Abstract
The composition and antimicrobial activity of the essential oils which were obtained from agarwood originated from Aquilaria sinensis (Lour.) Gilg stimulated by the chemical method (S1) were characterized, taking wild agarwood (S2) and healthy trees (S3) respectively as the positive and negative controls. The chemical composition of S1 was investigated by gas chromatography-mass spectrometry (GC-MS). The essential oil of S1 showed a similar composition to that of S2, being rich in sesquiterpenes and aromatic constituents. However, the essential oil of S3 was abundant in fatty acids and alkanes. Essential oils of S1 and S2 had better inhibition activities towards Bacillus subtilis and Staphyloccus aureus, compared with essential oil of S3. Escherichia coli was not sensitive to any of them.
Keywords: Aquilaria sinensis (Lour.) Gilg, healthy trees, agarwood, essential oil, GC-MS, antimicrobial activity
1. Introduction
Agarwood, a highly valuable resinous and fragrant heartwood, is used as incense for religious ceremonies, perfumes in the Arab world, ornamental materials, and medicinal components in oriental medicine [1,2]. It comes from the damage caused to healthy trunks or branches of the trees of some Aquilaria species in the family Thymelaeaceae by mold. As a healthy tree Aquilaria is worth next to nothing, but high-quality agarwood can fetch as much as US$1,000 per kilogram [3]. In a natural environment, it often takes several years for a wild damaged Aquilaria species plant to form agarwood [3]. The over-use of agarwood has seriously affected the natural resources of all Aquilaria species capable of producing agarwood, thus making these endangered species listed in Appendix II of the Convention on Internal Trade in Endangered Species of Wild Fauna and Flora (CITES) since 2004 [4].
In order to meet the demand for agarwood and protect the wild Aquilaria trees, many countries have been developing Aquilaria plantationsf [5,6,7]. A. sinensis, the main plant resource in China for agarwood, is chiefly distributed in South China [8]. A. sinensis trees are now widely cultivated in Hainan and Guangzhou provinces, with the planting area estimated to cover more than 700 acres.
The main active compounds in agarwood have been revealed to be sesquiterpenes and 2-(2-phenylethyl) chromone derivatives [9]. In order to improve the planting value of Aquilaria trees, great efforts have been made to induce healthy trees to produce these sesquiterpenes and 2-(2-phenylethyl) chromone derivatives, consequently forming argarwood [5,10]. The common methods now used in China and other countries include the deliberate wounding of trees with large knives and the hammering of nails into tree trunks. A chemical treatment method has also been developed recently [11]. Meanwhile, some studies have been carried out to compare the quality of man-treated and wild agarwood. Tamuli and Bhuiyan studied the quality of agarwood (A. agallocha Roxb.) formed through fungal infection by GC-MS [12,13]. Dai Haofu et al. evaluated the quality of three Chinese agarwood (A. sinensis) samples produced by the methods of nail insetting, holing and trunk breaking, respectively, through GC-MS [14], but there are no reports about agarwood formed by chemical methods. In this study, in order to test the quality of the agarwood originated from A. sinensis stimulated by the chemical method (S1), its chemical composition and relative amount of essential oils were measured by GC-MS, taking the wild agarwood (S2) and healthy trees (S3) as controls. The antimicrobial activities of essential oils of the agarwood originating from A. sinensis were also determined.
2. Results and Discussion
2.1. Chemical Composition of the Essential Oils
The yields of essential oils obtained after hydrodistillation of S1, S2 and S3 were 0.042% (w/w), 0.32% (w/w) and 0.0128% (w/w), respectively. They showed different colors and states (Figure 1). At room temperature, the essential oil of S1 was yellow, aromatic and a liquid, similar to that of S2, but different from that of S3. The essential oil of S2 was green, aromatic and liquid. The essential oil of S3 had an acidic smell and was a solid at room temperature.
Figure 1.
Color and state of the essential oils of three tested samples at room temperature.
A total of sixty-one essential compounds were identified from the three samples (Table 1 and Figure 2). Forty-two components were identified in S1, representing 90.01% of the total volatiles, with the major constituents being sesquiterpenes and aromatics, such as guaia-1(10),11-dien-9-one (10.89%), guaiol (9.34%), benzylacetone (7.91%) and hinesol (6.34%). Thirty-six components were identified in S2, representing 92.16% of the total volatiles. The dominant compounds were baimuxinal (14.78%), guaiol (10.67%), α-copaen-11-ol (10.22%) and 1,2,5,5,8a-pentamethyl-1,2,3,5,6,7,8,8a-octahydro-naphthalen-1-ol (5.82%). Thirty components were identified in the essential oil of S3, representing 95.68% of the total volatiles. N-hexadecanoic acid and oleic acid accounted for 59.65% of the total essential oil, which explained its smell and state.
Table 1.
Chemical compositions and relative amounts of the essential oils from S1, S2 and S3.
| No. | Compounds | RI a | Relative amount (%) c | Identification | |||
|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | |||||
| Sesquiterpenes and aromatics | 80.00 | 89.01 | 8.93 | ||||
| 1 | Benzylacetone * | 1257 | 7.91 | 2.34 | - b | RI,MS, | |
| 2 | Vanillin | 1418 | - | - | 0.46 | RI[15],MS | |
| 3 | α-Humulene | 1464 | 0.30 | - | - | RI,MS,[16,17] | |
| 4 | α-Selinene | 1493 | 0.45 | 3.71 | - | RI[18],MS | |
| 5 | α-Agarofuran | 1535 | - | 0.24 | - | RI[18],MS | |
| 6 | Elemol | 1556 | 0.32 | 0.71 | - | RI[19,24],MS | |
| 7 | 2,6-Dimethyl-10-methylene-12-oxatricyclo[7.3.1.0(1,6)]tridec-2-ene | 1576 | 0.37 | 0.61 | - | MS | |
| 8 | 5β,7βH,10α-Eudesm-11-en-1α-ol | 1583 | 0.54 | 0.66 | - | MS | |
| 9 | Caryophyllene oxide | 1588 | 2.22 | 2.12 | - | RI[19,24],MS | |
| 10 | 2H-Benzocyclohepten-2-one, 3,4,4a,5,6,7,8,9-octahydro-4a-methyl-, (S)- | 1593 | - | 0.20 | - | MS | |
| 11 | Isoaromadendrene epoxide | 1606 | 0.94 | 2.77 | - | RI[20],MS | |
| 12 | γ-Eudesmol | 1632 | 1.06 | 2.84 | 0.50 | RI[21],MS[22] | |
| 13 | Hinesol | 1638 | 6.34 | 0.34 | - | RI[23],MS | |
| 14 | Agarospirol | 1643 | 0.80 | 4.03 | 0.85 | RI[24],MS | |
| 15 | Cubenol | 1647 | 2.21 | 1.97 | - | RI[25],MS | |
| 16 | cis-Z-α-Bisabolene epoxide | 1651 | 0.83 | 0.78 | - | RI[26],MS | |
| 17 | (-)-Aristolene | 1654 | 0.61 | 4.70 | 1.31 | MS[22] | |
| 18 | Guaiol | 1661 | 9.34 | 10.67 | 2.18 | MS[27,28,29] | |
| 19 | Eudesm-7(11)-en-4α-ol | 1666 | 4.35 | 2.09 | - | RI,MS[30] | |
| 20 | Aromadendrene oxide (1) | 1674 | 1.27 | 1.41 | - | RI[31],MS | |
| 21 | 6-Isopropenyl-4,8a-dimethyl-1,2,3,5,6,7,8,8a-octahydro-naphthalen-2-ol | 1678 | 1.19 | 1.33 | - | RI[20],MS | |
| 22 | α -Copaen-11-ol | 1686 | 4.06 | 10.22 | - | RI,MS[32] | |
| 23 | 4,4,11,11-tetramethyl-7-tetracyclo-[6.2.1.0(3.8)0(3.9)]undecanol | 1690 | 0.53 | 1.50 | - | MS | |
| 24 | Bicyclo[4,4,0]dec-2-ene-4-ol,2-methyl-9-[prop-1-en-3-ol-2-yl] | 1697 | 3.11 | 0.52 | - | MS | |
| 25 | Diepi-α-cedrene epoxide | 1701 | 6.00 | 0.38 | - | MS | |
| 26 | Aromadendrene oxide (2) | 1705 | - | 1.88 | - | RI[33],MS | |
| 27 | Baimuxinal | 1707 | 2.44 | 14.78 | 1.52 | MS[27,28,32] | |
| 28 | Selina-3,11-dien-14-al | 1733 | 5.50 | 0.38 | - | RI[18], MS | |
| 29 | 5(1H)-Azulenone, 2,4,6,7,8,8a-hexahydro-3,8-dimethyl-4-(1-methylethylidene)-, (8S-cis)- | 1736 | 0.21 | 1.03 | - | RI,MS | |
| 30 | Guaia-1(10),11-dien-9-one | 1753 | 10.89 | - | - | RI[18],MS | |
| 31 | 1,2,5,5,8a-Pentamethyl-1,2,3,5,6,7,8,8a-octahydronaphthalen-1-ol | 1755 | - | 5.82 | - | RI[34],MS | |
| 32 | 6-Isopropenyl-4,8a-dimethyl-3,5,6,7,8,8a-hexahydro-2(1H)-naphthalenone | 1769 | 1.65 | 0.54 | - | RI[34],MS | |
| 33 | Eremophila-7(11),9-dien-8-one | 1811 | 4.54 | 5.42 | 2.11 | RI[34],MS | |
| 34 | Acetic acid, 3-hydroxy-6-isopropenyl-4,8a,dimethyl-1,2,3,5,6,7,8,8a-octahydronaphthalen-2-yl ester | 1847 | - | 3.04 | - | RI[34],MS | |
| Fatty acid and Alkanes | 5.75 | 0.64 | 79.31 | ||||
| 35 | Tetradecanoic acid | 1772 | - | - | 2.36 | RI[35],MS | |
| 36 | Nonanoic acid | 1278 | - | - | 1.50 | RI[36],MS | |
| 37 | n-Decanoic acid | 1371 | - | - | 0.52 | RI[36],MS | |
| 38 | cis-5-Dodecenoic acid | 1863 | 0.20 | - | - | RI[34],MS | |
| 39 | Pentadecanoic acid | 1878 | - | - | 4.87 | RI[34],MS | |
| 40 | cis-9-Hexadecenoic acid | 1955 | - | - | 2.87 | RI[34],MS | |
| 41 | n-Hexadecanoic acid | 1982 | 0.30 | 0.06 | 49.47 | RI[34],MS | |
| 42 | Hexadecanoic acid, ethyl ester | 1996 | - | - | 1.13 | RI[34],MS | |
| 43 | Eicosane | 1999 | 0.22 | 0.58 | - | RI[34],MS | |
| 44 | Heptadecanoic acid | 2073 | - | - | 0.37 | RI[34],MS | |
| 45 | Heneicosane | 2100 | 0.51 | - | 1.09 | RI[34],MS | |
| 46 | Oleic Acid | 2153 | - | - | 10.18 | RI[34],MS | |
| 47 | Docosane | 2200 | 0.80 | - | 0.53 | RI[34],MS | |
| 48 | Tricosane | 2300 | 0.97 | - | 0.80 | RI[34],MS | |
| 49 | Tetracosane | 2400 | 0.79 | - | 0.86 | RI[34],MS | |
| 50 | Pentacosane | 2500 | 0.70 | - | 0.87 | RI[34],MS | |
| 51 | Hexacosane | 2600 | 0.62 | - | 0.80 | RI[34],MS | |
| 52 | Heptacosane | 2700 | 0.45 | - | 0.57 | RI[34],MS | |
| 53 | Octacosane | 2800 | 0.20 | - | 0.54 | RI[34],MS | |
| Others | 4.26 | 2.51 | 7.44 | ||||
| 54 | 2-Hydroxycyclopentadecanone | 1851 | 0.24 | 0.30 | 2.32 | RI[37],MS | |
| 55 | 1,2-Benzenedicarboxylic acid, bis(2-methylpropyl) ester | 1869 | 0.69 | - | 0.85 | RI[37],MS | |
| 56 | Dibutyl phthalate | 1962 | 2.13 | 1.23 | 2.47 | RI[37],MS | |
| 57 | 1,2,3,4-Tetrahydro-1-nonylnaphthalene | 2021 | 0.64 | - | - | MS | |
| 58 | 8,9-Dehydro-9-formyl-cycloisolongifolene | 2082 | 0.56 | 0.98 | - | MS | |
| 59 | γ-Palmitolactone | 2111 | - | - | 0.99 | RI[34],MS | |
| 60 | 4,8,12,16-Tetramethylheptadecan-4-olide | 2357 | - | - | 0.42 | RI[34],MS | |
| 61 | 1,2-Benzenedicarboxylic acid, mono(2-ethylhexyl) ester | 2541 | - | - | 0.39 | RI[37],MS | |
| TOTAL | 90.01 | 92.16 | 95.68 | ||||
Compounds are listed in the order of elution; a RI indicates the retention indices which were calculated against C8-C40 n-alkanes on the non-polar VF-5MS column; b not detected; c Relative amount indicates the relative amount (the peak area relative to the total peak area); * verified by the authentic compound.
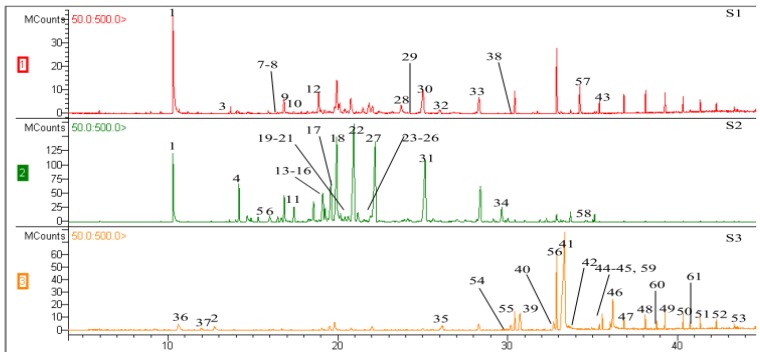
Figure 2.
GC chromatograms of the three essential oils. Component numbers in the chromatogram come from Table 1.
The investigation showed that the essential oil of S1 had similar components to those of S2. They were both rich in sesquiterpenes and aromatics, which reached 80.00% in S1 and 89.01% in S2. Thirty-one common compounds were identified in S1 and S2. Benzylacetone, cubenol, guaiol, eudesm-7(11)-en-4α-ol, α-copaen-11-ol, and baimuxinal were found to be the six compounds (in bold in Table 1) that had high relative amounts both in S1 and S2. They might be used as the reference compounds in determining the quality of agarwood.
The essential oil of S1 and S2 had significantly different components from that of S3, which had abundant fatty acid and alkanes. For instance, a trace of n-hexadecanoic acid was found in the oils of S1 and S2, whereas it reached 49.47% in S3; oleic acid was 10.18% in S3, but it was totally absent in S1 and S2. As to the alkanes, S1 and S3 were very similar, and most of the alkanes identified in S3 were also identified in S1, whereas no alkanes were detected in S2. This may affect the complex process of agar accumulation and the prolonged duration of accumulation, as well as the contents of fatty acid and alkanes [13]. A higher oil yield would require a longer time for resin formation.
It has been reported that different artificial methods used to stimulate agarwood formation in Aquilaria result in different agarwood qualities. Tamuli and Bhuiyan both showed that the essential oils obtained from the plants (A. agallocha Roxb.) inoculated with fungus, i.e., Chaetomium globosum, for 30 days or from plants injected artificial screws showed similar component distributions with that of healthy trees according to GC-MS [12,13]. Dai et al. found that the essential oils of the agarwood produced by nail insetting and holing for two years were full of sesquiterpenes and aromatic constituents, while the essential oil of agarwood formed through trunk breaking for two years was full of fatty acids [14]. Comparing our results with the above results, we come to the conclusion that the chemical stimulation method is a simple and efficient way of inducing Aqularia plants to form resinous material.
As to the total of 8.93% sesquiterpene compounds identified in essential oil of healthy trees (S3), it was probably because the cutting process was sufficient damage to initiate the resinous material formation process. During the drying period at room temperature, cells might be still alive, and the sesquiterpene metabolic pathways were thus initiated.
2.2. Antimicrobial Activities
Table 2 shows the antimicrobial activities of the three essential oils. The results indicated that all of the three essential oils had some activity against almost all the tested bacteria. All the essential oils were more active against Gram-positive bacterial strains (S. aureus and B. subtilis) than against the Gram-negative bacterial strain E. coli. Taken together, especially the results of MICs and MBCs, the essential oils of S1 and S2 had better antimicrobial activities than that of S3. This is probably because the essential oils of S1 and S2 were full of sesquiterpenes and aromatics, which may include some active components. It’s known that sesquiterpenes usually possess antimicrobial activity. It is critical to note that, if active components are isolated and purified, their antimicrobial activities could become stronger.
Table 2.
Screening results for antimicrobial activity of the three essential oils.
| Essential oil | E. coli | S. aureus | B. subtilis | |
|---|---|---|---|---|
| S1 | AWD a | 8.14 ± 0.05 | 10.29 ± 0.20 | 11.68 ± 0.26 |
| MIC b | 6.25 | 3.125 | 0.39 | |
| MBC c | 25 | 6.25 | 12.5 | |
| S2 | AWD a | 7.00 ± 0.02 | 11.25 ± 0.02 | 11.57 ± 0.02 |
| MIC b | 6.25 | 0.195 | 0.195 | |
| MBC c | 12.5 | 6.25 | 12.5 | |
| S3 | AWD a | 7.52 ± 0.26 | 8.10 ± 0.24 | 9.39 ± 0.30 |
| MIC b | 12.5 | 1.56 | 0.78 | |
| MBC c | >25 | 12.5 | >25 | |
| Gentamicin | AWD a | 23.08 ± 0.88 | 21.45 ± 1.77 | 23.73 ± 0.32 |
| MIC b | 0.487 | 0.487 | 0.487 | |
| MBC c | 0.487 | 0.487 | 0.487 | |
| DMSO | AWD a | 0 | 0 | 0 |
| ddH2O | AWD a | 0 | 0 | 0 |
a AWD = agar well diffusion method. The diameters of the inhibition zone, including the well diameters, are 6 mm; b MIC = minimum inhibitory concentration. The values of the three oil samples are given in mg/mL, and the values of gentamicin are given in μg/mLp; c MBC = minimum bactericidal concentration. The values of the three oil samples are given in mg/ml, and the values of Gentamicin are given in μg/mL.
The negative-control experiments, including the antimicrobial test for DMSO and ddH2O, indicated no microbial contamination in the essential oils and media, as shown by the data in Table 2. 5% v/v DMSO, the maximum concentration used for dissolving essential oil, showed no inhibition on the microbial growth.
This is the first report concerning the antimicrobial activities to the three bacterial strains of Chinese agarwood oil from A. sinensis. In previous studies, Mei [28] showed that the essential oil from Chinese agarwood had anti-MRSA activity; Wetwitayaklung [18] found that the essential oil of agarwood (A. crassna) had antimicrobial activities against S. aureus and C. albicans, but it was not active against E. coli at the maximum study concentration (2 mg/mL). In this study, the MICs and MBCs to E. coli were more than 6.25 and 12.5 mg/mL, respectively, consistent with the report by Wetwitayaklung.
3. Experimental
3.1. Plant Material
The three types of samples included artificially chemically stimulated plants, wild agarwood and stems of six-year-old healthy trees (Table 3). A. sinensis trees were cultivated wild in Haikou City, Hainan Province. The chemical treatment method was used to stimulate the formation of resinous in A. sinensis trees. A chemical reagent (NaCl) of a certain concentration was injected slowly into the xylem part of a tree. Because of water transport, the chemical reagent was transported to the whole body of the tree, thus forming an overall injury in the tree. This would stimulate the whole tree to produce resinous material to defend from the damage. Healthy trees without any treatment were collected as the negative control. The wild agarwood was purchased from Guangxi Yulin Market and identified by Dr. Jianhe Wei.
Table 3.
Materials used in this study.
| Brief Name | Stimulating method | Characterization | Age | Plant origin |
|---|---|---|---|---|
| S1 | chemical method | agarwood | 6 years | A. sinensis |
| S2 | unknown natural factor | agarwood | unknown | A. sinensis |
| S3 | no damage | healthy trees | 6 years | A. sinensis |
3.2. Isolation of Essential Oils
Three samples were powdered, passed through 20 mesh sieves, soaked in water overnight and then subjected to hydrodistillation for 12 h using a Clevenger apparatus. The distilled oil was dried over anhydrous sodium sulfate and stored in a freezer at −20 °C until analysis.
3.3. GC-MS Analysis
GC-MS analysis was performed with a Varian 450 gas chromatograph (USA) equipped with a VF-5MS capillary column (30 m × 0.25 mm i.d., flim thickness 0.25 μm) and a Varian 300 mass spectrometer with an ion trap detector in full scan mode under election impact ionization (70 eV). The carrier gas was helium, at a flow rate of 1 mL/min. The injections were performed in splitless mode at 250 °C. 1 μL essential oil solution in hexane (HPLC grade) was injected. The operating parameters were the temperature program of 50 °C for 1 min, ramp of 10 °C/min up to 155 °C (15 min), subsequent increase to 280 °C with an 8 °C/min heating ramp, and keeping at 280 °C for 10 min. The scan range was 50–500 amu under full scan. 1 μL C8-C40 n-alkanes was injected separately and ran in the same program as the essential oils.
3.4. Identification of Components
Most constituents were identified via gas chromatography by comparing their Kovats retention indices (RI) with those from the literature, and computer matching against the NIST 08 and NIST Chemistry WebBook (http://webbook.nist.gov/chemistry/) databases. The Kovats retention indices were determined in relation to a homologous series of n-alkanes (C8–C40) under the same operating conditions. AMDIS software was used to calculate Kovats retention indices. Further identification was made by comparing their mass spectra with these stored in NIST 08 and with mass spectra from the literature [15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37]. The relative concentrations of the components were obtained by peak areas normalization without applying correction factors.
3.5. Antimicrobial Activity
3.5.1. Test Microorganisms
Three clinical bacteria, Staphylococcus aureus ATCC 25923, Bacillus subtilis ACCC11060 and Escherichia coli ATCC25922, were used as test organisms in the screening. The microbial strains were obtained from the College of Life Science, Capital Normal University, Beijing, China.
3.5.2. Determination of Diameters of Inhibition Zone
Simple susceptibility screening test through agar well diffusion method was used [28]. The inocula of the bacterial strains were adjusted to 0.5 McFarland standard turbidity (approximately 108 CFU/mL) [38,39]. One hundred μL of a suspension containing approximately 108 CFU/mL of each microorganism was spread on nutrient agar (NA). Six-millimeter diameter wells were cut from the agar using a sterile cork-borer, and 50 μL of the oil solution in a concentration of 50 mg/mL (dissolved in DMSO) were delivered into the wells. Negative controls were prepared using DMSO. Gentamincin (100 μg/mL) were used as the positive reference standards. The plates were incubated for 18–24 h at 37°C. The antimicrobial activity was evaluated by measuring the zone of inhibition against the test organisms.
3.5.3. Determination of Minimum Inhibitory Concentration (MIC)
The MICs of the samples against the test bacterial strains were determined by the micro-well dilution method. The inocula of the bacterial strains were adjusted to 0.5 McFarland standard turbidity (approximately 108 CFU/mL). The essential oils were first dissolved in 10% DMSO, and serial two-fold dilutions of the oil were prepared in a 96-well plate, ranging from 3.9 mg/mL to 50 mg/mL. The MIC was defined as the lowest concentration of the essential oil at which the microorganism does not demonstrate visible growth [38,39,40]. Microorganism growth was indicated by turbidity. The MICs of the standard (gentamicin) were also determined in the same experiments.
3.5.4. Determination of Minimum Bactericidal Concentration (MBC)
Referring to the results of the MIC assays, the wells showing complete absence of growth were identified and 10 μL of each well was transferred to agar plates and incubated at 37 °C for 24 h. The MBC was defined as the lowest concentration of the juniper essential oil that allows no growth of microorganisms [40,41,42]. The MBCs of the standard (gentamicin) were also determined in the same experiments.
3.5.5. Data Analysis
All experiments were repeated at least twice. The data were recorded as means±standards and were analyzed with SPSS (version 13.0 for windows, SPSS Inc.).
4. Conclusions
The characterization of the essential oil obtained from the agarwood originated from A. sinensis stimulated by the chemical method has very high similarity with that of the essential oil of wild agarwood, both in chemical composition and antimicrobial activity. This suggests that agarwood could be produced by the artificially chemically stimulation method. What chemical agents and duration would be suitable for inducing better agarwood formation need further studies. This is the first report concerning the analysis of essential oils from chemically stimulated agarwood.
Acknowledgments
We are very grateful to Pan Ruile and Cao Li (Institute of Medicinal Plant Development, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China) for technical support and advice. This work was financially supported by the National Key Project of Scientific and Technical Supporting Programs funded by the Ministry of Science & Technology of China (No. 2007BAI27B01), the Program for New Century Excellent Talents in University of China (No. 2008), and the National Natural Science Foundation of China (31000136), both of which were granted to Jianhe Wei.
Footnotes
Sample Availability: Samples of the compounds are available from the authors.
References and Notes
- 1.Okudera Y., Ito M. Production of agarwood fragrant constituents in Aquilaria calli and cell suspension cultures. Plant Biotechnol. 2009;26:307–315. doi: 10.5511/plantbiotechnology.26.307. [DOI] [Google Scholar]
- 2.Kakino M., Tazawa S., Maruyama H., Tsuruma K., Araki Y., Shimazawa M., Hara H. Laxative effects of agarwood on low-fiber diet-induced constipation in rats. BMC Comple. Altern. Med. 2010;10:68–75. doi: 10.1186/1472-6882-10-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gerard A.P. Agarwood: the life of a wounded tree. Newsletter. 2007;45:24–25. [Google Scholar]
- 4.CITES. Amendments to Appendices I and II of CITES; Proceedings of Thirteenth Meeting of the Conference of the Parties; Bangkok, Thailand. 3–14 October 2004; Unpublished. [Google Scholar]
- 5.Barden A., Anak N.A., Mulliken T., Song M. Heart of the matter: Agarwood use and trade and CITES Implementation for Aquailaria malaccencis. Traffic Network Rep. 2000;46:17–18. [Google Scholar]
- 6.Mohd F.M., Mohd R.Y., Lim H.F., Alias R. Costs and benefits analysis of Aquilaria species on plantation for agarwood production in Malaysia. Int. J. Busi. Soc. Sci. 2010;1:162–174. [Google Scholar]
- 7.Blanchette R.A., Heuveling V.B.H. Cultivated agarwood. US 7,638,145 B2. 2009 Dec 29;
- 8.Qi S.Y. Aquilaria species: in vitro culture and the production of eaglewood (agarwood) (Medicinal and Aromatic Plants III) In: Bajaj Y.P.S., editor. Biotechnology in Agriculture and Forestry. Vol. 8. Springer Verlag; Berlin, Germany: 1995. pp. 36–44. [Google Scholar]
- 9.Chen H.Q., Wei J.H., Yang J.S., Zhang Z., Yang Y. Chemical constituents of agarwood originating from the endemic genus Aquilaria plants. Chem. Biodiver. 2011 doi: 10.1002/cbdv.201100077. [DOI] [PubMed] [Google Scholar]
- 10.Pojanagaroon S., Kaewrak C. Mechanical methods to stimulate aloes wood formation in Aquilaria crassna Pierre ex H. Lec. (Kritsana) trees. In: Jatisatienr A., Paratasilpin T., Elliott S., Anusarnsunthorn V., Wedge D., Craker L.E., Gardner Z.E., editors. Congress on Medicinal and Aromatic Plants. Vol. 2. Acta Horticulturae; Leuven, Thailand: 2003. pp. 161–166. [Google Scholar]
- 11.Zhang Z., Yang Y., Meng H., Sui Ch., Wei J.H., Chen H.Q. Advances in studies on mechanism of agarwood formation in Aquilaria sinensis and its hypothesis of agarwood formation induced by defense response. Chin. Tradit. Herbal Drugs. 2010;41:156–160. [Google Scholar]
- 12.Tamuli P., Boruah P., Nath S.C., Leclercq P. Essential oil of eaglewood tree: a product of pathogenesis. J. Essent. Oil Res. 2005;17:601–604. doi: 10.1080/10412905.2005.9699008. [DOI] [Google Scholar]
- 13.Bhuiyan M.N.I., Begum J., Bhuiyan M.N.H. Analysis of essential oil of eaglewood tree (Aquilaria agallocha Roxb.) by gas chromatography mass spectrometry. Bangladesh J. Pharm. 2009;4:24–28. [Google Scholar]
- 14.Lin F., Mei W.L., Wu J., Dai H.F. GC-MS analysis of volatile constituents from Chinese eaglewood produced by artificial methods. J. Chin. Med. Mater. 2010;33:222–225. [PubMed] [Google Scholar]
- 15.Jarunrattanasri A., Theerakulkait C., Cadwallader K.R. Aroma components of acid-hydrolyzed vegetable protein made by partial hydrolysis of rice bran protein. J. Agric. Food Chem. 2007;55:3044–3050. doi: 10.1021/jf0631474. [DOI] [PubMed] [Google Scholar]
- 16.Ito M., Okimoto K., Yagura T., Honda G. Induction of sesquiterpenoid production by Methyl Jasmonate in Aquilaria sinensis cell suspension culture. J. Essent. Oil Res. 2005;17:175–180. doi: 10.1080/10412905.2005.9698867. [DOI] [Google Scholar]
- 17.Kumeta Y., Ito M. Characterization of δ-guaiene synthases from cultured cells of Aquilaria, responsible for the formation of the sesquiterpenes in agarwood. Plant Physiol. 2010;154:1998–2007. doi: 10.1104/pp.110.161828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wetwitayaklung P., Thavanapong N., Charoenteeraboon J. Chemical constituents and antimicrobial activity of essential oil and extracts of heartwood of Aquilaria crassna obtained from water distillation and supercritical fluid carbon dioxide extraction. Silpakorn Univ. Sci. Tech. J. 2009;3:25–33. [Google Scholar]
- 19.Skaltsa H.D., Mavrommati A., Constantinidis T. A chemotaxonomic investigation of volatile constituents in Stachys subsect. Swainsonianeae (Labiatae) Phytochemistry. 2001;57:235–244. doi: 10.1016/s0031-9422(01)00003-6. [DOI] [PubMed] [Google Scholar]
- 20.Vujisic L., Vuckovic I., Tesevic V., Dokovic D., Ristic M.S., Janackovic P., Milosavljevic S. Comparative examination of the essential oils of Anthemis ruthenica and A. arvensis wild-growing in Serbia. Flavour Fragr. J. 2006;21:458–461. doi: 10.1002/ffj.1681. [DOI] [Google Scholar]
- 21.Moreira D.d.L., Guimaraes E.F., Kaplan M.A.C. Non-polar constituents from leaves of piper lhotzkyanum. Phytochemistry. 1998;49:1339–1342. doi: 10.1016/S0031-9422(98)00067-3. [DOI] [PubMed] [Google Scholar]
- 22.Yu F., Harada H., Yamasaki K., Okamoto S., Hirase S., Tanaka Y., Misawa N., Utsumi R. Isolation and functional characterization of a beta-eudesmol synthase, a new sesquiterpene synthase from Zingiber zerumbet Smith. FEBS Lett. 2008;582:565–567. doi: 10.1016/j.febslet.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 23.Lazari D.M., Skaltsa H.D., Constantinidis T. Volatile constituents of Centaurea pelia DC., C. thessala Hausskn. subsp. drakiensis (Freyn & Sint.) Georg. and C. zuccariniana DC. from Greece. Flavour Fragr. J. 2000;15:7–11. doi: 10.1002/(SICI)1099-1026(200001/02)15:1<7::AID-FFJ860>3.0.CO;2-3. [DOI] [Google Scholar]
- 24.Saroglou V., Dorizas N., Kypriotakis Z., Skaltsa H.D. Analysis of the essential oil composition of eight Anthemis species from Greece. J. Chromatogr. A. 2006;1104:313–322. doi: 10.1016/j.chroma.2005.11.087. [DOI] [PubMed] [Google Scholar]
- 25.Barra A., Coroneo V., Dessi S., Cabras P., Angioni A. Characterization of the volatile constituents in the essential oil of Pistacia lentiscus L. from different origins and its antifungal and antioxidant activity. J. Agric. Food Chem. 2007;55:7093–7098. doi: 10.1021/jf071129w. [DOI] [PubMed] [Google Scholar]
- 26.Zakaria C.A., Marcelline A., Sylvies B., Christian B., Timothée O., Eewan P., Pierre C. Composition, and antimicrobial and remarkable antiprotozoal activities of the essential oil of Rhizomes of Aframomum sceptrum K. SCHUM. (Zingiberaceae) Chem. Biodiver. 2011;8:658–667. doi: 10.1002/cbdv.201000216. [DOI] [PubMed] [Google Scholar]
- 27.Mei W.L., Zeng Y.B., Liu J., Dai H.F. GC-MS analysis of volatile constituents from five different kinds of Chinese eaglewood. J. Chin. Med. Mater. 2007;30:551–555. [PubMed] [Google Scholar]
- 28.Mei W.L., Zeng Y.B., Liu J.W., Cui H.B., Dai H.F. Chemical Composition and Anti-MRSA Activity of the Essential Oil from Chinese Eaglewood. J. Chin. Pharm. Sci. 2008;17:225–229. [Google Scholar]
- 29.Liang Z.Y., Zhang D.L., Liu C.S., Yang J.F. Determination of Chemical Composition of the Essential Oil from Aquilaria sinensis (Lour.) Gilg by CGC-MS. Nat. Sci. J. Hainan Univ. 2005;23:228–232. [Google Scholar]
- 30.Toyota M., Saito T., Asakawa Y. The absolute configuration of eudesmane-type sesquiterpenoids found in the Japanese liverwort Chiloscyphus polyanthus. Phytochemistry. 1999;51:913–920. doi: 10.1016/S0031-9422(99)00159-4. [DOI] [Google Scholar]
- 31.Kristiawan M., Sobolik V., Al-Haddad M., Allaf K. Effect of pressure-drop rate on the isolation of cananga oil using instantaneous controlled pressure-drop process. Chem. Eng. Proc. 2008;47:66–75. doi: 10.1016/j.cep.2007.08.011. [DOI] [Google Scholar]
- 32.Yang J.S., Chen Y.W. Studies on the constituents of Aquilaria sinensis (Lour.) Gilg. I. Isolation and structure elucidation of two new sesquiterpenes, baimuxinic acid and baimuxinal. Acta Pharm. Sinica. 1983;18:191–198. [PubMed] [Google Scholar]
- 33.Raal A., Kaur H., Orav A., Arak E., Kailas T., Muurisepp M. Content and composition of essential oils in some Asteraceae species. Proc. Estonian Acad. Sci. 2011;60:55–63. doi: 10.3176/proc.2011.1.06. [DOI] [Google Scholar]
- 34.Tret'yakov K.V. Retention Data. NIST Mass Spectrometry Data Center. NIST Mass Spectrometry Data Center. 2008. [(accessed on 6 May 2011)]. Available online: http://chemdata.nist.gov/mass-spc/pubs/pittcon-2000/index.htm.
- 35.Kundakovic T., Fokialakis N., Kovacevic N., Chinou I. Essential oil composition of Achillea lingulata and A. umbellata. Flavour Fragr. J. 2007;22:184–187. doi: 10.1002/ffj.1778. [DOI] [Google Scholar]
- 36.Alissandrakis E., Tarantilis P.A., Harizanis P.C., Polissiou M. Comparison of the volatile composition in thyme honeys from several origins in Greece. J. Agric. Food Chem. 2007;55:8152–8157. doi: 10.1021/jf071442y. [DOI] [PubMed] [Google Scholar]
- 37.Zeng Y.X., Zhao C.X., Liang Y.Z., Yang H., Fang H.Z., Yi L.Z., Zeng Z.D. Comparative analysis of volatile components from Clematis species growing in China. Anal. Chim. Acta. 2007;595:328–339. doi: 10.1016/j.aca.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 38.Sokmen A., Gulluce M., Akpulat H.A., Daferera D., Tepe B., Polissiou M., Sokmen M., Sahin F. The in vitro antimicrobial and antioxidant activities of the essential oils and methanol extracts of endemic Thymus spathulifolius. Food Control. 2004;15:627–634. doi: 10.1016/j.foodcont.2003.10.005. [DOI] [Google Scholar]
- 39.Cao L., Si J.Y., Liu Y., Sun H., Jin W., Li Z., Zhao X.H., Pan R.L. Essential oil composition, antimicrobial and antioxidant properties of Mosla chinensis Maxim. Food Chem. 2009;115:801–805. doi: 10.1016/j.foodchem.2008.12.064. [DOI] [Google Scholar]
- 40.Unlü M., Vardar-Unlü G., Vural N., Dönmez E., Ozbaş Z.Y. Chemical composition, antibacterial and antifungal activity of the essential oil of Thymbra spicata L. from Turkey. Nat. Prod. Res. 2009;23:572–579. doi: 10.1080/14786410802312316. [DOI] [PubMed] [Google Scholar]
- 41.Suttiwet C. Antimicrobial activity of essential oil from Nelumbo Nucifera Gaertn. Pollen. Int. J. Pharm. 2009;5:98–100. doi: 10.3923/ijp.2009.98.100. [DOI] [Google Scholar]
- 42.Wang J.H., Zhao J.L., Liu H., Zhou L.G., Liu Z.L., Wang J.G., Han J.G., Yu Z., Yang F.Y. Chemical analysis and biological activity of the essential oils of two valerianaceous species from China: Nardostachys chinensis and Valeriana officinalis. Molecules. 2010;15:6411–6422. doi: 10.3390/molecules15096411. [DOI] [PMC free article] [PubMed] [Google Scholar]