Abstract
Pentylphenols 1 and 2, cyclopropane fatty acid 3, and cyclopentenones 4 and 5, were isolated from an ascidian, Diplosoma sp. The structures of 1−5 were determined by spectroscopic analysis and/or synthesis. Compound 1 inhibited the division of fertilized sea urchin eggs and compound 4 showed mild cytotoxity against HCT116 cells (human colorectal cancer cell).
Keywords: ascidian, Diplosoma sp., cytotoxity, NMR
1. Introduction
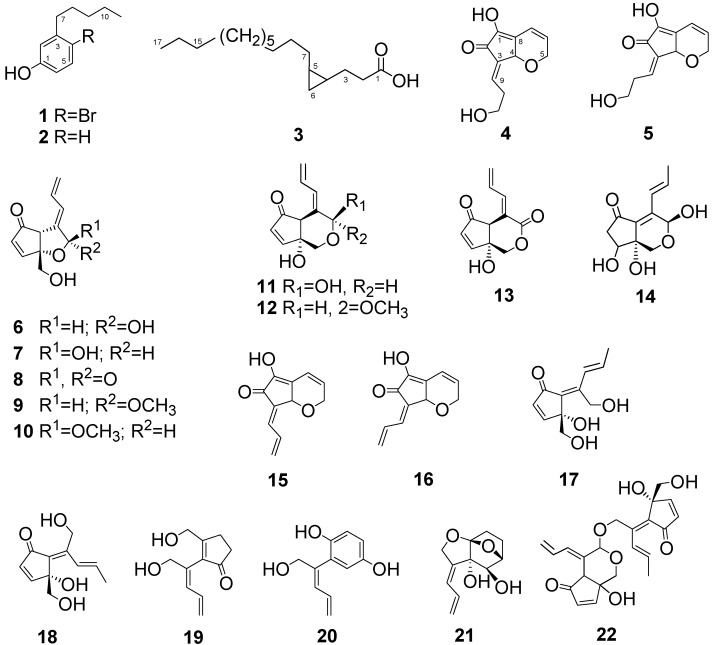
Ascidians are a rich source of novel bioactive secondary metabolites, including a diverse array of amino acid-derived alkaloids, cyclic peptides, and acetogenins [1,2,3,4]. The biomedical potential of ascidian metabolites has resulted in a focused interest in these primitive chordates. A series of C11 compounds having the distinctive exo-allylidene-lactone named didemnenones were isolated from didemnid ascidians, Trididemnum cyanophorum [didemnenones A (6) and B (7)] and Didemnum voeltzkowi (didemnenones C and D) [5]. They showed a wide range of biological activities, including toxicity against leukemia cells as well as antimicrobial and antifungal activities. Their structures were determined based on an X-ray investigation of the corresponding methylacetal and from synthetic results [5,6,7]. As described previously, as part of our ongoing research aimed at the isolation of biologically active metabolites from marine organisms living in the tidal zone, we have isolated fourteen C11 compounds, dinemnenone congeners 6–18 and 22 from the didemnid ascidians Lissoclinum sp. and Diplosoma spp. [8,9,10] (Figure 1).
Figure 1.
C11 compounds and a cyclopropane fatty acid from marine organisms.
As part of our continuing chemical studies of Okinawan marine organisms, we examined the constituents of the ascidian Diplosoma sp. A crude ethyl acetate (EtOAc) extract of this organism strongly inhibited cell division of fertilized sea urchin eggs [11]. Bioassay-guided fractionation of the extract, prepared from the first collection of the ascidian in April, 2003, led to the isolation of a new compound, 4-bromo-3-pentylphenol (1) [12], the known 3-pentylphenol (2) [12,13], and a new cyclopropane fatty acid 3. In addition, rapid fractionation of the extract made from a second collection in April 2006 gave new unstable C11 compounds 4[12] and 5.
2. Results and Discussion
The brown, encrusting ascidian Diplosoma sp. was collected by hand from the coast of Hateruma Island, Okinawa, and stored at −15 °C before being extracted with acetone. The acetone extract was partitioned between EtOAc and water. The EtOAc extract from the first collection of the ascidian completely inhibited the first cleavage of fertilized sea urchin eggs at 20 ppm. Bioassay-guided fractionation of the toxic extract by a series of chromatographic processes, including silica gel column chromatography (CC), high performance thin layer chromatography (HPTLC) and high performance liquid chromatography (HPLC), yielded compounds 1 (0.00010%), 2 (0.000050%), and cyclopropane fatty acid 3 (0.00020%). The sample from the second collection was immediately extracted with acetone after transportation to our laboratory, and the extract was partitioned between water and ethyl acetate (EtOAc). The EtOAc extract was suspended in MeOH/H2O (1:1) and successively extracted with hexanes and CHCl3. The CHCl3 extract was quickly separated by HPLC on ODS to yield unstable compounds 4 (0.37%) and 5 (0.057%).
LREIMS of 1 showed the M+ ion at m/z 242.0 and an ion of equal intensity at m/z 244.0, indicating the presence of a single bromine atom. Analysis of 1 by 13C-NMR (Table 1) and LREIMS provided the molecular formula C11H15BrO, which accounted for four degrees of unsaturation.The presence of a 1,3,5-trisubstituted benzene ring was deduced from 1H-NMR and 13C-NMR data [δH 6.07 (dd), δC 114.8 (d); δC 114.8 (s); δH 6.32 (d), δC 117.5 (d); δH 7.21 (d), δC 133.6 (d); δC 143.5 (s); δC 155.6 (s)].
Table 1.
1H- and 13C-NMR data for compounds 1, 4 and 5.
| C no. | 1 a | 4 | 5 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| δ C | δH (mult, J in Hz) | δC b | δH (mult, J in Hz) b | δC c | δH (mult, J in Hz) b | |||||
| 1 | 155.6 | 147.7 | 147.4 | |||||||
| 2 | 117.5 | 6.32 (1H, d, 3.0) | 188.0 | 188.8 | ||||||
| 3 | 143.5 | 135.5 | 135.5 | |||||||
| 4 | 114.8 | 70.8 | 4.88 (br s) | 71.8 | 4.63 (br s) | |||||
| 5 | 133.6 | 7.21 (1H, d, 8.5) | 66.9 | 4.40 (1H, ddd, 2.5, 2.5, 18.5) | 67.0 | 4.37 (1H, ddd, 2.5, 4.5, 18.5) | ||||
| 4.52 (1H, ddd, 1.6, 4.5, 18.5) | 4.48 (1H, ddd, 1.6, 4.5, 18.5) | |||||||||
| 6 | 114.8 | 6.07 (1H, dd, 3.0, 8.5) | 133.1 | 6.07 (1H, ddd, 2.5, 4.5, 10.0) | 134.2 | 6.02 (1H, ddd, 2.5, 4.5, 10.0) | ||||
| 7 | 36.4 | 2.57 (2H, t, 8.0) | 118.5 | 6.73 (1H, ddd, 1.6, 2.5, 10.0) | 118.3 | 6.70 (1H, ddd, 1.6, 2.5, 10.0) | ||||
| 8 | 29.8 | 1.50 (2H, quin., 8.0) | 130.5 | 128.8 | ||||||
| 9 | 31.8 | 1.21 (2H, m) | 134.7 | 6.61 (1H, dt, 1.6, 8.8) | 134.7 | 6.22 (1H, dt, 1.6, 8.8) | ||||
| 10 | 22.8 | 1.21 (2H, m) | 32.1 | 2.60 (1H, m) | 31.0 | 2.90 (1H, m) | ||||
| 2.70 (1H, m) | 2.90 (1H, m) | |||||||||
| 11 | 14.2 | 0.83 (3H, t, 7.3) | 60.5 | 3.70 (2H, m)) | 61.9 | 3.65 (2H, m)) | ||||
| OH | 3.96 (1H, br s) | |||||||||
a 1H-NMR (500 MHz) and 13C-NMR (125 MHz) recorded in C6D6; b 1H-NMR (500 MHz) or 13C-NMR (100 MHz) recorded in 5% CD3OD in CDCl3. c 13C-NMR (100 MHz) recorded in CDCl3.
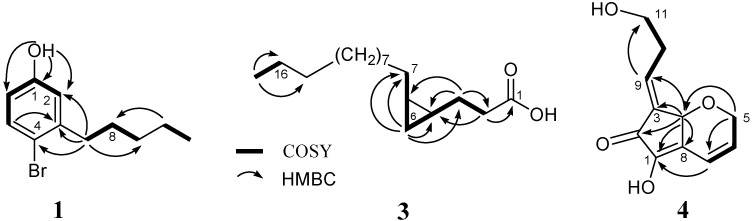
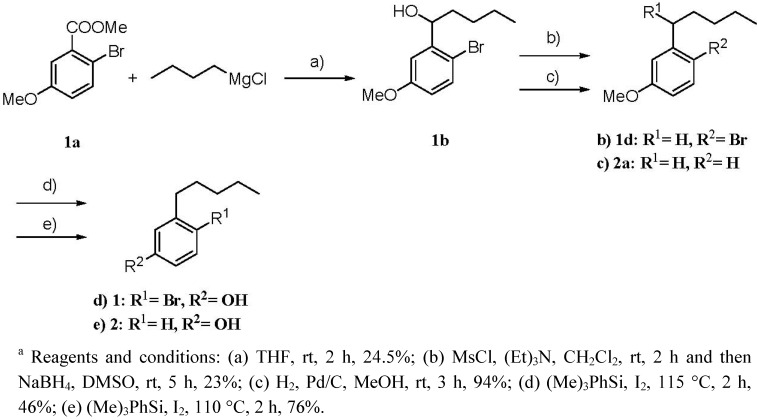
The 1H- and 13C-NMR spectra also contained five highfield signals [δC 14.2 (q), δH 0.83 (3H, t, J = 7.3 Hz); δC 22.8 (t), δH 1.21 (2H, m); δC 29.8 (t), δH (2H, quin., J = 8.0 Hz); δC 31.8 (t), δH 1.21 (2H, m); δC 36.4 (t), δH (2H, t, J = 8.0 Hz)]. The methyl protons at δH 0.83 were coupled to the methylene protons at δH 1.21. The methylene protons at δH 2.57 were coupled to the methylene protons at δH 1.50, which were in turn coupled to the other methylene protons at δH 1.21 (Table 1, Figure 2). Further detailed interpretation of the 1H-NMR, 13C-NMR, 1H−1H COSY and HMQC spectral data revealed the presence of an n-pentyl moiety in 1 (Figure 2). The proton at δH 3.96 (s) did not show any HMQC correlations, but HMBC correlations to two olefinic carbons, suggesting thepresence of an OH group coupled with the molecular formula of 1. Bromo, n-pentyl and hydroxyl positions on the benzene ring were determined by comparison with calculated 1H and 13C chemical shift values, and by HMBC correlations of OH/C-1, OH/C-2, H-2/C-1, H-2/C-7, H-5/C-3, H-5/C-4, H-5/C-6, H-7/C-2, H-7/C-3, H-7/C-4. Since decomposition of 1 prevented us from characterizing this compound completely, synthesis of 1 was attempted (Scheme 1). The synthesis started with methyl 2-bromo-5-methoxybenzoate (1a). The Grignard reaction of 1a with pentylmagnesium chloride afforded alcohol 1b. Mesylation of 1b, followed by reduction of the mesylate with NaBH4, gave 4-bromo-3-pentylanisole (1d). Cleavage of the ether 1d with phenyltrimethylsilane/iodine yielded 1 as the sole product. 1H-NMR data of the product were identical with those of the naturally occurring compound. The known 3-pentylphenol (2) was identified by comparison of its NMR data with those of synthetic 2 (Scheme 1) [12]. Reduction of 2b with H2/Pd-C gave 3-pentylanisole (2e). Cleavage of the ether 2e with phenyltrimethylsilane/iodine yielded 2 as the sole product. 1H-NMR data of the product were in agreement with those of natural compound 2. Compound 1 completely inhibited the first cleavage of fertilized sea urchin eggs at 1 ppm.
Figure 2.
Structures of compounds 1, 3 and 4 based on 2D NMR data.
Scheme 1.
Synthesis of compounds 1 and 2a.
Analysis of the 13C-NMR and HRFABMS data [m/z 269.2462 (M + H)+, Δ −1.9 mmu] for compound 3 provided the molecular formula C17H32O3, which accounted for two degrees of unsaturation. In the 13C-NMR spectrum, 17 carbon signals were observed, including a carbonyl carbon, two methine carbons, 13 methylene carbons and a methyl carbon. The 13C-NMR resonance at δC 176.9 indicated the presence of a carboxyl group, which was substantiated by IR absorption bands at 1700 and 3300 cm−1. Four upfield shifted signals [δH 0.71 (1H, m, H-4), δC 15.1 (d); 0.71 (1H, m, H-5), δC 15.9 (d); 0.60 (1H, ddd, J = 8.0, 8.0, 5.0 Hz, H-6a) and −0.27 (1H, ddd, J = 5.0, 5.0, 5.0 Hz, H-6b), δC 10.7 (t)] in the NMR spectra suggested that 3 should contain a cyclopropane ring. These data revealed compound 3 to be a cyclopropane fatty acid. Analysis of COSY, HMQC and HMBC spectra permitted assignment of the protons of the cyclopropane ring (Figure 2). Geometric configuration of the cyclopropane was assigned to be cis by analysis of the coupling constants (J4, 6a = 8.0 Hz, J4, 6b= 5.0 Hz, J5, 6a= 8.0 Hz, J5, 6b= 5.0 Hz, J6a, 6b= 5.0 Hz). This was confirmed by comparison of the 1H- and 13C-NMR chemical shifts within the cyclopropane ring for 3with those of cis- and trans-1,2-disubstituted cyclopropanes [14,15,16]. Compound 3 showed no activity at 1, 5 and 10 ppm in sea urchin eggs assay.
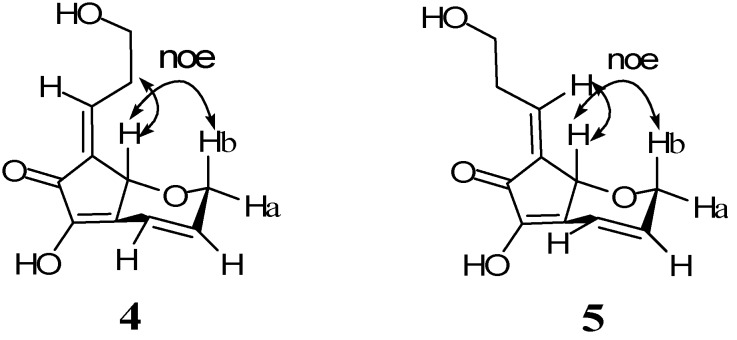
Analysis of 13C-NMR (Table 1) and HRESIMS data [m/z 231.0698 (M + Na)+, Δ −6.4 mmu; m/z 191.0688 (M + H − H2O)+, Δ −2.0 mmu] for compound 4 provided the molecular formula C11H12O4, which indicated six degrees of unsaturation. The IR absorption bands at 1,680 and 3,250 cm−1 indicated the presence of carbonyl and hydroxyl groups. 1H- and 13C-NMR data analysis indicated the presence of a carbonyl carbon (δC 188.0), a cis double bond [δH 6.07 (1H, ddd, J = 10.0, 4.5, 2.5 Hz), δC 133.1 (d); δH 6.73 (1H, ddd, J = 10.0, 2.5, 1.6 Hz ), δC 118.5 (d)], a tetrasubstituted double bond [δC 130.5 (s); 147.7(s)], a trisubstituted double bond [δH 6.61 (1H, td, J = 8.8, 1.6 Hz), δC 134.7 (d); δC 135.5 (s)], an oxygenated methine [δH 4.88 (1H, br s), δC 70.8 (d)], two oxygenated methylenes [δH 4.40 (1H, ddd, J = 18.5, 2.5, 2.5 Hz) and 4.52 (1H, ddd, J = 18.5, 4.5, 1.6 Hz), δC 66.9 (t); δH 3.70 (2H, m), δC 60.5 (t)] and a methylene [δH 2.60 (1H, m) and 2.70 (1H, m), δC 32.1 (t)]. The NMR data of 4 showed close similarity to those of the known compound 15[9,17]. However, in contrast to 15, 4 contained an oxygenated methylene and a methylene instead of a terminal double bond. The oxygenated methylene protons at δH 4.40 (1H) and 4.52 (1H) were coupled to the olefinic proton at δH 6.07. The oxygenated methylene protons were also coupled to the olefinic proton at δH 6.73 with small coupling constants of 2.5 Hz and 1.6 Hz, respectively. The other oxygenated methylene protons at δH 3.70 were coupled to the methylene protons at δH 2.60 (1H) and δH 2.70 (1H), which were in turn coupled to the olefinic proton at δH 6.61 (Table 1, Figure 2). Further detailed interpretation of the 1H-NMR, 13C-NMR, 1H−1H COSY and HMQC spectral data revealed the presence of partial structures, C-5−C-7, C-9−C-11, C-1−C-8, C-3−C-9, C-2 and C-4 (Figure 2). The connectivity of the partial structures was established from the HMBC correlations of H-4/C-1, H-4/C-2, H-4/C-3, H-4/C-8, H-4/C-9, H-5/C-4, H-5/C-7, H-7/C-1, H-9/C-11, as shown in Figure 2, to describe the entire carbon framework of 4. The double bond stereochemistry of compound 4 was established by NOE Differential Spectroscopy (NOEDS) experiments (Figure 3). Irradiation of H-4 resulted in enhancement of H-10 and H-5b, thereby supporting the E-configuration of the double bond between C-3/C-9. The proton H-9 shifts down field at δH 6.61. Irradiation of H-9 showed no significant enhancement on H-4, and a very small enhancement with H-11. The absolute configuration of C-4 is yet to be determined.
Figure 3.
Selected NOEs of compounds 4 and 5.
Analysis of 13C-NMR (Table 1) and HREIMS data [m/z 191.0736 (M + H − H2O)+, Δ −2.8 mmu] for compound 5 provided the molecular formula C11H12O4. Spectroscopic data for 5 showed close similarity to those of 4 (Table 1). The largest difference in chemical shifts observed between 4 and 5 were for H-9. The chemical shift (δH 6.61) of H-9 in 4 was at lower field than that (δH 6.38) in 5 owing to the magnetic anisotropy effect of the carbonyl group, suggesting an E configuration for the C-3,9 double bond of 4 and thus a Z configuration for that of 5. This was confirmed by NOEDS experiments. Irradiation of H-4 of 4 resulted in enhancement of H-9 and H-5b, and H-4 of 5 showed NOEs to H-10 and H-5b. Compound 4 showed weak cytotoxity against HCT116 cells (human colorectal cancer cells) in a dose dependent manner (IC50: >20 ppm). We could not evaluate the activity of unstable compound 5 because of the loss of 5 with the formation of insoluble material.
To date, a variety of C11 compounds 6–21have been isolated from ascidians, sponges and cyanobacteria [5,9,10,17,18,19,20]. C11 cyclopentenones (didemnenones) 13, 14 and 19, the related compound 20, and compound 21, have been isolated from ascidians (Lissoclinum spp.), cyanobacteria, and a sponge, respectively. Compound 18 has been isolated from an ascidian (Diplosoma virens) and a sponge (Ulosa sp.) [9,17]. Isolation of a series of C11 compounds, including compounds 4 and 5, from unrelated marine organisms supports the potential microbial origin of these compounds. From this perspective, it was assumed that the ascidian Diplosoma sp. might not be the real producer of compounds 4 and 5; rather, these compounds could originate from a microbial source such as a Prochloron sp., an obligatory symbiont of ascidians [21,22,23]. The Prochloron sp. was isolated from ascidian Diplosoma sp. by squeezing through a plankton net, followed by acetone extraction. 1H-NMR spectra of the crude extract showed the same peaks as those of pure compounds 4 and 5. Therefore, it was concluded that a microorganism, probably a Prochloron sp. was the actual producer of 4 and 5.
Most C11 compounds are derived from polyketides [(six acetates − C1) or (five acetates + C1)]. Pentylphenols are known to be formed with a loss of CO2 from a C12 parent (six acetates) [24]. In the previous paper, we proposed that didemnenone-related compounds 6-22 should be derived from 4-methyldecane via various types of cyclization [10,24]. Investigation of the biogenesis of compounds 4 and 5, and related compounds 6−18 is in progress in our laboratory.
3. Experimental
3.1. General Experimental Procedures
Optical rotations were measured on a JASCO P-1020 polarimeter. UV spectra of the methanol solutions were measured on a JASCO V-550 spectrophotometer. IR spectra were recorded on a JASCO FT/IR-300 spectrometer. The 1H-, 13C-, and 2D-NMR spectra were recorded on a JEOL lambda 400 or a JEOL α-500 spectrometer, and 1H and 13C chemical shifts were referenced to the solvent peaks (δH 7.24 and δC 77.0 in CDCl3; δH 7.16 and δC 128.6 in C6D6). Mass spectra were measured on a Waters Quattro micro API triple quadruple mass analyzer. Open column chromatography was performed on Kieselgel 60 (70–230 mesh, Merck). HPLC was performed using a COSMOSIL-packed ODS HPLC column (C18, 10 × 250 mm) or COSMOSIL Si60 HPLC column (5SL, 10 × 250 mm). Analytical TLC was performed using Kieselgel 60 F254 DC-fertigplatten (Merck). All solvents used were reagent grade.
3.2. Animal Material
The small brown tunicate was collected at low tide from the coast of Hateruma Island, Okinawa, Japan in April, 2003 and in April, 2006, and identified as Diplosoma sp. by Professor Euichi Hirose, University of the Ryukyus, Japan. A voucher specimen was deposited at the University of the Ryukyus (specimen no. 030412).
3.3. Extraction and Isolation
The brown tunicate collected from Hateruma Island was kept frozen during transportation. Theascidian Diplosoma sp. (1 kg, wet weight) was extracted with acetone (1.5 L) twice. After filtration, the extracts were concentrated in vacuo to give an acetone extract. The acetone extract was partitioned between H2O (200 mL) and EtOAc (300 mL × 2). The EtOAc extract (21.3 g) completely inhibited the first cell division of fertilized sea urchin eggs at 20 ppm. The extract was first chromatographed on silica gel using hexanes with increasing proportions of EtOAc [hexanes (600 mL) → hexanes/EtOAc (5:1, 600 mL → 3:1, 600 mL → 1:1, 600mL → 3:1, 600 mL) and then EtOAc with increasing proportions of MeOH [EtOAc (600 mL) → EtOAc/MeOH (9:1, 600 mL → 7:1, 600 mL)] to give 12 fractions. An active fifth fraction (240 mg) was subjected to further separation by CC on silica gel using the gradient solvent mixture hexanes-CH2Cl2-MeOH to give 19 fractions. Active fractions were combined and the mixture (100 mg) was subjected to ODS column chromatography (100% MeOH) to give 14 fractions. The second fraction (10.6 mg) was purified by HPTLC on silica gel using hexanes-EtOAc (6:1) to afford 1 (1.0 mg) and 2 (0.5 mg). The fifth fraction (21 mg) was separated by HPLC on silica gel using hexanes-CHCl3-isopropanol (50:10:3) to afford crude 3 (12 mg), which was purified by reversed phased HPLC on ODS using 0.01 M NH4Cl in MeCN-MeOH-H2O (50:48:2) to give 3 (2.0 mg). The sample of second collection (7 g, wet weight) was soaked in 50 mL acetone at room temperature (rt) and left in the dark for 8 h. After filtration, the residue was again extracted with acetone. The acetone extracts were concentrated under reduced pressure to give a residual oil. The oil was quickly partitioned between H2O and EtOAc three times to give 123.2 mg of EtOAc extract. This extract was suspended in MeOH and H2O (1:1) and then successively extracted with hexanes and chloroform (CHCl3), which were evaporated to give hexanes extract and CHCl3 extract. The CHCl3 part was subjected to reversed phased HPLC on ODS using MeOH-H2O (7:3) to furnish compound 4 (20 mg) in pure form, and a mixture of compound 4 and related compound 5 (10 mg). Further purification of the mixture by HPLC on ODS using MeOH-H2O (7:3) to give 4 (6 mg) and 5 (2 mg).
4-Bromo-3-pentylphenol (1). Colorless oil; 1H- and 13C-NMR (CDCl3) data, see Table 1; LREI(+)MS (relative intensity) m/z 244 (M+, 36), 242 (M+ + 2, 36), 109 (M+ − C4H9 − Br + H, 100).
cis-3-(2-Undecylcyclopropyl)propionic acid (3). Colorless powder; [α]25D + 11 (c 0.026 CHCl3); FT/IR (film) νmax 2950, 2830, 1700 cm−1; 1H-NMR (CDCl3, 500 MHz) δ 2.44 (2H, t, J = 7.5 Hz, H2), 1.75 (1H, m, H3), 1.49 (1H, m, H3), 1.36 (1H, m, H7), 1.2–1.3 (14H), 1.15 (1H, m, H7), 0.86 (3H, t, J = 6.5 Hz, H17), 0.71 (1H, m, H4), 0.71 (1H, m, H5), 0.60 (1H, ddd, J = 8.0, 8.0, 5.0 Hz, H6a), −0.27 (1H, ddd, J = 5.0, 5.0, 5.0 Hz, H6b); 13C-NMR (CDCl3, 125 MHz) δ 176.85, 33.98, 31.87, 30.09, 29.65, 29.60, 29.57, 29.30, 29.18, 28.59, 24.10, 22.63, 15.95, 15.08, 14.05, 10.74; HRAPCIMS m/z (M + H)+ 269.2462 (calcd for C17H32O3, 269.2481).
(E)-5-Hydroxy-7-(3-hydroxypropylidene)-7,7a-dihydrocyclopenta[b]pyran-6(2H)-one (4). Colorless oil; [α]29D + 2.4 (c 0.060 CHCl3); UV (MeOH) λmax 227 (logε 4.0), 330 (logε 3.7) nm; FT/IR (film) νmax 3350, 2920, 2850, 1685, 1420, 1150, 1046 cm−1; 1H-NMR (CDCl3, 500 MHz) and 13C-NMR (CDCl3) data, see Table 1; HRESI(+)MS m/z (M + Na)+ 231.0698 (calcd for C11H12O4Na, 231.0634), m/z (M + H − H2O)+ 191.0688 (calcd for C11H11O3, 191.0708).
(Z)-5-Hydroxy-7-(3-hydroxypropylidene)-7,7a-dihydrocyclopenta[b]pyran-6(2H)-one (5). Colorless oil; [α]25D + 5.4 (c 0.058 CHCl3); UV (MeOH) λmax 228 (logε 4.0), 330 (logε 3.6) nm; FT/IR (film) νmax 3350, 2920, 2850, 1684, 1420, 1151, 1058 cm−1; 1H- and 13C-NMR (CDCl3) data, see Table 1; HRESI(+)MS m/z (M + H − H2O)+ 191.0736 (calcd for C11H11O3, 191.0708).
4-Bromo-3-(1-hydroxybutyl)anisole (1b). To a solution of methyl 2-bromo-5-methoxybenzoate (1a) [243 µL (369 mg), 1.51 mmol, Tokyo Kasei Co., Ltd.] in tetrahydrofuran (THF, 2.0 mL) was added butylmagnesium chloride (0.91 M solution in THF, 2.4 mL, 2.12 mmol, Kanto Chemical Co., Inc.) via syringe at rt. The mixture was stirred at rt for 6 h and quenched with saturated NH4Cl solution. The products were extracted with CHCl3. The CHCl3 solution was dried (Na2SO4) and concentrated in vacuo. The residual oil (430 mg) was purified by column chromatography on silica gel (20 g) using hexanes-EtOAc (9:1) to afford alcohol 1b (101 mg, 24.5%). Colorless solid; mp 42−44 °C; UV (MeOH)max 228 (logε 4.0), 281 (logε 3.2) nm; FT IR νmax (KBr) 3300, 3005, 2960, 2920, 2830, 100, 1580, 1460 cm−1; 1H-NMR (500 MHz, CDCl3) δ 7.35 (1H, d, J = 8.8 Hz), 7.08 (1H, d, J = 3.2 Hz), 6.65 (1H, dd, J = 8.8, 3.2 Hz), 4.97 (1H, d, J = 8.3, 4.1 Hz), 3.77 (3H, s), 1.72 (1H, m), 1.61 (1H, m), 1.45 (1H, m), 1.35 (1H, m), 0.89 (3H, t, J = 7.3 Hz); 13C-NMR (125 MHz, CDCl3) δ 159.3, 145.0, 133.2, 114.7, 112.5, 112.2, 73.0, 55.5, 37.4, 28.0, 22.5, 14.0; LRESI(+)MS (relative intensity) m/z 295 [(M + Na)+, 100] and 297 [(M + 2 + Na)+, 100].
4-Bromo-3-butylanisole (1d). To a solution of alcohol 1b (43 mg, 0.16 mmol) and triethylamine [(183 µL) 132 mg, 1.30 mmol) in dichloromethane (2.0 mL) was added methanesulfonyl chloride [100 µL (148 mg), 1.29 mmol] via syringe at rt. After being stirred at rt for 2 h, the mixture was quenched with methanol (0.1 mL) and stirred at rt for 1 h. The mixture was diluted with H2O (5 mL) and the products were extracted with CHCl3. The CHCl3 solution was dried (Na2SO4) and concentrated in vacuo. The residual oil (75 mg) was then separated by CC [silica gel (500 mg), 100% hexanes] to give crude mesylate 1c (47 mg). The mesylate (47 mg) was dissolved in dimethyl sulfoxide (DMSO, 2 mL). To the solution was added a solution of NaBH4 (46 mg, 1.2 mmol) in DMSO (2 mL) via syringe. The mixture was stirred at rt for 24 h and quenched with 8 drops of acetone. After being stirred for 1 h, H2O (10 mL) was added. The products were extracted with hexanes (5 mL × 3). The hexanes solution was dried over Na2SO4 and concentrated in vacuo. The residue (34 mg) was purified by CC on silica gel (500 mg, 100% hexanes) to afford 1d (9.2 mg, 23%). UV (MeOH)max 228 (logε 3.9), 281 (logε 3.3) nm; FT IR νmax (KBr) 3010, 2960, 2930, 2860, 1630, 1560, 1460 cm−1; 1H-NMR (500 MHz, CDCl3) δ 7.37 (1H, d, J = 8.5 Hz), 6.74 (1H, d, J = 2.2 Hz), 6.59 (1H, dd, J = 8.5, 2.2 Hz), 3.76 (3H, s), 2.65 (2H, t, J = 8.1 Hz), 1.59 (2H, m), 1.33 (4H, m), 0.89 (3H, t, J = 6.6 Hz); 13C-NMR (100 MHz, C6D6) δ 160.1, 144.0, 134.1, 117.3, 115.9, 113.7, 55.4, 37.3, 32.4, 30.6, 23.4, 14.8; LRESI(−)MS (relative intensity) m/z 241 [(M − CH3)−, 100] and 243[(M + 2 − CH3)−, 100].
4-Bromo-3-pentylphenol (1). A solution of ether 1d (3.0 mg, 0.012 mmol), phenyltrimethylsilane [50 µL (44 mg), 0.29 mmol] and iodine (10.0 mg, 0.0394 mmol) was heated to 115 °C for 2 h. The mixture was concentrated in vacuo and the residual oil was purified by preparative TLC [silica gel, hexanes-EtOAc (9:1)] to give 4-bromo-3-pentylphenol (1) (1.3 mg, 46%). Colorless oil; UV (MeOH)max 228 (logε 3.9), 281 (logε 3.3) nm; FT IR νmax (KBr) 3300, 2960, 2920, 2840, 1600, 1575, 1460 cm−1; 1H-NMR (500 MHz, CDCl3) δ 7.33 (1H, d, J = 8.5 Hz), 6.69 (1H, d, J = 3.0 Hz), 6.52 (1H, dd, J = 8.5, 3.0 Hz), 2.62 (2H, t, J = 8.8 Hz), 1.55 (2H, m), 1.32 (4H, m), 0.89 (3H, t, J = 6.8 Hz). 1H NMR (500 MHz, C6D6) δ 7.20 (1H, d, J = 8.5 Hz), 6.32 (1H, d, J = 3.0 Hz), 6.06 (1H, dd, J = 8.5, 3.0 Hz), 3.98 (1H, br s), 2.58 (2H, t, J = 8.0 Hz), 1.52 (2H, quin., J = 8.0 Hz), 1.21 (4H, m), 0.83 (3H, t, J = 7.3 Hz); 13C-NMR (100 MHz, C6D6) δ 156.0, 143.9, 134.0, 117.9, 115.3, 115.2, 36.8, 32.1, 30.2, 23.2, 14.5; LRESI(−)MS (relative intensity) m/z 241 [(M − H)−, 100] and 243 [(M + 2 − H)−, 100].
3-Butylanisole (2a). To a solution of alcohol 1b (9.0 mg, 0.0329 mmol) in methanol (1.0 mL) was added a small amount of 5% palladium on activated carbon. The suspension was stirred at rt under an atmosphere of H2 for 8 h, the mixture was filtered through Celite and concentrated in vacuo. The filtrate was evaporated and the resulting residue (7.5 mg) was then separated by preparative TLC to give ether 2a (5.5 mg, 94%). Colorless oil; 1H-NMR (500 MHz, CDCl3) δ 7.17 (1H, t, J = 7.6 Hz), 6.75 (1H, br d, J = 7.6 Hz), 6.70 (2H, m), 3.76 (3H, s), 2.56 (2H, t, J = 7.6 Hz), 1.59 (2H, m), 1.30 (4H, m), 0.87 (3H, t, J = 6.6 Hz).
3-Pentylphenol (2). A solution of ether 2a (5.0 mg, 0.028 mmol), phenyltrimethylsilane [100 µL (87 mg), 0.579 mmol] and iodine (15 mg, 0.039 mmol) was heated to 110 °C for 2 h. The mixture was concentrated in vacuo and the residual oil was purified by preparative TLC [hexanes-EtOAc (10:1)] to give 4-pentylphenol (2) (3.5 mg, 76%). Colorless oil; 1H-NMR (500 MHz, CDCl3) δ 7.12 (1H, t, J = 7.6 Hz), 6.73 (1H, br d, J = 7.6 Hz), 6.73 (2H, m), 4.70 (1H, br s), 2.53 (2H, t, J = 7.5 Hz), 1.56 (2H, m), 1.29 (4H, m), 0.87 (3H, t, J = 6.6 Hz); (500 MHz, C6D6) δ 7.01 (1H, t, J = 8.0 Hz), 6.67 (1H, br d, J = 8.0 Hz), 6.43 (1H, br s), 6.40 (1H, br d, J = 8.0 Hz), 3.96 (1H, br s), 2.41 (2H, t, J =7.8 Hz), 1.49 (2H, quin., J = 7.8 Hz), 1.22 (4H, m), 0.83 (3H, t, J = 7.3 Hz); 13C-NMR (100 MHz, C6D6) δ 156.8, 145.2, 129.9, 121.2, 116.0, 113.2, 36.5, 32.1, 31.7, 23.2, 14.6.
4. Conclusions
We have isolated pentylphenols 1 and 2, cyclopropane fatty acid 3, and cyclopentenones 4 and 5 from an ascidian, Diplosoma sp. The structures of 1−5 were determined by spectroscopic analysis and/or synthesis. Compounds 1, 3, 4 and 5 were new. Compound 1 inhibited the division of fertilized sea urchin eggs and compound 4 showed mild cytotoxity against HCT116 cells (human colorectal cancer cell). 1H-NMR spectra of the extract of the separated Prochloron sp. from the body of the ascidian Diplosoma sp. showed the presence of the same peaks as present in those of 4 and 5, suggesting that Prochloron sp. is the actual producers of cyclopentenones.
Acknowledgements
We would like to thank Professor Euichi Hirose, University of the Ryukyus, for identifying the ascidian.
Footnotes
Sample Availability: Samples of the stable compounds are available from authors.
References and Notes
- 1.Davidson B.S. Ascidians: Producer of amino acid-derived metabolites. Chem. Rev. 1993;93:1771–1791. doi: 10.1021/cr00021a006. [DOI] [Google Scholar]
- 2.Rinehart K.L. Antitumor compounds from tunicates. Med. Res. Rev. 2000;20:1–27. doi: 10.1002/(SICI)1098-1128(200001)20:1<1::AID-MED1>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 3.Faulkner D.J. Marine natural products. Nat. Prod. Rep. 2002;19:1–48. doi: 10.1039/b009029h. and previous reports in this series. [DOI] [PubMed] [Google Scholar]
- 4.Blunt J.W., Copp B.R., Hu W.-P., Munro M.H.G., Northcote P.T., Prinsep M.R.J. Marine natural products. Nat. Prod. Rep. 2011;28:196–268. doi: 10.1039/c005001f. and previous reports in this series. [DOI] [PubMed] [Google Scholar]
- 5.Lindquist N., Fenical W., Sesin D.F., Ireland C.M., Duyne G.D.V., Forsyth C.J., Clardy J. Isolation and structure determination of the didemnenones, novel cytotoxic metabolites from tunicates. J. Am. Chem. Soc. 1988;110:1308–1309. doi: 10.1021/ja00212a059. [DOI] [Google Scholar]
- 6.Forsyth C.J., Clardy J. Total synthesis of (+)-didemnenones A and B. Absolute configurations of the didemnenones. J. Am. Chem. Soc. 1988;110:5911–5912. doi: 10.1021/ja00225a059. [DOI] [Google Scholar]
- 7.Beil W., Gores M., Nerenz F., Winterfeldt E. Total synthesis of tumor inhibiting didemnenone analogues. J. Prakt. Chem. 1999;341:384–390. doi: 10.1002/(SICI)1521-3897(199905)341:4<384::AID-PRAC384>3.0.CO;2-1. [DOI] [Google Scholar]
- 8.Margiastuti P., Ogi T., Teruya T., Taira J., Suenaga K., Ueda K. An unusual iodinated 5'-deoxyxylofuranosyl nucleoside from an Okinawan ascidian, Diplosoma sp. Chem. Lett. 2008;37:448–449. doi: 10.1246/cl.2008.448. [DOI] [Google Scholar]
- 9.Ogi T., Taira J., Margiastuti P., Ueda K. Cytotoxic metabolites from the Okinawan ascidian Diplosoma virens. Molecules. 2008;13:595–602. doi: 10.3390/molecules13030595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ogi T., Margiastuti P., Teruya T., Taira J., Suenaga K., Ueda K. Isolation of C11 cyclopentenones from two didemnid species, Lissoclinum sp. and Diplosoma sp. Mar. Drugs. 2009;7:816–832. doi: 10.3390/md7040816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fusetani N. In: Bioorganic Marine Chemistry. Scheuer P.J., editor. Springler-Verlag; Berlin/Heidelberg, Germany: 1987. pp. 61–92. [Google Scholar]
- 12.Maarisit W., Rob T., Ogi T., Taira J., Ueda K. Bioactive metabolites from Okinawan marine organisms. J. Mareine Fish. Postoharvesst Biotechnol. 2009;4:17–27. [Google Scholar]
- 13.Attygalle A.B., Siegel B., Vostrowsky O., Bestmann H.J., Maschwitz U. Chemical composition and function of metapleural gland secretion of the ant, Crematogaster deformis Smith (Hymenoptera: Myrmicinae) J. Chem. Ecol. 1989;15:317–328. doi: 10.1007/BF02027793. [DOI] [PubMed] [Google Scholar]
- 14.Knothe G. NMR Characterization of dihydrosterculic acid and its methyl ester. Lipids. 2006;41:393–396. doi: 10.1007/s11745-006-5110-x. [DOI] [PubMed] [Google Scholar]
- 15.Jing B., Tokutake N., McCullough D.H., III, Regen S.L. A quantitative assessment of permanent kinks on the mixing behavior of phospholipids in cholesterol-rich bilayers. J. Am. Chem. Soc. 2004;126:15344–15345. doi: 10.1021/ja044517c. [DOI] [PubMed] [Google Scholar]
- 16.Solladie-Cavallo A., Isarno T. Unambiguous and rapid cis/trans assignment of aryl-carboxy disubstituted cyclopropanes using NMR. Tetrahedron Lett. 1999;40:1579–1582. doi: 10.1016/S0040-4039(98)02649-5. [DOI] [Google Scholar]
- 17.Wratten S.J., Faulkner D.J. Antimicrobial metabolites from the marine sponge Ulosa sp. Tetrahedron Lett. 1978;19:961–964. doi: 10.1016/S0040-4039(01)85425-3. [DOI] [Google Scholar]
- 18.Nagle D.G., Gerwick W.H. Nakienones A-C and nakitriol, new cytotoxic cyclic C11 metabolites from an okinawan cyanobacterial (Synechocystis sp.) overgrowth of coral. Tetrahedron. Lett. 1995;36:849–852. doi: 10.1016/0040-4039(94)02397-T. [DOI] [Google Scholar]
- 19.Teruya T., Nakagawa S., Koyama T., Suenaga K., Uemura D. Terpiodiene: A novel tricyclic alcohol from the Okinawan sponge Terpios hoshinota. Chem. Lett. 2002:38–39. [Google Scholar]
- 20.Guzii G.A., Makar’eva N.T., Denisenko A.V., Dmitrenok S.P., Dmitrenok S.A., Grebnev B.B., Stonik A.V. Diosphenol from the ascidian Diplosoma sp. Chem. Nat. Comp. 2008;4:372–373. [Google Scholar]
- 21.Lewin R.A. Prochlorophyta as a proposed new division of algae. Nature. 1976;261:697–698. doi: 10.1038/261697b0. [DOI] [PubMed] [Google Scholar]
- 22.Withers N., Vidaver W., Lewin R.A. Pigment composition, photosynthesis and fine structure of a non-blue-green prokaryotic algal symbiont (Prochloron sp.) in a didemnid ascidian from Hawaiian waters. Phycologia. 1978;17:167–171. doi: 10.2216/i0031-8884-17-2-167.1. [DOI] [Google Scholar]
- 23.Oka T.A., Hirose E. Some Didemnid ascidians harboring prokaryotic algae from the reef shores in the Yaeyama islands, Okinawa, Japan. Biol. Mag. Okinawa. 2005;43:45–52. [Google Scholar]
- 24.Mann J. Secondary Metabolites Derived from Acetate Fatty Acid and Polyketides. In: Atkins P.W., Holker J.S.E., Holiday A.K., editors. Secondary Metabolism. Oxford University Press; Oxford, UK: 1990. pp. 55–58. [Google Scholar]