Abstract
A new 3, 3”-biflavanone, neochamaejasmin C (1), was isolated from the roots of Stellera chamaejasme L., together with four known compounds. Their structures and configurations were elucidated by spectroscopic methods, including 2D-NMR techniques.
Keywords: Stellera chamaejasme, Thymelaeaceae, biflavanone
1. Introduction
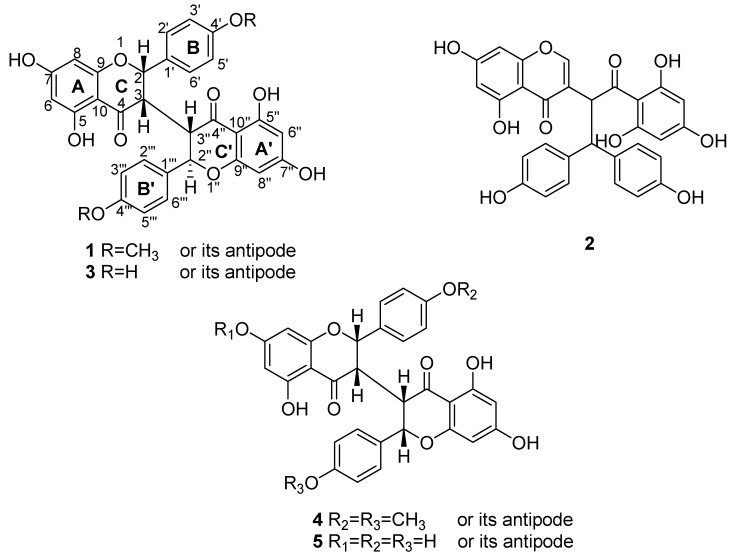
The plant Stellera chamaejasme L. (Thymelaeaceae) is a well-recognized traditional Chinese herbal medicine, widely distributed in the north and southwest of China. Its roots are commonly used for the treatment of scabies, tinea, stubborn skin ulcers, chronic tracheitis, and tuberculosis [1]. Previous studies on the chemical constituents of this plant have resulted in reports on the presence of groups of biflavonoids, lignans, diterpenes, etc. [2,3,4,5,6,7,8,9]. To achieve a deeper understanding of the chemistry of this crude drug, further phytochemical research on this plant was carried out by our group. One new 3,3”-biflavanone, neochamaejasmin C (1, Figure 1), together with four known compounds, namely chamaechromone (2), neochamaejasmin B (3), chamaejasmenin B (4), and chamaejasmenin C (5) (Figure 1) were isolated from the roots of Stellera chamaejasme L. Details of the isolation and structure elucidation of the compound 1 are presented herein.
Figure 1.
Chemical Structures of 1-6.
2. Results and Discussion
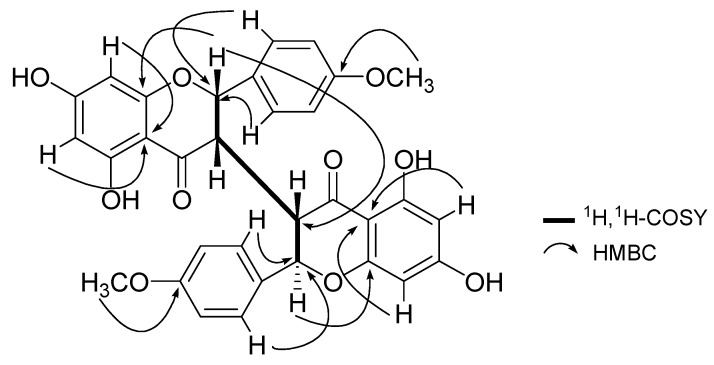
Neochamaejasmin C (1) was obtained as an amorphous powder. Its molecular formula C32H26O10was established by HR-ESI-MS (m/z 569.1446 [M-H]-). The color development reaction with HCl-Mg reagent showed a red color, indicating that 1 is a flavonoid. The 1H-NMR spectrum of 1 (Table 1) displayed signals of two methoxyl groups (δH 3.79, 3.82, s, each 3H), two protons of H-2 (δH 5.77, 1H, d, J = 4.8 Hz), H-2” (δH 5.22, 1H, d, J = 8.8 Hz), and two protons of H-3 (δH 3.24, 1H, br s), H-3” (δH 4.03, 1H, dd, J = 8.8, 3.4 Hz). The aromatic proton signals (δH 5.86-7.36, 12H) indicated the presence of two sets of typical 5,7-dioxygenated A rings (δH 5.95, 6.08, each 1H, d, J = 2.0 Hz; δH 5.87, 5.86, each 1H, d, J = 2.4 Hz), and two sets of para-oxygenated B rings (δH 7.36, 6.98, each 2H, d, J = 8.6 Hz; δH 7.10, 6.84, each 2H, d, J = 8.6 Hz). From the 13C-NMR data (Table 1), two carbonyl (δC 195.0, 197.2) and two methoxyl groups (δC 54.8) were also observed. On the basis of these observations, the structure of compound 1 was determined to consist of two flavanone units [2,3,4,5,6,7]. The partial (-CH-CH-CH-CH-) structure inferred from the 1H-1H COSY spectrum (bold line in Figure 2) suggested that the linkage of the two flavanones was possible only at the C-3 and C-3” positions, which was supported by the comparison of the 1H- and 13C-NMR data of 1 with those known 3,3”-biflavanones [2,3,4,5,6,7,8,9], and further confirmed by the HMBC correlations of H-2 (δH 5.77) with C-3”(δC 49.3). The linkage of the B ring to the C ring was established at C-2 by HMBC (Figure 2) experiment, in which H-2’ and H-6’ (δH 7.36) correlated with C-2 (δC 79.9). In the same way, linkage of the B’ ring to C-2” of the C’ ring was deduced by the correlations of H-2”’ and H-6”’ (δH 7.10) with C-2” (δC 81.8). The HMBC cross-peaks between two methoxyl groups with C-4’ and C-4”’ on B and B’ rings indicated that two methoxyl groups were connected to C-4’ and C-4”’ respectively.
Table 1.
NMR data of compound 1 in acetone-d6 (500 MHz for 1H, 125 MHz for 13C).
| No. | δH mult (J = Hz) | δC |
|---|---|---|
| 2 | 5.77 d (4.8) | 79.9 d |
| 3 | 3.24 br s | 48.0 d |
| 4 | - | 197.2 s |
| 5 | - | 164.2 s |
| 6 | 5.95 d (2.0) | 96.2 d |
| 7 | - | 166.5 s |
| 8 | 6.08 d (2.0) | 95.3 d |
| 9 | - | 163.9 s |
| 10 | - | 104.0 s |
| 1’ | - | 128.7 s |
| 2’ | 7.36 d (8.6) | 127.4 d |
| 3’ | 6.98 d (8.6) | 114.1 d |
| 4’ | - | 159.8 s |
| 5’ | 6.98 d (8.6) | 114.1 d |
| 6’ | 7.36 d (8.6) | 127.4 d |
| 2” | 5.22 d (8.8) | 81.8 d |
| 3” | 4.03 dd (8.8, 3.4) | 49.3 d |
| 4” | - | 195.0 s |
| 5” | - | 164.5 s |
| 6” | 5.87 d (2.4) | 96.0 d |
| 7” | - | 166.7 s |
| 8” | 5.86 d (2.4) | 94.9 d |
| 9” | - | 162.1 s |
| 10” | - | 102.8 s |
| 1”’ | - | 128.8 s |
| 2”’ | 7.10 d (8.6) | 129.1 d |
| 3”’ | 6.84 d (8.6) | 113.8 d |
| 4”’ | - | 160.1 s |
| 5”’ | 6.84 d (8.6) | 113.8 d |
| 6”’ | 7.10 d (8.6) | 129.1 d |
| 4’-OCH3 | 3.82 s | 54.8 q |
| 4’”-OCH3 | 3.79 s | 54.8 q |
Figure 2.
key 1H-1H COSY and HMBC correlations of 1.
The stereochemistry at the C-2/C-3 and C-2”/C-3” positions in 1 were determined as cis-trans geometry by comparison of the J values (JH-2 = 4.8 Hz and JH-2” = 8.8 Hz) with those of the known 3,3”-biflavanones. The relative configuration of 1 was assigned to be identical to that of neochamaejasmin B (3) due to the similarity of their chemical shifts and coupling constants of all H-atoms on H-2, H-3, H-2”, and H-3”, the key NOESY correlations between H-2” (δH 5.22) with H-2’(H-6’) (δH 7.36) further confirm the conclusion above. Thus, on the basis of the above evidence, the structure of 1 was established to be 5,5’,7,7’-tetrahydroxy-2-(4-methoxyphenyl)-2’-(4-methoxy-phenyl) [3,3’-bi-2H-1-benzopyran]-4,4’(3H,3’H)-dione, and named neochamaejasmin C. The known compounds were identified as chamaechromone (2) [4], neochamaejasmin B (3) [3], chamaejasmenin B (4) [6], and chamaejasmenin C (5) [6] by comparison of their 1H- and 13C-NMR and MS data with published data.
3. Experimental
3.1. General
Melting points were measured on a Thermal Values analytical microscope and were uncorrected. Optical rotations were recorded on a Perkin-Elmer 341 polarimeter. IR spectra were recorded on a Nicolet FI-IR 200SXY spectrophotomer. 1H- and 13C-NMR spectra were measured in (D6) acetone with TMS as the internal standard on a Bruker DMX-500 NMR instrument. Silica gel G254 and H (Qingdao Sea Chemical Factory, China) were used for TLC and column chromatography, respectively.
3.2. Plant Material
The roots of Stellera chamaejasme L. were collected in Kunming, Yunnan Province, China, in October, 2010. The plant was identified by Le Cai (Yunnan University). A voucher specimen was deposited with the Zhejiang University City College.
3.3. Extraction and Isolation
Air-dried powdered roots (3.0 kg) of S. chamaejasme were extracted exhaustively with 95% aq. EtOH (5 L) at r. t. for four times. After concentration in vacuo, a crude extract (360 g) was obtained, which was suspended in 1 L H2O, and the suspension was extracted three times successively with petroleum ether (PE) (1 L), AcOEt (1 L), and BuOH (1 L) at r. t. to yield 45, 160 and 86 g of each extract, respectively. The EtOAc extract was subjected to column chromatography (CC) with PE/AcOEt gradient system of increasing polarity (9:1, 8:2, 7:3, 6:4, 5:5) to give six fractions (Fr. 1-6). Fraction 3 was chromatographed repeatedly over a SiO2 column with MeOH/H2O (7:3, 8:2, 9:1) to afford 5 (18 mg), 4 (30 mg), and 1 (24 mg). Fraction 7 was rechromatographed on a SiO2 column with CHCl3/MeOH (99:1, 98:2, 94:6) to give 3 (16 mg). Fraction 10 was chromatographed over a SiO2 column with CHCl3/MeOH (99:1, 98:2, 95:5) to yield 2 (170 mg).
Neochamaejasmin C (1). Yellow amorphous powder, mp 216–218 °C; [α]+226 (c=0.65, MeOH); IR (KBr) cm−1: 3382, 1640; 1H-NMR and 13C-NMR data, see Table 1; HR-ESI-MS: m/z 569.1446 [M-H]−, calcd for C32H25O10, 569.1448.
4. Conclusions
In conclusion, one new biflavanone, 5,5’,7,7’-tetrahydroxy-2-(4-methoxyphenyl)-2’-(4-methoxy-phenyl) [3,3’-bi-2H-1-benzopyran]-4,4’(3H,3’H)-dione (1), together with four known compounds, chamaechromone (2), neochamaejasmin B (3), chamaejasmenin B (4), and chamaejasmenin C (5), were isolated from the EtOH extract of the roots of Stellera chamaejasme L.
Acknowledgements
This work was supported by the funds of the Teachers Research Fund of Zhejiang University City College (No. J-11016).
Footnotes
Sample Availability: Available from the authors.
References
- 1.Xu Z.H., Qin G.W., Li X.Y., Xu R.S. New biflavanones and bioactive compounds from Stellera chamaejasme L. Acta Pharm. Sin. 2001;36:668–671. [PubMed] [Google Scholar]
- 2.Niwa M., Takamizawa H., Tatematsu H., Hirata Y. Piscicidal constituents of Stellera chamaejasme L. Chem. Pharm. Bull. 1982;30:4518–4520. doi: 10.1248/cpb.30.4518. [DOI] [Google Scholar]
- 3.Niwa M., Tatematsu S., Liu G.Q., Hirata Y. Isolation and structures of two new C-3/C-3’-biflavanones, neochamaejasmin A and neochamaejasmin B. Chem. Lett. 1984:539–542. [Google Scholar]
- 4.Niwa M., Liu G.Q., Tatematsu S., Hirata Y. Chamaechromone, a novel rearranged biflavonoid from Stellera chamaejasme L. Tetrahedron Lett. 1984;25:3735–3738. doi: 10.1016/0040-4039(84)80118-5. [DOI] [Google Scholar]
- 5.Tatematsu H., Kurokawa M., Niwa M., Hirata Y. Piscicidal constituents of Stellera chamaejasme L. II. Chem. Pharm. Bull. 1984;32:1612–1613. doi: 10.1248/cpb.32.1612. [DOI] [Google Scholar]
- 6.Liu G.Q., Tatematsu H., Kurokawa M., Niwa M., Hirata Y. Novel C-3/C-3”-biflavanones from stellera chamaejasme L. Chem. Pharm. Bull. 1984;32:362–365. doi: 10.1248/cpb.32.362. [DOI] [Google Scholar]
- 7.Niwa M., Otsuji S., Tatematsu S., Liu G.Q., Chen S.F., Hirata Y. Stereostructures of two biflavanones from Stellera chamaejasme L. Chem. Pharm. Bull. 1986;34:3249–3251. doi: 10.1248/cpb.34.3249. [DOI] [Google Scholar]
- 8.Jiang Z.H., Tanaka T., Sakamoto T., Kouno I., Duan J.A., Zhou R.H. Biflavanones, diterpenes, and coumarins from the roots of Stellera chamaejasme L. Chem. Pharm. Bull. 2002;50:137–139. doi: 10.1248/cpb.50.137. [DOI] [PubMed] [Google Scholar]
- 9.Yang G.H., Liao Z.X., Xu Z.Y., Zhang H.P., Chen D.F. Antimitotic and antifungal C-3/C-3’-biflavanones from Stellera chamaejasme. Chem. Pharm. Bull. 2005;53:776–779. doi: 10.1248/cpb.53.776. [DOI] [PubMed] [Google Scholar]