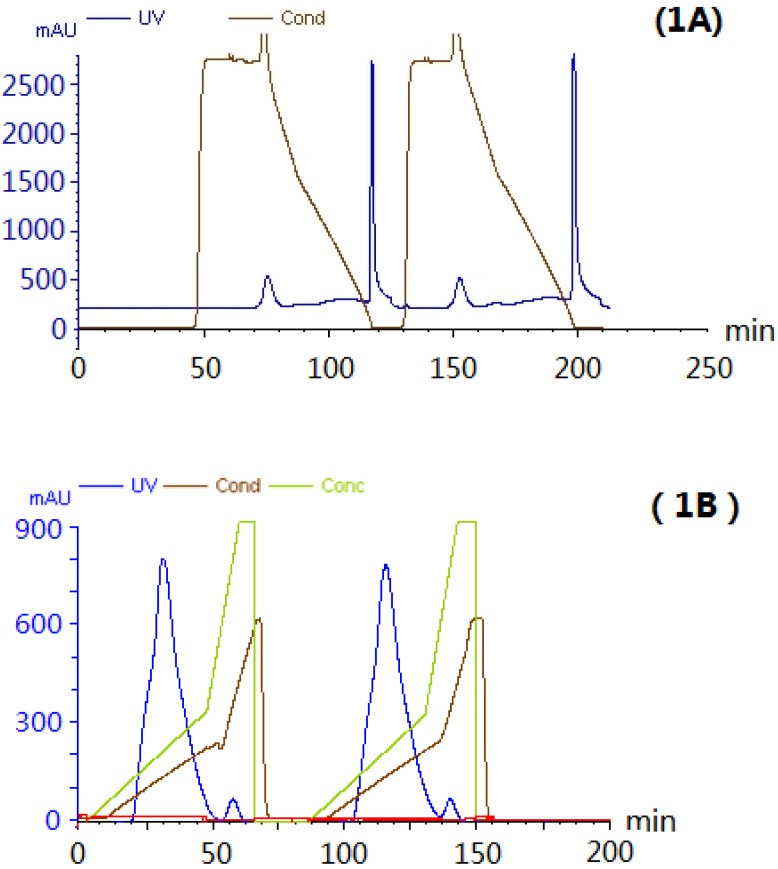
Figure 1.
The purification of AEL by hydrophobic interaction chromatography and ion exchange chromatography. The elution profiles were monitored at 280 nm. (A) Hydrophobic interaction chromatography of protein on HiprepTM Phenyl FF column (10 mL). The bound protein was eluted with a linear gradient of 0.6–0.3 mol/L (NH4)2SO4 for 15 min, then with 0.3–0 mol/L (NH4)2SO4 for 30 min, finally with H2O at a flow rate of 1 mL/min; (B) Ion exchange chromatography on HiTrapTM column pre-equilibrated with Tris-HCl buffer [pH 8.0]. The main peak which was obtained by hydrophobic interaction chromatography was eluted with a linear gradient of 0–0.4mol/L NaCl at a flow rate of 1 mL/min.