Abstract
Based on an established common pharmacophore of HIV-1 non-nucleoside reverse transcriptase inhibitors (NNTTIs), a series of quinolin-2-one derivatives were synthesized and assayed for their in vitro activities against HIV-1 reverse transcriptase (RT) for the first time. Some of the tested compounds were active against HIV-1 RT. Compounds 4a2 and 4d2 showed inhibitory activities with IC50 values of 0.21 and 0.15 μM, respectively, with a mode of interaction with RT residues of the allosteric pocket similar to that of efavirenz.
Keywords: quinolin-2-one, alkaloid, synthesis, HIV-1 RT, activity
1. Introduction
AIDS (Aquired Immune Deficiency Syndrome), caused by human immunodeficiency virus (HIV), a RNA dependent retrovirus, remains one of the major causes of death in the World. HIV-1 reverse transcriptase (RT) is one of the enzymes crucial for the HIV virus replication cycle. Upon entering a host cell, the single stranded viral RNA is converted to double-stranded DNA catalyzed by RT and then the virus DNA inserts into the genome of the host cell. Inhibition of reverse transcriptase (RT), the HIV-encoded polymerase which directs both RNA and DNA synthesis, has been proven to be one of the most effective ways to block viral multiplication [1,2,3,4,5].
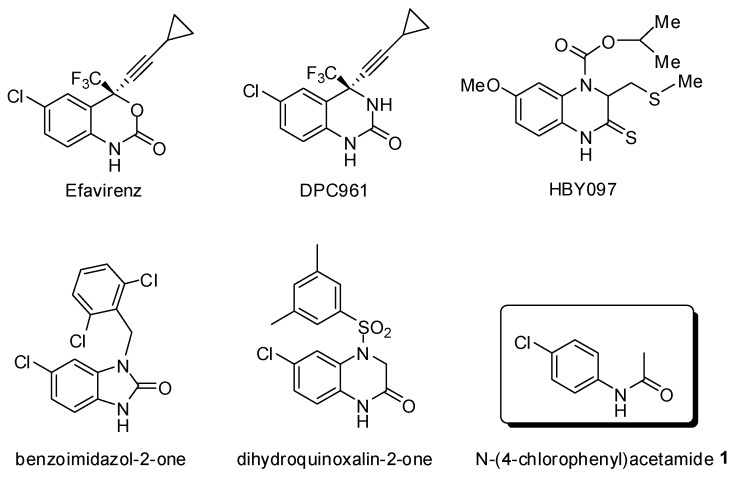
Based on the ready availability of X-ray crystal structures of inhibitor-HIV RT complexes, Freeman and colleagues have identified the common pharmacophore N-(4-chlorophenyl)acetamide (1) from an array of known non-nucleoside reverse transcriptase inhibitors [6] such as efavirenz, DPC961, HBY097 and the newly reported benzoimidazol-2-one [7] and dihydroquinoxalin-2-one [8] NNTRIs (Figure 1).
Figure 1.
NNRTI pharmacophore N-(4-chlorophenyl)acetamide (1) and related anti-HIV-1 RT active compounds.
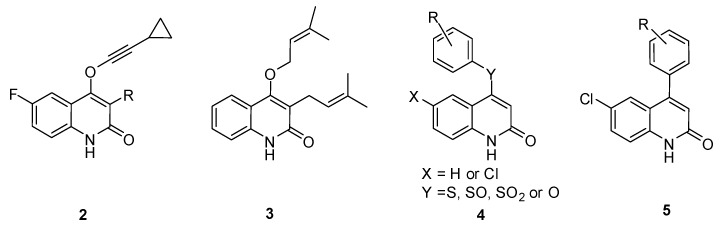
A series of substituted 2-quinolones (2, Figure 2) were synthesized and evaluated as HIV-1 inhibitors in Freeman’s research. As to natural products, a similar quinolin-2-one alkaloid 3 isolated from Euodia roxburghiana reported by McCormick also showed inhibitory activity with an IC50 of 8 μM in an HIV-1 reverse transcriptase (RT) assay [9].
Figure 2.
quinolin-2-one NNRTIs and newly designed derivatives.
Based on the pharmacophore established by Freeman, we designed and synthesized a series of 4-substituted quinolin-2-one alkaloids 4 and 5, as shown in Figure 2. We envisaged that replacement of the cyclopropylethynyloxy moiety with a hydrophobic aromatic ring would retain the inhibitory activity against HIV-1 RT [4,10,11,12,13]. In compounds 4, the group Y could be oxygen, sulfur, sulfinyl or sulfonyl units, which were flexible linkers to connect the hydrophobic phenyl group with the quinolin-2-one scaffold. To identify the importance of the linker, compounds 5 were synthesized with an aromatic ring directly connected to quinoline-2-one scaffold. In this paper, we report the synthesis of compounds 4 and 5 and the evaluation of the synthesized compounds for their in vitro inhibitory activities against HIV-1 RT for the first time.
2. Results and Discussion
2.1. Chemistry
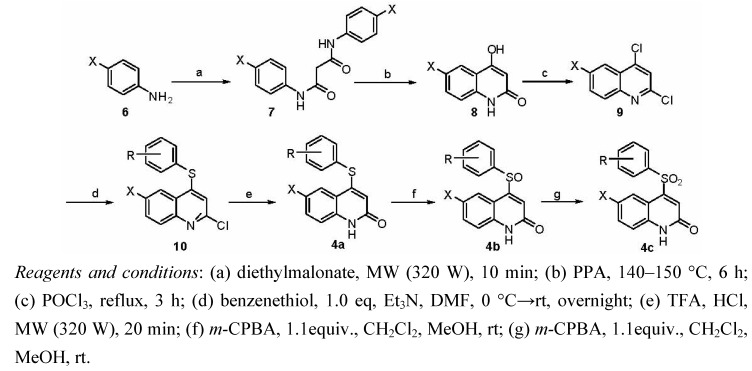
The syntheses of compounds 4 is summarized in Scheme 1 and Scheme 2, respectively. 3-Hydroxy-quinolin-2-one (8, Scheme 1) has been the subject of intensive synthetic studies for a long time. Among the synthetic strategies used is the Friedel-Crafts reaction [6]. Freeman reported that a treatment with aniline or 4-chloroaniline 6 and excess of diethyl malonate in diphenyl ether at 250 °C for 24 h afforded cyclized compound 8 directly. But in our study, target compound 8 was not obtained under these conditions and only some amide intermediates could be detected by HPLC-ESIMS. We thus irradiated a mixture of aniline and diethylmalonate (2:1 molar ratio) under microwaves and prepared corresponding dimalonamide 7 in good yield. Heating N,N’-di(4-chlorophenyl)malonamide in polyphosphoric acid (PPA) at 140–150 °C afforded the 4-hydroxy-2-quinolone 8 (Scheme 1) [14]. Refluxing compound 8 in POCl3 provided 2,4,6-trichloroquinoline 9 in good yield. A phenylthio group was then installed on the quinoline ring to afforded compounds 10, which were converted to target compounds 4a through a microwave assisted hydrolysis in mixture of TFA and HCl [15]. Compounds 4a were oxidized to target compounds 4b as racemates, and these were then converted to compounds 4c by reaction with another 1.1 equiv. of 3-chlorobenzoperoxoic acid (m-CPBA).
Scheme 1.
Synthesis of target compounds 4a–4c.
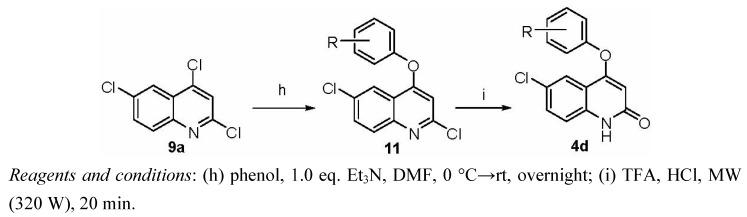
The synthesis of compounds 4d shared the same synthetic precursor 2,4,6-trichloroquinoline 9 (Scheme 2). Through treatment of compound 9a with various phenols followed by hydrolysis in acid mixtures, compounds 4d were synthesized in moderate yields using the conditions described above without need for further optimization.
Scheme 2.
Synthesis of target compounds 4d.
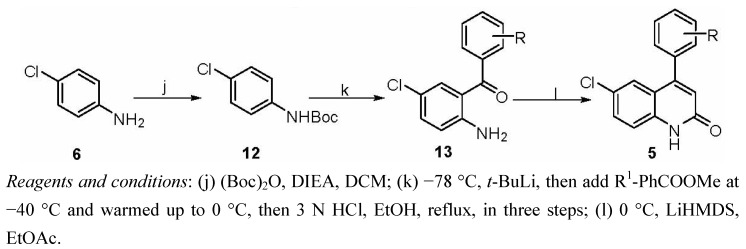
The synthetic route of target compounds 5 was summarized in Scheme 3. The synthesis of 5 by various methods has been reviewed. The majority of compounds 13 were prepared by the reaction of aryl esters with ortho-lithiated Boc-protected 4-chloroaniline via the formation of the dianion species with t-BuLi (2.2 equiv) followed by deprotection of the Boc group (Scheme 3) [16,17]. A tandem amidation/Knoevengel condensation of compounds 13 with ethyl acetate gave compounds 5 in good yields.
Scheme 3.
Synthesis of target compounds 5.
2.2. Enzymatic Activities
Compounds 4a–4d were tested in RT inhibition assays and proved to be active as inhibitors of HIV-1 RT (Table 1). The enzymatic data highlighted that derivative 4a2 (IC50 = 0.21 μM) and 4d2 (IC50 = 0.15 μM) turned out to be more active than 4a3 (IC50 = 13 μM), respectively, and suggested that the presence of a chlorine substituent at C-6 may lead to an increase of potency and influence the affinity to the enzyme. This finding is in agreement with the N-(4-chlorophenyl)acetamide pharmacophore established by Freeman, and new type of anti-HIV-1 RT compounds could be designed based on the pharmacophore model.
Table 1.
HIV-1 RT inhibitory activities of compounds 4–5. a
| Compounds | X | Y | R | IC50 (μM) b,c |
|---|---|---|---|---|
| 4a1 | Cl | S | H | 5.6 |
| 4a2 | Cl | S | 3,5-CH3 | 0.21 |
| 4a3 | H | S | 3,5-CH3 | 18 |
| 4b1 | Cl | SO | H | 8.9 |
| 4b2 | Cl | SO | 3,5-CH3 | 2.6 |
| 4c1 | Cl | SO2 | H | 49 |
| 4c2 | Cl | SO2 | 3,5-CH3 | 10 |
| 4d1 | Cl | O | H | 3.0 |
| 4d2 | Cl | O | 3,5-CH3 | 0.15 |
| 5a | Cl | / | H | >100 |
| 5b | Cl | / | 2-F | >100 |
| 5c | Cl | / | 2-Cl | >100 |
a All values are the mean of two independent experiments; b The inhibition of recombinant HIV-1 RT activity was performed with a commercially available ELISA kit (Roche) according to the instructions of the manufacturer. IC50 = effective concentration that inhibits 50% of HIV-1 RT; c Efavirenz: an HIV-1 RT inhibitor used as positive control. IC50 = 6 nM.
In addition, the type of linker which connected the aromatic ring with the quinoline scaffold might be crucial for potent anti-HIV-1 RT activity in the title compounds. Comparing compounds 4a2 (IC50 = 0.21 μM), 4b2 (IC50 = 2.6 μM) and 4c2 (IC50 = 10 μM), a decrease of activity against RT could be observed as the degree of oxidation of the sulfur atom increased. The role of the substituents of the phenyl ring on the biological activities was also investigated, as shown in Table 1. In compounds 4a1 (IC50 = 5.6 μM) and 4a2 (IC50 = 0.21 μM), two hydrophobic methyl group on the phenyl ring led to potent enzyme activity, and may contribute to the ability to bind with the hydrophobic site of RT. In contrast, compounds 5a–5c exhibited obviously decreased activities towards the enzyme. We envisagd that hydrophobic interaction ability was influenced by the rigid conjugation system between the phenyl ring and the quinolin-2-one scaffold.
2.3. Docking Studies
To investigate the binding mode of the synthesized quinolin-2-ones, computational modeling was performed using CDOCK. The CHARMM force field was used for the energy minimizations in the docking process. The coordinates of the RT-wfavirenz complex (PDB code: 1FK9) [12,18] were downloaded, and the molecular structure of the most potent compounds 4a2 and 4d2 were docked into its active site. The best docked conformation of compounds 4a2 and 4d2 were shown in Figure 3, together with the experimental position of efavirenz and the 3D common-feature pharmacophore model. Compounds 4a2 and 4d2 interact with RT residues of the allosteric pocket in a fashion similar to known NNRTIs. The p-chloroaniline moiety has a hydrophobic contact with the surrounding residues such as Tyr318, Pro236, Leu234 and Val106. The carboxamide group of the molecule interacts with the main-chain of Lys101 by hydrogen bond interactions. The 3,5-dimethylphenyl fragment occupies a hydrophobic pocket consisting of Pro95, Tyr181, Tyr188 and Trp229, which is occupied by the cyclopropyl-propynyl of efavirenz in the complex. One of the two methyl groups on the benzene ring adopts a similar orientation to the cyclopropyl group of efavirenz.
Figure 3.
CDOCK-modeled binding mode of 4a2 (left) and 4d2 (right) in comparison with the crystal structure (1FK9 in PDB) of efavirenz (orange colored carbon atoms). The key hydrogen bond is illustrated with green lines.
3. Experimental
3.1. Chemistry
3.1.1. Instruments and Materials
Column chromatography silica gel (200-300 mesh) and TLC plate (Qingdao Meijin Chemical Inc.; Qingdao; China); MS data were obtained on an AutoSpec-3000 or Agilent HPLC-ESIMS; 1H-NMR spectra were recorded on Bruker 300M or DRX-500 spectrometers and chemical shifts were given in δ with TMS as an internal reference. All the reagents are commercial available. Tetrahydrofuran (THF) was refluxed over Na before use.
3.1.2. General Procedure for Preparation of Compounds 4
3.1.2.1. Synthesis of Intermediates 7
Aniline or 4-chloroaniline (100 mmol), and diethyl malonate (50 mmol) were properly mixed in a 250 mL beaker. The obtained mixture was irradiated in a microwave oven at the power of 320 W for 5 min. After irradiation, the crude product was recrystallised in ethanol to afford the desired product.
bis(4-Chlorophenyl)malonamide (7a): yield 95%, white solid, ESIMS: m/z: 323 (M+H)+
Diphenylmalonamide (7b): yield 91%, white solid, ESIMS: m/z: 255 (M+H)+
3.1.2.2. Synthesis of Intermediates 8
A mixture of 7a or 7b (2.0 mmol) with 5–6 times by weight polyphosphoric acid were stirred in an oil bath at 140–150 °C for 6 h. Then the mixture was cooled, diluted with water and the resultant gum solidified by standing over night. The solid was filtered and dried in air. It was recrystallised from ethanol to afford corresponding hydroxyquinolones as creamy crystals.
6-Chloro-4-hydroxyquinolin-2(1H)-one (8a): white solid, yield: 82%, 1H-NMR (300 MHz, DMSO-d6, δ ppm): 5.80 (s, 1H), 7.68 (s, 1H), 7.27 (dd, 1H, J = 8.6, 4.8 Hz), 7.53 (d, 1H, J = 8.6 Hz), 11.35 (brs, 1H, NH).
4-Hydroxyquinolin-2(1H)-one (8b): white solid, yield: 85%, 1H-NMR (300 MHz, DMSO-d6, δ ppm): 5.83 (s, 1H), 7.51–7.68 (m, 3H), 7.23 (d, 1H, J = 8.0 Hz), 11.33 (brs, 1H, NH).
3.1.2.3. Synthesis of Intermediates 9
A mixture of 8 (84.47 mmol) in POCl3 (150 mL) was heated under reflux for 3h. After cooling down, the mixture was slowly added to crushed ice while shaking. The precipitate was collected by filtration and washed several times with water. The product was dried under vacuum overnight to give the products as white solid which are pure enough for further use.
2,4,6-Trichloroquinoline (9a): white solid, yield 70%, 1H-NMR (500 MHz, CDCl3, δ ppm): 8.00 (s, 1H), 7.94 (d, J = 9.0 Hz, 1H), 7.66 (d, J = 9.0 Hz, 1H), 7.56 (s, 1H); ESIMS: m/z: 232 (M+H)+.
2,4-Dichloroquinoline (9b): white solid, yield 66%, 1H-NMR (500 MHz, CDCl3, δ ppm): 8.16 (d, J = 8.0 Hz, 1H), 8.01 (d, J = 8.0 Hz, 1H), 7.80 (t, J = 8.0 Hz, 1H), 7.65 (t, J = 8.0 Hz, 1H), 7.47 (s, 1H); ESIMS: m/z: 198 (M+H)+.
3.1.2.4. Synthesis of Intermediates 10
To a solution of 9a or 9b (10.0 mmol) in DMF (10 mL) was added Et3N (2.78 mL, 20 mmol) and a solution of thiol (10 mmol) in DMF (10 mL) dropwise at 0 °C. The reaction was warmed up after 10 min and stirred overnight at rt. 200 mL of EtOAc was added to the mixture and washed with water and brine successively. The organic layer was separated and dried over Na2SO4. The product was purified on a column after removal of the solvent under vacuum. In most of cases, less than 7% of dithio-substituted quinoline was formed.
2,6-Dichloro-4-(phenylthio)quinoline (10a): amorphous powder, yield: 69%. 1H-NMR (300 MHz, CDCl3, δ ppm): 8.11 (s, 1H), 7.88–7.50 (m, 2H), 7.18–7.09 (m, 5H), 6.72 (s, 1H); ESIMS: m/z: 306 (M+H)+.
2,6-Dichloro-4-(3,5-dimethylphenylthio)quinoline (10b): amorphous powder, yield: 69%. 1H-NMR (300 MHz, CDCl3, δ ppm): 8.13 (s, 1H), 7.88–7.50 (m, 2H), 7. 24 (s, 2H), 7.19 (s, 1H), 6.62 (s, 1H), 2.34 (s, 6H); ESIMS: m/z: 334 (M+H)+.
2-chloro-4-(3,5-dimethylphenylthio)quinoline (10c): amorphous powder, yield: 59%. 1H-NMR (300 MHz, CDCl3, δ ppm): 8.23 (d, J = 8.0 Hz, 1H), 7.99–7.57 (m, 3H), 7.23 (s, 1H), 7.21 (s, 1H), 7.16 (s, 1H), 6.63 (s, IH), 2.33 (s, 6H); ESIMS: m/z: 282 (M+H)+.
3.1.2.5. Synthesis of Target Compounds 4a
A solution of compound 10 (0.50 mmol) in TFA (2 mL) and HCl (6 N, 2 mL) was heated under microwave radiation at 120 °C for 20 min. After cooling down, water (20 mL) was added. The precipitate was collected by filtration and washed with water several times. The product was air dried (71–85%) and was pure enough without further purification. The solubility of the products in organic solvents is poor.
6-Chloro-4-(phenylthio)quinolin-2(1H)-one (4a1): amorphous powder, yield: 71%. 1H-NMR (300 MHz, DMSO-d6, δ ppm): 11.13 (bs, 1H), 7.81 (s, 1H), 7.68–7.30 (m, 2H), 7.08–6.89 (m, 5H), 5.72 (s, 1H); ESIMS: m/z: 288 (M+H)+; HRESIMS: calc for C15H11ClNOS [M+H]+ 288.0250, found 288.0261.
6-Chloro-4-(3,5-dimethylphenylthio)quinolin-2(1H)-one (4a2): amorphous powder, yield: 77%. 1H-NMR (300 MHz, DMSO-d6, δ ppm): 11.38 (bs, 1H)7.76 (s, 1H), 7.68–7.30 (m, 2H), 7. 22 (s, 2H), 7.18 (s, 1H), 5.82 (s, 1H), 2.34 (s, 6H); ESIMS: m/z: 316 (M+H)+; HRESIMS: calc for C17H15ClNOS 316.0563, found 316.0547.
4-(3,5-dimethylphenylthio)quinolin-2(1H)-one (4a3): amorphous powder, yield: 85%. 1H-NMR (300 MHz, DMSO-d6, δ ppm): 11.66 (bs, 1H), 7.88 (d, J = 8.0 Hz, IH), 7.89–7.43 (m, 3H), 7.251 (s, 2H), 7.13 (s, 1H), 5.88 (s, IH), 2.32 (s, 6H); ESIMS: m/z: 282 (M+H)+; HRESIMS: calc for C17H16NOS [M+H]+ 282.0953, found 282.0950.
3.1.2.6. Synthesis of Target Compounds 4b
To a solution of compound 4a (0.25 mmol) in a mixture of CH2Cl2 and MeOH (50 mL) was added 1.1 equiv. of 3-chlorobenzoperoxoic acid. After stirring overnight, water (50 mL) was added. The mixture was extracted with CH2Cl2 and evaporated to give a residue which were further purified on silica gel column to give compounds 4b (66–75%).
6-Chloro-4-(phenylsulfinyl)quinolin-2(1H)-one (4b1): amorphous powder, yield: 66%. 1H-NMR (300 MHz, DMSO-d6, δ ppm): 11.85 (bs, 1H), 7.91 (s, 1H), 7.88–7.47 (m, 7H), 5.92 (s, 1H), 2.39 (s, 6H); ESIMS: m/z: 304 (M+H)+; HRESIMS: calc for C15H11ClNO2S [M+H]+ 304.0199, found 304.0208.
6-Chloro-4-(3,5-dimethylphenylsulfinyl)quinolin-2(1H)-one (4b2): amorphous powder, yield: 75%. 1H-NMR (300 MHz, DMSO-d6, δ ppm): 11.40 (bs, 1H), 7.96 (s, 1H), 7.88–7.55 (m, 2H), 7. 47 (s, 1H), 7. 42 (s, 1H), 7.40 (s, 1H), 6.01 (s, 1H), 2.32 (s, 6H); ESIMS: m/z: 332 (M+H)+; HRESIMS: calc for C17H15ClNO2S [M+H]+ 332.0512, found 332.0509.
3.1.2.7. Synthesis of Target Compounds 4c
To a solution of compound 4b (0.2 mmol) in a mixture of CH2Cl2 and MeOH (50 mL) was added 1.1 equiv. 3-chlorobenzoperoxoic acid. After stirring overnight, 50 mL of water was added. The mixture was extracted with CH2Cl2 and evaporated to give a residue which were further purified on silica gel column to give compound 4b (60–66%).
6-Chloro-4-(phenylsulfonyl)quinolin-2(1H)-one (4c1): amorphous powder, yield: 64%. 1H-NMR (300 MHz, CDCl3): 11.51 (bs, IH), 8.10 (s, 1H), 7.91–7.49 (m, 5H), 7.39 (d, J = 8.0 Hz, 1H), 7.29 (d, J = 8.0 Hz, 1H), 6.12 (s, 1H); ESIMS: m/z: 320 (M+H)+; HRESIMS: calc for C15H11ClNO3S [M+H]+ 330.0148, found 320.0154.
6-Chloro-4-(3,5-dimethylphenylsulfonyl)quinolin-2(1H)-one (4c2): amorphous powder, yield: 60%. 1H-NMR (300 MHz, CDCl3, δ ppm): 11.86 (bs, IH), 8.00 (s, 1H), 7.98–7.69 (m, 2H), 7.57 (s, 2H), 7.39 (s, 1H), 5.95 (s, 1H), 2.35 (s, 6H); ESIMS: m/z: 348 (M+H)+; HRESIMS: calc for C17H15ClNO3S [M+H]+ 348.0461, found 348.0470.
3.1.2.8. Synthesis of Target Compounds 4d
To a solution of 9a (10.0 mmol) in DMF (10 mL) was added Et3N (2.78 mL, 20 mmol) and a solution of phenol (10 mmol) in DMF (10 mL) dropwise at 0 °C. The reaction was warmed up after 10 min and stirred for 24h at rt. 200 mL of EtOAc was added to the mixture and washed with water and brine successively. The organic layer was separated and dried over Na2SO4. The product 11 was purified on column after removal of solvent under vacuum with yield of 75–86%. A solution of compound 11 (0.50 mmol) in TFA (2 mL) and HCl (6 N, 2 mL) was then heated under microwave radiation at 120 °C for 20 min. After cooling down, 20 mL of water was added. The precipitate was collected by filtration and washed with water for several times. The product was air dried (71–85%) and was pure enough without further purification.
6-Chloro-4-phenoxyquinolin-2(1H)-one (4d1): amorphous powder, yield: 71%. 1H-NMR (300 MHz, DMSO-d6, δ ppm): 11.94 (bs, 1H), 7.71 (s, 1H), 7.60–7.10 (m, 2H), 7.01–6.67 (m, 5H), 5.70 (s, 1H); ESIMS: m/z: 272 (M+H)+; HRESIMS: calc for C15H11ClNO2 [M+H]+ 272.0478, found 272.0470.
6-Chloro-4-(3,5-dimethylphenoxy)quinolin-2(1H)-one (4d2): amorphous powder, yield: 77%. 1H-NMR (300 MHz, DMSO-d6, δ ppm ): 11.64 (bs, 1H)7.70 (s, 1H), 7.65–7.31 (m, 2H), 6.89 (s, 2H), 6.93 (s, 1H), 5.72 (s, 1H), 2.33 (s, 6H); ESIMS: m/z: 300 (M+H)+; HRESIMS: calc for C17H15ClNO2 [M+H]+ 300.0791, found 300.0775.
3.1.3. General Procedure for Preparation of Compounds 5
3.1.3.1. Synthesis of Intermediate 12
4-Chloroaniline (19.2 g, 0.1 mol), DIEA (0.12 mol) and toluene (40 mL) were placed in a 100 mL flask and stirred at rt, then Boc2O (26 g, 0.12 mol) was added, and the solution was stirred at rt until the completion of the reaction monitored by TCL. Then the solution was poured into water and extracted with DCM and then the organic layer was washed by water and dried over anhydrous Na2SO4. Then the organic solvent was removed under vacuum to give crude product which was further purified on Si gel column chromatography and eluted with petroleum ether (PET)-EtOAc (6:1) to give the compound tert-butyl 4-chlorophenylcarbamate (12) 21.3 g, white solid, yield 94%. 1H-NMR (CDCl3, δ ppm): 7.31–7.22 (m, 4H), 6.54 (brs, 1H, NH), 1.51 (s, 9H, CH3); positive FABMS m/z (%): 227 [M]+ (60), 172 (83), 57 (100).
3.1.3.2. Synthesis of Compound 13
Compound 12 was taken as an example. To a 100 mL flask, Boc2O (11.4 g, 50 mmol) and anhydrous THF (30 mL) were added under an atmosphere of N2. The solution was stirred and cooled to −78 °C, then t-BuLi solution in THF (110 mmol) was added dropwise under N2. The solution was kept at −78 °C under stirring for 1 h and then warmed up to −40°C. Methyl benzoate (60 mmol, in 10 mL THF) was added dropwise under N2 in 15 min. The solution was kept in −40 °C for another 1 h and warmed up to rt. The solution was poured into ice cooled NH4Cl solution and extracted with EtOAc. The combined organic layer was washed with brine and dried over anhydrous Na2SO4 and then evaporated to give crude N-Boc protected 13. Without further purification, the crude product was dissolved in 30 mL EtOH, and then 3 mol/L HCl solution (10 mL) was added. The mixture was refluxed until the completion of the reaction. Then the solution was adjusted to pH 8 using saturated NaHCO3 solution and extracted with DCM successively. The organic layer was washed with brine and dried over anhydrous Na2SO4 and then evaporated to give a residue which was further purified on Si CC to give compound 13 (3.46 g, yield: 30%).
(2-Amino-5-chlorophenyl)(phenyl)methanone (13a): 1H-NMR (DMSO-d6, δ ppm): 7.64–7.33 (m, 7H), 6.99 (d, J = 8.8 Hz, 1H), 6.08 (brs, 2H, NH2); positive FABMS m/z (%): 232 [M+1]+ (100).
(2-Amino-5-chlorophenyl)(2-fluorophenyl)methanone (13b): yellow amorphous powder, yield 27%, 1H-NMR (DMSO-d6, δ ppm): 7.50–6.15 (m, 6H), 6.67 (d, J = 8.8 Hz, 1H), 6.38 (brs, 2H, NH2); positive FABMS m/z (%): 250 [M+1]+ (100). 249 [M]+ (100).
(2-Amino-5-chlorophenyl)(2-chlorophenyl)methanone (13c): yellow amorphous powder, yield 31%, 1H-NMR (DMSO-d6, δ ppm): 7.46–7.11 (m, 6H), 6.68–6.65 (m, 1H), 6.47 (brs, 2H, NH2); positive FABMS m/z (%): 266 [M+1]+ (100).
3.1.3.3. Synthesis of Compounds 5
Compound 5a was taken as an example. A 30 mL, 2-necked flask was charged with compound 13a (1 mmol, 231 mg) and THF (5 mL). The resulting solution was cooled to 0 °C and LiHMDS (6 mL, 1M in THF) was added over 5 min. The internal temperature was controlled <5 °C for 10 min, then EtOAc (144 mg, 2 mmol) was added over 1 min. The reaction solution was allowed to warm up to rt and stirred at rt for 2h. Then water (5 mL) was added, and the reaction mixture was stirred at rt for another 24 h. The solution was poured into 50 mL ice cooled water, and a water suspension formed. The suspension was filtered and the solid was further purified on Si CC eluted with CHCl3/CH3OH (97:3) to give white amorphous powder 5a (212 mg, yield: 83%).
6-Chloro-4-phenylquinolin-2(1H)-one (5a): white amorphous powder, yield:70% 1H-NMR (DMSO-d6, δ ppm): 12.02 (s, 1H, NH), 7.60–7.39 (m, 7H), 7.26 (s, 1H), 6.45 (s, 1H); positive FABMS m/z (%): 256 [M+1]+ (50), 73 (100). HRESIMS: calc for C15H11ClNO [M+H]+ 256.0529, found 256.0548.
6-Chloro-4-(2-fluorophenyl)quinolin-2(1H)-one (5b): white amorphous powder, yield: 77%, 1H-NMR (CDCl3-CD3OD, δ ppm): 8.16–8.10 (m, 3H), 8.01–7.96 (m, 3H), 7.89–7.87 (m, 2H); EI-MS m/z (%): 275 [M+2]+ (6), 274 [M+1]+ (4), 273 [M]+ (18), 246 (17), 210 (65), 131 (100), 117 (50), 105 (21), 91 (84): HRESIMS: calc for C15H10ClFNO [M+H]+ 274.0435, found 274.0448.
6-Chloro-4-(2-chlorophenyl)quinolin-2(1H)-one (5c): white amorphous powder, yield: 80%, 1H-NMR (DMSO-d6, δ ppm): 12.11 (s, 1H, NH), 7.67–7.40 (m, 4H), 6.84 (d, J = 2.2 Hz, 1H), 6.47 (s, 1H); EI-MS m/z (%): 293 [M+4]+ (4), 293 [M+3]+ (3), 291 [M+2]+ (10), 289 [M]+ (20), 167 (30), 149 (100); HRESIMS: calc for C15H10Cl2NO [M+H]+ 290.0139, found 290.0151.
3.2. General Procedure for HIV-1 RT Inhibitory Assay
HIV-1 reverse transcriptase (RT) activity was measured using a commercially available ELISA RT kit (Roche) according to the instructions of the manufacturer. The compounds were incubated with DIG-labeled reaction mixture at 37 °C for 2 h, then anti-DIG-POD solution was added, followed by substrate ABTS. Efaviren was used as a positive control. The absorbency at 405 nm/490 nm (A405/490) was read on Bio-Tek ELx 800 ELISA reader.
3.3. General Procedures for Docking Study
CDOCK was used for the docking study. The CHARMM force field was used for the energy minimizations in the docking process. The X-ray crystal structure of reverse transcriptase complexed with efavirenz was retrieved from PDB (PDB code: 1FK9). The active site was defined as all residues within 6.5 Å radius of the cocrystallized efavirenz. Starting from the ligand configuration, a set of 10 different orientations was randomly generated and ranked according to their binding free energy. The docking mode was chosen on the basis of binding affinity rank.
4. Conclusions
In summary, a group of quinoneline-2-one analogues with activity against HIV-1 RT were synthesized based on the SAR established by Freeman and known crystal structures. The SAR and docking studies of the titled compounds were analyzed. Analogues 4a2 and 4d2 demonstrated the most activity among these analogues with a similar mode of interaction with RT residues of the allosteric pocket as known NNRTIs. Further structure modifications and cell-based in vitro anti-HIV-1 assay studies of these quinolin-2-one derivatives will be reported in due course.
Acknowledgments
The authors are grateful to Qiong Gu’s research group for the docking study performed at the School of Pharmaceutical Sciences, Sun Yat-sen University. This work was partly supported by the Talent Introduction Project in Hunan Agricultural University (11YJ04).
Footnotes
Samples Availability: Samples of the all compounds are available from the authors.
References
- 1.Mehellou Y., Clercq E.D. Twenty-six years of anti-HIV drug discovery: Where do we stand and where do we go? J. Med. Chem. 2010;53:521–538. doi: 10.1021/jm900492g. [DOI] [PubMed] [Google Scholar]
- 2.Ragno R., Coluccia A., Regina G.L., Martino G.D., Piscitelli F., Lavecchia A., Novellino E., Bergamini A., Ciaprini C., Sinistro A., et al. Design, molecular modeling, synthesis, and anti-HIV-1 activity of new indolyl aryl sulfones. Novel derivatives of the indole-2-carboxamide. J. Med. Chem. 2006;49:3172–3184. doi: 10.1021/jm0512490. [DOI] [PubMed] [Google Scholar]
- 3.Barreca M.L., Rao A., Luca L.D., Iraci N., Monforte A., Maga G., Clercq E.D., Pannecouque C., Balzarini J., Chimirri A. Discovery of novel benzimidazolones as potent non-nucleoside reverse transcriptase inhibitors active against wild-type and mutant HIV-1 strains. Bioorg. Med. Chem. Lett. 2007;17:1956–1960. doi: 10.1016/j.bmcl.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 4.Regina G.L., Coluccia A., Piscitelli F., Bergamini A., Sinistro A., Cavazza A., Maga G., Samuele A., Zanoli S., Novellino E., et al. Indolyl aryl sulfones as HIV-1 non-nucleoside reverse transcriptase inhibitors: Role of two halogen atoms at the indole ring in developing new analogues with improved antiviral activity. J. Med. Chem. 2007;50:5034–5038. doi: 10.1021/jm070488f. [DOI] [PubMed] [Google Scholar]
- 5.Jones L.H., Allan G., Barba O., Burt C., Corbau R., Dupont T., Knochel T., Irving S., Middleton D. S., Mowbray C. E., et al. Novel indazole non-nucleoside reverse transcriptase inhibitors using molecular hybridization based on crystallographic overlays. J. Med. Chem. 2009;52:1219–1923. doi: 10.1021/jm801322h. [DOI] [PubMed] [Google Scholar]
- 6.Freeman G.A., Andrews C.W., III., Hopkins A.L., Lowell G.S., Schaller L.T., Cowan J.R., Gonzales S.S., Koszalka G.W., Hazen R.J., Boone L.R., et al. Design of non-nucleoside inhibitors of HIV-1 reverse transcriptase with improved drug resistance properties. 2. J. Med. Chem. 2004;47:5923–5936. doi: 10.1021/jm040072r. [DOI] [PubMed] [Google Scholar]
- 7.Barreca M.L., Rao A., Luca L.D., Zappalà M., Monforte A., Maga G., Pannecouque C., Balzarini J., Clercq E.D., Chimirri A., Monforte P. Computational strategies in discovering novel non-nucleoside inhibitors of HIV-1 RT. J. Med. Chem. 2005;48:3433–3437. doi: 10.1021/jm049279a. [DOI] [PubMed] [Google Scholar]
- 8.Monforte A., Logoteta P., Ferro S., Luca L.D., Iraci N., Maga G., Clercq E.D., Pannecouque C., Chimirri A. Design, synthesis, and structure-activity relationships of 1,3-dihydrobenzimidazol-2-one analogues as anti-HIV agents. Bioorg. Med. Chem. Lett. 2009;17:5962–5967. doi: 10.1016/j.bmc.2009.06.068. [DOI] [PubMed] [Google Scholar]
- 9.Mccormick J.L., McKee T.C., Cardellina J.H., II., Boyd M.R. HIV inhibitory natural products. 26. quinoline alkaloids from Euodia roxburghiana. J. Nat. Prod. 1996;59:469–471. doi: 10.1021/np960250m. [DOI] [PubMed] [Google Scholar]
- 10.Guillemont J., benjahad A., Oumouch S., Decrane L., Palandjian P., Vernier D., Queguiner L., Andries K., Béthune M., Hertogs K., et al. Synthesis and biological evaluation of C-5 methyl substituted 4-arylthio and 4-aryloxy-3-Iodopyridin-2(1H)-one type anti-HIV agents. J. Med. Chem. 2009;52:7473–7487. doi: 10.1021/jm900802y. [DOI] [PubMed] [Google Scholar]
- 11.Mowbray C.E., Burt C., Corbau R., Perros M., Tran I., Stupple P.A., Webster R., Wood A. Pyrazole NNRTIs 1: Design and initial optimisation of a novel template. Bioorg. Med. Chem. Lett. 2009;19:5599–5602. doi: 10.1016/j.bmcl.2009.08.039. [DOI] [PubMed] [Google Scholar]
- 12.Xu. B., Sun. Y., Guo Y., Cao Y., Yu T. Synthesis and biological evaluation of N4-(hetero)arylsulfonylquinoxalinones as HIV-1 reverse transcriptase inhibitors. Bioorg. Med. Chem. 2009;17:2767–2774. doi: 10.1016/j.bmc.2009.02.039. [DOI] [PubMed] [Google Scholar]
- 13.Ren J., Chamberlain P.P., Stamp A., Short S.A., Weaver K.L., Romines K.R., Hazen R., Freeman A., Ferris R.G., Andrew C.W., et al. Structural basis for the improved drug resistance profile of new generation benzophenone non-nucleoside HIV-1 reverse transcriptase inhibitors. J. Med. Chem. 2008;51:5000–5008. doi: 10.1021/jm8004493. [DOI] [PubMed] [Google Scholar]
- 14.O’llah E., Rufchahi M. Synthesis of 6-chloro and 6-fluoro-4-hydroxyl-2-quinolone and their azo disperse dyes. Chin. Chem. Lett. 2010;21:542–546. doi: 10.1016/j.cclet.2010.01.017. [DOI] [Google Scholar]
- 15.Zhu Q., Reza F., Yang Z., Anthony A., Liu Y., Joon C.H., Richard W. 4-Thio substituted quinoline and naphthyridine compounds. Patent EP 2,061,466. 2009
- 16.Whisler M.C., MacNeil S., Snieckus V., Beak P. Beyond thermodynamic acidity: A perspective on the complex-induced proximity effect (CIPE) in deprotonation reactions. Angew. Chem. Int. Ed. Engl. 2004;43:2206–2225. doi: 10.1002/anie.200300590. [DOI] [PubMed] [Google Scholar]
- 17.Hewawasam P., Fan W., Knipe J., Moon S.L., Boissard C.G., Gribkoff V.K., Starrett J.E. The synthesis and structure-activity relationships of 4-aryl-3-aminoquinolin-2-ones: A new class of calcium-dependent, large conductance, potassium (maxi-K) channel openers targeted for post-stroke neuroprotection. Bioorg. Med. Chem. Lett. 2002;12:1779–1783. doi: 10.1016/S0960-894X(02)00240-8. [DOI] [PubMed] [Google Scholar]
- 18.Ren J., Milton J., Weaver K.L., Short S.A., Stuart D.I. Structural basis for the resilience of efavirenz (DMP-266) to drug resistance mutations in HIV-1 reverse transcriptase. Structure. 2000;8:1089–1094. doi: 10.1016/S0969-2126(00)00513-X. [DOI] [PubMed] [Google Scholar]