Abstract
A new neolignan, 3,4-dimethoxy-3′,4′-methylenedioxy-2,9-epoxy-6,7-cyclo-1,8-neolign-11-en-5(5H)-one, which has been named (+)-kunstlerone (1), together with six known alkaloids: (+)-norboldine (2), (+)-N-methylisococlaurine (3), (+)-cassythicine (4), (+)-laurotetanine (5), (+)-boldine (6) and (-)-pallidine (7), were isolated from the leaves of Beilschmiedia kunstleri. The structures were established through various spectroscopic methods notably 1D- and 2D-NMR, UV, IR and LCMS-IT-TOF. (+)- Kunstlerone (1) showed a strong antioxidant activity, with an SC50 of 20.0 µg/mL.
Keywords: Beilschmiedia kunstleri, alkaloid, neolignan, lauraceae, antioxidant
1. Introduction
The Lauraceae family normally, with 30 genera and over 2,000 species, occurs throughout Southeast Asia and tropical America [1,2]. In Malaysia, its contribution is about 213 species, from 16 genera [2]. Beilschmiedia species are known to produce many types of phytochemicals [3,4,5,6,7,8,9,10,11,12] with varied biological activities such as O,O-dimethylcoclaurine isolated from Malaysian B. brevipes, which exhibited significant cytotoxicity against P-388 murine leukemia cells with an IC50 value of 6.5 μg/mL [13]. Besides the alkaloids, epoxyfuranoid lignans were also reported in the leaves of B. tsangii [10]. Neolignans, whose precursors are the di- and trioxygenated cinnamic acids, have never been reported so far in the species of Beilschmiedia; however they were detected in parts of other species of Lauraceae such as in the fruits of Aniba riparia and in the leaves of Ocotea catharinensis [14,15,16].
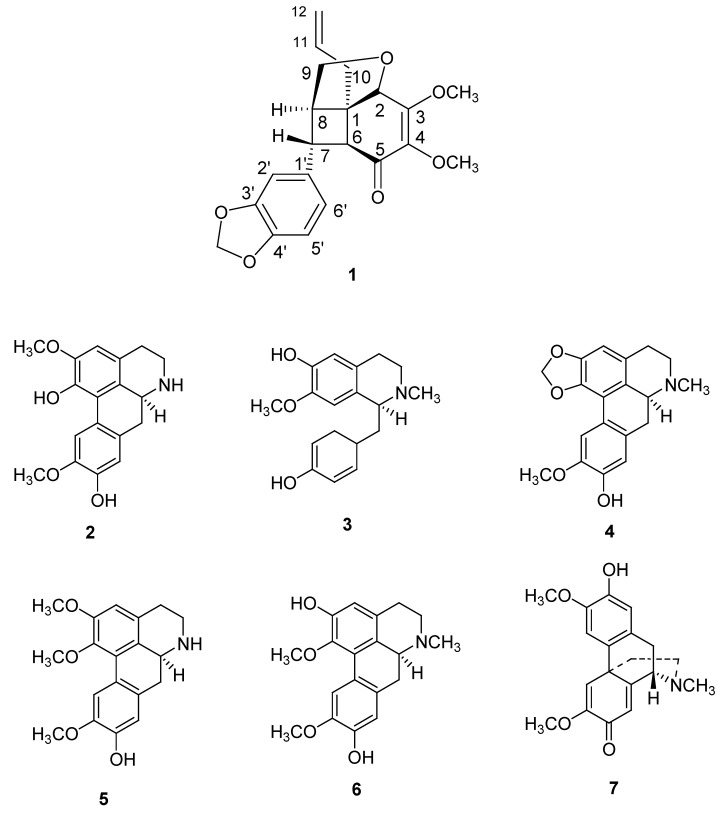
In continuation of our search for new bioactive compounds from Malaysian flora [13,17], we have performed a phytochemical study on the leaves of a Malaysian Lauraceae, Beilschmiedia kunstleri, which has led to the isolation of a new neolignan; (+)-kunstlerone (1). In addition, six known isoquinoline alkaloids, namely (+)-norboldine (2) [18], (+)-N-methylisococlaurine (3) [19], (+)-cassythicine (4) [20], (+)-laurotetanine (5) [21], (+)- boldine (6) [17] and (-)-pallidine (7) [22] were also isolated (Figure 1). This paper describes the structural elucidation and the DPPH radical scavenging activity of (+)-kunstlerone (1).
Figure 1.
Chemical structures of compounds 1-7.
2. Results and Discussion
(+)-Kunstlerone, = +163.63° was obtained as a white amorphous solid. The LCMS-IT-TOF revealed a pseudomolecular ion peak at m/z 371.1490 [M+H]+, thus suggesting a molecular formula of C21H23O6 (calc. 371.1495). The IR spectrum revealed absorption bands at 1,615 and 1,725 cm−1 due to the C=C and C=O stretching vibrations, respectively [14]. The 1H-NMR spectrum (see Table 1) established the presence of three ABX type phenyl protons at δ 6.76 (1H, d, J = 8.1 Hz, H-5′), 6.71 (1H, br d, J = 8.1 Hz, H-6′), and 6.80 (1H, br s, H-2′)], two methoxyl groups at δ 4.08 and 3.69, a methylenedioxy (δ 5.91, s, 2H), and protons of a propenyl group; H-11( δ 5.66, m), Htran-12 (δ 5.11, br d, 16.5 Hz) and Hcis -12 (δ 5.13, br d,11.0 Hz), respectively. The proton at C-7 of a cyclobutane moiety showed significantly as a dd at δ 3.21 with coupling constants of 7.8 and 8.5 Hz, which indicated that it is trans to H-8 (δ 2.83 m) and H-6 (δ 2.90 br d, 8.5 Hz) [23,24].
Table 1.
1H-NMR and 13C-NMR spectral data of (+)-kunstlerone (1) in CDCl3 (δ in ppm, J in Hz).
| Position | 1H | 13C |
|---|---|---|
| C-1′ | - | 132.12 |
| C-2′ | 6.80, br s | 107.25 |
| C-3′ | - | 147.97 |
| C-4′ | - | 146.39 |
| C-5′ | 6.76, d, (8.1) | 108.32 |
| C-6′ | 6.71, br d, (8.1) | 119.69 |
| C-1 | - | 46.01 |
| C-2 | 4.32, s | 79.11 |
| C-3 | - | 159.47 |
| C-4 | - | 138.46 |
| C-5 | - | 193.10 |
| C-6 | 2.90, d, (8.5) | 50.16 |
| C-7 | 3.21, dd, (7.8, 8.5) |
44.58 |
| C-8 | 2.83, m | 49.63 |
| C-9 | 3.72, dd, (9.5, 3.9) 3.90, d, (9.5) |
70.84 |
| C-10 | 2.38, m | 41.76 |
| C-11 | 5.66, m | 136.51 |
| C-12 | 5.13, d, (Jcis = 11.0), 5.11, d, (Jtrans = 16.5) |
119.41 |
| OCH2O | 5.91, s | 101.08 |
| 3-OCH3 | 4.08, s | 58.46 |
| 4-OCH3 | 3.69, s | 60.57 |
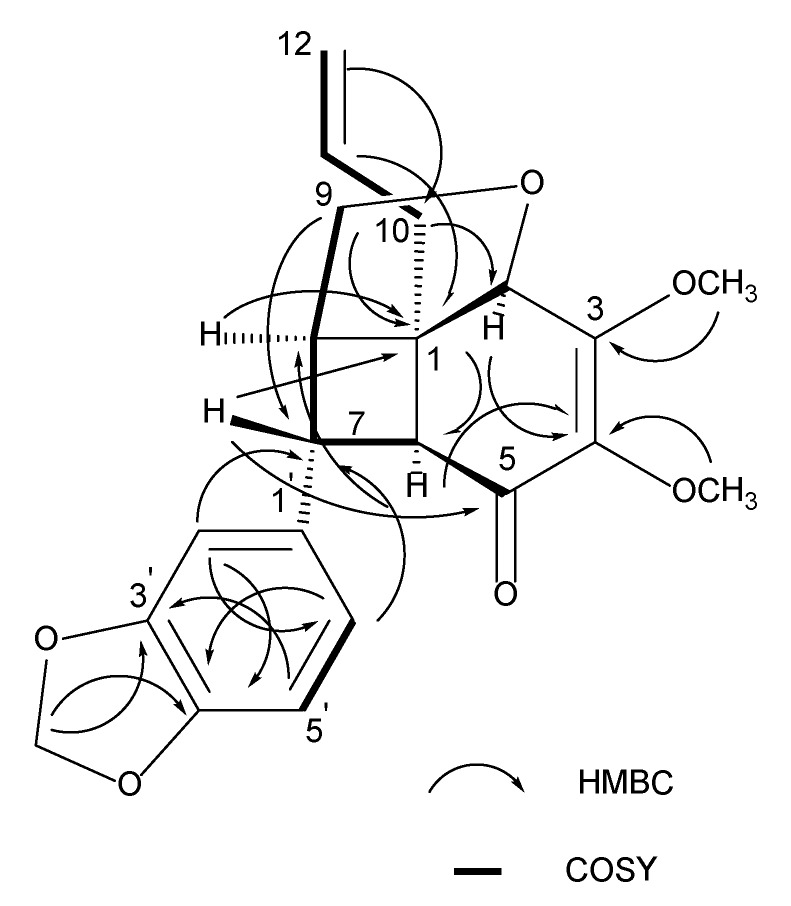
The 13C-NMR spectrum (Table 1) indicated the presence of 21 carbon signals; two methyls, four methylenes, four sp2 methines, four sp3 methines, six quaternary carbons and one carbonyl. The sp3 quaternary carbon, C-1 bearing a propenyl group gave a signal at δ 46.0, while C-5 carbonyl peak was observed at δ 193.1. The correlations of H-7/C-1, H-9/C-1 and H-11/C-1 in the HMBC spectrum further confirmed the position of propenyl fragment at C-1. In addition, the cross-peaks in the COSY spectrum were observed between H-5′/H-6′, H-7/H-6, H-7/H-8, H-8/H-9, H-10/H-11and H-11/H-12 as shown in Figure 2. Finally, thorough analysis of the COSY, HMQC and HMBC spectra allowed the complete assignments of all protons and carbons of compound 1 (Table 1).
Figure 2.
Selected HMBC and COSY correlations of (+)-kunstlerone (1).
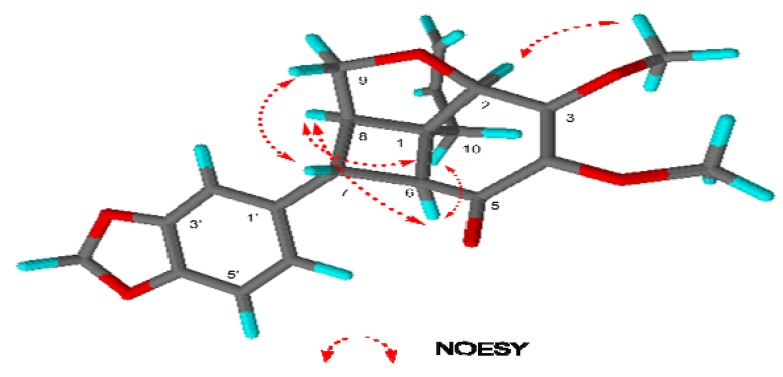
The relative configuration of the five stereogenic centers of compound 1 was determined by the correlations between H-6 (δ 2.90)/H-8 (δ 2.83), H-2/3-OCH3 (δ 4.08), H-6/H-10(δ 2.38) and H-7/H-9(δ 3.90) in the NOESY spectrum. Therefore, the relative configurations were assigned as 7R, 8R, 1R, 6S and 2R, as depicted in Figure 3 [24].
Figure 3.
Selected NOESY correlation of (+)- kunstlerone (1).
Antioxidant activity
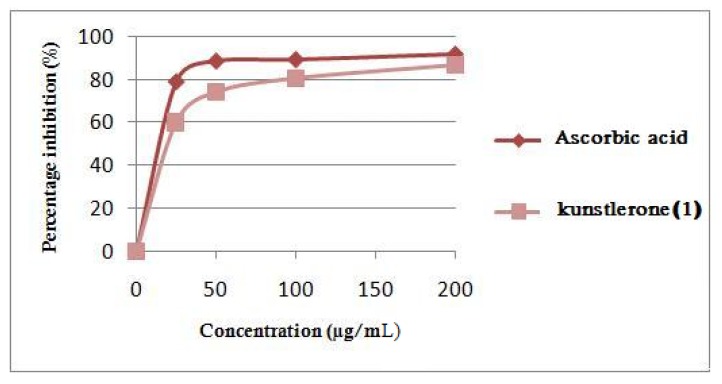
(+)- Kunstlerone (1) was tested for antioxidant activity using a DPPH radical scavenging activity assay. The results have shown that it possesses a potent antioxidant activity, with scavenging capacity (SC50 = 20.0 µg/mL) comparable to that of ascorbic acid (SC50 = 14.0 µg/mL, Figure 4). The antioxidant activities of neolignan glycosides from Verbascum salviifolium Boiss and neolignan phenols from Nectandra grandiflora have been reported previously [25]. The mechanism of action for the strong scavenging capacity of compound 1 is under investigation.
Figure 4.
DPPH radical scavenging activity of the isolated new neolignan; (+)- kunstlerone (1) with an SC50 = 20.0 µg/mL.
3. Experimental
3.1. General
The optical rotations were recorded on a JASCO (Japan) P1020 Polarimeter equipped with a tungsten lamp; MeOH as solvent. The mass spectra were obtained from LCMS-IT-TOF, Shimadzu. The ultraviolet spectra were obtained in MeOH on a Shimadzu UV-310 ultraviolet-visible spectrometer. The Fourier Transform Infrared (FTIR) spectra were obtained with CHCl3 (NaCl window technique) on a Perkin Elmer 2000 instrument. The 1H-NMR and 13C-NMR spectra were recorded in deuterated chloroform on a JEOL 400 MHz spectrometer; chemical shifts are reported in ppm on δ scale, and the coupling constants are given in Hz. Mayer’s reagent was used for alkaloid screening. Aluminum TLC sheets and PTLC (20 × 20 cm Silica gel 60 F254) were used in the TLC analysis. The TLC and PTLC spots were visualized under UV light (254 and 366 nm) followed by spraying with Dragendorff’s reagent for an alkaloid detection. All solvents, except those used for bulk extraction, were AR grade.
3.2. Plant materials
The leaves of Beilschmiedia kunstleri (Lauraceae) was collected from Hutan Simpan Sungai Tekam, Jerantut, Pahang, Malaysia. The plant was identified by Mr. Teo Leong Eng. A voucher specimen (KL5627) was deposited at the Herbarium of the Department of Chemistry, University of Malaya, Kuala Lumpur, Malaysia and at the Herbarium of the Forest Research Institute, Kepong, Malaysia.
3.3. Extraction and isolation of neolignan and the alkaloids
The air-dried leaves (2.50 kg) of Beilschmiedia kunstleri Gamble were extracted exhaustively with hexane (10.0 L) for 72 hours. The residual plant material was dried and left for 4 h after moistening with 10% NH4OH. It was then macerated with CH2Cl2 (12.0 L) for four days. After filtration, the supernatant was concentrated to 500 mL at room temperature (30 °C) followed by acidic extraction with 5% HCl until a negative Mayer’s test result was obtained. The aqueous solution was made alkaline to pH 11 with NH4OH and re-extracted with CH2Cl2. This was followed by washing with distilled H2O, dried over anhydrous sodium sulphate, and evaporation to give a crude alkaloid (4.50 g). The crude alkaloid (4.00 g) was subjected to exhaustive column chromatography over silica gel (column diameter = 2 cm, length =75 cm, silica gel 60, 70–230 mesh ASTM; Merck 7734) using CH2Cl2 gradually enriched with methanol (1% → 80% MeOH; 450 mL portions of eluent were used for each percentage) to yield 12 fractions. Purification of combined fractions 3–5 (0.4 g) using preparative TLC (Merck KGaA silica gel 60 F254) afforded a neolignan identified as kunstlerone (1, 0.02%), pallidine (7, 0.08%, CH2Cl2-MeOH; 98:2) and norboldine (2, 0.07%, CH2Cl2-MeOH; 95:5). Fraction 9 afforded N-methylisococlaurine (3, 0.05%, CH2Cl2-MeOH; 98:2), and fractions 10–11 (0.3 g) produced cassythicine (4, 0.03%; CH2Cl2-MeOH; 97:3), laurotetanine (5, 0.01%, CH2Cl2-MeOH; 98:2) and boldine (6, 0.04%, CH2Cl2-MeOH; 98:2).
(+)-Kunstlerone (1, Figure 1). = +163.63° (C = 2.37 × 10−3 M, MeOH), was obtained as a white amorphous solid; UV max (MeOH): 404 (1.36) and 332 (4.00) nm; IR bands (KBr): 1,725, 1,654, 1,615, 1,491, 1,231, and 1,038 cm−1; 1H-NMR (400 MHz, CDCl3) and 13C-NMR (100 MHz, CDCl3): see Table 1; LCMS-IT-TOF, m/z: 371.1490 [M+H]+ (calc. 371.1495 for C21H23O6).
3.4. Antioxidant assay
The free radical scavenging activity was determined using DPPH as described by Shimada et al. [26]. The DPPH radical scavenging activity assay is a decolorization assay that determines the activity of antioxidants to directly react with DPPH stable free radical by observing its absorbance at 517 nm with a spectrophotometer. A purple colored 1,1-diphenyl-2-picryl hydrazyl (DPPH), a stable free radical which is reduced to α,α-diphenyl-β-picryl hydrazine and give yellow color when reacts with antioxidant. The decolorization of purple color indicates the potential of antioxidants of the samples in which increased decolorization shows the higher scavenging activity of the samples.
Briefly, 0.1 mM DPPH (1 mL) dissolved in ethanol was added to an ethanol solution (3 mL) of the tested compound at different concentrations (25, 50, 100, 150, 200 µg/mL). An equal volume of ethanol was added in the control test. The mixture was shaken vigorously and allowed to stand at room temperature for 30 min. Then the absorbance at 517 nm was measured with a UV–VIS spectrophotometer. Lower absorbance of the reaction mixture indicated higher free radical scavenging activity. The percentage of scavenging of DPPH was calculated using the following equation:
where A° is the absorbance of the control reaction and A1 is the absorbance in the presence of the sample.
4. Conclusions
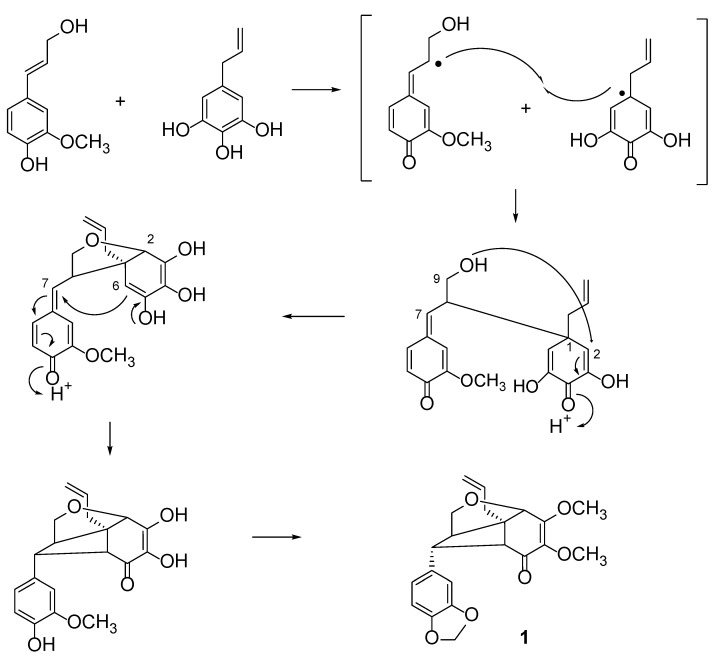
This is the first communication on neolignans from the Beilschmiedia Kunstleri. To the knowledge of the authors, kunstlerone (1) is the first neolignan reported in the family of Lauraceae bearing a propenyl group at C-1 and the ether ring that is attached to C-2 and C-9. (+)-Kunstlerone (1) showed a strong antioxidant activity with an SC50 of 20.0 µg/mL. This compound was also detected in neutral fractions, showing that it could be isolated using an acid and base extraction. A proposed plausible biogenetic pathway for 1 as shown in Scheme 1. Apparently, it results from the oxidative coupling between the two precursors coniferyl alcohol and allyl-3,4,5-trihydroxybenzene. The primary hydroxyl would give a Michael-type addition to α,β-carbonyl function and then, an enol addition to the quinone methide (attack of C-6 to C-7) would give the cyclobutane ring. Finally, the dimer was O-methylated at C-3 and C-4 and the methylenedioxy group was formed between the hydroxyl and methoxyl groups of the coniferyl alcohol moiety. Alkaloids 2-7, which were identified in this study, belong to the aporphine, benzylisoquinoline and morphinandienone type of alkaloids.
Scheme 1.
Biogenetic pathway for kunstlerone (1).
Acknowledgements
The authors gratefully acknowledge the financial support provided by University of Malaya Research Grant (UMRG RG045/11BIO), Centre of Natural Products and Drugs Development (CENAR), Postgraduate Research Grant of University of Malaya (PS366/2010B) and CNRS Grant. We also acknowledge the support given by Din Mohd Nor, Hasri Abdullah and Rafly Syamsir from the Herbarium Group, University of Malaya. This work was carried out within the framework of an official agreement between the CNRS and the University of Malaya (Malaysia).
Footnotes
Sample Availability: Samples of the compounds are not available from the authors.
References
- 1.Kochummen K.M. In: Tree Flora of Malaya: A manual For Foresters. Ng F.S.P., editor. Volume 4. Longman; Selangor, Malaysia: 1989. p. 98. [Google Scholar]
- 2.Corner E.J.H. Wayside Trees of Malaya. 4th ed. United Selangor Press; Kuala Lumpur, Malaysia: 1997. p. 371. [Google Scholar]
- 3.Lenta B.N., Tantangmo F., Devkota K.P., Wansi J.D., Chouna J.R., Soh R.C.F., Neumann B., Stammler H.G., Tsamo E., Sewald N. Bioactive constituents of the stem bark of Beilschmiedia zenkeri. J. Nat. Prod. 2009;72:2130–2134. doi: 10.1021/np900341f. [DOI] [PubMed] [Google Scholar]
- 4.Guinaudeau H., Leboeuf M., Cavé A. Aporphine alkaloids. Lloydia. 1975;38:275–337. [PubMed] [Google Scholar]
- 5.Chouna J.R., Nkeng-Efouet P.A., Lenta B.N., Devkota K.P., Neumann B., Stammler H.G., Kimbu S.F., Sewald N. Antibacterial endiandric acid derivatives from Beilschmiedia anacardioides. Phytochemistry. 2009;70:684–688. doi: 10.1016/j.phytochem.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 6.Harborne J.B., Méndez J. Flavonoids of Beilschmiedia miersii. Phytochemistry. 1969;8:763–764. doi: 10.1016/S0031-9422(00)85849-5. [DOI] [Google Scholar]
- 7.Yang P.S., Cheng M.J., Peng C.F., Chen J.J., Chen I.S. Endiandric acid analogs from the roots of Beilschmiedia erythrophloia. J. Nat. Prod. 2009;72:53–58. doi: 10.1021/np800504w. [DOI] [PubMed] [Google Scholar]
- 8.Yang P.S., Cheng M.J., Chen J.J., Chen I.S. Two new endiandric acid analogs, a new benzopyran and a new benzenoid from the root of Beilschmiedia erythrophloia. Helv. Chim. Acta. 2008;91:2130–2138. doi: 10.1002/hlca.200890229. [DOI] [Google Scholar]
- 9.Tchiegang C., Parmentier M. Chemical composition and nutritional evaluation of two Cameroonian soup thickeners: Belschmiedia Jacques felexii and Belschmiedia anacardiodes. Int. J. Food Sci. Technol. 2008;45:187–189. [Google Scholar]
- 10.Chen J.J., Chou E.T., Peng C.F., Chen I.S., Yang S.Z., Huang H.Y. Novel epoxyfuranoid lignans and antitubercular constituents from the leaves of Beilschmiedia tsangii. Planta Med. 2007;73:567–571. doi: 10.1055/s-2007-967195. [DOI] [PubMed] [Google Scholar]
- 11.Setzer W.N., Haber W.A. Leaf essential oil composition of five species of Beilschmiedia from Monteverde, Costa Rica. Nat. Prod. Commun. 2007;2:79–83. [Google Scholar]
- 12.Kitagawa L., Minagawa K., Zhang R.S., Hori K., Doi M., Inoue M., Ishida T., Kimura M., Uji T., Shibuya H. Dehatrine, an antimalarial bisbenzylisoquinoline alkaloid from the Indonesian medicinal plant Beilschmiedia madang, isolated as a mixture of two rotational isomers. Chem. Pharm. Bull. 1993;41:997–999. doi: 10.1248/cpb.41.997. [DOI] [PubMed] [Google Scholar]
- 13.Pudjiastuti P., Mukhtar M.R., Hadi A.H.A., Saidi N., Morita H., Litaudon M., Awang K. (6, 7-Dimethoxy-4-methylisoquinolinyl)-(4′-methoxyphenyl)-methanone, a new benzylisoquinoline alkaloid from Beilschmiedia brevipes. Molecules. 2010;15:2339–2346. doi: 10.3390/molecules15042339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rossi M.H., Yoshida M., Maia J.G.S. Neolignans, styrylpyrones and flavonoids from an Aniba species. Phytochemistry. 1997;45:1263–1269. doi: 10.1016/S0031-9422(97)00075-7. [DOI] [Google Scholar]
- 15.Barbosa Filho J.M., Yoshida M., Gottlieb O.R., Barbosa R.C.B.C., Giesbrecht A.M., Young C.M. Benzoyl esters and amides, styrylpyrones and neolignans from the fruits of Aniba riparia. Phytochemistry. 1987;26:2615–2617. doi: 10.1016/S0031-9422(00)83890-X. [DOI] [Google Scholar]
- 16.Funasaki M., Lordello A.L., Viana A.M., Santa-Catarina C., Floh E.I.S., Yoshida M., Kato M.J. Neolignans and sesquiterpenes from leaves and embryogenic cultures of Ocotea catharinensis (Lauraceae) J. Braz. Chem. Soc. 2009;20:853–859. doi: 10.1590/S0103-50532009000500008. [DOI] [Google Scholar]
- 17.Rachmatiah T., Mukhtar M.R., Nafiah M.A., Hanafi M., Kosela S., Morita H., Litaudon M., Awang K., Omar H., Hamid A.H.A. (+)-N-(2-Hydroxypropyl)lindcarpine: A new cytotoxic aporphine isolated from Actinodaphne pruinosa Nees. Molecules. 2009;14:2850–2856. doi: 10.3390/molecules14082850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tewari S., Bhakuni D.S., Dhar M.M. The aporphine alkaloids of Litsea glutenosa. Phytochemistry. 1972;11:1149–1152. doi: 10.1016/S0031-9422(00)88469-1. [DOI] [Google Scholar]
- 19.Yuan L.K., Chung H.C., Shoei S.L. Chemical constituents from Phoebe minutiflora II. Nat. Prod. Res. 2006;20:1199–1206. doi: 10.1080/14786410600899068. [DOI] [PubMed] [Google Scholar]
- 20.Gözler B., Öziç P., Freyer A.J., Shamma M. Morphinandienone alkaloids from Roemeria refracta. J. Nat. Prod. 1990;53:986–988. doi: 10.1021/np50070a035. [DOI] [PubMed] [Google Scholar]
- 21.Babcock P.A., Segelman A.B. Alkaloids of Lindera benzoin (L.) Blume (Lauraceae) I: Isolation and identification of laurotetanine. J. Pharm. Sci. 1974;63:1495–1496. doi: 10.1002/jps.2600630944. [DOI] [PubMed] [Google Scholar]
- 22.Guinaudeau H., Leboeuf M., Cavé A. Aporphinoid alkaloids, III. J. Nat. Prod. 1983;46:761–835. doi: 10.1021/np50030a001. [DOI] [Google Scholar]
- 23.Nozaki H., Hayashi K.I., Kido M., Kakumoto K., Ikeda S., Matsuura N., Tani H., Takaoka D., Iinuma M., Akao Y. Pauferrol A, a novel chalcone trimer with a cyclobutane ring from Caesalpinia ferrea mart exhibiting DNA topoisomerase II inhibition and apoptosis-inducing activity. Tet. Lett. 2007;48:8290–8292. doi: 10.1016/j.tetlet.2007.09.130. [DOI] [Google Scholar]
- 24.Engler T.A., Wei D., Letavic M.A., Combrink K.D., Reddy J.P. Regioselective Lewis acid-directed reactions of 2-alkoxy-5-alkyl-1,4-benzoquinones with styrenes: Synthesis of burchellin and guianin neolignans. J. Org. Chem. 1994;59:6588–6599. doi: 10.1021/jo00101a017. [DOI] [Google Scholar]
- 25.Ribeiro A.B., Bolzani V.S., Yoshida M., Santos L.S., Eberlin M.N., Silva D.H.S. A new neolignan and antioxidant phenols from Nectandra grandiflora. J. Braz. Chem. Soc. 2005;16:526–530. doi: 10.1590/S0103-50532005000400005. [DOI] [Google Scholar]
- 26.Shimada K., Fujikawa K., Yahara K., Nakamura T. Antioxidative properties of xanthin on autoxidation of soybean oil in cyclodextrin emulsion. J. Agric. Food Chem. 1992;40:945–948. doi: 10.1021/jf00018a005. [DOI] [Google Scholar]