Abstract
Plesiomonas shigelloides is an emerging pathogen with damaging effects on human health such as gastroenteritis and extraintestinal infections. Here, we carried out a bibliometric survey that aimed to examine publication trends in Plesiomonas-related research by time and place, international collaborative works, identify gaps and suggest directions for future research. The search term “Plesiomonas shigelloides” was used to retrieve articles published between 1990 and 2017 from the Web of Science database. Only primary research articles were included in the analysis. A total of 155 articles were published within the survey period, with an average of 5.54±2.66 articles per year and an annual growth rate of −0.8%. Research output peaked in 2000 and 2006 (each accounting for 7.7% of the total). The United States ranked first in terms of numbers of articles (n = 29, 18.1%) and total citations (n = 451). Cameroon, Canada, Cuba, Switzerland and Turkey co-shared the 10th position each with 2 articles (1.3%). Research collaboration was low (collaboration index = 3. 32). In addition to Plesiomonas shigelloides (n = 82, 52.9%), the top Authors Keywords and research focus included lipopolysaccharide and nuclear magnetic resonance (n = 13, 8.4%). Diarrhea (n = 43, 27.7%), Aeromonas species (n = 41, 26.5%) and infections (n = 31, 20.0%) were also highly represented in Keywords-Plus. Authors’ collaborations and coupling networks formed two mega-clusters which nodes were shared solely by authors from high-income countries. The common conceptual framework in retrieved articles determined by K-means clustering revealed three clusters with sizes of 7, 16, and 29, representing research responses focused on extraintestinal and gastroenteritis, P. shigelloides lipopolysaccharide structure, and co-infections, respectively. Our bibliometric analysis revealed a global diminishing research in Plesiomonas; greater research outcomes from high-income countries compared to others and low collaboration with developing countries.
Introduction
Plesiomonas shigelloides is a bacterium that has been labeled as an emerging pathogen for over three decades. There are many outstanding questions regarding its pathogenic potential, despite evidence for its detrimental effects on human health such as gastroenteritis and extraintestinal diseases [1–7]. Also, some food and waterborne outbreaks have been traced to P. shigelloides [8–13], and the incidence of Plesiomonas infections linked to immunocompromised health [14] is increasing, especially in light of present-day lifestyles [15]. Climate change and global warming are also predicted to contribute to increased incidence of waterborne infectious diseases including Plesiomonas infections [16–19].
Accurate estimates of the incidence of Plesiomonas-related gastroenteritis and extraintestinal infections both globally and at the level of individual countries remain unknown [1,2,4–7,10,20–37]. Retrospective reviews of infections due to P. shigelloides in China and Hong Kong have been published [38], and cases of P. shigelloides co-infection with viral and bacterial diarrheal pathogens are common in the literature [39]. Prevalence of P. shigelloides gastroenteritis varies considerably across regions, with lower rates reported from North America and Europe and higher estimates from Southeast Asia and Africa [40]. Nonetheless, there is a general underestimation of P. shigelloides infection, in part because it shares some clinical manifestations with other pathogens [38]. P. shigelloides is not routinely examined in clinical settings, and as such, awareness regarding this pathogen remains limited.
Bibliometric analysis is a statistical method for assessing both the quantitative and qualitative scope and adequacy of research efforts attained in an area of interest [41]. It can be used to determine national and international research focus and evaluate research performance in order to identify future research priorities, funding sources, and interdisciplinary collaborations [42–44]. It also provides a resource to policy-makers for implementing necessary prophylactic measures in case the analysis reveals a sharp increase in case reports or articles regarding a health issue in a particular geographic area [45,46]. Bibliometric reviews can additionally help international health agencies to identify priorities (e.g. by nations) for disbursing aid [47] and awarding research grants.
There have been a few recent reviews on P. shigelloides [2,14,48] but no comprehensive surveys of published studies on P. shigelloides have been yet conducted. On the other hand, bibliometric analyses have been applied to global disease research on viral agents such as dengue virus [49], Ebola virus [50], John Cunningham virus [51], Mayaro virus [52], Middle East respiratory syndrome coronavirus [53][34], yellow fever virus [54], West Nile virus [55], and Zika virus [56]; and bacterial agents such as Campylobacter [45], Leishmania species [57], and Mycobacterium tuberculosis [58]. Other bibliometric analyses have addressed Plasmodium species and resistant malaria vectors [59,60], Toxocara species [43], and antifungal triazole resistance (especially in Candida and Aspergillus species) [46].
Here we carried out a bibliometric analysis of studies on P. shigelloides published between 1990 to 2017. The articles were evaluated in terms of annual and country-specific output, theme, domain clusters, international collaboration networks, citations, topical evolution related to keywords and co-occurrence networks, co-authorship, and funding. The aim of the survey was to evaluate international participation in P. shigelloides research—with a special interest in regions where Plesiomonas infections have higher prevalence rates (i.e., Africa and Southeast Asia)—in order to address knowledge gaps and provide a resource that can help identify present and future research priorities.
Methods
Preamble and terms definition
Bibliometrix package is a suite of tools for accurate publication data processing such as file conversion, term extraction, duplicate matching and merging, descriptive analysis, matrix building and similarity normalization for network analysis [61]. Matrices are built from publication dataset (e.g.; authors, words, countries, references, keywords) for coupling, co-citation, collaboration, conceptual framework and multiple correspondence analyses. Bibliographic coupling occurs between two articles ⅈ and ⅉ when their reference lists cited at least one common source [62]. But, in a collaboration network, the nodes comprise authors and the links co-authorships [63]. The number of bibliographic coupling that occurs between articles ⅈ and ⅉ or co-authorship in scientific collaboration network denotes the strength of the network [61]. A network depicts relationships in a system as a set of nodes (components) and links (relationships) [64]. Co-Word or conceptual framework analysis explore K-means clustering and other dimensionality reduction techniques to identify clusters of common concepts known in a bibliographic collection. It relies on word co-occurrences in a publication dataset [61,64]. Scientific productivity or an author’s contributions in a field is evaluated in term of Lotka’s law [65]. The Lotka’s law is an inverse square law that describes how often authors published in a field [65].
Data retrieval
Published peer-reviewed articles on P. shigelloides were retrieved from the Web of Science (WoS) database on August 19, 2018. The WoS is among the most reliable and comprehensive databases for bibliometric studies and hosts a wide range of quality and high-impact scientific studies (12 million articles in over 12,000 journals) [44]. We used the search term “Plesiomonas shigelloides” to identify primary research articles published between 1990 and 2017. All available information was retrieved. To obtain subject-specific results and for the sake of accuracy (in order to avoid false-positive results), only article titles were searched. A title-specific search has been reported to increase recovery and specificity with a minimal loss of sensitivity compared to a topic search [44,45,66]. Articles were downloaded in the BibTeX file format. In order to account for variations in country population on rate of scientific production, the world population was retrieved from the World Bank website (https://data.worldbank.org/indicator/SP.POP.TOTL) and the mid-period population corresponding to the top 19 countries was extracted for calculation of article per million populations. England population data was retrieved from Office for National Statistics website (https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/timeseries/enpop/pop).
Data processing and analysis
We analyzed the retrieved data for bibliometric indicators using Rstudio v.3.4.1 software (2017-06-30) with bibliometrix R-package (http://www.bibliometrix.org) [61]. Data were imported into RStudio and converted to a bibliographic data frame and normalized for duplicate marching. The duplicated article was reduced to one record in the analysis. The data frame as typical columns named after the standard ISI WoS Field Tag codify.
Further, authors' names, authors' keywords (DE), and Keywords-Plus (ID) were extracted for standardization. Authors' names were extracted twice as two different sets (A and B). Each set was checked for variant names, spelling errors and matched with affiliations. We achieved normalized authors' names when |A ∩ B| ≡ |A ∪ B|. For keywords (DE) and Keywords-Plus (ID), a primary term was assigned to words with similar meanings (e.g., “Plesiomonas shigelloides", "Plesiomonas", "shigelloides", "Aeromonas-shigelloides", and "Plesiomonas-shigelloides" were allotted to “Plesiomonas shigelloides”). Multiple occurrences of a keyword or a similar keyword in an article were regarded as one. Co-occurrence of a term in authors' keywords (DE set) and Keywords-Plus (ID set) in the dataset was assessed as a set made of the intersect of the two sets (DE ⋂ ID||). All set-based test was performed using a Venn diagram software (http://bioinformatics.psb.ugent.be/webtools/Venn/). An annual number of articles and total citations were also graphed.
Data were analysed for descriptive output, citation analysis, authors’ h-index and scientific productivity using the relevant functions of the bibliometrix R-package. Bibliometric networks (e.g., citation, author, country, author keyword, and Keywords-Plus networks) and bibliographic coupling (co-citation and keyword co-occurrences) were computed and visualized from bibliometric two-way (bipartite) network of rectangular matrices of Articles × Attributes. A typical bibliometric network is expressed as Network(N) = X × NT where X is a bipartite network matrix composed of Articles × Attribute (e.g. Authors, keywords, citations, and Countries) and N is a symmetrical matrix N = NT.
We created a graphic model of all networks using force-directed algorithms (Fruchterman) implemented in the networkPlot function of the bibliometrix R-package. All networks were standardized using the Simpson’s coefficient (inclusion index), proximity index (association strength), the Jaccard’s similarity index, and the Salton’s cosine coefficient among nodes of a network [61]. In addition, k-means clustering were performed on keywords to evaluate concepts in Plesiomonas field of research using the function conceptualStructure of the package. The function implements Porter’s stemming algorithm [67] to modulate inflected words to their root form. For detailed search Boolean for articles identification from WoS, see supplementary file (S1 Appendix). Other bibliometric indicators such as language and affiliation were determined using the content search of BibTeX file.
Results
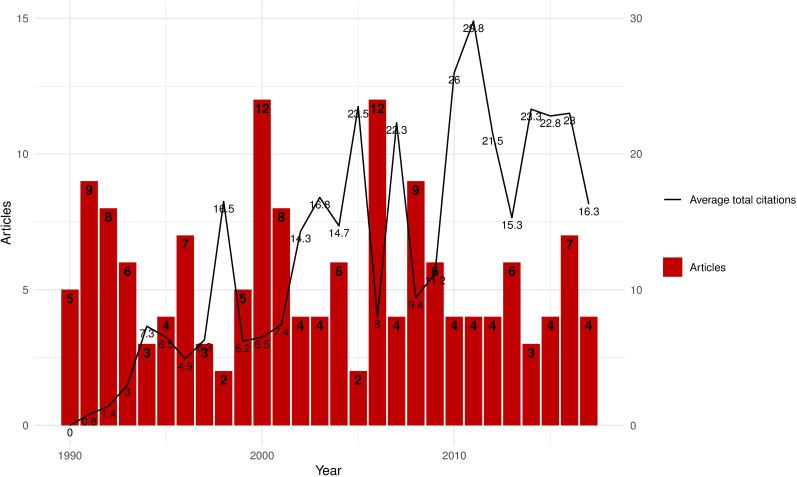
A total of 155 articles were published within the survey period; their attributes are presented in Table 1. The studies involved 493 authors, with 0.31 article/author (3.18 authors/article), 4.34 co-authors/article, and a collaboration index of 3.32. With the exception of two authors publishing solo, all 491 authors were involved in multi-author articles. An average of 11.49 citations/article was recorded during the study period. The scientific output related to P. shigelloides research by Lotka’s law showed a beta coefficient and constant of 2.30 and 0.44, respectively, with a Kolmogorov-Smirnoff goodness-of-fit of 0.92 (P = 0.46, two-sample t-test). The lack of a statistical difference between the theoretical and observed Lotka’s distributions indicates that Lotka’s law does not apply to P. shigelloides research productivity. Published studies on P. shigelloides from 1990 to 2017 and average total citations of articles by year are shown in Fig 1. The annual growth rate was −0.8%, with an overall mean of 5.54±2.66, suggesting that research on P. shigelloides has been decreasing overtime. Research output fluctuated during the survey period, peaking in 2000 and 2006 (each accounting for 7.7% [12/155] of the total). Similarly, average total citations of articles published fluctuated over the years and peaked in the year 2011 (average = 29.8).
Table 1. Summary information on retrieved P. shigelloides studies, 1990–2017.
| Descriptions | Counts and rates |
|---|---|
| No. of articles | 155 |
| No. of authors | 493 |
| Involved in single-author articles | 2 |
| Involved in multi-author articles | 491 |
| Articles/author | 0.31 |
| Authors/article | 3.18 |
| Co-author appearances | 673 |
| Co-authors/article | 4.34 |
| Collaboration index (CI) | 3.32 |
| Average no. of citations/article | 11.49 |
| Study source (journals) | 90 |
| Keywords-Plus (ID) | 398 |
| Author’s keywords (DE) | 259 |
| Language | |
| English | 147 |
| German | 4 |
| Spanish | 4 |
Fig 1. Published studies on P. shigelloides from 1990 to 2017.
ATC, average total citations of articles published in a year. The annual growth rate was −0.8%. Research output fluctuated during the study period and peaked in 2000 and 2006 (12 articles [7.7%] in each of those years; mean: 5.54±2.66 per year, range: 2.0–12.0).
Table 2 shows the top 20 most productive authors in the field. K. Krovacek (Sweden) ranked first, co-authoring 12 (7.7%) articles; and I. Ciznar (Slovak Republic) was second with 11 (7.1%) articles. The h_index (total citations) was 7 (162) for K. Krovacek, and 6 (148) for I. Ciznar. It is worth noting that the topmost active authors were affiliated with institutions in developed nations, including Sweden (n = 6), USA (n = 5), Japan (n = 3), Spain (n = 5), Poland (n = 3), Czech Republic (n = 1), and Slovak Republic (n = 1).
Table 2. Top 20 productive authors on P. shigelloides.
| Rank | Author | Affiliation | Nation | Articles | % of 155 | h_index | TC |
|---|---|---|---|---|---|---|---|
| 1 | Krovacek, K. | Sveriges Lantbruksuniversitet Biomedical Centre | Sweden | 12 | 7.7 | 7 | 162 |
| 2 | Ciznar, I. | Institute of Preventive and Clinical Medicine | Slovak Republic | 11 | 7.1 | 6 | 148 |
| 3 | Aldova, E. | National Institute of Public Health Prague Czechia | Czech Republic | 10 | 6.5 | 7 | 125 |
| 3 | Lugowski, C. | Polish Academy of Sciences and University of Opole | Poland | 10 | 6.5 | 7 | 142 |
| 4 | Gonzalez-Rey, C. | Sveriges Lantbruksuniversitet, Biomedical Centre | Sweden | 8 | 5.2 | 5 | 88 |
| 4 | Lukasiewicz, J. | Polish Academy of Sciences | Poland | 8 | 5.2 | 5 | 97 |
| 4 | Niedziela, T. | Swedish University of Agricultural Sciences | Sweden | 8 | 5.2 | 6 | 130 |
| 5 | Levin, R.E. | University of Massachusetts | USA | 7 | 4.5 | 3 | 24 |
| 6 | Jachymek, W. | Swedish University of Agricultural Sciences | Sweden | 6 | 3.9 | 6 | 123 |
| 6 | Kaszowska, M. | Polish Academy of Sciences | Poland | 6 | 3.9 | 3 | 29 |
| 6 | Tomas, J.M. | Universidad de Barcelona | Spain | 6 | 3.9 | 4 | 55 |
| 7 | Gu, W. | University of Massachusetts | USA | 5 | 3.2 | 2 | 16 |
| 7 | Kenne, L. | Swedish University of Agricultural Sciences | Sweden | 5 | 3.2 | 5 | 115 |
| 7 | Merino, S. | Universidad de Barcelona | Spain | 5 | 3.2 | 3 | 39 |
| 8 | Henderson, D.P. | University of Texas at Austin | USA | 4 | 2.6 | 4 | 67 |
| 8 | Okawa, Y. | Tohoku Pharmaceutical University | Japan | 4 | 2.6 | 4 | 53 |
| 8 | Shimada, T. | National Institute of Infectious Diseases | Japan | 4 | 2.6 | 3 | 36 |
| 8 | Svenson, S.B. | Swedish University of Agricultural Sciences | Sweden | 4 | 2.6 | 3 | 58 |
| 8 | Tsugawa, H. | Tohoku Pharmaceutical University | Japan | 4 | 2.6 | 4 | 53 |
| 9 | *Aquilini, E. | University of Barcelona | Spain | 3 | 1.9 | 2 | 12 |
Ranking based on the number of articles; TC, total citations.
*Shared with 9 others: Bravo, L. (Cuba), Corsaro, M.M. (Italy), Garcia-Lopez, M.L. (Spain), Hernandez, P. (Venezuela), Hostacka, A.(Slovakia), Lanzetta, R. (Italy), Obi, C.L. (Nigeria/South Africa), Otero, A. (Spain), Parrilli, M. (Italy), Pelayo, J.S. (Brazil), Pieretti, G. (Italy), Qadri, F. (Bangladesh), Sack, D.A. (USA), Santos, J.A. (Spain), Saridakis, H.O. (Brazil), Schneerson, R. (USA), Stock, I. (Germany),Yuen, K.Y. (China).
The 20 top cited articles on P. shigelloides are listed in S1 Table. These studies spanned the fields of infection, immunity, clinical microbiology, and biochemistry. The total no. of citations of the top-cited articles ranged from 35 to 111; most of these were the result of funded research.
Research output related to P. shigelloides for the top 20 most active countries is shown in Table 3. United States ranked first in terms of total number of articles (n = 29, 18.7%) and citations (n = 451), followed by Sweden (n = 14, 9.0%) and Germany (n = 10, 6.5%). The frequency of publication varied among the top countries from 1.3 to 18.7%. Sweden had highest productivity (1.563 article/million population) when normalized for population size using mid-period population (2003). The rank order of these countries changed when productivity was measured based on the number of citations per country, with only United States and Sweden remaining in the same positions. Other countries that made up the top 20 based on citations per country were Hong Kong (27.0), Australia and Finland (23.0–23.3). Asian countries in the top 20 list were Japan (n = 9), China (n = 7), Bangladesh (n = 5), and India (n = 3). Nigeria (n = 4) and Cameroon (n = 2) were the only African countries in the top 20 list.
Table 3. Most productive countries in terms of P. shigelloides research.
| Productivity based on no. of articles | Productivity based on no. of citations per country | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rank | Country | Articles | SCP | MCP | Frequency (%) | A/MP | Rank | Country | TC | ACC | |
| 1 | USA | 29 | 23 | 6 | 18.7 | 0.100 | 1 | USA | 451 | 15.6 | |
| 2 | Sweden | 14 | 1 | 13 | 9.0 | 1.563 | 2 | Sweden | 244 | 17.4 | |
| 3 | Germany | 10 | 9 | 1 | 6.5 | 0.121 | 3 | Australia | 93 | 23.3 | |
| 4 | Japan | 9 | 8 | 1 | 5.8 | 0.236 | 4 | Japan | 91 | 10.1 | |
| 4 | Poland | 9 | 5 | 4 | 5.8 | 0.071 | 5 | Brazil | 74 | 10.6 | |
| 5 | Brazil | 7 | 5 | 2 | 4.5 | 0.038 | 6 | Germany | 70 | 7.0 | |
| 5 | China | 7 | 6 | 1 | 4.5 | 0.005 | 7 | England | 68 | 17.0 | |
| 6 | Czech Republic | 6 | 5 | 1 | 3.9 | 0.589 | 8 | Poland | 62 | 6.9 | |
| 6 | Spain | 6 | 5 | 1 | 3.9 | 0.073 | 9 | China | 54 | 7.7 | |
| 7 | Bangladesh | 5 | 4 | 1 | 3.2 | 0.036 | 10 | Czech Republic | 50 | 8.3 | |
| 8 | Australia | 4 | 4 | 0 | 2.6 | 0.201 | 10 | Spain | 50 | 8.3 | |
| 8 | England | 4 | 3 | 1 | 2.6 | 0.070 | 11 | France | 49 | 16.3 | |
| 8 | Italy | 4 | 0 | 4 | 2.6 | 0.030 | 12 | Italy | 46 | 11.5 | |
| 8 | Nigeria | 4 | 4 | 0 | 2.6 | 0.001 | 13 | Bangladesh | 43 | 8.6 | |
| 9 | France | 3 | 2 | 1 | 1.9 | 0.558 | 14 | Hong Kong | 27 | 27.0 | |
| 9 | India | 3 | 3 | 0 | 1.9 | 0.116 | 15 | Finland | 23 | 23.0 | |
| 9 | Slovakia | 3 | 1 | 2 | 1.9 | 0.048 | 16 | Canada | 20 | 10.0 | |
| 9 | Venezuela | 3 | 3 | 0 | 1.9 | 0.003 | 17 | Netherlands | 16 | 16.0 | |
| 10* | Cameroon | 2 | 2 | 0 | 1.3 | 0.121 | 18 | Nigeria | 15 | 3.8 | |
| 10* | Canada | 2 | 0 | 2 | 1.3 | 0.063 | 19 | India | 13 | 4.3 | |
SCP: single country publications; MCP: multiple country publications; A/MP: Articles per million populations (2003 population); TC: Total Citations; AAC: Average Article Citations
*co-shared with Cuba, Switzerland and Turkey.
The top 20 journals with the most published articles on P. shigelloides are listed in S2 Table. These journals cover a range of subjects including carbohydrates, microbiology, food science, infectious disease, immunology, and biochemistry, reflecting active areas in Plesiomonas research. Carbohydrate Research ranked first (n = 9, 5.8%), followed by Journal of Clinical Microbiology, Folia Microbiologica and Food Biotechnology each with 6 articles (3.9%).
Table 4 shows the most relevant keywords related to Plesiomonas studies, including both author keywords (DE) and Keywords-Plus (ID). Both Author Keywords (DE) and Keywords-Plus (ID) have 10 keywords in common (lipopolysaccharide, Aeromonas species, antigens, oligosaccharide, disease, children, diarrhea, Shigella, virulence, and water). Fourteen keywords were unique to Author Keywords (Plesiomonas shigelloides, nuclear magnetic resonance (NMR), PCR, structure, MALDI-TOF, fish, pathogenicity, media, meningoencephalitis, resistance, enterotoxin, gastroenteritis, serotyping, and sepsis), and 13 keywords were unique to Keywords-Plus (infections, Escherichia, septicemia, environments, in-vitro, polysaccharide, Vibrio species, bacteria, biological repeating unit, iron, meningitis, humans, and strains). The unique Author Keywords primarily described medium of transmission (fish) and methods involved in isolation and characterization of the microorganism (NMR, MALDI-TOF, PCR, enterotoxin, resistance, pathogenicity, serotyping, and structure) and specific infections (meningoencephalitis, gastroenteritis, and sepsis). Author keyword terms associated with identification methods of P. shigelloides included polymerase chain reaction (PCR, n = 8, 5.2%), matrix-assisted laser desorption/ionization–time-of-flight mass spectrometry (MALDI-TOF, n = 6, 3.9%), and serotyping (n = 3, 1.9%). A total of 82 (52.9%) articles reported author keywords (DE) related to Plesiomonas shigelloides. Keywords in articles focusing on analysis of P. shigelloides cell wall structure included lipopolysaccharide (n = 13—, 8.4% (DE); n = 21, 13.6% (ID)), (NMR; n = 13, 8.4% (DE)), structure (n = 7, 4.5% (DE)), antigens (n = 6, 3.9% (DE), n = 19, 12.3% (ID)), biological repeating unit (n = 11, 7.1% (ID), and oligosaccharide (n = 6, 3.9, n = 11, 7.1% (ID)). The Keyword analysis identified diarrhea in 4 (2.6%) and 43 (27.7%) articles by author keyword and keyword pus respectively. Co-infection with Escherichia coli (n = 24, 15.5% (ID)) and Aeromonas species (n = 6, 3.9% (DE), n = 41, 26.5% (ID)) was represented. Keywords linked to extraintestinal P. shigelloides infection included septicemia (n = 24, 15.5% (ID)), meningoencephalitis (n = 4, 2.6 (DE)), Sepsis (n = 3, 1.9% (DE)), and meningitis (n = 10, 6.5% (ID)), which ranked 5th, 11th, and 11th respectively.
Table 4. Most relevant keywords.
| Rank | Author keywords (DE) | Freq. (% of 155) | Rank | Keywords-Plus (ID) | Freq. (% of 155) |
|---|---|---|---|---|---|
| 1 | Plesiomonas shigelloides | 82(52.9) | 1 | Diarrhea | 43(27.7) |
| 2 | Lipopolysaccharide | 13(8.4) | 2 | Aeromonas species | 41(26.5) |
| 2 | NMR | 13(8.4) | 3 | Infections | 31(20.0) |
| 3 | PCR | 8(5.2) | 4 | Escherichia | 29(18.7) |
| 4 | Structure | 7(4.5) | 5 | Septicemia | 24(15.5) |
| 5 | Aeromonas species | 6(3.9) | 4 | Lipopolysaccharide | 21(13.6) |
| 6 | Antigens | 6(3.9) | 5 | Disease | 20(12.9) |
| 6 | MALDI-TOF | 6(3.9) | 5 | Antigens | 19(12.3) |
| 7 | Oligosaccharide | 6(3.9) | 6 | Water | 15(9.7) |
| 8 | Disease | 5(3.2) | 7 | Environments | 14(9.0) |
| 9 | Fish | 5(3.2) | 8 | In-vitro | 13(8.4) |
| 11 | Pathogenicity | 5(3.2) | 8 | Polysaccharide | 13(8.4) |
| 11 | Children | 4(2.6) | 9 | Vibrio species | 12(7.7) |
| 11 | Diarrhea | 4(2.6) | 10 | Bacteria | 11(7.1) |
| 11 | Media | 4(2.6) | 10 | Biological repeating unit | 11(7.1) |
| 11 | Meningoencephalitis | 4(2.6) | 10 | Humans | 11(7.1) |
| 11 | Resistance | 4(2.6) | 10 | Oligosaccharide | 11(7.1) |
| 12 | Enterotoxin | 3(1.9) | 10 | Shigella | 11(7.1) |
| 12 | Gastroenteritis | 3(1.9) | 11 | Children | 10(6.5) |
| 12 | Sepsis | 3(1.9) | 11 | Iron | 10(6.5) |
| 12 | Serotyping | 3(1.9) | 11 | Meningitis | 10(6.5) |
| 12 | Shigella | 3(1.9) | 11 | Strains | 10(6.5) |
| 12 | Virulence | 3(1.9) | 11 | Virulence | 10(6.5) |
| 12 | Water | 3(1.9) |
MALDI–TOF MS, matrix-assisted laser desorption/ionization–time-of-flight mass spectrometry; NMR, nuclear magnetic resonance; PCR, polymerase chain reaction.
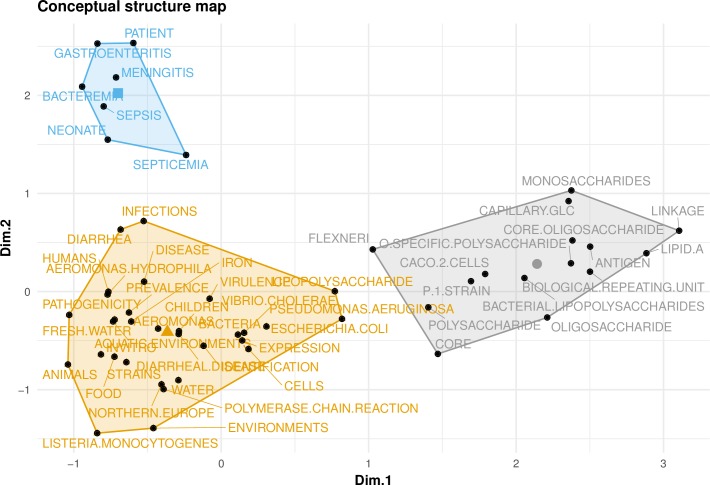
The common conceptual frames in retrieved articles determined by K-means clustering with three clusters of 8, 14, and 39 elements showed research responses focused on neonates (children) extraintestinal infections and gastroenteritis, elucidation of cell wall structure (lipopolysaccharides, core oligosaccharide, o-specific polysaccharide etc.), and co-infections of P. shigelloides with other pathogens, respectively (Fig 2). The 33-element cluster explained the co-occurrence and co-infection of P. shigelloides with other bacteria. Other indicators of frequently represented concepts and frameworks related to P. shigelloides included co-occurrence of terms and keywords. S1 Fig shows the co-occurrence network of the top 20 terms associated with P. shigelloides studies, while S2 Fig shows the co-occurrence networks of keywords. These concept-related frameworks or terms included virulence, meningoencephalitis, Aeromonas, newborn, antigen, pathogenicity, sepsis, diarrhea, infections, Escherichia coli, septicemia, biological repeating unit, diarrheal disease/gastroenteritis, lipopolysaccharide, water, iron, polysaccharide, bacteremia, meningitis, septicemia, lipid-A, strains, and aquatic environments.
Fig 2. Common conceptual frames associated with P. shigelloides studies.
The 155 retrieved articles showed K-means clustering with three clusters of sizes 8, 13, and 33 reflecting concepts frequently linked to P. shigelloides.
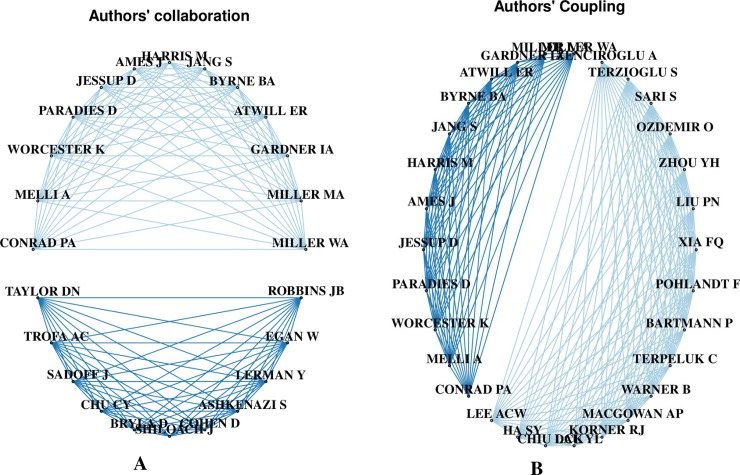
The top 20 authors’ collaboration and coupling networks on P. shigelloides studies were divided into two mega-clusters or spheres with nodes occupied solely by researchers from high-income countries (Fig 3A and 3B). The first sphere of the authors’ network comprised 13 nodes (authors) with no fewer than 10 linkages, while the second sphere included 10 nodes (authors) with the number of collaboration linkages ranging from nine to 10. Similarly, the two separate authors’ coupling network spheres included 13 and 17 authors’ networks, respectively; collaborative conjugation ranged from eleven nodes (authors) in the former and 18 in the latter.
Fig 3. The top 20 authors’ collaboration and coupling networks on P. shigelloides studies.
A. Top 20 authors’ collaboration networks on P. shigelloides studies. Each node in the network represents a different author’ collaboration with other authors. Connecting lines represent collaboration pathways between authors. The number of lines from a node corresponds to a number of co-authorship. B. Top 20 authors’ coupling networks on P. shigelloides studies. Each node in the network represents a different author coupling with other authors. Connecting lines represent coupling pathways between authors. The number of lines from a node corresponds to the number of articles that co-listed the author in their reference list.
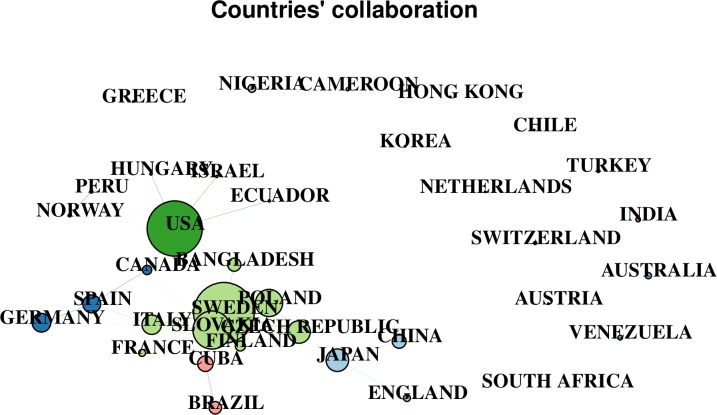
Fig 4 shows 50 countries’ collaboration networks on P. shigelloides studies. Collaboration pathways ranged from 1 to 9. The Sweden had a high number of collaborations (n = 9), followed by United States (n = 8), Slovakia (n = 8), and Italy (n = 7). Other countries had no collaboration networks. Prominent network color code: green, USA network; purple, Spain network; light green, Sweden network; pink, Cuba-Brazil network; blue, Japan-China network.
Fig 4. Fifty (50) countries’ collaboration networks on P. shigelloides studies.
Each node in the network represents a different nation and the node’s diameter corresponds to the strength of a nation’s collaboration with other countries. Lines represent collaboration pathways between countries.
Discussion
The present bibliometric analysis of P. shigelloides examined global research trends between 1990 and 2017 based on data retrieved from WoS. We found that the number of research articles on P. shigelloides increased non-linearly from 5 to 155 articles. However, a negative trend in rate of increase was noted (−0.8%) suggesting that research on P. shigelloides has not been of broad interest in the past 27 years, likely due to discontinuation of Plesiomonas-related research by certain authors and differences in regional distribution of the microorganism. Furthermore, the function estimates and goodness-of-fit indicated that scientific output on P. shigelloides does not follow Lotka’s law, suggesting that the number of articles related to Plesiomonas research will further decline in the future. In general, emerging and re-emerging bacterial pathogens are not accorded the same degree of attention as their viral counterparts. Health emergencies (e.g., outbreaks of infection) relating to emerging viral pathogens including Zika and Chikungunya viruses have driven the generation of new scientific knowledge, resulting in a significant increase in the number of research articles on these subjects [68]. For instance, in 2005 only eight articles were published on Chikungunya virus, but by 2014, the number had reached 302 [69]. Similarly, only 43 articles on Ebola virus were published in 2013 prior to the Ebola outbreaks in West Africa, but this increased to more than 600 articles in 2014 [70].
As is the case with other research areas, most of the leading authors in the Plesiomonas research field were from developed nations such as the United States, Sweden, Austria, Japan, Spain, Poland, Czech Republic, and the Slovak Republic, with few from low-income countries, thus follow the similar trend of low productivity of the region in other research areas. It has been suggested that the economic strength (growth) of a nation influences the research output [43,71–73]. The higher prevalence of Plesiomonas infections in developing countries [74] should motivate researchers in countries most affected to carry out more studies on this pathogen.
Some of the most frequently cited studies pertained to health and identification of the pathogen—for example, efforts to produce a multivalent vaccine using O-specific polysaccharides from Shigella spp. and P. shigelloides; iron uptake in Plesiomonas shigelloides; and comparative analysis of P. shigelloides and E. coli (Shigella) sonnei O-antigen gene clusters. Others focused on P. shigelloides hemolytic expression; serological analysis of the Plesiomonas core; and multilocus sequence typing of Plesiomonas and its pathogenic potential. The United States and Sweden dominated the list of top 20 countries most actively researching Plesiomonas in terms of numbers of articles and citations. In addition to economic strength and availability of research facilities and funding [43,71–73], this productivity can be ascribed to a high level of intra-national and possibly multinational collaboration with other institutions, which can impact research visibility and citation frequency [43,73,75]. In particular, the dominance of the United States has been noted in other fields of research [42,54,76,77]. Also, authors’ multiple affiliations influence country collaboration network. Conversely, the low contributions from developing countries including countries from Africa characterized by a high frequency of self-funded or independent studies [78], mirror the situation of research in other fields. The shift in rank among the top 20 nations most active in the Plesiomonas research field when productivity was measured based on total citation per country ought not be regarded as a precise measure of productivity. Citation rate does not reflect publication output of an author or country [76], since the smaller the number of articles used for estimation, the larger the impact of a few frequently cited articles [76]. Self-citations and inaccurate citations can also provide false quality metrics [76].
The most frequently mentioned keywords and research areas (including publication outlets) associated with Plesiomonas studies reflect the research hotspot during the survey period, which included cell wall (Carbohydrate research), co-infection, and extraintestinal infections such as septicemia, bacteremia, and meningitis. These findings reveal the most pressing health issues related to Plesiomonas-induced gastroenteritis and extraintestinal infections, and co-infections with other pathogens and effort to gain an understanding of the structural architect of the pathogen’s cellwall; this was supported by other conceptual framework indicators such as co-words or keyword co-occurrence networks. However, important topics such as strain-based delineation and identification, including detection of pathogenic and non-pathogenic strains, that are necessary for infection management were lacking and were not evident from the bibliometric analyses. Newer research themes such as molecular and genomic-level studies as an alternative or complementary to traditional experimentation (which has some limitations) necessary to clarify the pathogenesis of Plesiomonas infection were not apparent throughout this study. A bibliometric survey complemented with a narrative review or meta-analysis may be beneficial in Plesiomonas research. Future research is needed to answer questions related to what particular strain of the microorganisms are pathogenic and how to differentiate pathogenic variants from non-pathogenic ones.
The two mega-clusters or spheres in the top authors’ collaboration and coupling networks on P. shigelloides studies showed collaboration pathways mainly among authors from high-income countries, which is similar to trends observed in collaboration network analyses of human immunodeficiency virus and human papilloma virus studies [78]. Alliances between developing and developed countries are rare in a number of scientific areas [78]. Among researchers in the United States, collaboration pathways were largely intra-national, as suggested by a large number of publications but 6 multiple country publications. In contrast, collaborations by authors in Cuba, Finland, Czech Republic, Italy, Slovakia, and Sweden tended to be multi-national, which is more valuable for the epidemiological control of pathogens. The absence of collaboration pathway in Venezuela, India, Nigeria, and other African nations is consistent with the low number of publications from these countries. Intra- and international collaborations between developed and developing nations could provide opportunities for the division of labor and resources to address important scientific questions.
There were some limitations related to the bibliometric survey adopted in this study, including the use of a single database (the WoS), the low sensitivity and strictness of the search terms and search strategy used, and the exclusions of other document types (e.g., meeting abstract, note and proceeding papers etc.) and articles published in non-English Language (e.g in Chinese). Also, the current analysis did not allow for a narrative review and judgment on contents and results of the articles [79]. As noted earlier, emerging themes and recent research focus in Plesiomonas are not easily recognized in bibliometric studies due to low frequency of appearance in keywords. Notwithstanding these limitations, this is the first bibliometric study on Plesiomonas-related research contributing to the evidence base and would help direct future research. Also, the WoS has a larger coverage compared to other database, reliable indexing technology that minimizes the ‘‘indexer effect” and is well accepted among scientific communities [80].
Conclusion
Our bibliometric analysis revealed a global diminishing research in Plesiomonas, greater research output from high-income countries compared to low- and middle-income countries and limited collaboration with developing countries. The low productivity in developing countries in Plesiomonas research mirror the state of affairs in other research fields. A better understanding of the clinical features, epidemiology and Plesiomonas-associated diseases is needed in countries with high infection rates. Emerging themes and recent research focus in Plesiomonas research are not easily recognized in bibliometric studies due to low frequency of appearance in keywords and, hence, the need for future studies guided by narrative reviews.
Supporting information
Each node in the network represents a different term. The node’s diameter corresponds to the frequency of co-occurrence with other terms. Lines depict co-occurrence pathways between terms.
(TIF)
Each node in the network represents one of the top 20 keywords. The node’s diameter corresponds to the keyword’s frequency of co-occurrence with other keywords. Lines depict co-occurrence pathways between keywords.
(TIF)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
The authors thank the South Africa Medical Research Council (SAMRC) and the National Research Foundation, The World Academy of Science (NRF-TWAS) for financial support. Conclusions arrived at and opinions expressed in this article are those of the authors and are not necessarily to be attributed to SAMRC or NRF-TWAS.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
We are grateful to the South Africa Medical Research Council (SAMRC), The World Academy of Science and the National Research Foundation for financial support. Conclusions arrived at, and opinions expressed are those of the authors and are not necessarily to be attributed to the NRF-TWAS or SAMRC.
References
- 1.Eschenbach DA. Treating spontaneous and induced septic abortions. Obstet Gynecol. 2015; 10.1097/AOG.0000000000000795 [DOI] [PubMed] [Google Scholar]
- 2.Hustedt JW, Ahmed S. Plesiomonas shigelloides Periprosthetic Knee Infection After Consumption of Raw Oysters. Am J Orthop (Belle Mead NJ). 2017; [PubMed] [Google Scholar]
- 3.Chen M, Xu J, Yao H, Lu C, Zhang W. Isolation, genome sequencing and functional analysis of two T7-like coliphages of avian pathogenic Escherichia coli. Gene. Elsevier B.V.; 2016;582: 47–58. 10.1016/j.gene.2016.01.049 [DOI] [PubMed] [Google Scholar]
- 4.Pence MA. Wound Infection with Plesiomonas shigelloides following a Freshwater Injury. J Clin Microbiol. 1752 N ST NW, WASHINGTON, DC 20036–2904 USA: AMER SOC MICROBIOLOGY; 2016;54: 1180–1182. 10.1128/JCM.02651-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Novoa-Farías O, Frati-Munari AC, Peredo MA, Flores-Juárez S, Novoa-García O, Galicia-Tapia J, et al. Susceptibility of bacteria isolated from acute gastrointestinal infections to rifaximin and other antimicrobial agents in Mexico. Rev Gastroenterol Mex. Asociación Mexicana de Gastroenterología; 2016;81: 3–10. 10.1016/j.rgmx.2015.07.003 [DOI] [PubMed] [Google Scholar]
- 6.Patel S, Gandhi D, Mehta V, Bhatia K, Epelbaum O. Plesiomonas shigelloides: an extremely rare cause of Spontaneous Bacterial Peritonitis. Acta Gastroenterol Belg. 2016; [PubMed] [Google Scholar]
- 7.Bowman JK, Zhang XC, Hack JB. Plesiomonas shigelloides meningitis in an adult in the ED. The American Journal of Emergency Medicine 2016. p. 1329.e1–1329.e2. 10.1016/j.ajem.2015.12.046 [DOI] [PubMed] [Google Scholar]
- 8.Tsukamoto T, Kinoshita Y, Shimada T, Sakazaki R. No Title. 1978; 10.1017/S0022172400053638 [DOI] [PMC free article] [PubMed]
- 9.Newsom R, Gallois C. Diarrheal disease caused by Plesiomonas shigelloides. Clin Microbiol Newsl. 1982;4: 158–159. 10.1016/S0196-4399(82)80049-4 [Google Scholar]
- 10.Rutala WA, Sarubbi FA, Finch CS, Maccormack JN, Steinkraus GE. OYSTER-ASSOCIATED OUTBREAK OF DIARRHOEAL DISEASE POSSIBLY CAUSED BY PLESIOMONAS SHIGELLOIDES. The Lancet. 1982. 10.1016/S0140-6736(82)92647-2 [DOI] [PubMed] [Google Scholar]
- 11.Medema G, Schets C. Occurrence of Plesiomonas shigelloides in surface water: relationship with faecal pollution and trophic state Zentralbl Hyg Umweltmed; 1993; [PubMed] [Google Scholar]
- 12.Wouafo M, Pouillot R, Kwetche PF, Tejiokem M-C, Kamgno J, Fonkoua M-C. An acute foodborne outbreak due to Plesiomonas shigelloides in Yaounde, Cameroon. Foodborne Pathog Dis. 2006; 10.1089/fpd.2006.3.209 [DOI] [PubMed] [Google Scholar]
- 13.Van Houten R, Farberman D, Norton J, Ellison J, Kiehlbauch J, Morris T, et al. Plesiomonas shigelloides and Salmonella serotype Hartford infections associated with a contaminated water supply—Livingston County, New York, 1996 (Reprinted from MMWR, vol 47, pg 394–396, 1998). Infect Med. 134 W 29TH ST, NEW YORK, NY 10001–5304 USA: SCP COMMUNICATIONS INC; 1998;15: 495–497. [PubMed] [Google Scholar]
- 14.Janda MJ, Abbott SL, McIver CJ. Plesiomonas shigelloides revisited. Clin Microbiol Rev. 2016; 10.1128/CMR.00103-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allerberger F, Wagner M. Listeriosis: A resurgent foodborne infection. Clinical Microbiology and Infection. 2010. 10.1111/j.1469-0691.2009.03109.x [DOI] [PubMed] [Google Scholar]
- 16.Patz JA, Campbell-Lendrum D, Holloway T, Foley JA. Impact of regional climate change on human health. Nature. 2005. 10.1038/nature04188 [DOI] [PubMed] [Google Scholar]
- 17.Lafferty KD. The ecology of climate change and infectious diseases. Ecology. 2009; 10.1890/08-0079.1 [DOI] [PubMed] [Google Scholar]
- 18.Altizer S, Ostfeld RS, Johnson PTJ, Kutz S, Harvell CD. Climate change and infectious diseases: From evidence to a predictive framework. Science. 2013. 10.1126/science.1239401 [DOI] [PubMed] [Google Scholar]
- 19.Baker-Austin C, Trinanes J, Gonzalez-Escalona N, Martinez-Urtaza J. Non-Cholera Vibrios: The Microbial Barometer of Climate Change. Trends Microbiol. Elsevier Ltd; 2017;25: 76–84. 10.1016/j.tim.2016.09.008 [DOI] [PubMed] [Google Scholar]
- 20.Clark RB, Randolph Westby G, Spector H, Soricelli RR, Young CL. Fatal Plesiomonas shigelloides septicaemia in a splenectomised patient. J Infect. 1991; 10.1016/0163-4453(91)94217-8 [DOI] [PubMed] [Google Scholar]
- 21.Gopal V, Burns FE. Cellulitis and compartment syndrome due to Plesiomonas shigelloides: a case report. Mil Med. 1991; [PubMed] [Google Scholar]
- 22.Alcaniz JP, de Cuenca Moron B, Rubio MG, Albares JLM, Alvarez JG. Spontaneous bacterial peritonitis due to Plesiomonas shigelloides. Am J Gastroenterol. 1995; [PubMed] [Google Scholar]
- 23.Jönsson I, Monsen T, Wiström J. A case of plesiomonas shigelloides cellulitis and bacteraemia from Northern Europe. Scand J Infect Dis. 1997; 10.3109/00365549709035909 [DOI] [PubMed] [Google Scholar]
- 24.Wong TY, Tsui HY, So MK, Lai JY, Lai ST, Tse CW, et al. Plesiomonas shigelloides infection in Hong Kong: retrospective study of 167 laboratory-confirmed cases. Hong Kong Med J. 2000;6: 375–380. [PubMed] [Google Scholar]
- 25.Ampofo K, Graham P, Ratner A, Rajagopalan L, Della-Latta P, Saiman L. Plesiomonas shigelloides sepsis and splenic abscess in an adolescent with sickle-cell disease. Pediatr Infect Dis J. 530 WALNUT ST, PHILADELPHIA, PA 19106–3621 USA: LIPPINCOTT WILLIAMS & WILKINS; 2001;20: 1178–1179. 10.1097/00006454-200112000-00019 [DOI] [PubMed] [Google Scholar]
- 26.Roth T, Hentsch C, Erard P, Tschantz P. Pyosalpinx: Not always a sexual transmitted disease? Pyosalpinx cuased by Plesiomonas shigelloides in an immunocompetent host. Clin Microbiol Infect. European Society of Clinical Infectious Diseases; 2002;8: 803–805. 10.1046/j.1469-0691.2002.00443.x [DOI] [PubMed] [Google Scholar]
- 27.Tzanetea R, Konstantopoulos K, Xanthaki A, Kalotychou V, Spiliopoulou C, Michalopoulos A, et al. Plesiomonas shigelloides sepsis in a thalassemia intermedia patient. Scand J Infect Dis. 2002; 10.1080/00365540210147877 [DOI] [PubMed] [Google Scholar]
- 28.Schneider F, Lang N, Reibke R, Michaely HJ, Hiddemann W, Ostermann H. Plesiomonas shigelloides pneumonia. Med Mal Infect. 2009;39: 397–400. 10.1016/j.medmal.2008.11.010 [DOI] [PubMed] [Google Scholar]
- 29.Ozdemir O, Sari S, Terzioglu S, Zenciroglu A. Plesiomonas shigelloides Sepsis and Meningoencephalitis in a Surviving Neonate. J Microbiol Immunol Infect. Taiwan Society of Microbiology; 2010;43: 344–346. 10.1016/S1684-1182(10)60053-9 [DOI] [PubMed] [Google Scholar]
- 30.Hustedt JW, Ahmed S. Infection After Consumption of Raw Oysters. 2017; 32–34. [PubMed] [Google Scholar]
- 31.Auxiliadora-Martins M, Bellissimo-Rodrigues F, Viana JM, Teixeira GCA, Nicolini EAAAAAA, Cordeiro KSMKSMKSMKSMKSMKSMKSM, et al. Septic shock caused by Plesiomonas shigelloides in a patient with sickle beta-zero thalassemia. Hear Lung J Acute Crit Care. 2010;39: 335–339. 10.1016/j.hrtlng.2009.06.015 [DOI] [PubMed] [Google Scholar]
- 32.Klatte JM, Dastjerdi MH, Clark K, Harrison CJ, Grigorian F, Stahl ED. HYPERACUTE INFECTIOUS KERATITIS WITH PLESIOMONAS SHIGELLOIDES FOLLOWING TRAUMATIC LAMELLAR CORNEAL LACERATION. Pediatr Infect Dis J. 530 WALNUT ST, PHILADELPHIA, PA 19106–3621 USA: LIPPINCOTT WILLIAMS & WILKINS; 2012;31: 1200–1201. 10.1097/INF.0b013e318266b61f [DOI] [PubMed] [Google Scholar]
- 33.Pfeiffer ML, DuPont HL, Ochoa TJ. The patient presenting with acute dysentery—A systematic review. J Infect. Elsevier Ltd; 2012;64: 374–386. 10.1016/j.jinf.2012.01.006 [DOI] [PubMed] [Google Scholar]
- 34.Bonatti H, Sifri C, Sawyer RG. Successful Liver Transplantation from Donor with Plesiomonas shigelloides Sepsis after Freshwater Drowning: Case Report and Review of Literature on Gram-Negative Bacterial Aspiration during Drowning and Utilization of Organs from Bacteremic Donors. Surg Infect (Larchmt). 140 HUGUENOT STREET, 3RD FL, NEW ROCHELLE, NY 10801 USA: MARY ANN LIEBERT INC; 2012;13: 114–120. 10.1089/sur.2010.018 [DOI] [PubMed] [Google Scholar]
- 35.Xia F-QQ, Liu P-NN, Zhou Y-HH. Meningoencephalitis caused by Plesiomonas shigelloides in a Chinese neonate: case report and literature review. Ital J Pediatr. 236 GRAYS INN RD, FLOOR 6, LONDON WC1X 8HL, ENGLAND: BIOMED CENTRAL LTD; 2015;41: 3 10.1186/s13052-014-0107-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Claesson BE, Holmlund DE, Lindhagen CA, Mätzsch TW. Plesiomonas shigelloides in acute cholecystitis: a case report. J Clin Microbiol. 1984;20: 985–7. Available: http://www.ncbi.nlm.nih.gov/pubmed/6511879%0Ahttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC271489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fischer K, Chakraborty T, Hof H, Kirchner T, Wamsler O. Pseudoappendicitis caused by Plesiomonas shigelloides. J Clin Microbiol. 1988;26: 2675–2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen Y, Chen X, Yu F, Wu M, Wang R, Zheng S, et al. Serology, virulence, antimicrobial susceptibility and molecular characteristics of clinical Vibrio parahaemolyticus strains circulating in southeastern China from 2009 to 2013. Clin Microbiol Infect. Elsevier Ltd; 2016;22: 258.e9–258.e16. 10.1016/j.cmi.2015.11.003 [DOI] [PubMed] [Google Scholar]
- 39.Bhavnani D. Diarrheal Disease in Northwestern Ecuador: Prevalence, Pathogenicity, and Transmission of Enteric Pathogens Across the Region University of Michigan; 2012. [Google Scholar]
- 40.Michael Janda J, Abbott SL, McIver CJ, Janda MJ, Abbott SL, McIver CJ, et al. Plesiomonas shigelloides revisited. Clin Microbiol Rev. 2016;29: 349–374. 10.1128/CMR.00103-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ellegaard O, Wallin JA. The bibliometric analysis of scholarly production: How great is the impact? Scientometrics. 2015; 10.1007/s11192-015-1645-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Geaney F, Scutaru C, Kelly C, Glynn RW, Perry IJ. Type 2 diabetes research yield, 1951–2012: Bibliometrics analysis and density-equalizing mapping. PLoS One. 2015; 10.1371/journal.pone.0133009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zyoud SH. Global toxocariasis research trends from 1932 to 2015: A bibliometric analysis. Heal Res Policy Syst. 2017; 10.1186/s12961-017-0178-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zyoud SH, Waring WS, Al-Jabi SW, Sweileh WM. Global cocaine intoxication research trends during 1975–2015: A bibliometric analysis of Web of Science publications. Subst Abus Treat Prev Policy. 2017; 10.1186/s13011-017-0090-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sweileh WM, Al-Jabi SW, Sawalha AF, AbuTaha AS, Zyoud SH. Bibliometric analysis of publications on Campylobacter: (2000–2015). J Health Popul Nutr. Journal of Health, Population and Nutrition; 2016;35: 39 10.1186/s41043-016-0076-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sweileh WM, Sawalha AF, Al-Jabi S, Zyoud SH. Bibliometric analysis of literature on antifungal triazole resistance: 1980–2015. GERMS. 2017; doi: 10.11599/germs.2017.1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sweileh WM. Global research trends of World Health Organization’s top eight emerging pathogens. Global Health. 2017; 10.1186/s12992-017-0233-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ekundayo, Cyrus Temitope and Okoh AI. Plesiomonas shigelloides seventy years of systematics and taxonomy in perspective of the present-day diagnostic demands. Res J Med Sci. 2017;11: 103–113. [Google Scholar]
- 49.Zyoud SH. Dengue research: A bibliometric analysis of worldwide and Arab publications during 1872–2015. Virol J. 2016; 10.1186/s12985-016-0534-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yi F, Yang P, Sheng H. Tracing the scientific outputs in the field of Ebola research based on publications in the Web of Science. BMC Res Notes. 2016; 10.1186/s13104-016-2026-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zheng HC, Yan L, Cui L, Guan YF, Takano Y. Mapping the history and current situation of research on John Cunningham virus—A bibliometric analysis. BMC Infect Dis. 2009; 10.1186/1471-2334-9-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Patiño-Barbosa AM, Bedoya-Arias JE, Cardona-Ospina JA, Rodriguez-Morales AJ. Bibliometric assessment of the scientific production of literature regarding Mayaro. Journal of Infection and Public Health. 2016. 10.1016/j.jiph.2015.10.001 [DOI] [PubMed] [Google Scholar]
- 53.Zyoud SH. Global research trends of Middle East respiratory syndrome coronavirus: A bibliometric analysis. BMC Infect Dis. 2016; 10.1186/s12879-016-1600-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bundschuh M, Groneberg DA, Klingelhoefer D, Gerber A. Yellow fever disease: Density equalizing mapping and gender analysis of international research output. Parasites and Vectors. 2013; 10.1186/1756-3305-6-331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Al-Jabi SW. Global research trends in West Nile virus from 1943 to 2016: A bibliometric analysis. Global Health. 2017; 10.1186/s12992-017-0284-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Martinez-Pulgarin DF, Acevedo-Mendoza WF, Cardona-Ospina JA, Rodríguez-Morales AJ, Paniz-Mondolfi AE. A bibliometric analysis of global Zika research. Travel Medicine and Infectious Disease. 2016. 10.1016/j.tmaid.2015.07.005 [DOI] [PubMed] [Google Scholar]
- 57.Ramos JM, González-Alcaide G, Bolaños-Pizarro M. Bibliometric analysis of leishmaniasis research in Medline (1945–2010). Parasites and Vectors. 2013; 10.1186/1756-3305-6-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Groneberg DA, Weber E, Gerber A, Fischer A, Klingelhoefer D, Brueggmann D. Density equalizing mapping of the global tuberculosis research architecture. Tuberculosis. 2015; 10.1016/j.tube.2015.05.003 [DOI] [PubMed] [Google Scholar]
- 59.Lewison G, Srivastava D. Malaria research, 1980–2004, and the burden of disease. Acta Trop. 2008; 10.1016/j.actatropica.2008.01.009 [DOI] [PubMed] [Google Scholar]
- 60.Sweileh WM, Sawalha AF, Al-Jabi SW, Zyoud SH, Shraim NY, Abu-Taha AS. A bibliometric analysis of literature on malaria vector resistance: (1996–2015). Global Health. 2016; 10.1186/s12992-016-0214-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aria M, Cuccurullo C. bibliometrix: An R-tool for comprehensive science mapping analysis. J Informetr. Elsevier Ltd; 2017;11: 959–975. 10.1016/j.joi.2017.08.007 [Google Scholar]
- 62.Kessler MM. Bibliographic coupling between scientific papers. Am Doc. 1963; 10.1002/asi.5090140103 [Google Scholar]
- 63.Glänzel W. National characteristics in international scientific co-authorship relations. Scientometrics. 2001; 10.1023/A:1010512628145 [Google Scholar]
- 64.Zhang J, Xie J, Hou W, Tu X, Xu J, Song F, et al. Mapping the knowledge structure of research on patient adherence: Knowledge domain visualization based co-word analysis and social network analysis. PLoS One. 2012;7: 1–7. 10.1371/journal.pone.0034497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lotka AJ. The frequency distribution of scientific productivity. J Washingt Acad Sci. 1926 [Google Scholar]
- 66.Aleixandre JL, Aleixandre-Tudó JL, Bolaños-Pizarro M, Aleixandre-Benavent R. Mapping the scientific research in organic farming: a bibliometric review. Scientometrics. 2015; 10.1007/s11192-015-1677-4 [Google Scholar]
- 67.Porter MF. An algorithm for suffix stripping. Program. 1980. 10.1108/eb046814 [Google Scholar]
- 68.Albuquerque PC, Castro MJC, Santos-Gandelman J, Oliveira AC, Peralta JM, Rodrigues ML. Bibliometric Indicators of the Zika Outbreak. PLoS Negl Trop Dis. 2017; 10.1371/journal.pntd.0005132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vera-Polania F, Muñoz-Urbano M, Bañol-Giraldo AM, Jimenez-Rincón M, Granados-Álvarez S, Rodriguez-Morales AJ. Bibliometric assessment of scientific production of literature on chikungunya. Journal of Infection and Public Health. 2015. 10.1016/j.jiph.2015.03.006 [DOI] [PubMed] [Google Scholar]
- 70.Cruz-Calderón S, Nasner-Posso KM, Alfaro-Toloza P, Paniz-Mondolfi AE, Rodríguez-Morales AJ. A bibliometric analysis of global Ebola research. Travel Medicine and Infectious Disease. 2015. 10.1016/j.tmaid.2015.02.007 [DOI] [PubMed] [Google Scholar]
- 71.Zhang L, Wang MH, Hu J, Ho YS. A review of published wetland research, 1991–2008: Ecological engineering and ecosystem restoration. Ecol Eng. 2010; 10.1016/j.ecoleng.2010.04.029 [Google Scholar]
- 72.Peng Y, Lin A, Wang K, Liu F, Zeng F, Yang L. Global trends in DEM-related research from 1994 to 2013: a bibliometric analysis. Scientometrics. 2015; 10.1007/s11192-015-1666-7 [Google Scholar]
- 73.Liu X, Zhang L, Hong S. Global biodiversity research during 1900–2009: A bibliometric analysis. Biodivers Conserv. 2011; 10.1007/s10531-010-9981-z [Google Scholar]
- 74.Chen X, Chen Y, Yang Q, Kong H, Yu F, Han D, et al. Plesiomonas shigelloides infection in Southeast China. PLoS One. 2013;8 10.1371/journal.pone.0077877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li T, Ho Y-S, Li C-Y. Bibliometric analysis on global Parkinson’s disease research trends during 1991–2006. Neurosci Lett. 2008; 10.1016/j.neulet.2008.06.044 [DOI] [PubMed] [Google Scholar]
- 76.Fricke R, Uibel S, Klingelhoefer D, Groneberg DA. Influenza: A scientometric and density-equalizing analysis. BMC Infect Dis. 2013; 10.1186/1471-2334-13-454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brüggmann D, Mäule LS, Klingelhöfer D, Schöffel N, Gerber A, Jaque JM, et al. World-wide architecture of osteoporosis research: density-equalizing mapping studies and gender analysis. Climacteric. 2016; 10.1080/13697137.2016.1200548 [DOI] [PubMed] [Google Scholar]
- 78.Vanni T, Mesa-Frias M, Sanchez-Garcia R, Roesler R, Schwartsmann G, Goldani MZ, et al. International scientific collaboration in HIV and HPV: A network analysis. PLoS One. 2014; 10.1371/journal.pone.0093376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Law J, Bauin S, Courtial JP, Whittaker J. Policy and the mapping of scientific change: A co-word analysis of research into environmental acidification. Scientometrics. 1988; 10.1007/BF02020078 [Google Scholar]
- 80.He Q. Knowledge Discovery Through Co-Word Analysis. Libr Trends. 1999 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Each node in the network represents a different term. The node’s diameter corresponds to the frequency of co-occurrence with other terms. Lines depict co-occurrence pathways between terms.
(TIF)
Each node in the network represents one of the top 20 keywords. The node’s diameter corresponds to the keyword’s frequency of co-occurrence with other keywords. Lines depict co-occurrence pathways between keywords.
(TIF)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.