, H-17), 3.97 (q, 2H, H-5), 1.71 (m, 2H, H-7), 1.36 (m, 2H, H-6), 0.84 (t, 3H, H-8); 13C-NMR (pyridine-d5) δ 178.31 (C-3), 140.29 (C-11), 130.94 (C-13), 138.45 (C-15), 125.41 (C-20), 123.44 (C-17), 122.45 (C-18), 121.29 (C-19), 112.71 (C-16), 112.56 (C-12), 44.26 (C-5), 32.05 (C-6), 20.43 (C-7), 14.01 (C-8).
Indole-3-carboxaldehyde-4-hexyl-thiosemicarbazone (23). Beige solid; m.p. 170–172 °C [22]; yield: 52%; FT-IR (KBr, υ cm−1): 3354 (N-H), 3,259 (N-H), 3,195 (N-H), 2,925 (C-H), 1,544 (C=N), 1,105 (C=S); 1H-NMR (pyridine-d5) δ 12.84 (s, 1H, H-14), 12.34 (s, 1H, H-2), 8.77 (s, 1H, H-11), 8.65 (d, 1H, H-19), 8.47 (s, 1H, H-4), 7.90 (d, 1H, H-13), 7.58 (d, 1H, H-16), 7.34 (t, 1H, H-18), 7.29 (t, 1H, H-17), 3.99 (q, 2H, H-5), 1.75 (m, 2H, H-6), 1.35 (m, 2H, H-7), 1.21 (m, 4H, H-8, H-9), 0.8 (t, 3H, H-10); 13C-NMR (pyridine-d5) δ 178.25 (C-3), 140.30 (C-11), 138.42 (C-15), 130.96 (C-13), 125.36 (C-20), 123.42 (C-17), 122.43 (C-19), 121.25 (C-18), 112.68 (C-12), 112.53 (C-16), 44.54 (C-5), 31.75 (C-8), 29.60 (C-6), 26.95 (C-7), 22.85 (C-9), 14.06 (C-10).
Indole-3-carboxaldehyde-4-cyclohexyl-thiosemicarbazone (24). Beige solid; m.p. 203–205 °C; yield: 84%; FT-IR (KBr, υ cm−1): 3,410 (N-H), 3,354 (N-H), 3,219 (N-H), 2,923 (C-H), 1544 (C=N), 1,105 (C=S); 1H-NMR (pyridine-d5) δ 12.88 (s, 1H, H-14), 12.39 (s, 1H, H-2), 8.75 (s, 1H, H-11), 8.60 (d, 1H, H-19), 8.03 (s, 1H, H-4), 7.92 (d, 1H, H-13), 7.60 (d, 1H, H-16), 7.44 (t, 1H, H-18), 7.37 (t, 1H, H-17), 4.77 (m, 1H, H-5), 1.65-1.10 (m, 10H, H-6 to H-10); 13C-NMR (pyridine-d5) δ 177.04 (C-3), 140.10 (C-11), 130.98 (C-13), 138.47 (C-15), 123.47 (C-17), 125.41 (C-20), 122.04 (C-18), 121.44 (C-19), 112.69 (C-16), 112.63 (C-12), 52.58 (C-5), 32.96 (C-6, C-10), 25.74 (C-7), 25.02 (C-7, C-9). Anal. Calcd. for C16H20N4S (300.42); C, 63.97; H, 6.71; N, 18.65%. Found: C, 63.94; H, 6.68; N, 18.71%.
Figure 3.
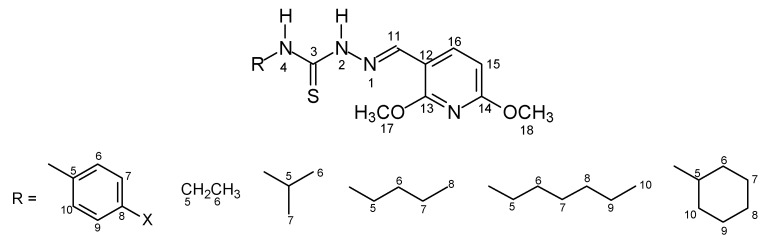
2,6-Dimethoxypyridinecarboxaldehyde series 25–33.
2,6-Dimethoxypyridine-3-carboxaldehyde-4-phenylthiosemicarbazone (25). Yellow solid; m.p. 211–212 °C; yield: 82%; FT-IR (KBr, υ cm−1): 3,307 (N-H), 3,128 (N-H), 2,978 (C-H), 1,595 (C=N), 1,326 (C-O), 1,205 (O-CH3), 1,004 (C=S); 1H-NMR (acetone-d6) δ 11.77 (s, 1H, H-4), 10.06 (s, 1H, H-2), 8.58 (d, 1-H, H-16), 8.32 (s, 1H, H-11), 7.53 (d, 2H, H-6, H-10), 7.35 (t, 2H, H-7, H-9), 7.19 (t, 1H, H-8), 6.45 (d, 1H, H-15), 3.95 (s, 3H, H-17), 3.90 (s, 3H, H-18); 13C-NMR (acetone-d6) δ 175.52 (C-3), 163.64 (C-13), 160.53 (C-14), 139.08 (C-5), 138.62 (C-16), 137.31 (C-11), 127.95 (C-7, C-9), 125.70 (C-6, C-10), 125.14 (C-8), 108.34 (C-12), 102.39 (C-15), 53.52 (C-17), 53.47 (C-18). Anal. Calcd. for C15H16N4O2S (316.38); C, 56.95; H, 5.10; N, 17.71%. Found: C, 56.98; H, 5.06; N, 17.79%.
2,6-Dimethoxypyridine-3-carboxaldehyde-4-(p-methylphenyl)-thiosemicarbazone (26). Yellow solid; m.p. 187–188 °C; yield: 70%; FT-IR (KBr, υ cm−1): 3,286 (N-H), 3,140 (N-H), 2,983 (C-H), 1,598 (C=N), 1,126 (C=S); 1H-NMR (acetone-d6) δ 11.72 (s, 1H, H-4), 9.99 (s, 1H, H-2), 8.58 (d, 1H, H-16), 8.31 (s, 1H, H-11), 7.38 (d, 2H, H-6, H-10), 7.14 (d, 2H, H-7, H-9), 6.45 (d, 1H, H-15), 3.94 (s, 3H, H-17), 3.90 (s, 3H, H-18), 2.49 (s, 3H, CH3); 13C-NMR (acetone-d6) δ 175.60 (C-3), 163.61 (C-13), 160.50 (C-14), 138.61 (C-16), 137.15 (C-11), 136.53 (C-8), 134.32 (C-5), 128.45 (C-6), 128.43 (C-10), 125.68 (C-7, C-9), 108.38 (C-12), 102.39 (C-15), 53.52 (C-17), 53.46 (C-18), 20.53 (CH3). Anal. Calcd. for C16H18N4O2S (330.40); C, 58.16; H, 5.49; N, 16.96%. Found: C, 58.21; H, 5.44; N, 17.02%.
2,6-Dimethoxypyridine-3-carboxaldehyde-4-(p-methoxyphenyl)-thiosemicarbazone (27). Yellow solid; m.p. 189–190 °C; yield: 78%; FT-IR (KBr, υ cm−1): 3,329 (N-H), 3,134 (N-H), 2,981 (C-H), 1,604 (C=N), 1,326 (C-O), 1,213 (O-CH3), 1,114 (C=S); 1H-NMR (acetone-d6) δ 11.69 (s, 1H, H-4), 9.96 (s, 1H, H-2), 8.58 (d, 1H, H-16), 8.30 (s, 1H, H-11), 7.35 (d, 2H, H-6, H-10), 6.90 (t, 2H, H-7, H-9), 6.44 (d, 1H, H-15), 3.94 (s, 3H, H-17), 3.90 (s, 3H, H-18), 3.75 (s, 3H, OCH3); 13C-NMR (acetone-d6) δ 175.94 (C-3), 163.59 (C-13), 160.48 (C-14), 156.86 (C-8), 138.59 (C-16), 137.02 (C-11), 132.00 (C-5), 127.45 (C-6, C-10), 113.20 (C-7, C-9), 108.43 (C-12), 102.38 (C-15), 55.20 (OCH3), 53.53 (C-17), 53.46 (C-18). Anal. Calcd. for C16H18N4O3S (346.40); C, 55.48; H, 5.24; N, 16.17%. Found: C, 55.41; H, 5.21; N, 16.23%.
2,6-Dimethoxypyridine-3-carboxaldehyde-4-(p-nitrophenyl)-thiosemicarbazone (28). Yellow solid; m.p. 209–211 °C; yield: 82%; FT-IR (KBr, υ cm−1): 3,257 (N-H), 3,122 (N-H), 2,968 (C-H), 1,598 (C=N), 1,332 (N=O), 1,107 (C=S), 844 (C-N); 1H-NMR (acetone-d6) δ 12.12 (s, 1H, H-4), 10.37 (s, 1H, H-2), 8.56 (d, 1H, H-16), 8.37 (s, 1H, H-11), 8.21 (d, 2H, H-7, H-9), 8.04 (d, 2H, H-6, H-10), 6.49 (d, 1H, H-15), 3.95 (s, 3H, H-17), 3.90 (s, 3H, H-18); 13C-NMR (acetone-d6) δ 174.71 (C-3), 163.92 (C-13), 160.79 (C-14), 145.41 (C-8), 143.32 (C-5), 138.69 (C-16), 138.62 (C-11), 124.17 (C-6, C-10), 123.64 (C-7, C-9), 107.99 (C-12), 102.52 (C-5), 53.58 (C-17), 53.54 (C-18). Anal. Calcd. for C15H15N5O4S (361.38); C, 49.86; H, 4.18; N, 19.38%. Found: C, 49.81; H, 4.23; N, 19.42%.
2,6-Dimethoxypyridine-3-carboxaldehyde-4-ethylthiosemicarbazone (29). White solid; m.p. 218–220 °C; yield: 99%; FT-IR (KBr, υ cm−1): 3,276 (N-H), 3,138 (N-H), 2,976 (C-H), 1,604 (C=N), 1,380 (C-O), 1,236 (O-CH3), 1,012 (C=S); 1H-NMR (pyridine-d5) δ 12.49 (s, 1H, H-2), 8.99 (s, 1H, H-4), 8.61 (s, 1H, H-11), 7.92 (d, 1H, H-16), 6.24 (d, 1H, H-15), 3.98 (m, 2H, H-5), 3.83 (s, 3H, H-17), 3.80 (s, 3H, H-18), 1.28 (t, 3H, H-6); 13C-NMR (pyridine-d5) δ 178.48 (C-3), 164.21 (C-13), 161.15 (C-14), 137.49 (C-16), 137.17 (C-11), 109.01 (C-12), 102.99 (C-15), 53.55 (C-17), 53.51 (C-18), 39.36 (C-5), 15.06 (C-6). Anal. Calcd. for C11H16N4O2S (268.33); C, 49.24; H, 6.01; N, 20.88%. Found: C, 49.28; H, 5.93; N, 20.92%.
2,6-Dimethoxypyridine-3-carboxaldehyde-4-isopropylthiosemicarbazone (30). White solid; m.p. 216–218 °C; yield: 99%; FT-IR (KBr, υ cm−1): 3,315 (N-H), 3,151 (N-H), 2,970 (C-H), 1,597 (C=N), 1,325 (C-O), 1,240 (O-CH3), 1,018 (C=S); 1H-NMR (pyridine-d5) δ 12.45 (s, 1H, H-2), 8.63 (s, 1H, H-11), 8.43 (s, 1H, H-4), 7.93 (d, 1H, H-16), 6.24 (d, 1H, H-15), 5.09 (m, 1H, H-5), 3.82 (s, 3H, H-17), 3.79 (s, 3H, H-18), 1.32 (s, 3H, H-6), 1.30 (s, 3H, H-7); 13C-NMR (pyridine-d5) δ 177.49 (C-3), 164.23 (C-13), 161.18 (C-14), 137.57 (C-16), 137.40 (C-11), 108.88 (C-12), 102.98 (C-15), 53.54 (C-17), 53.51 (C-18), 46.48 (C-5), 22.39 (C-6, C-7). Anal. Calcd. for C12H18N4O2S (282.36); C, 51.05; H, 6.43; N, 19.84%. Found: C, 51.12; H, 6.40; N, 19.89%.
2,6-Dimethoxypyridine-3-carboxaldehyde-4-butylthiosemicarbazone (31). White solid; m.p. 204–206 °C; yield: 94%; FT-IR (KBr, υ cm−1): 3,334 (N-H), 3,132 (N-H), 2,987 (C-H), 1,604 (C=N), 1,332 (C-O), 1,215 (O-CH3), 1,008 (C=S); 1H-NMR (acetone-d6) δ 10.28 (s, 1H, H-2), 8.29 (s, 1H, H-11), 8.21 (s, 1H, H-4), 6.36 (d, 1H, H-15), 3.98 (s, 3H, H-17), 3.93 (s, 3H, H-18), 3.65 (q, 2H, H-5),1.91 (m, 2H, H-7), 1.80 (m, 2H, H-6), 0.92 (t, 3H, H-8); 13C-NMR (acetone-d6) δ 178.84 (C-3), 167.93 (C-13), 160.83 (C-14), 141.43 (C-16), 137.29 (C-11), 109.45 (C-12), 104.42 (C-15), 53.92 (C-16, C-18), 44.39 (C-5), 32.17 (C-6), 20.67 (C-7), 14.14 (C-8). Anal. Calcd. for C13H20N4O2S (296.39); C, 52.68; H, 6.80; N, 19.90%. Found: C, 52.72; H, 6.76; N, 19.96%.
2,6-Dimethoxypyridine-3-carboxaldehyde-4-hexylthiosemicarbazone (32). Yellow solid; m.p. 130–132 °C; yield: 88%; FT-IR (KBr, υ cm−1): 3,309 (N-H), 3,126 (N-H), 2,952 (C-H), 1,602 (C=N), 1,328 (C-O), 1,238 (O-CH3), 1,012 (C=S); 1H-NMR (acetone-d6) δ 10.28 (s, 1H, H-2), 8.87 (t, 3H, H-10), 8.29 (s, 1H, H-11), 8.25 (s, 1H, H-4), 8.21 (d, 1H, H-16), 6.36 (d, 1H, H-15), 3.97 (s, 3H, H-17), 3.93 (s, 3H, H-18), 3.64 (q, 2H, H-5), 1.65 (m, 2H, H-6), 1.32 (m, 6H, H-7 to H-9); 13C-NMR (acetone-d6) δ 178.79 (C-3), 164.96 (C-14), 161.81 (C-13), 138.37 (C-16), 137.26 (C-11), 109.49 (C-12), 103.42 (C-15), 53.92 (C-17, C-18), 44.68 (C-5), 32.39 (C-8), 32.22 (C-9), 29.97 (C-6), 27.24 (C-7), 14.27 (C-10). Anal. Calcd. for C15H24N4O2S (324.44); C, 55.53; H, 7.46; N, 17.27%. Found: C, 55.57; H, 7.42; N, 17.34%.
2,6-Dimethoxypyridine-3-carboxaldehyde-4-cyclohexylthiosemicarbazone (33). Yellow solid; m.p. 241–243 °C; yield: 97%; FT-IR (KBr, υ cm−1): 3,332 (N-H), 3,113 (N-H), 2,978 (C-H), 1,602 (C=N), 1,328 (C-O), 1,222 (O-CH3), 1,014 (C=S); 1H-NMR (pyridine-d5) δ 12.49 (s, 1H, H-2), 8.63 (s, 1H, H-11), 8.37 (s, 1H, H-4), 8.01 (d, 1H, H-16), 6.29 (d, 1H, H-15), 4.79 (m, 1H, H-5), 3.83 (s, 3H, H-18), 3.80 (s, 3H, H-17), 1.62–0.99 (m, 10H, H-6 to H-10); 13C-NMR (pyridine-d5) δ 177.40 (C-3), 164.27 (C-13), 161.21 (C-14), 137.41 (C-11), 137.70 (C-16), 108.90 (C-12), 103.04 (C-15), 53.56 (C-17), 53.53 (C-18), 53.49 (C-5), 32.88 (C-6, C-10), 25.76 (C-8), 25.48 (C-7, C-9). Anal. Calcd. for C15H22N4O2S (322.43); C, 55.88; H, 6.88; N, 17.38%. Found: C, 55.94; H, 6.82; N, 17.42%.
Quinoline-4-carboxaldehyde-4-phenylthiosemicarbazone (34). Yellow solid; m.p. 195–198 °C (lit. [24] 200 °C); yield: 77%; FT-IR (KBr, υ cm−1): 3,338 (N-H), 3,132 (N-H), 1,525 (C=N), 1,195 (C=S); 1H-NMR (pyridine-d5) δ 13.28 (s, 1H, H-2), 11.02 (s, 1H, H-4), 9.09 (s, 1H, H-11), 8.38 (d, 1H, H-14), 8.34 (d, 1H, H-17), 8.06 (d, 2H, H-6, H-10), 7.73 (t, 1H, H-16), 7.72 (d, 1H, H-20), 7.54 (t, 1H, H-15),7.41 (t, 2H, H-7, H-9), 7.22 (t, 1H, H-8), 8.39 (d, 1H, H-19); 13C-NMR (pyridine-d5) δ 178.16 (C-3), 150.39 (C-19), 149.49 (C-18), 140.04 (C-5), 137.79 (C-11), 137.77 (C-12), 130.85 (C-16), 129.74 (C-4), 128.79 (C-9), 128.78 (C-7), 127.52 (C-15), 125.98 (C-6, C-10), 125.95 (C-8), 125.92 (C-13), 123.79 (C-17), 118.53 (C-20).
Quinoline-4-carboxaldehyde-4-(p-methylphenyl)-thiosemicarbazone (35). Yellow solid; m.p. 203–205 °C; yield: 84%; FT-IR (KBr, υ cm−1): 3,319 (N-H), 3,211 (N-H), 2,985 (C-H), 1,544 (C=N), 1,188 (C=S); 1H-NMR (pyridine-d5) δ 13.22 (s, 1H, H-2), 11.00 (s, 1H, H-4), 9.09 (s, 1H, H-11), 8.39 (d, 1H, H-19), 8.38 (d, 1H, H-14), 8.34 (d, 1H, H-17), 7.91 (d, 1H, H-6), 7.88 (d, 1H, H-10), 7.73 (t, 1H, H-16), 7.72 (d, 1H, H-20), 7.54 (t, 1H, H-15), 7.03 (d, 2H, H-7, H-9), 2.19 (s, 1H, H-20); 13C-NMR (pyridine-d5) δ 178.68 (C-3), 150.39 (C-19), 149.51 (C-18), 137.86 (C-8), 137.63 (C-11), 137.46 (C-12), 132.96 (C-5), 130.85 (C-16), 129.74 (C-14), 129.37 (C-6, C-10), 127.97 (C-7), 127.50 (C-15), 126.10 (C-9), 125.94 (C-13), 123.79 (C-17), 118.47 (C-20), 20.87 (CH3). Anal. Calcd. for C18H16N4S (320.41); C, 67.48; H, 5.03; N, 17.49%. Found: C, 67.44; H, 4.98; N, 17.54%.
Quinoline-4-carboxaldehyde-4-(p-methoxyphenyl)-thiosemicarbazone (36). Yellow solid; m.p. 186–189 °C; yield: 57%; FT-IR (KBr, υ cm−1): 3,309 (N-H), 3,201 (N-H), 2,929 (C-H), 1,550 (C=N), 1,247 (C-O), 1,184 (C=S), 1,035 (O-CH3); 1H-NMR (pyridine-d5) δ 13.23 (s, 1H, H-2), 11.02 (s, 1H, H-4), 9.10 (s, 1H, H-11), 8.87 (d, 1H, H-19), 8.39 (d, 4H, H-14, OCH3), 8.34 (d, 1H, H-17), 7.88 (d, 2H, H-7, H-9), 7.72 (d, 1H, H-20), 7.71 (t, 1H, H-16), 7.53 (t, 1H, H-15), 7.01 (d, 2H, H-6, H-10); 13C-NMR (pyridine-d5) δ 178.73 (C-3), 158.12 (C-8), 150.43 (C-19), 149.54 (C-18), 137.90 (C-12), 137.60 (C-11), 132.97 (C-5), 130.89 (C-16), 129.79 (C-14), 128.05 (C-6, C-10), 127.57 (C-15), 126.00 (C-13), 123.52 (C-17), 118.28 (C-20), 114.13 (C-7, C-9), 55.37 (OCH3). Anal. Calcd. for C18H16N4OS (336.41); C, 64.27; H, 4.79; N, 16.65%. Found: C, 64.32; H, 4.76; N, 16.72%.
Quinoline-4-carboxaldehyde-4-(p-nitrophenyl)-thiosemicarbazone (37). Yellow solid; m.p. 220–223 °C; yield: 79%; FT-IR (KBr, υ cm−1): 3,296 (N-H), 3,082 (N-H), 1,552 (C=N), 1,330 (N=O), 1,193 (C=S), 852 (C-N); 1H-NMR (pyridine-d5) δ 13.65 (s, 1H, H-2), 11.35 (s, 1H, H-4), 9.13 (s, 1H, H-11), 8.87 (d, 1H, H-19), 8.38 (d, 2H, H-7, H-14), 8.34 (d, 2H, H-9, H-17), 8.26 (d, 1H, H-10), 8.06 (d, 1H, H-6), 7.73 (t, 1H, H-16), 7.65 (d, 1H, H-20), 7.58 (t, 1H, H-15); 13C-NMR (pyridine-d5) δ 177.67 (C-3), 150.39 (C-19), 149.51 (C-18), 146.03 (C-8), 138.97 (C-11), 137.46 (C-12), 130.92 (C-16), 129.83 (C-14), 127.64 (C-15), 125.92 (C-13), 124.73 (C-7, C-10), 124.36 (C-6, C-9), 123.80 (C-17), 118.66 (C-20). Anal. Calcd. for C17H13N5O2S (351.38); C, 58.11; H, 3.73; N, 19.93%. Found: C, 58.19; H, 3.68; N, 20.01%.
Quinoline-4-carboxaldehyde-4-ethylthiosemicarbazone (38). Yellow solid; m.p. 175–177 °C; yield: 70%; FT-IR (KBr, υ cm−1): 3,373 (N-H), 3,149 (N-H), 2,958 (C-H), 1,533 (C=N), 1,230 (C=S); 1H-NMR (pyridine-d5) δ 12.86 (s, 1H, H-2), 9.31 (s, 1H, H-4), 9.01 (s, 1H, H-11), 8.84 (d, 1H, H-19), 8.35 (d, 1H, H-14), 8.32 (d, 1H, H-17), 7.69 (t, 1H, H-16), 7.66 (d, 1H, H-20), 7.50 (t, 1H, H-15), 4.01 (m, 2H, H-5), 1.32 (t, 3H, H-6); 13C-NMR (pyridine-d5) δ 179.08 (C-3), 150.34 (C-19), 149.50 (C-18), 138.04 (C-12), 136.94 (C-11), 130.82 (C-16), 129.68 (C-14), 127.41 (C-15), 125.91 (C-13), 123.47 (C-7), 118.22 (C-20), 39.62 (C-5), 14.83 (C-6). Anal. Calcd. for C13H14N4S (258.34); C, 60.44; H, 5.46; N, 21.69%. Found: C, 60.47; H, 5.41; N, 21.73%.
Quinoline-4-carboxaldehyde-4-isopropylthiosemicarbazone (39). White solid; m.p. 193–195 °C; yield: 75%; FT-IR (KBr, υ cm−1): 3,303 (N-H), 3,136 (N-H), 2,976 (C-H), 1,541 (C=N), 1,236 (C=S); 1H-NMR (pyridine-d5) δ 12.86 (s, 1H, H-2), 9.28 (s, 1H, H3), 8.99 (s, 1H, H-11), 8.83 (d, 1H, H-19), 8.72 (s, 1H, H-4), 8.32 (d, 2H, H-14, H-17), 7.70 (t, 1H, H-16), 7.63 (d, 1H, H-20), 7.54 (t, 1H, H-15), 5.09 (m, 1H, H-5), 1.37 (d, 3H, H-7), 1.35 (d, 3H, H-6); 13C-NMR (pyridine-d5) δ 178.15 (C-3), 150.37 (C-18), 149.48 (C-19), 137.97 (C-12), 137.04 (C-11), 130.87 (C-16), 129.69 (C-14), 127.44 (C-15), 125.91 (C-13), 123.42 (C-7), 118.28 (C-20), 46.84 (C-5), 22.23 (C-6, C-7). Anal. Calcd. for C14H16N4S (272.37); C, 61.74; H, 5.92; N, 20.57%. Found: C, 61.77; H, 5.87; N, 20.64%.
Quinoline-4-carboxaldehyde-4-butylthiosemicarbazone (40). Yellow solid; m.p. 104–108 °C; yield: 81%; FT-IR (KBr, υ cm−1): 3,379 (N-H), 3,205 (N-H), 2,927 (C-H), 1,529 (C=N), 1,211 (C=S); 1H-NMR (pyridine-d5) δ 12.89 (s, 1H, H-2), 9.26 (s, 1H, H-4), 9.01 (s, 1H, H-11), 8.86 (d, 1H, H-19), 8.38 (d, 1H, H-14), 8.33 (d, 1H, H-17), 7.70 (t, 1H, H-16), 7.69 (d, 1H, H-20), 7.50 (t, 1H, H-15), 4.00 (m, 2H, H-5), 1.79 (m, 2H, H-7), 1.37 (m, 2H, H-6), 0.85 (t, 2H, H-8); 13C-NMR (pyridine-d5) δ 179.24 (C-3), 150.36 (C-19), 149.50 (C-18), 138.05 (C-12), 137.02 (C-11), 130.82 (C-16), 129.69 (C-14), 127.41 (C-15), 125.88 (C-13), 123.55 (C-17), 118.35 (C-20), 44.56 (C-5), 31.75 (C-6), 20.41 (C-7), 14.00 (C-8). Anal. Calcd. for C15H18N4S (286.39); C, 62.91; H, 6.33; N, 19.56%. Found: C, 62.97; H, 6.28; N, 19.61%.
Quinoline-4-carboxaldehyde-4-hexylthiosemicarbazone (41). Yellow solid; m.p. 158–160 °C; yield: 57%; FT-IR (KBr, υ cm−1): 3,271 (N-H), 3,153 (N-H), 2,927 (C-H), 1,543 (C=N), 1,224 (C=S); 1H-NMR (pyridine-d5) δ 12.89 (s, 1H, H-2), 9.27 (s, 1H, H-4), 9.00 (s, 1H, H-11), 8.85 (d, 1H, H-19), 8.38 (d, 1H, H-14), 8.32 (d, 1H, H-17), 7.70 (t, 1H, H-16), 7.69 (d, 1H, H-20), 7.52 (t, 1H, H-15), 4.00 (q, 2H, H-5), 1.82 (m, 2H, H-6), 1.35 (m, 2H, H-7), 1.20 (m, 4H, H-8, H-9), 0.78 (t, 3H, H-10); 13C-NMR (pyridine-d5) δ 179.28 (C-3), 150.41 (C-19), 149.56 (C-18), 137.10 (C-11), 138.08 (C-12), 130.89 (C-16), 129.73 (C-14), 127.46 (C-15), 125.93 (C-13), 123.60 (C-17), 118.42 (C-20), 44.95 (C-5), 31.80 (C-8), 26.98 (C-7), 22.86 (C-9), 29.70 (C-6), 14.18 (C-10). Anal. Calcd. for C17H22N4S (314.45); C, 64.93; H, 7.05; N, 17.82%. Found: C, 65.02; H, 6.98; N, 17.89%.
Quinoline-4-carboxaldehyde-4-cyclohexylthiosemicarbazone (42). Yellow solid; m.p. 208–210 °C; yield: 62%; FT-IR (KBr, υ cm−1): 3,255 (N-H), 3,174 (N-H), 2,925 (C-H), 1,527 (C=N), 1,207 (C=S); 1H-NMR (pyridine-d5) δ 12.86 (s, 1H, H-2), 9.26 (s, 1H, H4), 8.98 (s, 1H, H-11), 8.90 (d, 1H, H-19), 8.62 (s, 1H, H-4), 8.38 (d, 1H, H-14), 8.34 (d, 1H, H-17), 7.70 (d, 1H, H-20), 7.69 (t, 1H, H-16), 7.56 (t, 1H, H-15), 3.85 (m, 1H, H-5), 1.66-1.04 (m, 10H, H-6 to H-10); 13C-NMR (pyridine-d5) δ 178.05 (C-3), 150.43 (C-19), 149.51 (C-18), 137.95 (C-11), 137.21 (C-12), 130.88 (C-16), 129.72 (C-14), 127.47 (C-15), 125.87 (C-13), 123.55 (C-17), 118.54 (C-20), 53.73 (C-5), 32.64 (C-6, C-10), 35.39 (C-9), 25.74 (C-8), 25.39 (C-7). Anal. Calcd. for C17H20N4S (312.43); C, 65.35; H, 4.45; N, 17.93%. Found: C, 65.41; H, 4.38; N, 18.02%.
3.7. Antifungal Activity
Determination of antifungal activity was performed as described in the M27-A2 document of Clinical and Laboratory Standards Institute (CLSI, 2002) for the yeast Candida albicans (ATCC 24433) and M38-A for the filamentous fungus Aspergillus parasiticus CMT 0334 provided by Mycological Collection of Trichocomaceae at IOC/FIOCRUZ-RJ, Brazil. Briefly, the broth microdilution method was performed by 96-well microtiter assay plate containing RPMI 1640 medium (Invitrogen, USA) at pH 7.0 buffered with MOPS 0.16 M. The 36 thiosemicarbazones were diluted in DMSO: Tween 20 (1:1 v/v) to obtain final concentrations ranging from 3.90 to 500 μg/mL and maximum concentration of organic solvent at 2.5%. Next, the yeast C. albicans and conidia of A. parasiticus suspensions were inoculated into the appropriate well at a final concentration of 0.5–2.5 × 103 CFU mL−1 and 0.4–5.0 × 104 CFU mL−1, respectively. The minimum inhibitory concentration (MIC) of each drug was determined visually after incubation at 35 °C for 48 h. The lowest concentration inhibiting growth of the organism was recorded as the MIC. Itraconazole (ITC, Sigma Chemical Co., St Louis, MO, USA) was used as reference compound. Each experiment was performed in triplicate.
4. Conclusions
In summary, four series of thiosemicarbazones derived from cinnamaldehyde, 3-indole-carboxaldehyde, 2,6-dimethoxypyridinecarboxaldehyde and 4-quinolinecarboxaldehyde and the corresponding thiosemicarbazides were prepared using microwave-assisted reactions in the presence of ethanol as solvent and under solvent free conditions, resulting in good yields, high purity and lower reaction times in comparison with the traditional reflux method, especially when solvent free conditions were utilized.
Acknowledgments
The authors thank CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico), CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) and FAPERJ (Fundação de Apoio a Pesquisa do Estado do Rio de Janeiro) for financial support and fellowships. Further, the authors thank the Departamento de Biologia (IOC-FIOCRUZ) for providing the strain of Aspergillus parasiticus to Mycological Collection of the Trichocomaceae.
Conflict of Interest
The authors declare that no conflicts of interest exist in this work.
Footnotes
Sample Availability: Samples of the compounds are available from the authors.
References and Notes
- 1.Rosu T., Gulea A., Nicolae A., Georgescu R. Complexes of 3dn metal ions with thiosemicarbazones: Synthesis and antimicrobial activity. Molecules. 2007;12:782–796. doi: 10.3390/12040782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biyala M.K., Fahmi N., Singh R.V. Antifertility and antimicrobial activities of palladium and platinum complexes of 6-nitro-3-(indolin-2-one) hydrazine carbothioamide and 6-nitro-3-(indolin-2-one)hydrazinecarboxamide. Indian J. Chem. 2006;45A:1999–2005. [Google Scholar]
- 3.Pirrung M.C., Pansare S.V., Sarma K.D., Keith K.A., Kern E.R. Combinatorial optimization of isatin-β-thiosemicarbazones as anti-poxvirus agents. J. Med. Chem. 2005;48:3045–3050. doi: 10.1021/jm049147h. [DOI] [PubMed] [Google Scholar]
- 4.Banerjee D., Yogeeswari P., Bhat P., Thomas A., Srividya M., Sriram D. Novel isatinylthiosemicarbazones derivatives as potential molecule to combat HIV-TB co-infection. Eur. J. Med. Chem. 2011;46:106–121. doi: 10.1016/j.ejmech.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 5.Kang I.J., Wang L.W., Hsu T.A., Yueh A., Lee C.C., Lee Y.C., Lee C.Y., Chao Y.S., Shih S.R., Chern J.H. Isatin-β-thiosemicarbazones as potent herpes simplex virus inhibitors. Bioog. Med. Chem. Lett. 2011;21:1948–1952. doi: 10.1016/j.bmcl.2011.02.037. [DOI] [PubMed] [Google Scholar]
- 6.Easmon J., Purstinger G., Heinisch G., Roth T., Fiebig H.H., Holzer W., Jäger W., Jenny M., Hofmann J. Synthesis, cytotoxicity, and antitumor activity of Copper(II) and Iron(II) complexes of 4N-azabicyclo[3.2.2]nonane thiosemicarbazones derived from acyl diazines. J. Med. Chem. 2001;44:2164–2171. doi: 10.1021/jm000979z. [DOI] [PubMed] [Google Scholar]
- 7.Patole J., Padhye S., Newton C.J., Christopher A., Powel A.K. Synthesis, characterization and in vitro anticancer activities of semicarbazone and thiosemicarbazone derivatives of salicylaldehyde and their copper complexes against human breast cancer cell line MCF-7. Indian J. Chem. 2004;43A:1654–1658. [Google Scholar]
- 8.Otero L., Vieites M., Boiani L., Denicola A., Rigol C., Opazo L., Olea-Azar C., Maya J.D., Morello A., Krauth-Siegel R.L., et al. Novel antitrypanosomal agents based on Palladium nitrofurylthiosemicarbazone complexes: DNA and redox metabolism as potential therapeutic targets. J. Med. Chem. 2006;49:3322–3331. doi: 10.1021/jm0512241. [DOI] [PubMed] [Google Scholar]
- 9.Khamis E., Ameer M.A., Alandis N.M., Al-Senan G. Effect of thiosemicarbazones on corrosion of steel in phosphoric acid produced by wet process. Corrosion. 2000;56:127–138. doi: 10.5006/1.3280528. [DOI] [Google Scholar]
- 10.Tadros A.B., El-Batouti M. Spectral study and antifouling assessment of some thiosemicarbazone derivatives. Anti-Corros. Methods Mater. 2004;51:406–413. doi: 10.1108/00035590410560958. [DOI] [Google Scholar]
- 11.Kappe C.O. Controlled microwave heating in modern organic synthesis. Angew. Chem. Int. Ed. 2004;43:6250–6284. doi: 10.1002/anie.200400655. [DOI] [PubMed] [Google Scholar]
- 12.Sanseverino A.M. Microondas em síntese orgânica. Quím. Nova. 2002;25:660–667. doi: 10.1590/S0100-40422002000400022. [DOI] [Google Scholar]
- 13.Polshettiwar V., Varma R.S. Microwave-assisted organic synthesis and transformations using benign reaction media. Acc. Chem. Res. 2008;41:629–639. doi: 10.1021/ar700238s. [DOI] [PubMed] [Google Scholar]
- 14.Esteves-Souza A., Echevarria A., Vencato I., Jimeno M.L., Elguero J. Unexpected formation of bis-pyrazolyl derivatives by solid support coupled with microwave irradiation. Tetrahedron. 2001;57:6147–6153. doi: 10.1016/S0040-4020(01)00604-4. [DOI] [Google Scholar]
- 15.Rodrigues-Santos C.E., Echevarria A. An efficient and fast synthesis of 4-aryl-3,4-dihydrocoumarins by (CF3SO3)3Y catalysis under microwave irradiation. Tetrahedron Lett. 2007;48:4505–4508. doi: 10.1016/j.tetlet.2007.04.144. [DOI] [Google Scholar]
- 16.Rodrigues-Santos C.E., Echevarria A. Convenient syntheses of pyrazolo[3,4-b]pyridin-6-ones using either microwave or ultrasound irradiation. Tetrahedron Lett. 2011;52:336–340. doi: 10.1016/j.tetlet.2010.11.054. [DOI] [Google Scholar]
- 17.Reis C.M., Echevarria-Lima J., Miranda A.F., Echevarria A. Improved synthesis of 1,3,4-thiadiazolium-2-phenylamines using microwave and ultrasound irradiation and their cytotoxic activity. J. Braz. Chem. Soc. 2011;22:1505–1510. doi: 10.1590/S0103-50532011000800014. [DOI] [Google Scholar]
- 18.Shah I.D., Trivedi J.P. Synthesis of thiazolidones. I. Synthesis of 4-oxo-3-aryl-5-substituted thiazolin-2-ylhydrazones. J. Indian Chem. Soc. 1963;40:889–893. [Google Scholar]
- 19.Shah I.D., Trivedi J.P. Synthesis of thiazolidones. II. 4-oxo-3-aryl-5-alkyl(aryl)-thiazolin-2-yl hydrazones. J. Indian Chem. Soc. 1964;41:704–706. [Google Scholar]
- 20.Liu L., Yang J., Zhao Z., Shi P., Liu X. Solvent-free synthesis of indole-based thiosemicarbazones under microwave irradiation. J. Chem. Res. 2010;34:57–60. doi: 10.3184/030823410X12628833270368. [DOI] [Google Scholar]
- 21.Husain K., Bhat A.R., Azam A. New Pd(II) complexes of the synthesized 1-N-substituted thiosemicarbazones of 3-indolecarboxaldehyde: Characterization and antiamoebic assessment against E.histolytica. Eur. J. Med. Chem. 2008;43:2016–2028. doi: 10.1016/j.ejmech.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 22.Fujikawa F., Yamashita I., Seno T., Sasaki M., Naito M., Tsukuma S. Chemotherapeutics for Mycobacterium tuberculosis. XVII. Synthesis and antibacterial activity on M. tuberculosis of indole-3-carboxaldehyde derivatives. Yakugaku Zasshi. 1966;86:801–804. doi: 10.1248/yakushi1947.86.9_861. [DOI] [PubMed] [Google Scholar]
- 23.Britto M.M., Almeida T.M.G., Leitão A., Donnici C.L., Lopes M.T.P., Montanari C.A. Synthesis of mesoionic 4-(p-substituted-phenyl)-5-(2,4-dichlorophenyl)-1,3,4-thiadiazolium-2-aminides by direct cyclization via acylation of thiosemicarbazides. Synth. Comm. 2006;36:3359–3369. doi: 10.1080/00397910600941398. [DOI] [Google Scholar]
- 24.Grammaticakis P. Absorption in the middle ultraviolet and the visible of 2-formyl- and 4-formylquinoline and their nitrogenous functional derivatives. Compt. Rend. 1959;248:3719–3721. [Google Scholar]