Abstract
Cardanol is a renewable, low cost natural material, widely available as a by-product of the cashew industry. It is a mixture of 3-n-pentadecylphenol, 3-(pentadeca-8-enyl)phenol, 3-(pentadeca-8,11-dienyl)phenol and 3-(pentadeca-8,11,14-trienyl)phenol. Olefin metathesis (OM) reaction on cardanol is an important class of reactions that allows for the synthesis of new olefins that are sometime impossible to prepare via other methods. The application of this natural and renewable material to both academic and industrial research will be discussed.
Keywords: Grubbs catalysts, metathesis, pentadecylphenol, cardanol, porphyrins, metallo-porphyrins, fullerenes, fulleropyrrolidines
1. Introduction
In recent years, among other natural compounds, the use of cardanol to form various new compounds and hybrid compounds has attracted the attention of scientists [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17]. Cardanol is a renewable and inexpensive organic natural resource that is easily obtained via the vacuum distillation of roasted cashew nut shell liquid (CNSL) obtained from the spongy mesocarp of cashew nut shells.
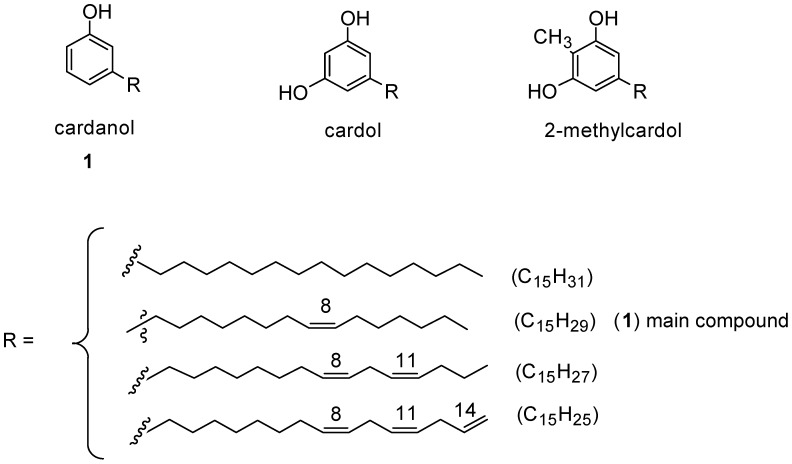
CNSL is a mixture of cardanol, cardol and 2-methylcardol. All these compounds possess a characteristic long alkyl chain in the meta position [1,2,3,4]. Cardanol is the main component (about 84%) of CNSL and is itself a mixture of 3-n-pentadecylphenol, 3-(-pentadeca-8-enyl)phenol, 3-(pentadeca-8,11-dienyl)phenol and 3-(pentadeca-8,11,14-trienyl)phenol (Figure 1). Depending on the purification-distillation of cardanol, the monoolefinic 3-(-pentadeca-8-enyl)phenol, can be the main component, accounting for almost 95% on the total; hence, in this work, the term cardanol refers to this monoolefin.
Figure 1.
Constituents of technical-grade cashew nut shell liquid.
The preparation of fine chemicals from natural and renewable materials has become an attractive topic of research, particularly when the purpose is to recycle the huge amount of agro-industrial wastes to produce, through environmentally sustainable processes, fine chemicals which can be used for different purposes. The extraction of fine chemicals from wastes, such as cardanol, is an old concept. In fact, the phrase “from waste to value”, which means making useful chemical products using the wastes of industry is a well known one. Further, cardanol has a special and unique characteristic: It contains in the meta position of the phenolic ring a long alkyl chain that confers attractive properties to cardanol derivatives such as good processability and high solubility in organic solvents; but, also the possibility to influence many chemical transformation introducing novel functionalities [3,4,5,6,7,8,9,10,11,12,13,14].
This paper presents an overview of the developments in olefin metathesis involving cardanol or cardanol-derived molecules for the preparation of new fine chemicals as well new hybrid functional materials, such as porphyrins, phthalocyanines and fullerenes.
2. Cardanol: Description and Oil Characteristic
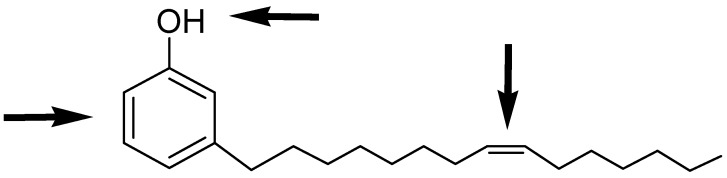
Cardanol is easily obtained by vacuum distillation of CNSL, which is a by-product of the cashew industry. Commercially available CNSL contains a mixture of cardanol (1), cardol and 2-methylcardol (Figure 1) in approximately 84, 11 and 5%, respectively. As reported in the literature, by further re-distillation of the liquid, it is possible to obtain a mixture that is rich (90% on average) in the monoolefinic and diolefinic components, together with minor amounts of the triolefin and 3-n-pentadecylphenol. The average composition of the mixture is: 20%–30% 3-n-pentadecyl phenol, 70%–80% 3-(pentadeca-8-enyl) phenol (1), nearly 5% 3-(pentadeca-8,11-dienyl) phenol and less than 5% 3-(pentadeca-8,11,14-trienyl) phenol. Cis and trans isomers of each component are present in the mixture, but usually the cis component is the major one. This mixture can be further purified by successive re-distillation/chromatography. In this way, the monoolefin component, 3-(penta-deca-8-enyl)phenol was obtained almost pure. For simplicity, this isolate will hereon be referred to as “cardanol” in this work. Several types of chemical reactions can be carried out involving the benzene ring, the olefin or the hydroxyl group (Figure 2).
Figure 2.
Possible sites for functionalization of the cardanol molecule.
The presence olefin group in the long chain attached to the meta position of the phenolic ring allows for the possibility for generating new cardanol-based compounds via metathesis reactions.
3. Preparation of Cardanol-Based Derivatives
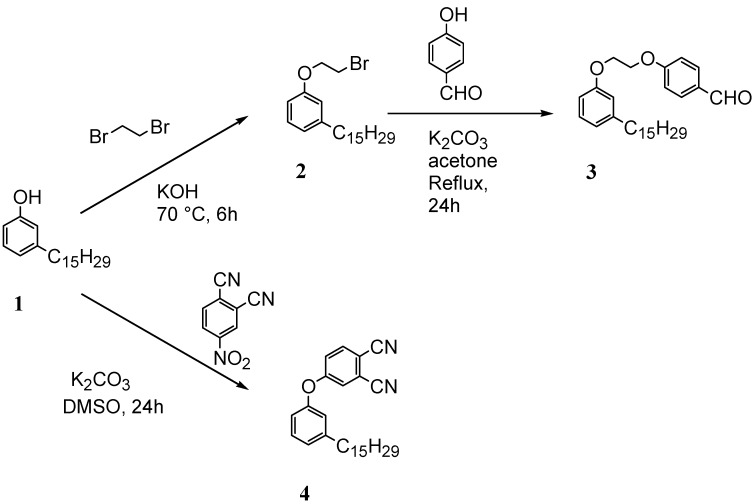
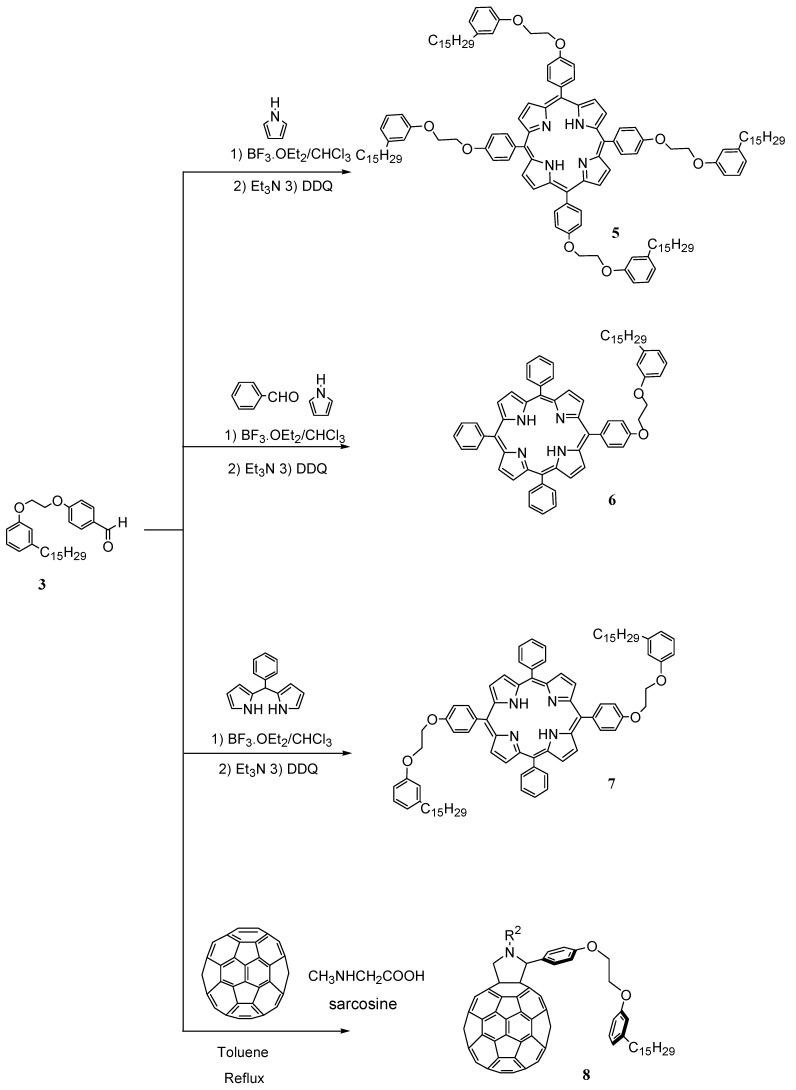
Scheme 1 and Scheme 2 depict the syntheses of several cardanol-based derivatives that have been used as precursors for metathesis reactions.
Scheme 1.
Molecular structure and synthesis of the cardanol-based precursors 1–4 used for metathesis reactions.
Scheme 2.
Synthesis of the cardanol-based porphyrins 5–7 and fulleropyrrolidine 8 used for metathesis reactions.
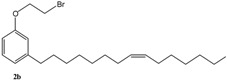
As summarized in Scheme 1, the compound 2 was prepared via the reaction of cardanol (1) with 1,2-dibromoethane in the presence of KOH at 70 °C for 6 h and isolated in 70% yield [15]. Successively, 2 was reacted with 4-hydroxybenzaldehyde in the presence of anhydrous potassium carbonate in acetone under reflux for 30 h to afford 3 in 40% isolated yield. The compound 4 was prepared in 90% isolated yield by reacting 1 with 1,2-dicyano-4-nitrobenzene in DMSO in the presence of K2CO3 at room temperature for 24 h [16].
As shown in the Scheme 2, 4-[2-(3-(pentadeca-8-enyl) phenoxy)-ethoxy]benzaldehyde 3 was reacted with pyrrole, benzaldehyde or fullerene according to the methods reported in the literature [3,15] in order to obtain cardanol-based porphyrins 5–7 and cardanol-based fulleropyrolidine 8 which can be used as starting materials for metathesis reactions.
In particular, cardanol-based porphyrin 5 was obtained by the reaction of 3 with pyrrole [15]; porphyrin 6 was synthesized by the acid-catalyzed condensation of 3 with pyrrole and benzaldehyde or with meso-phenyldipyrrolmethane [3].
The fulleropyrrolidine cardanol 8 was prepared by the cycloaddition of sarcosine (N-methylglycine) in the presence of 3 and C60 in refluxing toluene under nitrogen atmosphere for 24 h [17].
4. Metathesis on Cardanol and Its Hybrid Derivatives
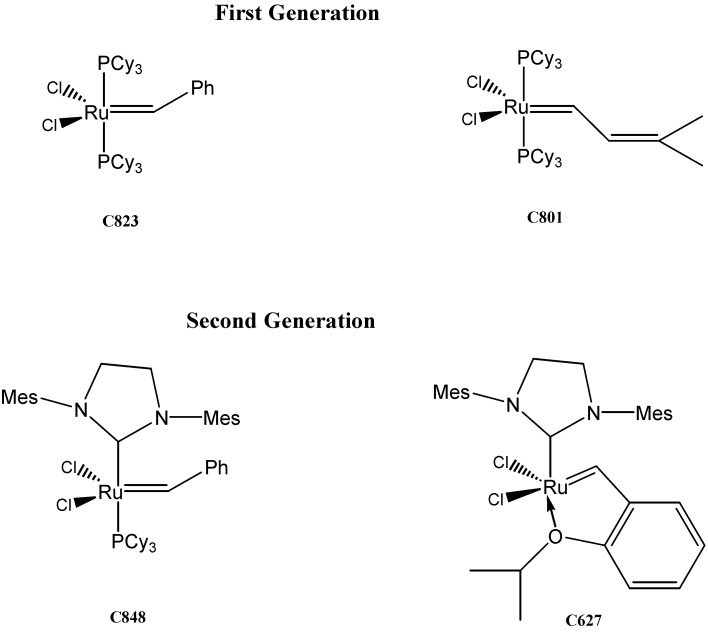
As mentioned previously, the olefin metathesis reaction is an organic reaction which offers the possibility to form new olefins [18,19,20,21]. Different commercially available ruthenium-catalysts, which are illustrated in Figure 3 and labelled according to their molecular weights (C627, C823, C801, C848), have been used.
Figure 3.
First- and second- generation of ruthenium-catalysts used for metathesis reactions.
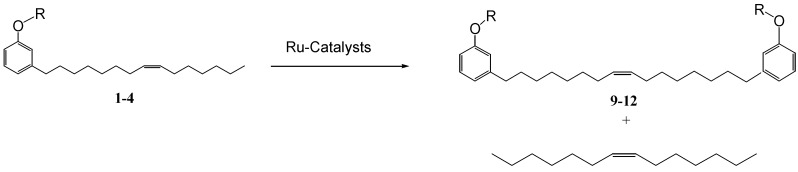
Initially, the mono-olefinic cardanol, 3-(pentadeca-8-enyl) phenol, was used as the starting material to prepare, using a Hoveyda-Grubbs’ catalyst (Scheme 3) [22].
Scheme 3.
Metathesis reaction involving cardanol 1 and its derivatives 2–4.
The metathesis reaction was then performed with other cardanol-based substrates to prepare the new derivatives listed in Table 1. The best metathesis conditions resulted used 2 mmol of 1, and 5 mol% of catalyst C627 in dichloromethane (1.2 mL) at 40 °C for 45–87 h under N2 atmosphere.
Table 1.
Metathesis performed on cardanol 1 and cardanol derivatives 2–4.
| Substrate | Product | Time (h) | Conv. (%) | Yield (%) |
|---|---|---|---|---|
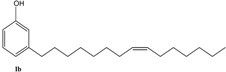 |
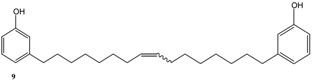 |
72 | 78 | 36 |
 |
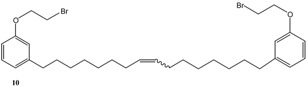 |
64 | 86 | 51 |
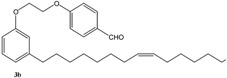 |
 |
92 | 62 | 20 |
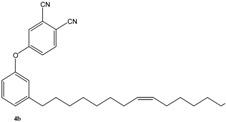 |
 |
87 | 55 | 36 |
Reaction conditions: Starting material (1.0 mmol), catalyst C627 (2 mol %); dichloromethane (1.67 M). The reaction mixture was refluxed under nitrogen atmosphere.
In all cases, the conversion of 1–4 into 9–12 reached only 42%–65% (isolated yields). This was attributed to the reaction’s reversibility [20,23,24]. All products were formed as a mixture of Z- and E-isomers, in which Z-isomers were dominant according with the results reported by Meek et al. [25].
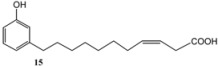
Subsequently, metathesis reactions were applied on cardanol in the presence of commercial olefins bearing –COOH and –COOEt moieties in order to prepare new cardanol-based carboxylic acid- (15) and ester-type (16) derivatives (Table 2) [26,27].
Table 2.
Cross-metathesis reaction involving cardanol.
| Substrate | Olefin | Product | Yield (%) |
|---|---|---|---|
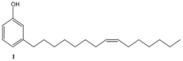
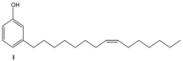 |
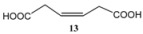 |
 |
50 |
 |
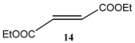 |
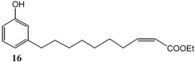 |
30 |
Reaction conditions: Substrate 1 (3.0 mmol), substrate 13 (or 14) (3.0 mmol), C627 catalyst (5 mol%); dichloromethane (15 mL). The reaction mixture was stirred under reflux in an oil bath.
In 2006, the preparation and characterization of new hybrid cardanol-based meso-tetraarylporphyrins bearing unsaturated chains were reported [15]. Metathesis reactions were then carried out on these porphyrins in order to prepare new cardanol-based products with different chemical and physical properties [15].
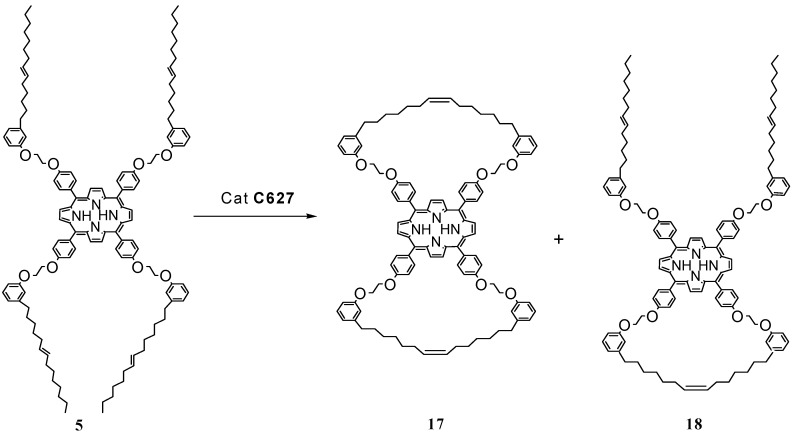
In 2009, new tetracardanol-based porphyrins were prepared, and an evaluation of an intra- or inter-molecular metathesis process was investigated [28]. On one of these compounds, intramolecular metathesis was performed in dichloromethane and in the presence of Ti(OiPr)4 and Grubbs catalyst, involving two or all four double bonds of porphyrin’s structure (Scheme 4). Two different products were isolated, one bearing a double-bridged moiety (compound 17), the other having two free alkyl chains (compound 18) [16].
Scheme 4.
Metathesis reaction involving the cardanol based porphyrin 5.
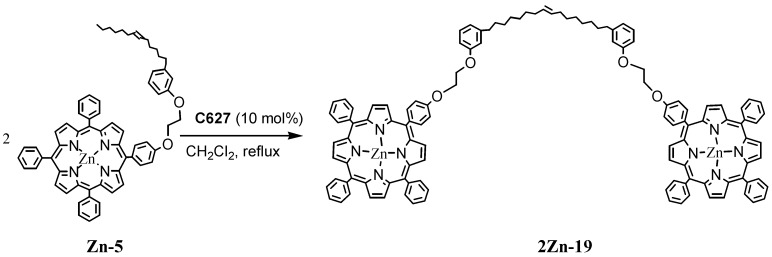
The 1H-NMR and LC-MS spectroscopic characterization of such compounds proved are in agreement with the proposed structure. It was possible to observe that the triplet at 0.9 ppm of the methyl protons present in 5 disappeared in 17 because of the double-bridged porphyrin-porphyrin structure (Scheme 4). In contrast, this triplet was present in the 1H-NMR spectrum of 18. When the metathesis reaction was performed on the porphyrin derivative Zn-5, which contains only one cardanol moiety, an inter-molecular coupling occurred to produce 2Zn-19 (Scheme 5) [28]. The linking of porphyrin molecules by covalent bonds can give rise to stable, multi-porphyrin architectures and well-defined arrays, and this is a very good way for creating novel molecular wires, light-harvesting systems, energy transduction devices for molecular recognition and many other applications [29].
Scheme 5.
Metathesis reaction involving the cardanol-based zinc porphyrin Zn-5.
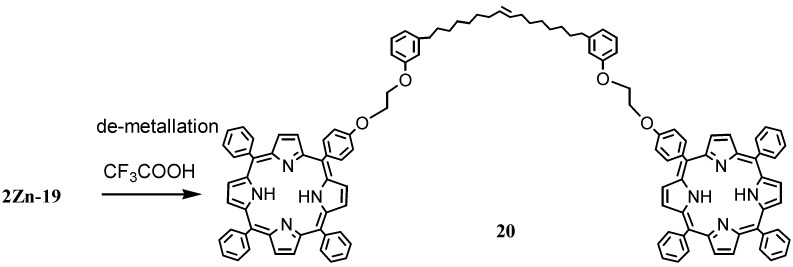
The route by which the zinc compound 2Zn-19 was prepared is depicted in Scheme 5. The compound 2Zn-19 was isolated in 35% yield [28]; it was successively de-metallated to obtain the non-metallic compound 20 (Scheme 6).
Scheme 6.
Metathesis reaction involving the cardanol based zinc porphyrin 2Zn-19.
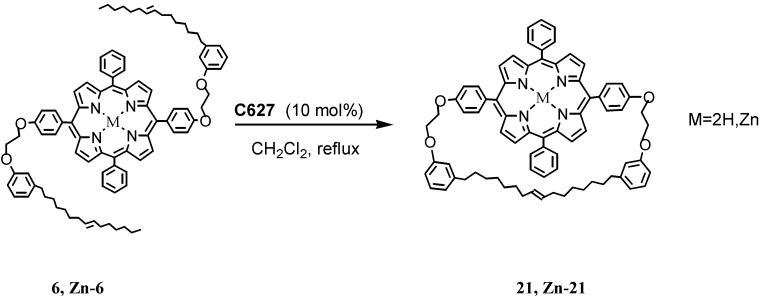
In the case of porphyrin derivatives with two symmetric cardanol moieties, 6 and Zn-6, using catalyst C627, produced the ring-closed products 21 and Zn-21, respectively in 20 and 30% yields (Scheme 7) [28].
Scheme 7.
Metathesis reaction involving the cardanol based porphyrins 6 and Zn-6.
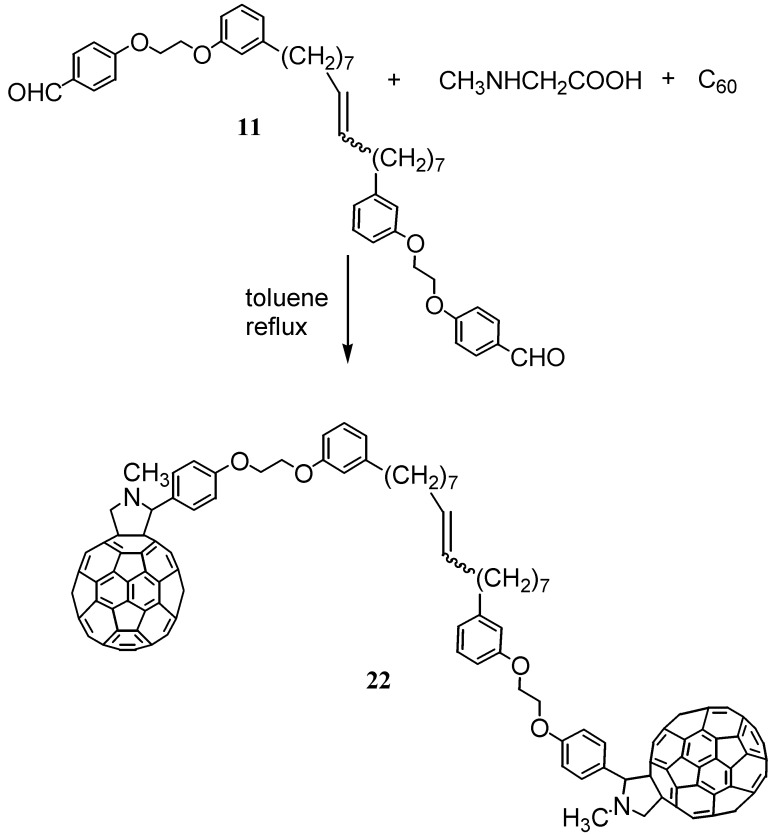
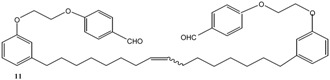
A few years ago, it was reported that new hybrid cardanol-based fulleropyrrolidines could be directly prepared via a three-component 1,3-dipolar cycloaddition reaction of fullerene (C60) and N-methyl glycine with various aldehydes in toluene. The reaction was found to proceed through the generation of azomethine ylides in situ [17,30]. So, in the same manner bis-fulleropyrrolidine 22 was prepared in 28% yield through a cycloaddition of the bis-azomethyne ylide in the presence of the cardanol based aldehyde precursors 11 and C60, as depicted in the Scheme 8.
Scheme 8.
Preparation of the bis-fulleropyrrolidine 22 from the precursor 11.
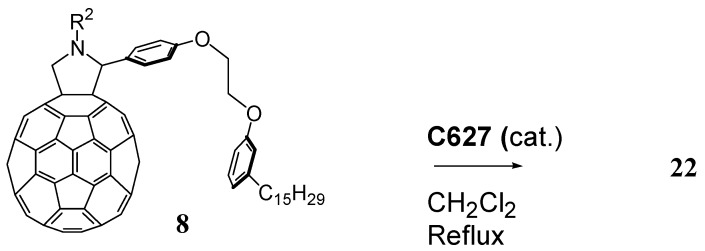
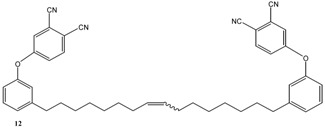
Alternatively, the bis-fulleropyrrolidine 22 could also be prepared according to Scheme 9.
Scheme 9.
Preparation of the bis-fulleropyrrolidine 22 through homo-cross metathesis reaction of the fulleropyrrolidine 8 using C627 as catalyst.
Compound 22 was prepared in 30% yield through the metathesis reaction of the cardanol-based fulleropyrrolidine 8, which was obtained via the cycloaddition of N-methylglycine in the presence of the cardanol-based aldehyde precursors 3 using C60. In a typical reaction, 40 mg (0.033 mmol) of fulleropyrrolidine 8 were dissolved in 21 mL of dichloromethane. Then, a solution of the catalyst C627 (0.42 mg in 1.5 mL of CH2Cl2, 0.02 equiv.) was used to perform the metathesis reaction [17]. The crude product was purified with silica gel using toluene as the eluent.
5. Conclusions
Cardanol a well-known by-product of the cashew industry has been successfully used as the starting material to perform olefin metathesis reactions. The olefin metathesis reaction is particularly convenient due to commercial availablility of many α-olefins as renewable starting materials. In this paper, it has been shown that olefin metathesis reactions allow for the preparation of new cardanol-derived olefins and hybrid materials, combined with porphyrins and fullerenes, which are sometimes impossible to prepare via other methods.
Acknowledgments
The authors are grateful to University of Salento for financial support (ex. 60%).
References
- 1.Tyman J.H.P. Non-isoprenoid long chain phenols. Chem. Soc. Rev. 1979;8:499–537. doi: 10.1039/cs9790800499. [DOI] [Google Scholar]
- 2.Tyman J.H.P. Synthetic and Natural Phenols. Elsevier; Amsterdam, The Netherlands: 1996. [Google Scholar]
- 3.Mele G., Vasapollo G. Fine chemicals and new hybrid materials from cardanol. Mini-Rev. Org. Chem. 2008;5:243–253. doi: 10.2174/157019308785161611. [DOI] [Google Scholar]
- 4.Mazzetto S.E., Lomonaco D., Mele G. Óleo Da Castanha De Caju: Oportunidades E Desafios No Contexto Do Desenvolvimento E Sustentabilidade Industrial. Quim. Nova. 2009;32:732–741. doi: 10.1590/S0100-40422009000300017. [DOI] [Google Scholar]
- 5.Lubi M.C., Thachil E.T. Cashew nut shell liquid (CNSL)—A versatile monomer for polymer synthesis. Des. Monomers Polym. 2000;3:123–153. doi: 10.1163/156855500300142834. [DOI] [Google Scholar]
- 6.Rodrigues F.H.A., Feitosa J.P.A., Ricardo N.M.P.S., de Franca F.C.F., Carioca J.O.B. Antioxidant activity of cashew nut shell liquid (CNSL) derivatives on the thermal oxidation of synthetic cis-1,4-polyisoprene. J. Braz. Chem. Soc. 2006;17:265–271. doi: 10.1590/S0103-50532006000200008. [DOI] [Google Scholar]
- 7.Kim Y.H., Won K., Kwon J.M., Jeong H.S., Park S.Y., An E.S., Song B.K. Synthesis of polycardanol from a renewable resource using a fungal peroxidase from Coprinus cinereus. J. Mol. Catal. B-Enzym. 2005;34:33–38. doi: 10.1016/j.molcatb.2005.04.005. [DOI] [Google Scholar]
- 8.Attanasi O.A., Berretta S., Favi G., Filippone P., Mele G., Moscatelli G., Saladino R. Tetrabromo hydrogenated cardanol: Efficient and renewable brominating agent. Org. Lett. 2006;8:4291–4293. doi: 10.1021/ol061637b. [DOI] [PubMed] [Google Scholar]
- 9.Maffezzoli A., Calò E., Zurlo S., Mele G., Tarzia A., Stifani C. Cardanol based matrix biocomposites reinforced with natural fibres. Comp. Sci. Tech. 2004;64:839–845. doi: 10.1016/j.compscitech.2003.09.010. [DOI] [Google Scholar]
- 10.Calò E., Maffezzoli A., Mele G., Martina F., Mazzetto S.E., Tarzia A., Stifani C. Synthesis of a novel cardanol-based benzoxazine monomer and environmentally sustainable production of polymers and bio-composites. Green Chem. 2007;9:754–759. doi: 10.1039/b617180j. [DOI] [Google Scholar]
- 11.Bhunia H.P., Jana R.N., Basak A., Lenka S., Nando G.B. Synthesis of polyurethane from cashew nut shell liquid (CNSL), a renewable resource. J. Polym. A-Poly. Chem. 1998;36:391–400. doi: 10.1002/(SICI)1099-0518(199802)36:3<391::AID-POLA3>3.0.CO;2-V. [DOI] [Google Scholar]
- 12.Coletta M., Filippone P., Fiorucci C., Marini S., Mincione E., Neri V., Saladino R. A new and efficient synthesis of ortho- and para-benzoquinones of cardanol derivatives by the catalytic system MeReO3-H2O2. J. Chem. Soc. Perkin Trans. 1. 2000:581–586. [Google Scholar]
- 13.Amorati R., Pedulli G.F., Valgimigli L., Attanasi O.A., Filippone P., Fiorucci C., Saladino R. Absolute rate constants for the reaction of peroxyl radicals with cardanol derivatives. J. Chem. Soc. Perkin Trans. 2. 2001:2142–2146. [Google Scholar]
- 14.Mele G., Del Sole R., Vasapollo G., García-López E., Palmisano L., Mazzetto S.E., Attanasi O.A., Filippone P. Polycrystalline TiO2 impregnated with cardanol-based porphyrins for the photocatalytic degradation of 4-nitrophenol. Green Chem. 2004;6:604–608. doi: 10.1039/b409510c. [DOI] [Google Scholar]
- 15.Guo Y.C., Xiao W.J., Mele G., Martina F., Margapoti E., Mazzetto S.E., Vasapollo G. Synthesis of new meso-tetraarylporphyrins bearing cardanol and further transformation of the unsaturated chains. J. Porphyr. Phtalocya. 2006;10:1071–1079. doi: 10.1142/S1088424606000430. [DOI] [Google Scholar]
- 16.Attanasi O.A., Del Sole R., Filippone P., Mazzetto S.E., Mele G., Vasapollo G. Synthesis of novel cardanol based porphyrins. J. Porphyr. Phtalocya. 2004;8:1276–1284. doi: 10.1142/S1088424604000635. [DOI] [Google Scholar]
- 17.Attanasi O.A., Mele G., Filippone P., Mazzetto S.E., Vasapollo G. Synthesis and characterization of novel cardanol based fulleropyrrolidines. ARKIVOC. 2009;VIII:69–84. [Google Scholar]
- 18.Trnka T.M., Grubbs R.H. The development of L2X2RudCHR olefin metathesis catalysts: An organometallic success story. Acc. Chem. Res. 2001;34:18–29. doi: 10.1021/ar000114f. [DOI] [PubMed] [Google Scholar]
- 19.Herrmann W.A. Heterocyclic carbenes: A new concept in organometallic catalysis. Angew. Chem. Int. Ed. 2002;41:1290–1309. doi: 10.1002/1521-3773(20020415)41:8<1290::AID-ANIE1290>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 20.Grubbs R.H. Olefin metathesis. Tetrahedron. 2004;60:7117–7140. doi: 10.1016/j.tet.2004.05.124. [DOI] [Google Scholar]
- 21.Choi T.L., Chatterjee A.C., Grubbs R.H. Synthesis of a,β-unsaturated amides by olefin cross-metathesis. Angew. Chem. Int. Ed. 2001;40:1277–1279. doi: 10.1002/1521-3773(20010401)40:7<1277::AID-ANIE1277>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 22.Guo Y.C., Mele G., Martina F., Margapoti E., Vasapollo G., Xiao W.J. An efficient route to biscardanol derivatives and cardanol-based porphyrins via olefin metathesis. J. Organomet. Chem. 2006;691:5383–5390. doi: 10.1016/j.jorganchem.2006.07.020. [DOI] [Google Scholar]
- 23.Astruc D. The metathesis reactions: From a historical perspective to recent developments. New J. Chem. 2005;29:42–56. doi: 10.1039/b412198h. [DOI] [Google Scholar]
- 24.Grubbs R.H. Olefin-metathesis catalysts for the preparation of molecules and Materials. Angew. Chem. Int. Ed. Engl. 2006;45:3760–3765. doi: 10.1002/anie.200600680. [DOI] [PubMed] [Google Scholar]
- 25.Meek S.J., O’Brien R.V., Llaveria J., Schrock R.R., Hoveyda A.H. Catalytic Z-selective olefin cross-metathesis for natural product synthesis. Nature. 2011;471:461–466. doi: 10.1038/nature09957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martina F. Ph.D. Thesis. University of Salento; Lecce, Italy: 2008. Synthesis and characterization of new derivatives from cardanol. [Google Scholar]
- 27.Vasapollo G., Mele G., Martina F. 2009. University of Salento. Unpublished work. [Google Scholar]
- 28.Mele G., Li J., Margapoti E., Martina F., Vasapollo G. Synthesis of novel porphyrins cardanol based via cross metathesis. Catal. Today. 2009;140:37–43. [Google Scholar]
- 29.Kadish K.M., Smith K.M., Guilard R., editors. Handbook of Porphyrin Science-With Applications to Chemistry, Physics, Materials Science, Engineering, Biology and Medicine. Volume 1-10 World Scientific Publishing Co.; Singapore: 2010. [Google Scholar]
- 30.Attanasi O.A., Del Sole R., Filippone P., Ianne R., Mazzetto S.E., Mele G., Vasapollo G. Synthesis of fullerene cardanol derivatives. Synlett. 2004;5:799–802. [Google Scholar]