Abstract
A series of different 1-monosubstituted and 1,1-disubstituted 1,2,3,4-tetrahydro-isoquinolines was synthesized in high yields from different ketoamides. We have developed a convenient method for the synthesis of disubstituted derivatives by interaction of ketoamides with organomagnesium compounds, followed by cyclization in the presence of catalytic amounts of p-toluenesulfonic acid (PTSA). A number of substituents at the C-1 in the isoquinoline skeleton were introduced varying either carboxylic acid or organomagnesium compound. Some of the obtained 1,1-dialkyl-1,2,3,4-tetrahydro-isoquinolines possess contractile activity against guinea pig’s gastric smooth muscle preparations.
Keywords: Grignard reagent, tetrahydroisoquinolines, contractile activity
1. Introduction
The pharmacological activity of certain tetrahydroisoquinolines has long been established [1]. The tetrahydroisoquinoline motif is present in a variety of natural products, including cactus alkaloids (peyoruvic acid) [2], mammalian alkaloids (salsoline carboxylic acid) [3,4,5], the esteinascidine family (ET743) [6,7,8,9] and spiro-benzoisoquinoline alkaloids (parfumine) [10,11]. Within the series of 1-substituted 6,7-dihydroisoquinolines there are several active sympathomimetic amines [12,13], one of which, trimetoquinol, is a potent bronchodilator [14,15]. Ohkubo and co-workers [16] synthesized a series of 1,2,3,4-tetrahydroisoquinolines, for example MK801 (disocilpine), and evaluated them for anticonvulsant activity against intracerebro-ventriculas N-methyl-D-aspartate (NMDA)-induced seizures in mice [17,18,19,20]. The authors [16] found that (+)-1-methyl-1-phenyl-1,2,3,4-tetrahydroiso-quinoline hydrochloride [(+)-FR115427)] was the most effective anticonvulsant, protected CA1 hippocampal neuronal degeneration in rats and also showed anti-hypoxic activity in mice. Some isoquinoline derivatives, specially 1,1-dialkyl-1,2,3,4-tetrahydroisoquinolines, have been found to have a peripheral vasodilatory effect, a sympathetic nerve stimulating effect, an analgesic effect, or an anticonvulsant effect, and a few of them have become available clinically [21]. The biological tests indicate that 1,1-dialkyl-1,2,3,4-tetrahydroisoquinolines have potent dopamine D2 receptor-blocking activity and an excellent safety profile. 1,1-Disubstituted tetrahydroisoquinoline derivatives, also were found in Aristolichia species (Aristolochiaceae). [22]. Kubota et al. [23] also synthesized different N-acyl 1,2,3,4-tetrahydroisoquinoline derivatives and evaluated their pharmacological activity as novel specific bradycardic agents.
The variety of biological activities of substituted 1,2,3,4-tetrahydroisoquinolines prompted us to synthesized a number of their derivatives. The most appropriate method for their synthesis is the Pictet-Spengler reaction. However, this classical reaction has some disadvantages, the main one of which is the ring closure after condensation of phenethylamines with an aldehyde in the classical variant (aldehydes give good yields while ketones tend not to give products at all). However, in the last several years 1,1-disubstituted tetrahydroisoquinolines have been synthesized from starting cyclic ketones using titanium(IV)isopropoxide and acetic-formic anhydride [24]. Later, Kumpaty [25] reported a selective and direct access to secondary amines by reductive mono-N-alkylation of primary amines in the presence of the Ti(i-PrO)4 and NaBH4. A new, environmentally friendly variation of the Pictet-Spengler reaction has been elaborated using a small pore size zeolite, Ersorb 4 [26]. Some authors have reported the synthesis and an application of a new planar-chiral Lewis acid based on a 1,2-azaborolyl framework [27]. Pictet-Spengler condensation of dopamine with (+)-menthyl pyruvate followed by acid hydrolysis furnished (-)-R-salsolinol-1-carboxylic acid in good yield [28,29,30]. The basic method, described in literature is preparation of 1,l-dimethyltetrahydroisoquinoline from 3,4-dihydro-6,7-dimethoxy-1-methylisoquinoline [31]. Recently Kałuza and co-workers described a facile synthesis of highly substituted, optically pure tetraydroisoquinolines with a quaternary carbon stereocenter [32]. Funabashi et al. [33] reported the first example of a catalytic enantioselective quaternary stereocenter construction through a Reissert-type reaction with quinolines, using a bifunctional catalyst. 1-Substituted isoquinolines or 3,4-dihydroisoquinolines were used as starting materials for the synthesis of Reisert compounds [34,35,36,37,38]. Stereodivergent synthesis of 1,10-cis- and -trans-thiazolo[4,3-a]isoquinolinones, starting from N-4,3-dimethoxyphenethylthiazolidinedione and using N-acyliminium ion or Parham cyclization, also was reported recently [39]. Kirkpatrick and Maclaren prepared 1,1,-disubstituted 1,2,3,4-tetrahydro-b-carbolines by action of trifluoracetic acid on enamines of tryptamine or tryptophan [40]. Later Bobowski [41,42] reported condensation of 1H-indole-3-ehtanamines with different 2,4-pentanediones and b-keto esters, followed by acid-catalyzed ring closure of resulting enamines to corresponding 1,1-disubtituted indoles.
2. Results and Discussion
The biological activity of isoquinoline derivatives, as analogues of various drugs, has provided great deal of interest for the synthesis of new compounds. Papaverine, for example, is a smooth muscle relaxant and vasodilator which acts directly on the heart muscle. The biological activity of papaverine attracted a great deal of our interest for the synthesis and investigations of 1-substituted isoquinoline derivatives, as potential new drugs.
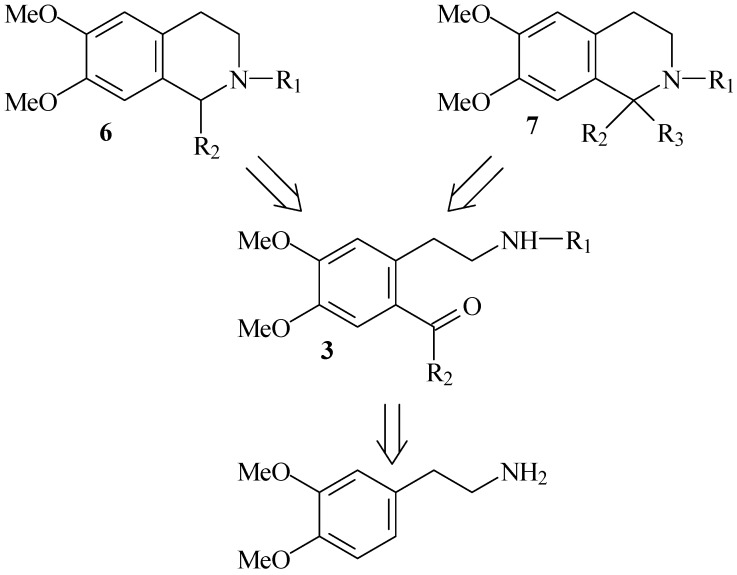
We report herein an alternative of the classical methods which includes ortho-acylation of 2-phenethylamines in polyphosphoric acid and following cyclization. In our previous reports we applied this protocol for the synthesis of variety O- and N-heterocycles and alkaloids [43,44,45,46,47]. Our synthetic approach to 1- and 1,1-disubstituted 1,2,3,4-tetrahydroisoquinolines is depicted in the Scheme 1, which shows the key steps as well as the main starting material.
Scheme 1.
Retrosynthetic scheme for the synthesis of both 1-monosubstituted and 1,1-disubstituted 1,2,3,4-tetrahydroisoquinolines.
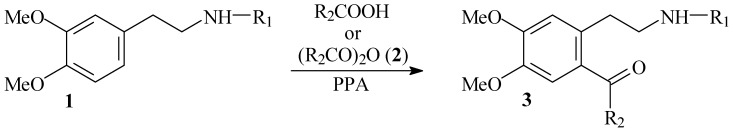
Our strategy is based on the acylation of the amides of homoveratrylamine 1 with carboxylic acids and application of obtained ketoamides 3 for the preparation of 1-substituted 6 or 1,1-disubstited 1,2,3,4-tetrahydroisoquinolines 7. Starting ketoamides of homoveratrylamine 3 were obtained by Friedel-Crafts-type acylation. The Friedel-Crafts acylation of activated benzene rings in the presence of polyphosphoric acid (PPA) is a very convenient method for direct synthesis of aromatic ketones [43], 1-substituted 3,4-dihydroisoquinolines [44], 1-substituted 3,4-dihydro-b-carbolines [45], quinazolinones [46], isochromanes [47], etc. The reaction of amides of homoveratrylamine 1 with acetic anhydride, benzoic or phenylacetic acid 2 in PPA gave the expected ketoamides 3 in high yield (Scheme 2, Table 1).
Scheme 2.
Synthesis of starting ketoamides.
Table 1.
Reaction conditions and yields for starting ketoamides 3.
| 3 | R1 | R2 | Reaction conditions | mp, °C | Yield [%] |
|---|---|---|---|---|---|
| a | СОCH3 | CH3 | 2 h, 80 °С | 124–125 | 92 |
| b | COC6H5 | CH3 | 2 h, 80 °С | 147–148 | 80 |
| c | COCH2C6H5 | CH3 | 2 h, 80 °С | 135–137 | 79 |
| d | COOC2H5 | CH3 | 3 h, 80 °С | 90–90.5 | 95 |
| e | SO2CH3 | CH3 | 2 h, 80 °С | 140–141 | 75 |
| f | CONHC6H5 | CH3 | 2 h, 60 °С | 118–121 | 75 |
| g | СОCH3 | C6H5 | 2 h, 80 °С | 212–213 | 87 |
| h | COC6H5 | C6H5 | 2 h, 80 °С | 117–121 | 85 |
| i | COCH2C6H5 | C6H5 | 4 h, 60 °С | 108–111 | 89 |
| j | COOC2H5 | C6H5 | 3 h, 80 °С | 97–98 | 89 |
| k | SO2CH3 | C6H5 | 3h, 80 °C | 84–86 | 83 |
| l | CONHC6H5 | C6H5 | 4 h, 60 °С | 128–131 | 76 |
| m | СОCH3 | CH2C6H5 | 20 h, 60 °С | 188–189 | 62 |
| n | COC6H5 | CH2C6H5 | 20 h, 60 °С | 141–141.5 | 82 |
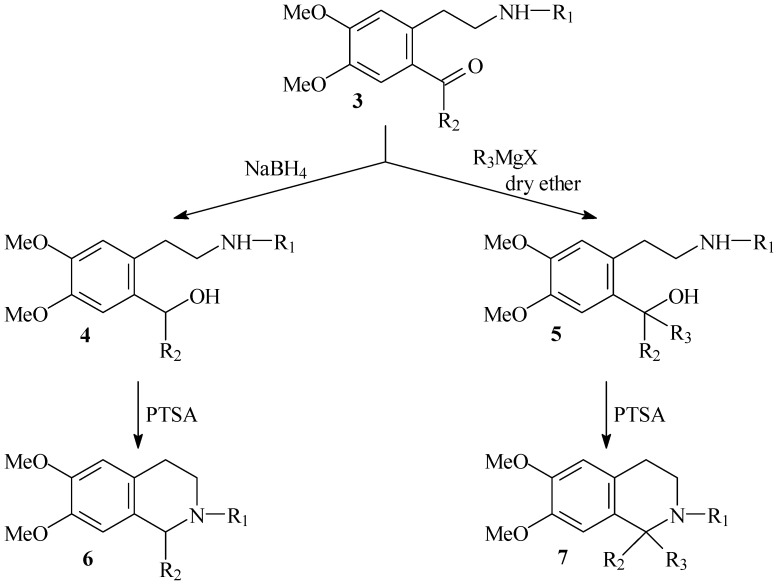
The next step in our synthesis was application of acylated ketoamides for the construction of isoquinoline ring system. We anticipated that 1-substituted 1,2,3,4-tetrahydroisoquinolines could be prepared trough reduction of ketoamides followed by cyclization of newly obtained hydroxyamides through p-toluenesulfonic acid. Respectively, 1,1-disubstituted 1,2,3,4-tetrahydroisoquinolines could be prepared from 4 and 5, obtained from ketoamides and Grignard reagents (Scheme 3).
Scheme 3.
Synthesis of 1-substituted and 1,1-disubstituted-1,2,3,4-tetrahydroisoquinolines.
For the next step, 3 were reduced with NaBH4 in methanol to give corresponding 4 with 85–90% yields (Table 2).
Table 2.
Synthesis of hydroxyamides 4.
| 4 | R1 | R2 | Yield [%] | mp, °C |
|---|---|---|---|---|
| a | СОCH3 | CH3 | 95 | 108–110 |
| b | COC6H5 | CH3 | 94 | 109–110 |
| c | COCH2C6H5 | CH3 | 92 | oil |
| d | COOC2H5 | CH3 | 92 | 85–87 |
| e | SO2CH3 | CH3 | 90 | oil |
| f | CONHC6H5 | CH3 | 91 | 147–150 |
| g | СОCH3 | C6H5 | 94 | 133–135 |
| h | COC6H5 | C6H5 | 90 | 58–60 |
| i | COCH2C6H5 | C6H5 | 91 | oil |
| j | COOC2H5 | C6H5 | 91 | 92–95 |
| k | SO2CH3 | C6H5 | 90 | oil |
| l | CONHC6H5 | C6H5 | 90 | 73–75 |
| m | СОCH3 | CH2C6H5 | 95 | oil |
| n | COC6H5 | CH2C6H5 | 91 | 117–118 |
Compounds 5 were prepared with good (50–56%) yields from starting ketoamides 3 and 5-fold excess of magnesium and equimolar amounts of alkyl- (or aryl-) halide. Reaction proceeded at room temperature in dry ether (Table 3).
Table 3.
Reaction of ketoamides with Grignard reagents.
| 5 | R1 | R2 | R3 | Yield [%] | mp, °C |
|---|---|---|---|---|---|
| a | СОCH3 | CH3 | CH3 | 52 | oil |
| b | COC6H5 | CH3 | CH3 | 50 | 160–162 |
| c | COCH2C6H5 | CH3 | CH3 | 50 | oil |
| d | COOC2H5 | CH3 | CH3 | - * | - |
| e | SO2CH3 | CH3 | CH3 | - * | - |
| n | COC6H5 | CH2C6H5 | CH3 | 50 | 48–50 |
| o | СОCH3 | CH3 | C2H5 | - * | - |
| p | COC6H5 | CH3 | C2H5 | 65 | 131–135 |
| r | SO2CH3 | CH3 | C2H5 | - * | - |
| s | COC6H5 | CH3 | C6H5 | 50 | 96–98 |
| t | SO2CH3 | C6H5 | C2H5 | 51 | 131–134 |
* compounds were directly cyclized.
The next step was cyclization of the newly synthesized compounds 4 and 5. We found that 4 in the presence of a catalytic amount of toluene-p-sulfonic acid for 30 min at rt in dichloromethane afforded the corresponding 1,2,3,4-tetrahydroisoquinolines 6 with high yield 90–97% (Table 4).
Table 4.
Synthesis of 1-substituted 1,2,3,4-tetrahydroisoquinolines.
| 6 | R1 | R2 | Yield [%] | mp, °C |
|---|---|---|---|---|
| a | СОCH3 | CH3 | 94 | 97–98 |
| b | COC6H5 | CH3 | 90 | 126–127 |
| c | COCH2C6H5 | CH3 | 92 | 115–116 |
| d | COOC2H5 | CH3 | 90 | 72–74 |
| e | SO2CH3 | CH3 | 92 | 105–106 |
| f | CONHC6H5 | CH3 | 91 | 178–180 |
| g | СОCH3 | C6H5 | 94 | 109–191 |
| h | COC6H5 | C6H5 | 93 | 143–144 |
| i | COCH2C6H5 | C6H5 | 95 | oil |
| j | COOC2H5 | C6H5 | 88 | oil |
| k | SO2CH3 | C6H5 | 90 | 183–184 |
| l | CONHC6H5 | C6H5 | 89 | 120–122 |
| m | СОCH3 | CH2C6H5 | 91 | 102–105 |
| n | COC6H5 | CH2C6H5 | 94 | 189–192 |
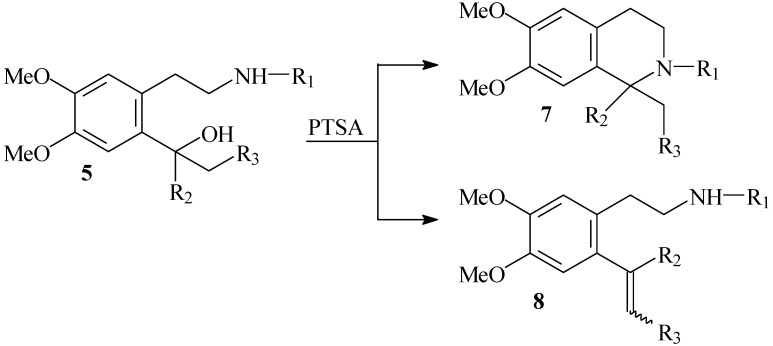
The same protocol can be readily used for the cyclization to 1,1-disubstituted 1,2,3,4-tetrahydro-isoquinolines 7 (Table 5).
Table 5.
Cyclisation to 1,1-disubstituted 1,2,3,4-tetrahydroisoquinolines.
| 7 | R1 | R2 | R3 | Yield [%] | mp, °C |
|---|---|---|---|---|---|
| a | СОCH3 | CH3 | CH3 | 96 | 123–125 |
| b | COC6H5 | CH3 | CH3 | 97 | 143–146 |
| c | COCH2C6H5 | CH3 | CH3 | 97 | oil |
| d | COOC2H5 | CH3 | CH3 | 90 | 55-56 |
| e | SO2CH3 | CH3 | CH3 | 95 | 123–125 |
| n | COC6H5 | CH2C6H5 | CH3 | 30 | 164–166 |
| o | СОCH3 | CH3 | C2H5 | 60 | 76–81 |
| p | COC6H5 | CH3 | C2H5 | 80 | 90–92 |
| r | SO2CH3 | CH3 | C2H5 | 60 | 100–102 |
| s | COC6H5 | CH3 | C6H5 | 60 | 103–133 |
| t | SO2CH3 | C6H5 | C2H5 | 60 | 138–141 |
We also found that when the substituent at the C-1 is ethyl or benzyl (in some cases also methyl), the styrene products 8 were formed also than expected cyclic 1,1-disubtituted product 7 (Scheme 4, Table 6).
Scheme 4.
Formation of styrenes 8.
Table 6.
Formation of styrenes.
| 8 | R1 | R2 | R3 | Yield [%] | mp, °C |
|---|---|---|---|---|---|
| g | COC6H5 | CH3 | CH3 | 65 | 72–75 |
| n | COC6H5 | CH3 | C6H5 | 50 | 48–50 |
| s | COC6H5 | C6H5 | H | 50 | 96–98 |
| t | SO2CH3 | C6H5 | CH3 | 30 | 145–148 |
2.1. Estimation of Gastric Smooth Muscle Contractile Activity for Some of the Newly Synthesized Compounds
The experiments were performed on gastric corpus smooth muscle preparations obtained from adult male guinea-pig. All experimental procedures were done in strict accordance with the current European regulations (86/609/EEC) regarding the protection of animals used for experimental purposes. The spontaneous contractile activity of the smooth-muscle strips were measured with the help of a tensotransducer measuring system at isometric conditions.
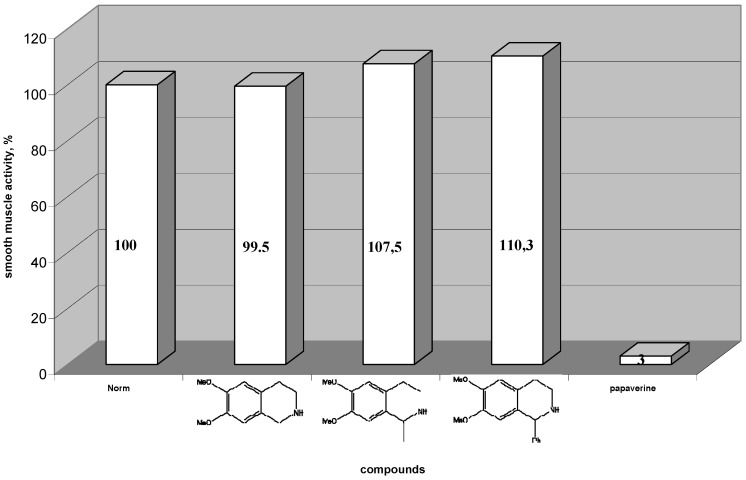
The biological activity of papaverine attracted a great deal of our interest for the synthesis and investigations of 1- and 1,1-disubstituted isoquinoline derivatives, as potential new drugs. The target compounds, being structural analogues of known bioactive leads, as papaverine and cryptostiline, are expected to show biological activity. For this purpose we tested three main groups of compounds for contractile activity. In search of the reason for activity, firstly we estimated 1,2,3,4-tetrahydroisoquinoline skeleton and 1-methyl- and 1-phenyl-6,7-dimetoxy-1,2,3,4-tetrahydroisoquinolines. We found that the absence of substituents devoided the compounds of contractile activity, as shown in Figure 1. The isoquinoline derivatives were less effective in contractile smooth muscle activity (−0.5%, +7.5% and +10.3% vs. control, respectively).
Figure 1.
Change of spontaneous contractile activity of gastric smooth muscles preparation after using 1-substituted 1,2,3,4-tetrahydroisoquinoline derivatives and papaverine, normal activity is taken for 100%.
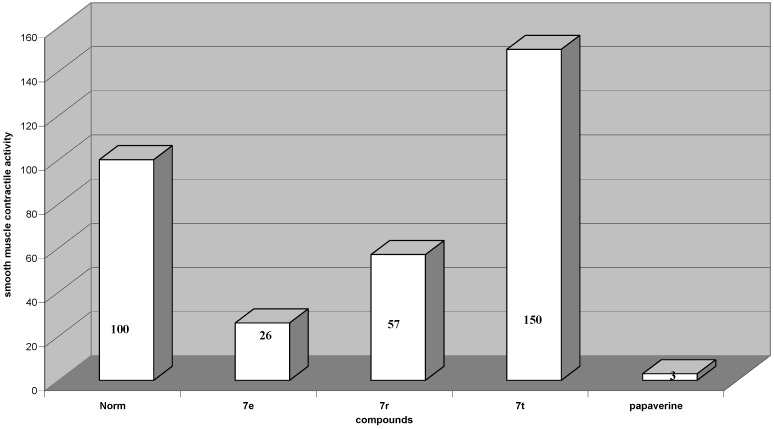
The second group included 1,1-disubstituted-6,7-dimetoxy-1,2,3,4-tetrahydroisoquinolines. The estimation of contractile activity showed that tested compounds have similar effect, as papaverine. The isoquinoline derivatives 7e and 7r were most effective in contractile smooth muscle activity (−74% and −43% vs. control, respectively) (Figure 2).
Figure 2.
Change of spontaneous contractile activity of gastric smooth muscles preparation after using 1,1-disubstituted 1,2,3,4-tetrahydroisoquinoline derivatives and papaverine, normal activity is taken for 100%.
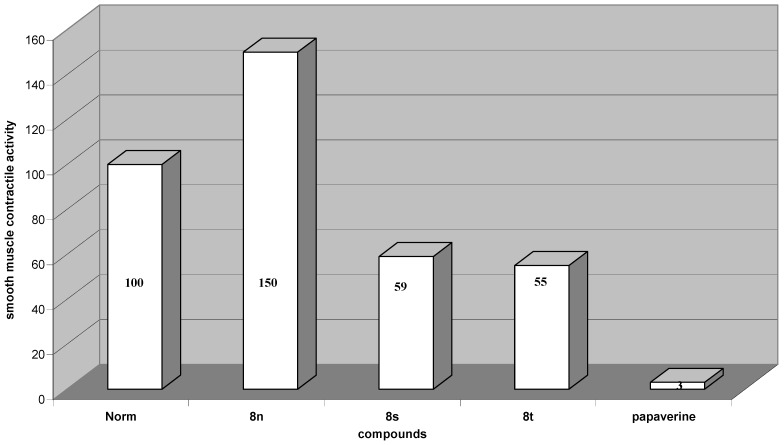
The third group included styrene products 8. As shown in Figure 3, styrenes 8s and 8t were most effective in contractile smooth muscle activity (−41% and −45% vs. control, respectively) (Figure 3). The contractile activity against smooth muscle preparations of these compounds were not as high as the activity of 7.
Figure 3.
Change of spontaneous contractile activity of gastric smooth muscles preparation after using styrenes and papaverine, normal activity is taken for 100%.
3. Experimental
Reagents and chemicals were purchased from commercial sources (Sigma-Aldrich S.A. and Riedel-de Haën) and used as received. Melting points were determined on a Boetius hot stage apparatus and are uncorrected. Spectra were recorded on a Bruker Avance DRX250 spectrometer. 1H-NMR and 13C-NMR spectra were taken in CDCl3 (unless otherwise specified) at 250 or 600 MHz and 62.5 MHz respectively. Chemical shifts were given in part per million (ppm) relative and were referenced to TMS (δ = 0.00 ppm) as an internal standard and coupling constants are indicated in Hz. All the NMR spectra were taken at rt (ac. 295 K). Elemental analyses were performed with a TruspecMicro. TLC was carried out on precoated 0.2 mm Fluka silica gel 60 plates, using diethyl ether:n-hexane:1:1 as the eluent system. Merck silica gel 60 (0.063–0.2 mm) was used for column chromatographic separation. Polyphosphoric acid was obtained from 85% phosphoric acid and P2O5 (1:1 w/w).
3.1. Typical Procedure for Preparation of N-[2-(2-Acyl-4,5-dimethoxyphenyl)-ethyl]amides 3
To a solution of amide 1 (3 mmol) and the corresponding carboxylic acid or their anhydrides 2 (5 mmol) in dichloromethane (10 mL) in an open flask polyphosphoric acid (7 g) was added. The mixture was stirred on a mechanical stirrer carefully at 60 °C or 80 °C then poured on crushed ice and extracted with CH2Cl2 (3 × 20 mL). The combined extracts were dried (Na2SO4), filtered on the short column filled with neutral Al2O3 and then concentrated.
N-(2-Acetyl-4,5-dimethoxyphenethyl)acetamide (3a): known compound [53,54,55,56].
N-(2-Acetyl-4,5-dimethoxyphenethyl)benzamide (3b): 1H-NMR: 2.61 (s, 3H), 3.10 (t, J = 6.4, 2H), 3.75 (dt, J = 6.6, 5.4, 2H), 3.88 (s, 3H), 3.90 (s, 3H), 6.80 (broad s, 1H, NH), 7.16 (s, 1H), 7.28 (s, 1H), 7.34–7.47 (m, 4H), 7.77–7.80 (m, 1H); 13C-NMR: 201.5, 181.6, 167.4, 152.1, 146.8, 134.5, 131.0, 128.3, 126.9, 114.0, 112.6, 56.1, 55.9, 42.4, 32.1, 29.4. Anal. calcd. for C19H21NO4: C, 69.71; H, 6.47; N, 4.28. Found: C, 69.87; H, 6.58; N, 4.18.
N-(2-Acetyl-4,5-dimethoxyphenethyl)-2-phenylacetamide (3c): 1H-NMR: 2.51 (s, 3H), 2.95 (t, J = 6.8, 2H), 3.47 (s, 2H), 3.51 (dd, J = 7.0, 5.2, 2H), 3.89 (s, 3H), 3.92 (s, 3H), 6.43 (broad s, 1H, NH), 6.70 (s, 1H), 7.12 (s, 1H), 7.14–7.27 (m, 5H); 13C-NMR: 200.3, 181.6, 171.2, 152.0, 146.7, 135.0, 134.3, 129.3, 128.7, 128.6, 126.9, 114.1, 112.97, 56.2, 55.97, 43.8, 41.5, 32.5, 29.2. Anal. calcd. for C20H23NO4: C, 70.36; H, 6.79; N, 4.10. Found: C, 70.48; H, 6.95; N, 3.90.
Ethyl 2-acetyl-4,5-dimethoxyphenethylcarbamate (3d): 1H-NMR: 1.21 (t, J = 7.1, 3H), 2.58 (s, 3H), 3.03 (t, J = 6.9, 2H), 3.43 (dd, J = 12.7, 6.6, 2H), 3.92 (s, 3H), 3.93 (s, 3H), 4.08 (q, J = 7.1, 2H), 5.31 (broad s, 1H, NH), 6.75 (s, 1H), 7.22 (s, 1H); 13C-NMR: 200.0, 156.7, 151.8, 146.8, 134.4, 129.7, 114.3, 113.2, 60.5, 56.3, 55.9, 42.5, 33.9, 29.3, 14.6. Anal. calcd. for C15H21NO5: C, 61.00; H, 7.17; N, 4.74. Found: C, 61.25; H, 7.45; N, 4.54.
N-(2-Acetyl-4,5-dimethoxyphenethyl)methanesulfonamide (3e): 1H-NMR (DMSO): 2.53 (s, 3H), 2.82 (s, 3H), 2.94 (t, J = 7.2, 2H), 3.12 (dd, J = 7.4, 6.1, 2H), 3.80 (s, 3H), 3.81 (s, 3H), 6.89 (s, 1H), 6.98 (t, J = 5.6, 1H, NH), 7.35 (s, 1H); 13C-NMR: 200.6, 151.5, 146.8, 133.1, 129.7, 115.1, 114.1, 56.1, 55.9, 44.2, 39.6, 34.1, 29.8. Anal. calcd. for C13H19NO5S: C, 51.81; H, 6.35; N, 4.65; S, 10.64. Found: C, 51.96; H, 6.15; N, 4.72; S, 10.45.
1-(2-Acetyl-4,5-dimethoxyphenethyl)-3-phenylurea (3f): 1H-NMR: 2.42 (s, 3H), 2.56 (s, 1H, CONH), 2.91 (dd, J = 6.5, 7.9, 2H), 3.28 (td, J = 6.4, 7.3, 2H), 3.73 (s, 3H), 3.75 (s, 3H), 5.78 (t, J = 5.8, 1H, NHCO), 6.64 (s, 1H), 6.77 (tt, J = 1.1, 7.7, 1H), 7.05 (dd, J = 1.6, 6.8, 1H), 7.09 (s, 1H), 7.26 (dd, J = 3.4, 5.6, 2H), 7.83 (s, 1H); 13C-NMR: 199.7, 156.1, 151.7, 146.6, 140.0, 134.7, 129.3, 128.7, 121.7, 118.6, 114.6, 113.4, 56.1, 55.9, 41.3, 34.8, 29.4. Anal. calcd. for C19H22N2O4: C, 66.65; H, 6.48; N, 8.18. Found: C, 66.86; H, 6.28; N, 8.32.
N-(2-Benzoyl-4,5-dimethoxyphenethyl)acetamide (3g): 1H-NMR: 1.95 (s, 3H), 2.86-2.88 (m, 2H), 3.59–3.52 (m, 2H), 3.80 (s, 3H), 3.97 (s, 3H), 6.84 (s, 1H), 6.89 (s, 1H), 7.01 (broad s, 1H, NH), 7.54–7.45 (m, 2H), 7.66–7.58 (m, 1H), 7.85–7.81 (m, 2H); 13C-NMR: 198.2, 170.6, 151.4, 146.5, 138.0, 133.4, 130.5, 130.3, 129.96, 128.5, 128.3, 113.3, 112.9, 56.2, 56.1, 41.9, 31.8, 23.2. Anal. calcd. for C19H21NO4: C, 69.71; H, 6.47; N, 4.28. Found: C, 69.98; H, 6.29; N, 4.34.
N-(2-Benzoyl-4,5-dimethoxyphenethyl)benzamide (3h): known compound [48].
N-(2-Benzoyl-4,5-dimethoxyphenethyl)-2-phenylacetamide (3i): 1H-NMR: 2.84 (t, J = 6.7, 2H), 3.51 (s, 2H), 3.56–3.60 (m, 2H), 3.80 (s, 3H), 3.95 (s, 3H), 6.76 (broad s, 1H, NH), 6.80 (s, 1H), 6.84 (s, 1H), 7.29–7.15 (m, 5H), 7.52–7.46 (m, 2H), 7.62–7.59 (m, 1H), 7.77–7.74 (m, 2H); 13C-NMR: 197.5, 171.3, 151.3, 146.3, 138.1, 135.1, 133.1, 130.4, 129.9, 129.3, 128.6, 128.4, 126.9, 113.2, 113.1, 56.1, 56.0, 43.8, 41.6, 31.6. Anal. calcd. for C25H25NO4: C, 74.42; H, 6.25; N, 3.47. Found: C, 74.78; H, 6.37; N, 3.25.
Ethyl 2-benzoyl-4,5-dimethoxyphenethylcarbamate (3j): known compound [44].
N-(2-Benzoyl-4,5-dimethoxyphenethyl)methanesulfonamide (3k): 1H-NMR: 2.79 (s, 3H), 2.94 (t, J = 6.6, 2H), 3.48 (ddd, J = 2.2, 5.9, 6.5, 2H), 3.79 (s, 3H), 3.99 (s, 3H), 5.66 (t, J = 5.1, 1H, NH), 6.87 (s, 1H), 6.91 (s, 1H), 7.49 (tdd, J = 1.4, 6.6, 8.2, 2H), 7.59–7.66 (m, 1H), 7.79 (t, J=1.8, 1H), 7.82 (t, J = 1.4, 1H); 13C-NMR: 197.7, 151.5, 146.6, 138.0, 133.2, 132.6, 130.4, 130.1, 128.4, 113.5, 58.7, 56.1, 45.0, 39.7, 32.9. Anal. calcd. for C18H21NO5S: C, 59.49; H, 5.82; N, 3.85; S, 8.82. Found: C, 59.70; H, 5.95; N, 3.64; S, 8.61.
1-(2-Benzoyl-4,5-dimethoxyphenethyl)-3-phenylurea (3l): 1H-NMR: 2.87 (t, J = 6.9, 2H), 3.51 (q, J = 6.8, 2H), 3.74 (s, 3H), 3.90 (s, 3H), 5.92 (t, J = 4.9, 1H, NHCO), 6.83 (s, 1H), 6.87 (s, 1H), 6.99 (tt, J = 1.3, 7.7, 1H), 7.08 (broad s, 1H, CONH), 7.18–7.31 (m, 4H), 7.44 (tt, J = 1.4, 6.8, 2H), 7.54–7.61 (m, 1H), 7.74–7.77 (m, 2H); 13C-NMR: 198.0, 156.0, 151.4, 146.4, 139.1, 138.2, 133.6, 133.1, 130.4, 129.9, 128.9, 128.4, 123.0, 120.3, 113.6, 113.3, 56.1, 56.0, 42.4, 33.3. Anal. calcd. for C24H24N2O4: C, 71.27; H, 5.98; N, 6.93. Found: C, 71.49; H, 6.19; N, 6.87.
N-(4,5-Dimethoxy-2-(2-phenylacetyl)phenethyl)acetamide (3m): known compound [44].
N-(4,5-Dimethoxy-2-(2-phenylacetyl)phenethyl)benzamide (3n): known compound [44].
3.2. Typical Procedure for the Preparation of Compounds 4a-n
To solution of the corresponding ketoamide 3 (1 mmol) in methanol (15 mL), NaBH4 (2 mmol, 0.1 g) was added portionwise. The solution was stirred 30 min at room temperature, than the solvent was removed under vacuum. Water (30 mL) was added to the residue and the solution was extracted with CH2Cl2 (3 × 20 mL), then the combined extracts were dried (Na2SO4). The products, after evaporation of the solvent, were obtained with 85–90% yields.
N-(2-(1-Hydroxyethyl)-4,5-dimethoxyphenethyl)acetamide (4a): 1H-NMR: 1.48 (d, J = 6.4, 3H), 1.85 (s, 3H), 2.72 (td, J = 7.1, 14.0, 1H), 2.86 (td, J = 6.9, 13.9, 1H), 3.13 (broad s, 1H, OH), 3.32 (td, J = 6.9, 13.3, 1H), 3.49 (td, J = 7.0, 13.4, 1H), 3.82 (s, 3H), 3.85 (s, 3H), 5.07 (q, J = 6.4, 1H), 6.28 (t, J = 4.6, 1H, NH), 6.60 (s, 1H), 7.00 (s, 1H); 13C-NMR: 170.5, 148.0, 147.8, 135.8, 127.9, 112.9, 108.9, 65.8, 55.9, 55.88, 41.0, 31.6, 24.4, 23.0. Anal. calcd. for C14H21NO4: C, 62.90; H, 7.92; N, 5.24. Found: C, 63.15; H, 8.06; N, 5.13.
N-(2-(1-Hydroxyethyl)-4,5-dimethoxyphenethyl)benzamide (4b): 1H-NMR: 1.84 (s, 3H), 2.07 (broad s, 1H, OH), 2.72 (td, J = 7.2, 14.1, 1H), 2.90 (td, J = 7.2, 14.1, 1H), 3.27–3.51 (m, 2H), 3.75 (s, 3H), 3.86 (s, 3H), 6.04 (d, J = 1.7, 1H), 6.18 (t, J = 4.9, 1H, NH), 6.67 (s, 1H), 6.83 (s, 1H), 7.25–7.35 (m, 5H); 13C-NMR: 170.6, 148.8, 147.5, 143.8, 134.2, 129.1, 128.3, 127.2, 126.5, 113.0, 111.4, 72.4, 55.9, 55.8, 40.9, 31.7, 22.9. Anal. calcd. for C19H23NO4: C, 69.28; H, 7.04; N, 4.25. Found: C, 69.43; H, 7.25; N, 4.13.
N-(2-(1-Hydroxyethyl)-4,5-dimethoxyphenethyl)-2-phenylacetamide (4c): 1H-NMR: 1.44 (d, J = 6.4, 3H), 2.76 (ddd, J = 7.1, 14.3, 32.3, 2H), 3.08 (broad s, 1H, OH), 3.43 (dtd, J = 7.4, 13.6, 13.4, 20.6, 2H), overpalled with 3.43 (s, 2H), 3.78 (s, 3H), 3.85 (s, 3H), 5.02 (q, J = 6.3, 6.4, 1H), 6.09 (t, J = 5.6, 1H, NH), 6.52 (s, 1H), 6.99 (s, 1H), 7.11–7.15 (m, 2H), 7.24–7.31 (m, 3H); 13C-NMR: 171.2, 147.8, 147.7, 135.9, 134.7, 129.2, 128.7, 127.5, 127.0, 112.8, 108.9, 65.6, 55.8, 43.4, 40.7, 31.4, 24.2. Anal. calcd. for C20H25NO4: C, 69.95; H, 7.34; N, 4.08. Found: C, 70.15; H, 7.53; N, 3.96.
Ethyl2-(1-hydroxyethyl)-4,5-dimethoxyphenethylcarbamate (4d): 1H-NMR: 1.19 (t, J = 7.1, 3H), 1.47 (d, J = 6.4, 3H), 2.62-2.90 (m, 2H), 3.36 (tt, J = 6.9, 13.6, 2H), 3.84 (s, 3H), 3.86 (s, 3H), 4.05 (q, J = 7.1, 2H), 4.92 (broad s, 1H, NH), 5.10 (q, J = 6.3, 1H), 6.60 (s, 1H), 7.03 (s, 1H); 13C-NMR: 156.8, 148.0, 148.0, 136.1, 127.4, 112.9, 108.8, 65.9, 60.8, 55.93, 55.91, 42.2, 32.4, 24.6, 14.6. Anal. calcd. for C15H23NO5: C, 60.59; H, 7.80; N, 4.71. Found: C, 60.73; H, 7.98; N, 4.56.
N-(2-(1-Hydroxyethyl)-4,5-dimethoxyphenethyl)methanesulfonamide (4e): 1H-NMR: 1.48 (d, J = 6.4, 3H), 1.91 (broad s, 1H, OH), 2.74 (s, 3H), 2.82 (td, J = 3.9, 6.7, 2H), 3.22–3.42 (m, 2H), 3.84 (s, 3H), 3.86 (s, 3H), 5.06 (q, J = 6.4, 1H), 5.31 (t, J = 6.5, 1H, NH), 6.65 (s, 1H), 6.99 (s, 1H); 13C-NMR: 148.3, 148.0, 135.7, 127.4, 112.9, 109.1, 66.0, 56.0, 55.9, 44.6, 39.9, 32.2, 24.4. Anal. calcd. for C13H21NO5S: C, 51.47; H, 6.98; N, 4.62; S, 10.57. Found: C, 51.68; H, 7.18; N, 4.44; S, 10.69.
1-(2-(1-Hydroxyethyl)-4,5-dimethoxyphenethyl)-3-phenylurea (4f): 1H-NMR: 1.43 (d, J = 6.4, 3H), 2.76 (q, J = 6.9, 1H), 2.87 (td, J = 7.1, 14.1, 1H), 3.31 (td, J = 7.0, 12.4, 1H), 3.58 (dq, J = 6.5, 13.3, 1H), 3.83 (s, 3H), 3.87 (s, 3H), 5.10 (dq, J = 3.5, 6.3, 1H), 5.80 (t, J = 5.7, 1H, NH), 6.66 (s, 1H), 6.93 (tt, J = 1.1, 7.5, 1H), 7.11 (s, 1H), 7.18–7.25 (m, 2H), 7.35 (dd, J = 1.1, 8.6, 2H); 13C-NMR: 155.7, 147.4, 147.3, 139.6, 136.8, 128.3, 127.6, 121.4, 118.2, 112.4, 108.6, 65.0, 55.5, 40.8, 31.8, 24.5. Anal. calcd. for C19H24N2O4: C, 66.26; H, 7.02; N, 8.13. Found: C, 66.47; H, 7.15; N, 8.01.
N-(2-(Hydroxy(phenyl)methyl)-4,5-dimethoxyphenethyl)acetamide (4g): 1H-NMR: 1.84 (s, 3H), 2.07 (broad s, 1H, OH), 2.72 (td, J = 7.2, 14.1, 1H), 2.90 (td, J = 6.8, 13.8, 1H), 3.27–3.51 (m, 2H), 3.75 (s, 3H), 3.86 (s, 3H), 6.04 (d, J = 1.7, 1H), 6.18 (t, J = 4.9, 1H, NH), 6.68 (s, 1H), 6.83 (s, 1H), 7.25–7.39 (m, 5H); 13C-NMR: 170.6, 148.3, 147.5, 143.7, 134.2, 129.1, 128.3, 127.2, 126.5, 113.0, 111.4, 72.4, 56.0, 55.8, 40.9, 31.7, 23.0. Anal. calcd. for C19H23NO4: C, 69.28; H, 7.04; N, 4.25. Found: C, 69.19; H, 7.24; N, 4.37.
N-(2-(Hydroxy(phenyl)methyl)-4,5-dimethoxyphenethyl)benzamide (4h): 1H-NMR: 1.81 (broad s, 1H, OH), 2.86 (td, J = 7.2, 14.3, 1H), 3.02 (td, J = 7.2, 14.0, 1H), 3.60 (dt, J = 7.2, 13.5, 2H), 3.75 (s, 3H), 3.80 (s, 3H), 6.0 (s,1H), 6.11 (broad s, 1H, NH), 6.71 (s, 1H), 6.82 (s, 1H), 7.50–7.25 (m, 8H), 7.70–7.67 (m, 2H); 13C-NMR: 167.8, 148.4, 147.6, 143.6, 134.4, 134.0, 131.4, 129.2, 128.5, 128.4, 127.3, 126.8, 126.5, 113.2, 111.6, 72.7, 55.9, 55.8, 41.3, 31.7. Anal. calcd. for C24H25NO4: C, 73.64; H, 6.44; N, 3.58. Found: C, 73.85; H, 6.23; N, 3.79.
N-(2-(Hydroxy(phenyl)methyl)-4,5-dimethoxyphenethyl)-2-phenylacetamide (4i): 1H-NMR: 1.90 (broad s, 1H, OH), 2.71–2.59 (m, 1H), 2.88 (ddd, J = 6.2, 7.4, 13.7, 1H), 3.54–3.25 (m, 2H), overlapped with 3.46 (s, 2H), 3.78 (s, 3H), 3.83 (s, 3H), 5.84 (t, J = 5.9, 1H, NH), 5.98 (d, J = 3.4, 1H), 6.59 (s, 1H), 6.82 (s, 1H), 7.17–7.14 (m, 2H), 7.37–7.25 (m, 8H); 13C-NMR: 171.4, 148.2, 147.6, 143.6, 134.8, 134.2, 129.4, 128.9, 128.7, 128.3, 127.2, 126.5, 113.1, 111.5, 72.4, 55.9, 55.8, 43.6, 40.7, 31.8. Anal. calcd. for C25H27NO4: C, 74.05; H, 6.71; N, 3.45. Found: C, 74.35; H, 6.97; N, 3.26.
Ethyl 2-(hydroxy(phenyl)methyl)-4,5-dimethoxyphenethylcarbamate (4j): 1H-NMR: 1.18 (t, J = 7.1, 3H), 2.70 (td, J = 7.4, 14.0, 1H), 2.89 (td, J = 6.9, 13.4, 1H), 3.07 (broad s, 1H, OH), 3.29 (dd, J = 8.7, 13.8, 2H), 3.76 (s, 3H), 3.85 (s, 3H), 4.04 (q, J = 7.1, 2H), 4.83 (t, J = 6.6, 1H, NH), 6.03 (s, 1H), 6.65 (s, 1H), 6.87 (s, 1H), 7.21–7.37 (m, 5H); 13C-NMR: 156.8, 148.3, 147.7, 142.6, 134.1, 128.7, 128.4, 128.3, 127.3, 126.6, 113.1, 111.2, 72.4, 60.8, 55.92, 55.89, 42.0, 32.6, 14.6. Anal. calcd. for C20H25NO5: C, 66.83; H, 7.01; N, 3.90. Found: C, 67.01; H, 7.26; N, 3.85.
N-(2-(Hydroxy(phenyl)methyl)-4,5-dimethoxyphenethyl)methanesulfonamide (4k): 1H-NMR: 1.48 (d, J = 6.4, 3H), 1.80 (broad s, 1H, OH), 2.74 (s, 3H), 2.79 (dd, J = 6.9, 13.8, 1H), 2.91 (td, J = 7.0, 14.0, 1H), 3.26 (dd, J = 5.9, 12.7, 2H), 3.76 (s, 3H), 3.86 (s, 3H), 5.12 (t, J = 5.7, 1H, NH), 6.00 (d, J = 1.8, 1H), 6.69 (s, 1H), 6.83 (s, 1H), 7.23–7.33 (m, 5H); 13C-NMR: 148.6, 147.7, 143.4, 134.0, 128.4, 127.5, 126.5, 113.1, 111.5, 72.7, 56.0, 55.9, 44.4, 39.9, 32.4. Anal. calcd. for C18H23NO5S: C, 59.16; H, 6.34; N, 3.83; S, 8.77. Found: C, 59.38; H, 6.15; N, 3.93; S, 8.95.
1-(2-(Hydroxy(phenyl)methyl)-4,5-dimethoxyphenethyl)-3-phenylurea (4l): 1H-NMR: 2.0 (broad s, 1H, OH), 2.66 (td, J = 6.7, 13.7, 1H), 2.88 (td, J = 7.0, 14.0, 1H), 3.34 (dq, J = 6.1, 13.3, 2H), 3.68 (s, 3H), 3.78 (s, 3H), 4.26 (s, 1H, NH), 5.53 (t, J = 6.1, 1H, NH), 6.02 (s, 1H), 6.62 (s, 1H), 6.77 (s, 1H), 6.97–7.04 (m, 1H), 7.17–7.31 (m, 9H); 13C-NMR: 156.4, 148.3, 147.5, 143.6, 138.6, 134.3, 129.3, 129.0, 128.3, 127.2, 126.4, 123.3, 120.4, 113.0, 111.5, 72.3, 55.9, 55.8, 41.5, 32.4. Anal. calcd. for C24H26N2O4: C, 70.92; H, 6.45; N, 6.89. Found: C, 71.23; H, 6.27; N, 6.95.
N-(2-(1-Hydroxy-2-phenylethyl)-4,5-dimethoxyphenethyl)acetamide (4m): 1H-NMR: 1.86 (s, 3H), 1.98 (broad s, 1H, OH), 2.65 (dt, J = 7.0, 14.0, 1H), 2.78 (dt, J = 7.0, 13.9, 1H), 3.06 (dd, J = 3.8, 6.6, 2H), 3.25 (dt, J = 7.0, 12.9, 1H), 3.44 (qd, J = 6.8, 6.9, 13.4, 1H), 3.86 (s, 3H), 3.89 (s, 3H), 5.01 (t, J = 6.7, 1H), 5.93(t, J = 4.9, 1H, NH), 6.60 (s, 1H), 7.05 (s, 1H), 7.17–7.30 (m, 5H); 13C-NMR: 170.4, 148.2, 147.8, 138.0, 133.8, 129.5, 128.5, 128.2, 126.6, 112.6, 109.6, 71.6, 55.9, 45.2, 40.7, 31.4, 23.0. Anal. calcd. for C20H25NO4: C, 69.95; H, 7.34; N, 4.08. Found: C, 70.17; H, 7.56; N, 3.89.
N-(2-(1-Hydroxy-2-phenylethyl)-4,5-dimethoxyphenethyl)benzamide (4n): 1H-NMR: 1.87 (broad s, 1H, OH), 2.65 (td, J = 4.7, 16.6, 1H), 2.88 (ddd, J = 6.3, 7.4, 13.6, 1H), 3.54–3.24 (m, 2H), overlapped with 3.45-3.47 (m, 2H), 3.78 (s, 3H), 3.83 (s, 3H), 5.83 (t, J = 5.7, 1H, NH), 5.98 (s, 1H), 6.59 (s, 1H), 6.82 (s, 1H), 7.17–7.14 (m, 2H), 7.37–7.22 (m, 8H); 13C-NMR: 171.4, 148.2, 147.6, 143.7, 134.8, 134.2, 129.4, 128.9, 128.7, 128.3, 127.2, 126.5, 113.1, 111.5, 72.4, 55.9, 55.8, 43.6, 40.7, 31.8. Anal. calcd. for C25H27NO4: C, 74.05; H, 6.71; N, 3.45. Found: C, 73.81; H, 6.43; N, 3.49.
3.3. Typical Procedure for the Preparation of Compounds 5
In a 100-mL round-bottomed flask fitted with a glass-rod, stirrer, reflux condenser and inlet for argon are placed magnesium turnings (0.36 g, 15 mmol) in dry diethyl ether (30 mL). The apparatus is flushed with argon. A slow stream of argon is introduced. About one-fifth of a solution of the corresponding halide (15 mmol) in dry ether (20 mL) is added to the vigorously stirred mixture. Reaction commences within 2–8 minutes and the remainder of the halide solution is then added steadily over about 12 minutes to the mixture. Stirring is continued for an additional 60 minutes followed by adding of solution of corresponding ketoamide 3 (3 mmol) in CH2Cl2 (10 mL). The reaction mixture is poured into water (the end of the reaction is proved with thin-layer chromatography) and extracted with CH2Cl2 (4 × 20 mL). If emulsion was formed, the saturated solution of ammonium chloride is added. The combined extracts were dried (Na2SO4) and concentrated. The corresponding product were isolated after column chromatography on silicagel with n-hexane:diethyl ether as eluent with 50–65% yields.
N-(2-(2-Hydroxypropan-2-yl)-4,5-dimethoxyphenethyl)acetamide (5a): 1H-NMR: 1.67 (s, 6H), 1.89 (s, 3H), 2.61 (broad s, 1H, OH), 3.14 (t, J = 7.1, 2H), 3.49 (dd, J = 12.4, 6.8, 2H), 3.87 (s, 6H), 5.31 (s, 1H, NH), 6.73 (s, 1H), 6.89 (s, 1H); 13C-NMR: 170.4, 147.7, 146.5, 138.0, 129.8, 114.7, 109.8, 56.0, 55.85, 42.1, 32.6, 29.4, 23.1. Anal. calcd. for C15H23NO4: C, 64.03; H, 8.24; N, 4.98. Found: C, 64.25; H, 8.05; N, 4.77.
N-(2-(2-Hydroxypropan-2-yl)-4,5-dimethoxyphenethyl)benzamide (5b): 1H-NMR: 1.71 (s, 6H), 2.74 (s, 1H, OH), 3.24 (t, J = 6.8, 2H), 3.69 (dd, J = 11.7, 6.9, 2H), 3.81 (s, 3H), 3.85 (s, 3H), 6.74 (s, 1H, NH), 6.86 (s, 1H), 7.26 (s, 1H), 7.33–7.42 (m, 3H), 7.69 (d, J = 1.6, 1H), 7.72 (t, J = 1.4, 1H); 13C-NMR: 167.6, 147.8, 146.6, 137.7, 134.6, 131.1, 129.98, 128.3, 126.9, 114.7, 109.8, 56.1, 55.8, 42.6, 32.7, 32.2. Anal. calcd. for C20H25NO4: C, 69.95; H, 7.34; N, 4.08. Found: C, 69.64; H, 7.33; N, 4.17.
N-(2-(2-Hydroxypropan-2-yl)-4,5-dimethoxyphenethyl)-2-phenylacetamide (5c): 1H-NMR: 1.58 (s, 6H), 1.88 (s, 1H, OH), 3.08 (t, J = 6.9, 2H), 3.49-3.53 (m, 4H), 3.85 (s, 3H), 3.88 (s, 3H), 6.68 (s, 1H, NH), 6.81 (s, 1H), 7.14 (s, 1H), 7.15–7.18 (m, 2H), 7.29–7.33 (m, 3H); 13C-NMR: 171.3, 147.7, 146.4, 137.9, 135.1, 129.7, 128.8, 127.1, 114.5, 109.6, 56.1, 55.8, 43.7, 42.1, 32.3, 31.9. Anal. calcd. for C21H27NO4: C, 70.56; H, 7.61; N, 3.92. Found: C, 70.34; H, 7.56; N, 4.22.
N-(2-(2-Hydroxy-1-phenylpropan-2-yl)-4,5-dimethoxyphenethyl)benzamide (5n): 1H-NMR: 1.65 (s, 3H), 2.65 (s, 1H, OH), 3.03 (td, J = 5.5, 13.2, 1H), 3.12(d, J = 13.2, 1H), 3.21 (d, J = 13.2, 1H), 3.50 (ddd, J = 6.2, 8.6, 14.4, 1H), 3.70 (tt, J = 4.5, 8.9, 2H), 3.78 (s, 3H), 3.83 (s, 3H), 6.70 (s, 1H), 6.76 (s, 1H), 7.08 (dd, J = 1.6, 8.2, 2H), 7.24-7.28 (m, 3H), 7.33 (t, J = 7.7, 2H) 7.41 (t, J = 7.4, 1H), 7.63 (t, J = 4.7, 1H, NH), 7.71 (dd, J = 1.1, 8.4, 2H); 13C-NMR: 167.5, 147.9, 146.5, 136.7, 136.3, 134.6, 131.1, 130.8, 130.3, 128.3, 128.2, 128.19, 127.0, 126.9, 114.5, 110.4, 76.2, 56.1, 55.7, 50.5, 42.8, 31.9, 30.1. Anal. calcd. for C26H29NO4: C, 74.44; H, 6.97; N, 3.34. Found: C, 74.25; H, 6.77; N, 3.53.
N-(2-(2-Hydroxybutan-2-yl)-4,5-dimethoxyphenethyl)benzamide (5p): 1H-NMR: 0.92 (t, J = 7.4, 3H), 1.71 (s, 3H), 1.76 (ddd, J = 0.9, 2.0, 6.6, 2H), 2.90 (dt, J = 4.6, 7.0, 2H), 3.70 (ddd, J = 5.7, 7.1, 19.5, 2H), 3.85 (s, 3H), 3.90 (s, 3H), 6.22 (broad s, 1H, NH), 6.64 (s, 1H), 6.75 (s, 1H), 7.42–7.49 (m, 3H), 7.70–7.79 (m, 2H); 13C-NMR: 167.5, 147.6, 138.6, 136.3, 131.4, 130.1, 128.6, 128.4, 126.8, 124.4, 112.5, 110.8, 106.0, 63.9, 56.2, 55.8, 41.4, 32.3, 18.8, 12.4. Anal. calcd. for C21H27NO4: C, 70.56; H, 7.61; N, 3.92. Found: C, 70.26; H, 7.43; N, 4.13.
N-(2-(1-Hydroxy-1-phenylethyl)-4,5-dimethoxyphenethyl)benzamide (5s): 1H-NMR: 1.98 (s, 3H), 2.68 (t, J = 6.9, 2H), 2.79 (dd, J = 7.3, 13.8, 1H), 3.27 (dt, J = 6.2, 13.4, 1H), 3.84 (s, 3H), 3.92 (s, 3H), 5.80 (d, J = 1.3,1H, NH) 6.77 (s, 1H), 7.15 (s, 1H), 7.24–7.48 (m, 10H); 13C-NMR: 167.6, 149.3, 148.8, 147.4, 146.3, 134.6, 131.4, 126.5, 125.3, 115.5, 114.6, 113.8, 112.7, 110.9, 56.2, 55.9, 41.4, 40.7, 33.6. Anal. calcd. for C25H27NO4: C, 74.05; H, 6.71; N, 3.45. Found: C, 74.32; H, 6.97; N, 3.37.
N-(2-(1-Hydroxy-1-phenylpropyl)-4,5-dimethoxyphenethyl)methanesulfonamide (5t): 1H-NMR: 0.90 (t, J = 7.3, 3H), 2.30 (dq, J = 7.3, 13.8, 2H), 2.53 (broad s, 1H, OH), 2.67 (s, 3H), 2.73 (dd, J = 4.9, 9.0, 2H), 2.86 (td, J = 5.9, 17.8, 1H), 2.94–3.04 (m, 1H), 3.88 (s, 3H), 3.94 (s, 3H), 4.60 (t, J = 5.3, 1H, NH), 6.66 (s, 1H), 7.18 (s, 1H), 7.20–7.32 (m, 5H); 13C-NMR: 148.2, 147.2, 146.5, 137.1, 130.4, 127.9, 126.7, 125.9, 114.7, 111.1, 105.9, 78.6, 56.2, 55.9, 44.4, 39.7, 36.2, 32.7, 8.1. Anal. calcd. for C20H27NO5S: C, 61.05; H, 6.92; N, 3.56; S, 8.15. Found: C, 60.81; H, 6.64; N, 3.65; S, 7.83.
3.4. Cyclization of 4 and 5 to the Corresponding Isoquinolines 6 and 7
To solution of the corresponding compound 4 or 5 (1 mmol) in dichloromethane (15 mL) a catalytic amount of p-toluensulfonic acid (PTSA) was added. The solution was stirred 30 min at room temperature, then the solution was filtered on a short column with neutral Al2O3. The products 6 or 7, after evaporation of the solvent, were obtained with 88–90% yields. When the substituent at the C-1 is ethyl or benzyl, the styrene products 8 were formed predominantly (~60%) than expected cyclic 1,1-disubtituted product 7 (~30%).
1-(6,7-Dimethoxy-1-methyl-3,4-dihydroisoquinolin-2(1H)-yl)ethanone (6а): known compound [49,50,51].
(6,7-Dimethoxy-1-methyl-3,4-dihydroisoquinolin-2(1H)-yl)(phenyl)methanone (6b): 1H-NMR (600 MHz): 1.60 (d, J = 6.7, 3H), 2.63 (d, J = 16.7, 1H), 2.94 (ddd, J = 5.3, 12.0, 17.3, 1H), 3.16 (td, J = 14.0, 30.6, 1H), 3.44 (dt, J = 3.2, 13.1, 1H), 3.88 (s, 6H), 5.72 (q, J = 5.7, 1H), 6.61 (s, 1H), 6.69 (s, 1H), 7.43–7.45 (m, 5H); 13C-NMR: 170.2, 147.9, 147.7, 136.7, 136.6, 129.5, 128.6, 126.6, 111.2, 109.8, 56.0, 55.9, 53.4, 40.9, 29.2, 21.5. Anal. calcd. for C19H21NO3: C, 73.29; H, 6.80; N, 4.50. Found: C, 72.95; H, 6.98; N, 4.45.
1-(6,7-Dimethoxy-1-methyl-3,4-dihydroisoquinolin-2(1H)-yl)-2-phenylethanone (6c): 1H-NMR (600 MHz): 1.40 (d, J = 6.8, 1H), 1.46 (d, J = 6.8, 3H), 2.52–2.60 (m, 2H), 3.40 (ddd, J = 5.0, 10.5, 13.5, 1H), 3.83 (d, J = 4.3, 2H), 3.85 (s, 3H), 3.87 (s, 3H), 5.63 (q, J = 6.8, 1H), 6.53 (s, 1H), 6.62 (s, 1H), 7.23–7.36 (m, 5H); 13C-NMR: 169.3, 147.9, 147.5, 135.2, 130.4, 128.9, 128.7, 126.8, 111.0, 109.8, 56.0, 55.9, 52.2, 41.8, 40.0, 28.8, 21.4. Anal. calcd. for C20H23NO3: C, 73.82; H, 7.12; N, 4.30. Found: C, 73.57; H, 7.31; N, 4.34.
Ethyl 6,7-dimethoxy-1-methyl-3,4-dihydroisoquinoline-2(1H)-carboxylate (6d): known compound [52,53,54,55].
6,7-dimethoxy-1-methyl-2-(methylsulfonyl)-1,2,3,4-tetrahydroisoquinoline (6e): known compound [56].
6,7-dimethoxy-1-methyl-N-phenyl-3,4-dihydroisoquinoline-2(1H)-carboxamide (6f): known compound [57].
1-(6,7-dimethoxy-1-phenyl-3,4-dihydroisoquinolin-2(1H)-yl)ethanone (6g): known compound [58,59,60].
(6,7-dimethoxy-1-phenyl-3,4-dihydroisoquinolin-2(1H)-yl)(phenyl)methanone (6h): known compound [58,59,60].
1-(6,7-dimethoxy-1-phenyl-3,4-dihydroisoquinolin-2(1H)-yl)-2-phenylethanone (6i): 1H-NMR (600 MHz): 2.61 (dd, J = 4.1, 7.7, 2H), 3.33 (td, J = 8.7, 14.3, 1H), 3.78 (s, 3H), overlapped with 3.74–3.79 (m, 1H), 3.83 (d, J = 2.0, 2H), 3.89 (s, 3H), 6.56 (s, 1H), 6.62 (s, 1H), 6.95 (s, 1H), 7.22–7.34 (m, 10H); 13C-NMR: 169.99, 147.6, 142.7, 142.5, 135.1, 128.8, 128.7, 128.2, 127.4, 126.7, 126.3, 111.4, 111.0, 92.2, 55.96, 55.88, 54.7, 41.4, 39.8, 28.4. Anal. calcd. for C25H25NO3: C, 77.49; H, 6.50; N, 3.61; Found: C, 77.68; H, 6.75; N, 3.56.
Ethyl 6,7-dimethoxy-1-phenyl-3,4-dihydroisoquinoline-2(1H)-carboxylate (6j): known compound [61,62,63].
6,7-Dimethoxy-2-(methylsulfonyl)-1-phenyl-1,2,3,4-tetrahydroisoquinoline (6k): 1H-NMR: 2.64 (s, 3H), 2.76 (ddd, J = 1.7, 4.5, 16.8, 1H), 3.09 (ddd, J = 6.3, 11.7, 16.3, 1H), 3.29 (ddd, J = 4.6, 11.8, 13.6, 1H), 3.77 (s, 3H), 3.82-3.91 (m, 1H), 3.92 (s, 3H), 6.02 (s, 1H), 6.48 (s, 1H), 6.71 (s, 1H), 7.27–7.36 (m, 5H); 13C-NMR: 148.5, 147.8, 140.9, 128.9, 128.5, 128.0, 125.8, 125.7, 111.5, 110.9, 58.7, 56.0, 55.9, 39.6, 38.5, 21.1. Anal. calcd. for C18H21NO4S: C, 62.23; H, 6.09; N, 4.03; S, 9.23. Found: C, 62.03; H, 6.27; N, 4.09; S, 9.15.
6,7-Dimethoxy-N,1-diphenyl-3,4-dihydroisoquinoline-2(1H)-carboxamide (6l): 1H-NMR (600 MHz): 1.56 (s, 1H), 2.71 (d, J = 16.4, 1H), 2.87 (dd, J = 7.3, 15.9, 1H), 3.51 (ddd, J = 2.9, 6.7, 11.7, 1H), 3.71 (s, 3H), 3.81 (s, 3H), 6.35 (s, 1H), 6.45 (broad s, 1H, NH), 6.55 (s, 1H), 6.61 (s, 1H), 6.95 (t, J = 7.2, 1H), 7.18–7.23 (m, 4H), 7.22–7.27 (m, 5H); 13C-NMR: 154.9, 148.1, 147.6, 142.7, 139.1, 128.9, 128.6, 127.9, 127.8, 127.6, 126.9, 123.1, 120.0, 111.2, 111.1, 57.4, 56.1, 56.0, 40.0, 28.1. Anal. calcd. for C24H24N2O3: C, 74.21; H, 6.23; N, 7.21. Found: C, 74.43; H, 6.45; N, 7.13.
1-(1-Benzyl-6,7-dimethoxy-3,4-dihydroisoquinolin-2(1H)-yl)ethanone (6m): 1H-NMR: 1.63 (s, 3H), 2.84 (ddd, J = 5.6, 10.1, 14.8, 2H), 3.09–3.17 (m, 2H), 3.50 (ddd, J = 3.4, 7.8, 10.8, 1H), 3.69 (td, J = 5.6, 12.9, 1H), 3.87 (s, 3H), 3.89 (s, 3H), 5.68 (dd, J = 5.3, 8.4, 1H), 6.14 (s, 1H), 6.48 (s, 1H), 7.17–7.27 (m, 5H); 13C-NMR: 169.5, 148.2, 147.3, 138.2, 129.6, 128.7, 128.2, 126.7, 125.5, 110.9, 110.1, 59.3, 56.0, 55.8, 43.2, 35.1, 28.6, 22.0. Anal. calcd. for C20H23NO3: C, 73.82; H, 7.12; N, 4.30. Found: C, 73.99; H, 7.05; N, 4.25.
(1-Benzyl-6,7-dimethoxy-3,4-dihydroisoquinolin-2(1H)-yl)(phenyl)methanone (6n): known compound [57].
1-(6,7-Dimethoxy-1,1-dimethyl-3,4-dihydroisoquinolin-2(1H)-yl)ethanone (7a): 1H-NMR: 1.82 (s, 6H), 2.21 (s, 3H), 2.79 (t, J = 5.5, 2H), 3.56 (dt, J = 5.5, 3.8, 2H), 3.87 (s, 3H), 3.89 (s, 3H), 6.57 (s, 1H), 6.77 (s, 1H); 13C-NMR: 170.3, 147.9, 147.1, 137.0, 126.3, 110.5, 109.6, 59.85, 56.1, 55.8, 44.1, 30.4, 27.99, 25.6. Anal. calcd. for C15H21NO3: C, 68.42; H, 8.04; N, 5.32. Found: C, 68.65; H, 8.18; N, 5.14.
(6,7-Dimethoxy-1,1-dimethyl-3,4-dihydroisoquinolin-2(1H)-yl)(phenyl)methanone (7b): 1H-NMR: 1.96 (s, 6H), 2.80 (t, J = 5.5, 2H), 3.52 (dt, J = 5.5, 3.5, 2H), 3.89 (s, 3H), 3.92 (s, 3H), 6.59 (s, 1H), 6.84 (s, 1H), 7.42–7.46 (m, 3H), 7.48–7.52 (m, 2H); 13C-NMR: 172.6, 147.9, 147.2, 138.9, 136.8, 129.6, 128.5, 126.7, 126.1, 110.8, 109.7, 59.9, 56.1, 55.9, 45.3, 30.4, 27.7; Anal. calcd. for C20H23NO3: C, 73.82; H, 7.12; N, 4.30. Found: C, 73.87; H, 7.05; N, 4.57.
1-(6,7-Dimethoxy-1,1-dimethyl-3,4-dihydroisoquinolin-2(1H)-yl)-2-phenylethanone (7c): 1H-NMR: 1.84 (s, 6H), 2.49 (t, J = 5.3, 2H), 3.47 (dt, J = 4.8, 6.0, 2H), 3.82 (s, 3H), overlapped with 3.83 (s, 2Н), 3.86 (s, 3H), 6.47 (s, 1H), 6.75 (s, 1H), 7.23–7.27 (m, 2H), 7.30–7.33 (m, 3H); 13C-NMR: 170.9, 147.8, 147.1, 136.8, 135.6, 128.6, 128.4, 126.6, 126.3, 110.5, 109.6, 60.1, 56.1, 55.8, 44.8, 43.8, 30.1, 27.9; Anal. calcd. for C21H25NO3: C, 74.31; H, 7.42; N, 4.13. Found: C, 74.26; H, 7.38; N, 4.20.
Ethyl 6,7-dimethoxy-1,1-dimethyl-3,4-dihydroisoquinoline-2(1H)-carboxylate (7d): 1H-NMR: 1.32 (dd, J = 6.4, 13.5, 3H), 1.79 (s, 6H), 2.75 (t, J = 5.5, 2H), 3.73–3.77 (m, 2H), 3.88 (s, 3H), 3.89 (s, 3H), 4.19 (q, J = 7.12, 7.09, 2H), 6.57 (s, 1H), 6.78 (s, 1H); 13C-NMR: 181.6, 147.7, 147.1, 136.5, 127.0, 110.6, 109.8, 60.8, 58.6, 56.1, 55.8, 47.8, 30.2, 28.8, 14.6. Anal. calcd. for C16H23NO4: C, 65.51; H, 7.90; N, 4.77. Found: C, 65.84; H, 7.76; N, 4.80.
6,7-Dimethoxy-1,1-dimethyl-2-(methylsulfonyl)-1,2,3,4-tetrahydroisoquinoline (7e): 1H-NMR: 1.86 (s, 6H), 2.81 (dd, J = 5.0, 6.0, 2H), 2.98 (s, 3H), 3.67–3.62 (m, 2H), 3.86 (s, 3H), 3.87 (s, 3H), 6.54 (s, 1H), 6.71 (s, 1H); 13C-NMR: 147.8, 147.5, 135.6, 125.9, 110.9, 109.3, 61.4, 56.2, 55.8, 43.0, 42.6, 30.4, 30.0. Anal. calcd. for C14H21NO4S: C, 56.16; H, 7.07; N, 4.68; S, 10.71. Found: C, 56.36; H, 7.18; N, 4.63; S, 10.97.
(1-Benzyl-6,7-dimethoxy-1-methyl-3,4-dihydroisoquinolin-2(1H)-yl)(phenyl)methanone (7n): 1H-NMR: 2.09 (s, 3H), 2.27 (ddd, J = 2.7, 4.8, 15.2, 1H), 3.07 (ddd, J = 8.0, 12.6, 22.8, 1H), 3.21 (td, J = 4.1, 13.3, 1H), 3.88 (s, 3H), 3.97 (s, 3H), 4.56 (d, J = 13.3, 1H), 6.42 (s, 1H), 6.68 (dd, J = 1.5, 8.6, 2H), 7.00 (s, 1H), 7.05 (t, J = 7.5, 2H), 7.11–7.13 (m, 1H), 7.24 (d, J = 5.6, 2H), 7.35–7.38 (m, 3H); 13C-NMR: 172.4, 147.9, 147.3, 139.1, 137.8, 133.7, 130.1, 129.3, 129.0, 128.5, 127.5, 127.4, 126.4, 126.2, 126.15, 110.2, 109.8, 64.2, 56.3, 56.2, 45.9, 45.3, 29.7, 26.4. Anal. calcd. for C26H27NO3: C, 77.78; H, 6.78; N, 3.49. Found: C, 77.53; H, 6.87; N, 3.52.
1-(1-Ethyl-6,7-dimethoxy-1-methyl-3,4-dihydroisoquinolin-2(1H)-yl)ethanone (7o): 1H-NMR: 0.55 (t, J = 7.4, 3H), 1.65 (dt, J = 7.3, 14.6, 1H), 1.78 (s, 3H), 2.24–2.26 (m, 1H), 2.70 (ddd, J = 3.4, 5.3, 15.6, 1H), 2.86 (ddd, J = 4.9, 10.1, 13.0, 1H), 3.29 (dd, J = 7.4, 14.1, 1H), 3.42 (ddd, J = 3.3, 9.4, 13.0, 1H), 3.89 (s, 6H), 6.58 (s, 1H), 6.76 (s, 1H); 13C-NMR: 185.6, 148.0, 147.0, 134.6, 128.1, 110.2, 109.2, 63.9, 56.1, 55.8, 44.7, 33.4, 26.7, 25.5, 8.5. Anal. calcd. for C16H23NO3: C, 69.29; H, 8.36; N, 5.05. Found: C, 69.13; H, 8.48; N, 5.12.
(1-Ethyl-6,7-dimethoxy-1-methyl-3,4-dihydroisoquinolin-2(1H)-yl)(phenyl)methanone (7p): 1H-NMR: 0.66 (t, J = 7.3, 3H), 1.77 (dd, J = 7.2, 14.1, 1H), 1.93 (s, 3H), 2.60 (ddd, J = 3.1, 4.0, 15.3, 1H), 2.94 (ddd, J = 3.6, 10.5, 14.5, 1H), 3.31 (ddd, J = 2.8, 10.5, 13.3, 1H), 3.43 (dd, J = 7.2, 14.1, 1H), 3.77 (td, J = 4.0, 13.3, 1H), 3.90 (s, 3H), 3.93 (s, 3H), 6.59 (s, 1H), 6.81 (s, 1H), 7.43–7.51 (m, 5H); 13C-NMR: 172.0, 148.0, 147.2, 139.0, 129.4, 128.5, 128.0, 126.6, 110.5, 109.3, 63.8, 56.1, 55.8, 45.8, 33.0, 30.3, 26.4, 8.5. Anal. calcd. for C21H25NO3: C, 74.31; H, 7.42; N, 4.13. Found: C, 74.11; H, 7.64; N, 4.03.
1-Ethyl-6,7-dimethoxy-1-methyl-2-(methylsulfonyl)-1,2,3,4-tetrahydroisoquinoline (7r): 1H-NMR: 0.72 (t, J = 7.3, 3H), 1.77 (dd, J = 8.1, 15.4, 1H), 1.84 (s, 3H), 2.74–2.80 (m, 2H), 2.83-2.89 (m, 1H), 2.99 (s, 3H), 3.42 (ddd, J = 3.4, 8.8, 12.3, 1H), 3.72 (ddd, J = 4.1, 5.7, 12.4, 1H), 3.88 (s, 6H), 6.56 (s, 1H), 6.69 (s, 1H); 13C-NMR: 148.0, 147.4, 133.4, 127.6, 110.6, 108.9, 65.5, 56.2, 55.8, 43.6, 41.2, 36.2, 30.2, 28.2, 8.6. Anal. calcd. for C15H23NO4S: C, 57.48; H, 7.40; N, 4.47; S, 10.23. Found: C, 57.24; H, 7.62; N, 4.54; S, 10.35.
(6,7-Dimethoxy-1-methyl-1-phenyl-3,4-dihydroisoquinolin-2(1H)-yl)(phenyl)methanone (7s): 1H-NMR: 2.26 (s, 3H), 2.85 (ddd, J = 3.1, 5.4, 15.4, 1H), 3.21 (ddd, J = 4.1, 9.4, 14.7, 1H), 3.59–3.69 (m, 1H), overlapped with 3.64 (s, 3H), 3.88 (s, 3H), 3.97 (ddd, J = 4.3, 5.2, 13.3, 1H), 6.28 (s, 1H), 6.61 (s, 1H), 7.39-7.45 (m, 5H), 7.47-7.51 (m, 5H). Anal. calcd. for C25H25NO3: C, 77.49; H, 6.50; N, 3.61. Found: C, 77.14; H, 6.73; N, 3.65.
1-Ethyl-6,7-dimethoxy-2-(methylsulfonyl)-1-phenyl-1,2,3,4-tetrahydroisoquinoline (7t): 1H-NMR: 0.80 (t, J = 7.2, 3H), 1.90 (s, 3H), 1.98 (dd, J = 6.6, 13.8, 1H), 2.82 (td, J = 2.7, 15.4, 1H), 3.14 (ddd, J = 4.1, 11.6, 15.6, 1H), 3.30 (dt, J = 2.4, 11.1, 11.3, 1H), 3.59 (s, 3H), 3.64 (dd, J = 7.3, 13.8, 1H), 3.91 (s, 3H), 4.05 (td, J = 3.8, 7.1, 1H), 6.09 (s, 1H), 6.61 (s, 1H), 7.29–7.40 (m, 3H), 7.48–7.53 (m, 2H); 13C-NMR: 148.0, 147.5, 144.0, 133.3, 128.9, 128.2, 128.1, 127.8, 111.2, 109.5, 69.1, 56.0, 55.8, 43.2, 37.1, 34.5, 30.0, 6.3. Anal. calcd. for C20H25NO4S: C, 63.97; H, 6.71; N, 3.73; S, 8.54. Found: C, 64.41; H, 6.88; N, 3.76; S, 8.14.
N-(2-(but-2-en-2-yl)-4,5-dimethoxyphenethyl)benzamide (8g): 1H-NMR: 1.76 (ddd, J = 0.9, 2.0, 6.6, 1H), 1.96 (td, J = 1.2, 2.2, 1H), 2.90 (dt, J = 4.5, 7.01, 2H), 3.29 (tdd, J = 6.2, 12.7, 19.5, 2H), 3.85 (s, 3H), 3.89 (s, 3H), 5.41 (dq, J = 1.45, 6.7, 1H), 6.22 (broad s, 1H, NH), 6.40 (s, 1H), 6.75 (s, 1H), 7.42–7.49 (m, 3H), 7.71–7.75 (m, 2H); 13C-NMR: 167.5, 147.9, 147.2, 147.1, 138.6, 136.3, 131.4, 128.6, 128.5, 127.0, 124.4, 112.5, 112.4, 56.2, 55.9, 41.4, 32.4, 18.8, 13.9. Anal. calcd. for C21H25NO3: C, 74.31; H, 7.42; N, 4.13. Found: C, 74.12; H, 7.54; N, 4.15.
N-(4,5-dimethoxy-2-(1-phenylprop-1-enyl)phenethyl)benzamide (8n): 1H-NMR: 2.22 (d, J = 1.1, 3H), 2.95 (t, J = 7.1, 2H), 3.70 (dd, J = 6.9, 13.1, 2H), 3.84 (s, 3H), 3.89 (s, 3H), 6.23 (t, J = 4.7, 1H, NH), 6.39 (s, 1H), 6.73 (s, 1H), 6.78 (s, 1H), 7.34–7.39 (m, 7H, Ar), 7.44–7.47 (m, 1H, Ar), 7.68 (dd, J = 1.2, 8.4, 2H); 13C-NMR: 167.4, 147.9, 147.3, 138.4, 137.6, 131.4, 129.9, 128.9, 128.5, 128.3, 128.2, 127.5, 126.8, 126.7, 126. 66, 112.5, 111.8, 56.0, 55.8, 41.4, 32.5, 21.1. Anal. calcd. for C26H27NO3: C, 77.78; H, 6.78; N, 3.49. Found: C, 77.85; H, 6.89; N, 3.55.
N-(4,5-dimethoxy-2-(1-phenylvinyl)phenethyl)benzamide (8s): 1H-NMR: 2.69 (t, J = 7.2, 2H), 3.52 (dd, J = 7.0, 12.9, 2H), 3.88 (s, 3H), 3.89 (s, 3H), 5.25 (d, J = 1.3, 1H), 5.81 (d, J = 1.4, 1H), 6.00 (t, J = 6.1, 1H, NH), 6.80 (s, 1H), 6.81 (s, 1H), 7.28–7.31 (m, 4H), 7.38–7.50 (m, 4H), 7.65–7.69 (m, 2H); 13C-NMR: 167.3, 148.8, 148.5, 147.3, 140.7, 134.6, 133.8, 131.3, 129.0, 128.5, 127.9, 126.7, 126.5, 115.5, 114.6, 113.8, 112.7, 56.0, 55.9, 40.8, 32.7. Anal. calcd. for C25H25NO3: C, 77.49; H, 6.50; N, 3.61. Found: C, 77.64; H, 6.65; N, 3.55.
N-(4,5-dimethoxy-2-(1-phenylprop-1-enyl)phenethyl)methanesulfonamide (8t): 1H-NMR: 0.81 (t, J = 7.3, 3H), 2.09 (ddd, J = 6.2, 8.6, 12.6, 2H), 2.97 (s, 3H), 3.23 (td, J = 1.7, 7.1, 2H), 3.46–3.50 (m, 1H), 3.86 (s, 3H), 3.88 (s, 3H), 6.43 (s, 1H), 6.71 (s, 1H), 6.82–6.86 (m, 2H), 7.05-7.13 (m, 3H); 13C-NMR: 147.9, 147.3, 139.1, 137.8, 133.7, 130.1, 129.3, 129.0, 128.5, 127.4, 126.4, 126.2, 110.2, 109.8, 56.2, 55.9, 45.9, 45.3, 29.7, 26.4. Anal. calcd. for C20H25NO4S: C, 63.97; H, 6.71; N, 3.73; S, 8.54. Found: C, 63.76; H, 6.81; N, 3.77; S, 8.24.
3.5. Estimation of Contractile Activity of Some of the Newly Synthesized Compounds
3.5.1. Collection and Preparation of Tissue Samples
Guinea-pigs (350 ± 50g) were used. Whole mount muscle preparations without mucosa were obtained from gastric corpus. The tissue was pinned flat in a dissecting dish containing preparation solution containing (mmol/L) Na+ - 143; K+ - 5.84; Ca2+ - 3.7 and preparations were cut longitudinal muscle fibres with a final size of (13.0 ± 1.5−1.0 ± 1.5) mm. Tissue samples were immediately rinsed with cooled (4 °C) preparation solution. The muscle preparations were suspended in fort individual organ baths containing 15 mL modified Krebs’ solution (KS) containing (mmol/L) Na+ - 143, K+ - 5.84, Ca2+ - 2.5, Mg2+ - 1.19, Cl− - 133, HCO3− - 16.7, H2PO4− - 1.2 and 11.5 glucose (35.5 ± 0.25 °C) each and constantly oxygenated with 95% O2 and 5% CO2. The preparations were connected to an isometric force transducer (TRI 201, LSi LETICA; Pnlab s.l., Barcelona, Spain). Preparations were allocated to the organ baths in random manner and were allowed an equilibration period of 1 h. Muscle tension was preset to 7 mN g in one step during the equilibration time. The mechanical activity was amplified with 4-channels tensiometrical interface system for registration and investigation of spontaneous muscle contractility of the muscle strips.
3.5.2. Estimation of SM Contractile Activity after Applications of Newly Synthesized Compounds
Preparations from 3 to 5 guinea-pigs were used for each experiment of studying of effect of application of isoquinoline derivatives. Basal tone (BT), frequency, mean amplitude (Amean) and area under the curve (AUC) were analysed after the equilibration time for a 5-min period. These values were defined as predrug (baseline period) and were used for further comparative analysis.
Again, at the end of each trial, the organ baths were flushed and 1 × 10−5 M acetylcholine was added to test the ability of the preparations to exert a contractile response after activation of cholinergic receptors. For each trial, four preparations from the same animal were used and compounds were assigned to the organ baths in random order.
The signal digitalisations ware achieved by 13 bit analogue to digital converter based on microcontroller. A logical level synchronization was developed by special controller for parallel PC port communication. The calibration curve was used to define the range of application of registration system 0–20 mN.
Special Visual-Basic program was assembled to visualization of spontaneous smooth muscle activity with options to dynamically alteration of amplification, offset value and printing ability of smooth-muscle parameter. It is possible to build in the first deviation of the signals and saving of incoming data in 4 dimensional matrixes with appropriated format to statistical analysis.
3.5.3. Parameters, Data Analysis and Statistics
The following parameters were analysed to describe contractility parameters for each application: BT, Amean, AUC and frequency. Basal tone of the muscle has been measured because an increase in this parameter can be independent of Amean of contractions or frequency of contractions. In addition to an increase or decrease in BT, changes in frequency of contractions or Amean indicate changes in contractility due to the drugs used. The variables were calculated by using the software ChartTM included in the PowerLab from ADInstruments Ltd, Australia. All results were expressed as percentage of the corresponding predrug measurement.
Statistical analysis was performed using Statistica 4.5 (StaSoft, Inc. Microsoft), SPSS Inc., Chicago, IL, USA, Excel VB for applications end PraphPad. Data were subjected to descriptive and comparative analyses.
Data of are presented as mean and standard error (SEM), and 25 and 75% percentiles. Wilcoxon signed rank test was used to compare predrug between solvent and drug. In case of no significant difference in predrug between specimens used for solvent and drug, further calculations were performed. Differences within the results were analysed by the Friedman test. If Friedman analysis revealed significant differences for compound and the corresponding control, Wilcoxon signed rank test was used.
4. Conclusions
In conclusion, new isoquinoline derivatives were synthesized, as type of compounds found in nature and among bioactive compounds of interest. We have developed a convenient method for their synthesis by interaction of ketoamides with organomagnesium compounds, followed by cyclization in acidic medium with a catalytic amount of PTSA. A variety of substituents at the C-1 in the isoquinoline skeleton can be readily introduced. The estimation of contractile activity against smooth muscle preparations showed that some of the obtained compounds, especially 1,1-dialkyl 1,2,3,4-tetrahydroisoquinolines with sulfonamide substitutent possess the most pronounced effect.
Acknowledgments
We acknowledge financial support from the National Fund for Scientific Research DO-02-195.
Footnotes
Sample Availability: Samples of the compounds are available from the authors.
References
- 1.Rodger I.W., Hersom A.S., Waigh R.D. Actions of two dopamine derivatives at adreno- and cholinoceptors. J. Med. Chem. 1979;22:117–119. doi: 10.1021/jm00187a027. [DOI] [PubMed] [Google Scholar]
- 2.Lundstrom J. In: The Alkaloids. Brossi A., editor. Vol. 21. Academic Press; New York, NY, USA: 1983. p. 255. [Google Scholar]
- 3.Collins M.A. In: The Alkaloids. Brossi A., editor. Vol. 21. Academic Press; New York, NY, USA: 1983. p. 329. [Google Scholar]
- 4.Chrzanowska M., Schönenberg B., Brossi A., Flippen-Anderson J.L. Mammalian alkaloids: Configurations of optically active salsoline- and 3′,4′-Dideoxynorlaudanosoline-1-carboxylic acids. Helv. Chim. Acta. 1987;70:1721–1731. doi: 10.1002/hlca.19870700707. [DOI] [Google Scholar]
- 5.Czarnocki Z., Suh D., MacLean D.B., Hultin P.G., Szarek W.A. Enantioselective synthesis of 1-substituted tetrahydroisoquinoline-1-carboxylic acids. Can. J. Chem. 1992;70:1555–1561. doi: 10.1139/v92-191. [DOI] [Google Scholar]
- 6.Corey E.J., Gin D. A convergent enantioselective synthesis of the tetrahydroisoquinoline unit in the spiro ring of ecteinascidin 743. Tetrahedron Lett. 1996;37:7163–7166. doi: 10.1016/0040-4039(96)01622-X. [DOI] [Google Scholar]
- 7.Zhou B., Guo J., Danishefsky S.J. Studies directed to the total synthesis of ET 743 and analogues thereof: An expeditious route to the ABFGH subunit. Org. Lett. 2002;4:43–46. doi: 10.1021/ol016844k. [DOI] [PubMed] [Google Scholar]
- 8.Scott J.D., Williams R.M. Chemistry and biology of the tetrahydroisoquinoline antitumor antibiotics. Chem. Rev. 2002;102:1669–1730. doi: 10.1021/cr010212u. [DOI] [PubMed] [Google Scholar]
- 9.Menchaca R., Martínez V., Rodríguez A., Rodríguez N., Flores M., Gallego P., Manzanares I., Cuevas C. Synthesis of natural ecteinascidins (ET-729, ET-745, ET-759B, ET-736, ET-637, ET-594) from cyanosafracin B. J. Org. Chem. 2003;68:8859–8866. doi: 10.1021/jo034547i. [DOI] [PubMed] [Google Scholar]
- 10.Blaskó G. In: The Alkaloids. Brossi A., editor. Vol. 48. Academic Press; New York, NY, USA: 1990. p. 249. [Google Scholar]
- 11.Sousek J., Guedon G., Adam T., Bochorakova H., Taborska E., Valka I., Simanek V. Alkaloids and organic acids content of eight Fumaria species. Phytochem. Anal. 1999;10:6–11. doi: 10.1002/(SICI)1099-1565(199901/02)10:1<6::AID-PCA431>3.0.CO;2-0. [DOI] [Google Scholar]
- 12.Craig P., Nabenhauer F., Williams P., Macko E., Toner J. Tetrahydroisoquinolines. I. 1-Alkyl-6,7-dihydroxy-1,2,3,4-tetrahydroisoquinolines. J. Am. Chem. Soc. 1952;74:1316–1317. doi: 10.1021/ja01125a051. [DOI] [Google Scholar]
- 13.Holtz P.H. Introductory remarks. Pharm. Rev. 1966;18:85–88. [Google Scholar]
- 14.Yamato E., Hirakura M., Sugasawa S. Synthesis of 6,7-dihydrox-1,2,3,4-tetrahydroisoquinoline derivatives. Tetrahedron. 1966;22:129–134. doi: 10.1016/S0040-4020(01)82177-3. [DOI] [PubMed] [Google Scholar]
- 15.Houston J., Rodger I. Actions of the sympathomimetic bronchodilator, trimetoquinol (AQL 208) on the cardiac, respiratory and skeletal muscle systems in the anaesthetized cat, and on cat isolated atrial and tracheal preparations. Clin. Exp. Pharmacol. Physiol. 1974;1:401–413. doi: 10.1111/j.1440-1681.1974.tb00562.x. [DOI] [Google Scholar]
- 16.Ohkubo M., Kuno A., Katsuta K., Ueda Y., Shirakawa K., Nakanishi H., Nakanishi I., Kinoshita T., Takasugi H. Studies on cerebral protective agents. IX. Synthesis of novel 1,2,3,4-Tetrahydroisoquinolines as N-Methyl-D-aspartate antagonists. Chem. Pharm. Bull. 1996;44:95–102. doi: 10.1248/cpb.44.95. [DOI] [PubMed] [Google Scholar]
- 17.Thompson W.J., Anderson P.S., Britcher S.F., Lyle T.A., Thies J.E., Magill C.A., Varga S.L., Schwering J.E., Lyle P.A., Christy M.E., et al. Synthesis and pharmacological evaluation of a series of dibenzo[a,d]cycloalkenimines as N-methyl-D-aspartate antagonists. J. Med. Chem. 1990;33:789–808. doi: 10.1021/jm00164a052. [DOI] [PubMed] [Google Scholar]
- 18.Olney J., Price M., Salles K.S., Labruyere J., Friedrich G. MK-801 powerfully protects against N-methyl aspartate neurotoxicity. Eur. J. Pharmacol. 1987;141:357–361. doi: 10.1016/0014-2999(87)90552-8. [DOI] [PubMed] [Google Scholar]
- 19.McDonald J.W., Silverstein F.S., Johnston M.V. MK-801 protects the neonatal brain from hypoxic-ischemic damage. Eur. J. Pharmacol. 1987;140:359–361. doi: 10.1016/0014-2999(87)90295-0. [DOI] [PubMed] [Google Scholar]
- 20.Gill R., Brazell C., Woodruff G.N., Kemp J.A. The neuroprotective action of dizocilpine (MK-801) in the rat middle cerebral artery occlusion model of focal ischaemia. Br. J. Pharmacol. 1991;103:2030–2036. doi: 10.1111/j.1476-5381.1991.tb12371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirobe M., Ohta S., Masukawa Y. Use of 1,1-dialkyl-1,2,3,4-tetrahydroisoquinolines for the manufacture of a medicament for the treatment of psychosis and pain. WO9736588. WIPO Patent Application. 1997 Kind Code: A2.
- 22.Francisco M., Nasser A.L., Lopes L. Tetrahydroisoquinoline alkaloids and 2-deoxyribonolactones from Aristolochia arcuata. Phytochemistry. 2003;62:1265–1270. doi: 10.1016/S0031-9422(02)00655-6. [DOI] [PubMed] [Google Scholar]
- 23.Kubota H., Watanabe T., Kakefuda A., Masuda N., Wada K., Ishii N., Sakamoto S., Tsukamoto S. Synthesis and pharmacological evaluation of N-acyl-1,2,3,4-tetrahydroisoquinoline derivatives as novel specific bradycardic agents. Bioorg. Med. Chem. 2004;12:871–882. doi: 10.1016/j.bmc.2003.12.032. [DOI] [PubMed] [Google Scholar]
- 24.Horiguchi Y., Kodama H., Nakamura M., Yoshimura T., Hanezi K., Hamada H., Saritoh T., Sano T. A convenient synthesis of 1,1-disubstituted 1,2,3,4-tetrahydroisoquinolines via Pictet-Spengler reaction using titanium(IV) isopropoxide and acetic-formic anhydride. Chem. Pharm. Bull. 2002;50:253–257. doi: 10.1248/cpb.50.253. [DOI] [PubMed] [Google Scholar]
- 25.Kumpaty H., Bhattacharyya S., Rehr E., Gonzalez A. Selective access to secondary amines by a highly controlled reductive mono-n-alkylation of primary amines. Synthesis. 2003:2206–2210. [Google Scholar]
- 26.Hegedüs A., Hell Z. One-step preparation of 1-substituted tetrahydroisoquinolines via the Pictet-Spengler reaction using zeolite catalysts. Tetrahedron Lett. 2004;45:8553–8555. doi: 10.1016/j.tetlet.2004.09.097. [DOI] [Google Scholar]
- 27.Liu S.-Y., Lo M., Fu G. The synthesis of an enantiopure planar-chiral Lewis acid complex via kinetic resolution and its application in stereoselective additions to imines. Tetrahedron. 2006;62:11343–11349. doi: 10.1016/j.tet.2006.06.048. [DOI] [Google Scholar]
- 28.Walker J.B., Walker M.S. The enzymatic reduction of hydroxyguanidine. J. Biol. Chem. 1959;234:1481–1484. [PubMed] [Google Scholar]
- 29.Bailey D., DeGrazia G., Lape H., Frering R., Fort D., Skulan T. Hydroxyguanidines. New class of antihypertensive agents. J. Med. Chem. 1973;16:151–156. doi: 10.1021/jm00260a015. [DOI] [PubMed] [Google Scholar]
- 30.Lee J., Chae J., Kim C., Kim J., Lim D., Lee J., Shon M., Jo D. Quinazoline deriviates for treating peptic ulcer. US Patent. :1997. [Google Scholar]
- 31.Gray A., Reit E., Ackerly J., Hava M. Conformational requirements for direct adrenergic stimulation. J. Med. Chem. 1973;16:1023–1027. doi: 10.1021/jm00267a014. [DOI] [PubMed] [Google Scholar]
- 32.Kałuza Z., Mostowicz D., Dołęga G., Wójcik R. A new route to optically pure highly functionalized tetrahydro-isoquinolines with a quaternary carbon stereocenter. Tetrahedron. 2008;64:2321–2328. doi: 10.1016/j.tet.2008.01.011. [DOI] [Google Scholar]
- 33.Funabashi K., Ratni H., Kanai M., Shibasaki M. Enantioselective construction of quaternary stereocenter through a reissert-type reaction catalyzed by an electronically tuned bifunctional catalyst: Efficient synthesis of various biologically significant compounds. J. Am. Chem. Soc. 2001;123:10784–10785. doi: 10.1021/ja016935c. [DOI] [PubMed] [Google Scholar]
- 34.Shamma M., Jones C. Dihydro Reissert compounds. J. Org. Chem. 1970;35:3119–3121. doi: 10.1021/jo00834a055. [DOI] [Google Scholar]
- 35.Cooney J.V. Reissert compounds and their open-chain analogs in organic synthesis. J. Heterocycl.Chem. 1983;20:823–837. doi: 10.1002/jhet.5570200401. [DOI] [Google Scholar]
- 36.Popp F.D., Soto A. Reissert compound studies. V. Nature of the acid chloride. J. Chem. Soc. 1963:1760–1763. doi: 10.1039/jr9630001760. [DOI] [Google Scholar]
- 37.Popp F.D., Blount W. Reissert compounds studies. III. Nature of the isoquinoline. J. Org. Chem. 1962;27:297–298. doi: 10.1021/jo01048a512. [DOI] [Google Scholar]
- 38.Berg M., Gibson H. Cyanoacylation of 1-substituted isoquinolines and 3,4-dihydroisoquinolines. J. Org. Chem. 1992;57:748–750. doi: 10.1021/jo00028a064. [DOI] [Google Scholar]
- 39.Osante I., Collado M., Lete E., Sotomayor N. Stereodivergent synthesis of hetero-fused isoquinolines by acyliminium and metallation methods. Eur. J. Org. Chem. 2001:1267–1277. [Google Scholar]
- 40.Kirkpatrick A., Maclaren J. Synthesis of 1,2,3,4-Tetrahydro-β-carbolines. Aust. J. Chem. 1983;36:833–838. doi: 10.1071/CH9830833. [DOI] [Google Scholar]
- 41.Bobowski G. Synthesis of 1-substituted-2,3,4,9-tetrahydro-(2-oxopropyl)-1H-pyrido [3,4-b] indoles and their base-catalyzed rearrangements to N-[2-[2-(1-alkyl-3-oxobutenyl)-1H-indol-3-yl] ethyl]acetamides. J. Heterocycl. Chem. 1983;20:267–272. doi: 10.1002/jhet.5570200202. [DOI] [Google Scholar]
- 42.Bobowski G., Shavel J., Jr. 1,1-Disubstituted-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indolecarboxylic acid esters and ketones. The base catalyzed transformation of 1-(2′,3′,4′,9′-tetrahydrospiro[cyclohexane-1,1′-[1H]pyrido[3,4-b]indol]-2-yl)alkanones into 2-(4,9-dihydro-3H-pyrido[3,4-b]indol-1-yl)-1 -alkylcyclohexanols. J. Heterocycl. Chem. 1985;22:1679–1688. doi: 10.1002/jhet.5570220643. [DOI] [Google Scholar]
- 43.Schellhammer W. In: Methoden der Organischen Chemie (Houben-Weyl) Műller E., editor. 7/2a. Thieme; Stuttgart, Germany: 1973. p. 281. [Google Scholar]
- 44.Venkov A., Ivanov I. Synthesis of isoquinolines from 2-phenylethylamines, amides, nitriles and carboxylic acids in polyphosphoric acid. Tetrahedron. 1996;52:12299–12308. doi: 10.1016/0040-4020(96)00716-8. [DOI] [Google Scholar]
- 45.Ivanov I., Nikolova S., Statkova-Abeghe S. A simple method for the synthesis of 1-Substituted β-Carboline derivatives from tryptamine and carboxylic acids in polyphosphoric acid. Heterocycles. 2005;65:2483–2492. doi: 10.3987/COM-05-10484. [DOI] [Google Scholar]
- 46.Ivanov I., Nikolova S., Kochovska E., Statkova-Abeghe S. Polyphosphoric acid-induced construction of quinazolinone skeleton from 1-(3,4-Dimethoxyphenyl)-3-phenylurea and carboxylic acids. Heterocycles. 2006;68:1443–1449. doi: 10.3987/COM-06-10753. [DOI] [Google Scholar]
- 47.Ivanov I., Nikolova S., Kochovska E., Statkova-Abeghe S. Application of ortho-acylated phenylacetic acid esters to the synthesis of 1-substituted isochromanes. ARKIVOC. 2007;xv:31–44. [Google Scholar]
- 48.Orito K., Matsuzaki T., Suginome H., Rodrigo R. Studies on Friedel-crafts acylation of N-acetylhomoveratrylamine and preparation of 1-substituted 3,4-dihydro-6,7-dimethoxyisoquinolines. Heterocycles. 1988;27:2403–2412. doi: 10.3987/COM-88-4647. [DOI] [Google Scholar]
- 49.Hromatka O., Graf W., Knollmüller M. Piperazinsubstituierte isochinolinderivate. Monatsh. Chem. 1966;97:19–32. doi: 10.1007/BF00905479. [DOI] [Google Scholar]
- 50.Dalton D., Ramey K.C., Gisler H.J., Jr., Lendvay L.J., Abraham A. Hindered rotation in substituted 1,2,3,4-tetrahydro-6,7-dimethoxyisoquinolines. J. Am. Chem. Soc. 1969;91:6367–6371. doi: 10.1021/ja01051a031. [DOI] [Google Scholar]
- 51.Rozwadowska M.D., Sulima A. Synthesis of isoquinoline alkaloid, (±)-Salsolidine, using Thiazolino[2,3-a]isoquinolinone S-Oxide as intermediate. Pol. J. Chem. 2001;75:1847–1852. [Google Scholar]
- 52.Venkov A., Statkova-Abeghe S. Application of the intermolecular α-amido-alkylation reaction for the synthesis of tertiary amides and 1-substituted 2-acyltetrahydroiso-quinolines. Synthesis of (±)-carnegine. Synth. Commun. 1995;25:1817–1824. doi: 10.1080/00397919508015425. [DOI] [Google Scholar]
- 53.Venkov A., Ivanov I. Reductive formylation of isoquinoline derivatives with formamide and synthesis of 2-formyltetrahydroisoquinolines. Synth. Commun. 1998;28:1433–1438. doi: 10.1080/00397919808006842. [DOI] [Google Scholar]
- 54.Kuhakarn C., Panyachariwat N., Ruchirawat S. A variation of the Pictet-Spengler reaction via a sequential reduction–cyclization reaction of N-acylcarbamates: synthesis of 1-substituted tetrahydroisoquinoline derivatives Tetrahedron Lett. 2007;48:8182–8184. [Google Scholar]
- 55.Venkov A., Lukanov L. Synthesis of 2-acyl-1-benzyl-, 1-phenylethyl- and spirobenzyltetrahydroisoquinolines. Synth. Commun. 1996;26:755–762. doi: 10.1080/00397919608086750. [DOI] [Google Scholar]
- 56.Kawase M., Motohashi N., Niwa M., Nozaki M. Use of the triflamide group for Friedel-Crafts acylation of N-(β-phenethyl)amino acids to 3-benzazepine derivatives. Heterocycles. 1997;45:1121–1129. doi: 10.3987/COM-97-7803. [DOI] [Google Scholar]
- 57.Nair M.D., Mehta S.R. Reaction of 1-methyl-3,4-dihydroisoquinolines with phenyl isocyanate and isothiocyanate. Indian J. Chem. 1969;7:684. [Google Scholar]
- 58.Mollov N., Venkov А. Eine neue methode zur synthese von 2-acyl-1-aryl-1,2,3,4-tetrahydroisochinolinen. Synthesis. 1978:62–63. doi: 10.1055/s-1978-24677. [DOI] [Google Scholar]
- 59.Gitto R., Barreca M.L., de Luca L., de Sarro G., Ferreri G., Quartarone S., Russo E., Constanti A., Chimirri A. Discovery of a novel and highly potent noncompetitive AMPA receptor antagonist. J. Med. Chem. 2003;46:197–200. doi: 10.1021/jm0210008. [DOI] [PubMed] [Google Scholar]
- 60.Youn S.W. Development of the Pictet-Spengler reaction catalyzed by AuCl3/AgOTf. J. Org. Chem. 2006;71:2521–2523. doi: 10.1021/jo0524775. [DOI] [PubMed] [Google Scholar]
- 61.Venkov A.P., Mollov N.M. Synthesis of Cryptostylines I and II, their analogous and derivatives by means of inner α-amidoalkylation reaction. Compt. Rend. Acad. bulg. Sci. 1979;32:895–897. Chem. Abstr. 1980, 92, 129142b. [Google Scholar]
- 62.de Luca L., Gitto R., Barreca M.L., Caruso R., Quartarone S., Citraro R., de Sarro G., Chimirri A. 3D Pharmacophore models for 1,2,3,4-tetrahydroisoquinoline derivatives acting as anticonvulsant agents. Arch. Pharm. 2006;339:388–400. doi: 10.1002/ardp.200600022. [DOI] [PubMed] [Google Scholar]
- 63.Venkov A., Boyadjieva A. Synthesis Of 2-acyltetrahydro-β-carbolines by an intramolecular α-amidoalkylation reaction. Synth. Commun. 1999;29:487–494. doi: 10.1080/00397919908085791. [DOI] [Google Scholar]