Abstract
Background
Despite the improvements in diagnostic tools for detection of Trypanosoma cruzi in human blood samples, the isolation of parasite from bloodstream in the chronic phase of Chagas disease is challenging. Thus, there is an increasing interest in the development of strategies that allow an accurate monitoring of the parasite load in bloodstream of Chagas disease patients. Given that, the comparison of a classical diagnostic method such as blood culture and multiplex quantitative real-time PCR (qPCR) was few explored so far. Therefore, this study aimed to compare the detection and quantification of T. cruzi load in the circulating blood of patients with chronic Chagas disease, using blood culture and qPCR techniques.
Methods⁄Principal findings
The multiplex real-time quantitative PCR assay (qPCR) based on TaqMan technology was evaluated in 135 blood samples from 91 patients with chronic Chagas disease presenting indeterminate (asymptomatic, n = 23) and cardiac (chronic cardiomyopathy, n = 68) forms, in comparison with the classical blood culture (BC) technique. The total positivity of qPCR and BC was 58.5% and 49.6%, respectively. The median parasite load of all positive patients was 1.18 [0.39–4.23] par. eq.⁄mL, ranging from 0.01 to 116.10 par. eq.⁄mL. We did not find significant differences between T. cruzi load with age and distinct clinical manifestations of patients.
Conclusions/Significance
Our data suggest that qPCR can be an auxiliary tool for studies that require T. cruzi isolation from the bloodstream of patients with chronic Chagas disease, after the establishment of a parasite load cut-off that guarantees a relative success rate of parasite isolation using BC technique.
Introduction
The diagnosis of Trypanosoma cruzi infection should be carried out using different methodologies, depending on the stage of the disease. In the acute phase of the infection, the parasitemia is high in the peripheral circulation and the diagnosis of Chagas disease can be performed by direct examination of the blood. In contrast, in the chronic phase of the disease the parasitemia is subpatent, transient and depends on the immune response of each patient [1]. Current methods available for parasitological and serological diagnosis have limitations in sensitivity and specificity, especially when applied for the diagnosis in chronic phase of the disease. The major limitation of serological methods is the low specificity, due to cross-reactions with other trypanosomatids present in endemic areas such as Leishmania sp. and Trypanosoma rangeli [2–4]. Another difficulty is that following anti-T. cruzi specific treatment, serological methods remain positive for several years. In the chronic phase of the disease, the post treatment serological reversion is less than 10%, which impairs the efficacy of therapeutic evaluation [5–10]. Indirect parasitological diagnostic methods such as xenodiagnosis and blood culture (BC) depend on the presence of at least one intact trypomastigote form for its growth in culture medium. The results of these methods can take up to 120 days and still be doubtful [6, 11–13]. Negative results of BC and/or xenodiagnosis may be due to low parasitemia observed in the chronic phase of Chagas disease and do not rules out the possibility of infection. On the other hand, a positive test has an absolute diagnostic value [14]. In individuals with inconclusive serology, BC is an important tool for identifying T. cruzi, and when positive it is possible the parasite isolation for biological, biochemical and molecular studies [15,16].
The main technique that has been tested for the research of T. cruzi directly in the blood of chronic-affected patients is the conventional PCR, based on the use of synthetic oligonucleotides that amplify specific DNA sequences of the parasite, presenting high sensitivity and promising results, although it is not feasible for a quantitative evaluation [17–20]. The difficulties in the diagnosis of Chagas disease in chronic phase justify the interest and the necessity of implementation of a direct and more sensitive method that allows monitoring the presence of the parasite and confirming the etiology of the disease.
In the last decade, the methodology used for the detection of genes and specific sequences of T. cruzi has been improved with the development of different real-time PCR systems. An automated quantitative approach based on the use of fluorogenic probes (TaqMan) or fluorescent dyes with DNA affinity (SYBRGreen) has been useful for demonstrating the absolute levels of T. cruzi circulating in infected individuals. This methodology represents a major advance in molecular diagnostic methods and gives support to research laboratories, particularly facilitating the quantification of DNA or RNA fragments in different biological samples and, capable of accurately estimates T. cruzi parasite load of patients in chronic phase of Chagas disease [21–34]. In addition, it allows the monitoring of disease progression, evaluation of parasitemia in response to specific treatment, congenital infection and early detection of reactivation [21–34].The qPCR is a more advantageous methodology when compared to conventional PCR and BC, since it presents higher sensitivity and early outcome for confirming the infection. Furthermore, it evaluates and quantifies the parasite load, being useful for medical decision regarding the introduction or not of specific therapeutic against T. cruzi infection. The aim of this study was to evaluate the qPCR (TaqMan system) and blood culture strategies for detecting T. cruzi load in asymptomatic and cardiac patients with chronic Chagas disease without previous etiological treatment, since the comparison of classical parasitological method BC with qPCR was few explored. Genotyping was performed to determine the genetic profile of T. cruzi in newly isolated strains of infected patients.
Materials and methods
Study population
This study included 91 patients in the chronic phase of Chagas disease from different endemic regions of the state of Minas Gerais (Southern Brazil). All patients were adults and had at least two positive conventional serological tests for T. cruzi and were selected at the Referral Outpatient Center for Chagas Disease at the Clinical Hospital of the Universidade Federal de Minas Gerais (UFMG). Patients were subjected to a standard screening protocol that included medical history, physical examination, ECG, laboratory and chest X-ray examinations, disease evolution by echocardiography and characterization according to the clinical classification of chronic Chagas disease [35]. None of the patients were undergoing etiological treatment nor had been previously treated for T. cruzi infection. Blood samples for BC (30 mL) and qPCR (5 mL) were collected at the same time for each patient. Amongst 91 patients, 44 subjects (48%) had two blood samples collected prospectively within a range interval of two and three years, aiming to evaluate the parasitemia over time in patients with chronic Chagas disease, with a total of 135 samples. This study comprises patients from a broad project on the clinical, parasitological, molecular and immunological studies that has been developed in our laboratory since 2011.
Ethical statement
The study was approved by the Research Ethic Committee of the Universidade Federal de Minas Gerais (protocol COEP-ETHIC 0559.0.203.000-11/2012/UFMG), and all participants provided written informed consent.
Blood culture
Blood culture (BC) was performed with 30 mL of venous blood collected in heparinized vacuum tubes and red cells were recovered from the plasma by centrifugation at 300 × g for 10 min at 4°C [12]. The packed red blood cells were washed once and re-suspended in 6 mL of LIT (Liver Infusion Tryptose), mixed and distributed into six plastic tubes (Falcon, USA) containing 3 mL of LIT. The plasma supernatant was centrifuged at 900 × g for 20 min at 4°C, and 5 mL LIT was added to the precipitated cells. All tubes were maintained at 28°C, mixed gently twice a week, and examined monthly for up to 120 days. Microscopic examination was carried out in 10 μL aliquots of each preparation under a 22-mm2coverslip at a magnification of 400×.
Genotyping of Trypanosoma cruzi isolates
T. cruzi was isolated from all clinical samples with positive BC and the genotyping was performed by conventional PCR and RFLP, using three different parasite molecular targets: D7 domain 24Sα ribosomal (rRNA) gene [36], mitochondrial cytochrome oxidase subunit 2 gene (COII) [37], and the intergenic region of spliced leader genes [38], as markers for the six discrete typing units (DTUs) [39]. The following, TcI (Col1.7G2 Colombiana clone), TcII (JG), TcIII (222), TcIV (CAN III clone), TcV (3253 Lages-Silva et al.: unpublished data), and TcVI (CL) were used as reference strains and DTU controls [37,39].
DNA processing for absolute quantification by qPCR assays
For each patient, five milliliters of venous blood were collected and immediately mixed with an equal volume of 6M Guanidine Hydrochloride / 0.2 M ethylenediaminetetraacetic acid buffer (EDTA) solution, pH 8.0. The Guanidine-EDTA Blood lysates (GEB) were boiled during 15 min and stored at 4°C, as previously described [40]. Extraction of DNA was processed from 300 μL GEB using the High Pure PCR Template Preparation kit, according to the instruction provided by the manufacturer (Roche Diagnostics Corp., Indiana, USA). A linearized p-Zero plasmid containing a sequence of Arabidopsis thaliana was used as exogenous internal reference (Internal Amplification Control, IAC) [23,25]. Each round of DNA extraction was performed using 12 blood samples, being 11 of patients and 1 of a negative control (GEB-) for the DNA extraction. After extraction, DNA was stored at -20° C until the time of use in qPCR.
Standard curves and positive controls
For the construction of the standard curve and the generation of positive controls used in qPCR, GEB- from healthy individuals were spiked with 106 epimastigote forms/mL of T. cruzi, Y strain (spiked GEB+). This strain corresponds to the discrete typing unit (DTU) II and was selected due to the high prevalence of this DTU and its association with human infection in the State of Minas Gerais/MG [16,41,42]. Total DNA was purified as previously described, followed by serial dilutions to obtain the concentrations of 104, 103, 102, 101, 100 and 0.5 par. eq./mL. As diluent, DNA extracted from blood sample of a healthy individual (GEB-) was used. Each dilution was correlated to one point of the standard curve for the absolute quantification of parasite load in the clinical samples. DNA extracted from GEB+ spiked with T. cruzi to reach the concentrations of 102 and 100 par. eq./mL were also used as positive controls for the qPCR, in each assay.
Absolute quantification by qPCR assays
The qPCR was performed according to a methodology previously proposed [25], using the multiplex TaqMan system targeting the T. cruzi nuclear satellite DNA and IAC. The qPCR reactions were carried out with 5 μL of DNA, using FastStart Universal Probe Master Mix (Roche Diagnostics GmbHCorp, Mannheim, Germany) in a final volume of 20 μL. The amplifications were carried out in the Step One Plus Real-Time PCR system (Applied Biosystems, USA) using 750 nM of Cruzi 1 and Cruzi 2 primers, 50 nM of Cruzi 3 probe, 100nM of IAC Fw and IAC Rv primers and 50 nM IAC Tq probe. The oligonucleotide sequences were: Cruzi 1 (ASTCGGCTGATCGTTTTCGA), Cruzi 2 (AATTCCTCCAAGCAGCGGATA) and Cruzi 3 probe (FAM-CACACACTGGACACCA-NFQ-MGB), IAC Fw (ACCGTCATGGAACAGCACGTA), IAC Rv (CTCCCGCAACAAACCCTATAAAT) and IAC Tq probe (VIC-AGCATCTGTTCTTGAAGGT-NFQ-MGB) [25]. PCR cycling conditions were: 95°C for 10 min, followed by 40 cycles at 95°C 15s and 58°C for 1min. To analyze the results, the threshold was set at 0.02. Clinical samples were tested in duplicate, and considered positive when the fluorescent signal of both technical replicates cross the threshold or negative when the fluorescent signal of both technical replicates did not cross the threshold.
Statistical analysis
Pearson’s correlation was used to verify the linear relationship between the parasite load of T. cruzi (par. eq./mL) detected in the clinical samples (qPCR), patient age and number of positive tubes in BC. Mann-Whitney-Wilcoxon and Kruskal-Wallis tests [43] were used, respectively, for comparison of T. cruzi parasite load (par. eq./mL) with the cardiac clinical form of patients and the different levels of heart disease. Pearson chi-squared test was used to compare the positivity of BC and qPCR in the clinical samples of patients with two blood collections (samples 1 and 2). Kappa coefficient concordance and 95% confidence intervals were used to quantify the degree of agreement between the results of BCs and qPCR [44,45] in clinical samples of patients with two blood collections. To confirm or refute the evidence found by the tests mentioned above, a 5% significance level was used.
Results
Characteristics of the study population
Overall, 25.3% (23/91) were patients with the chronic indeterminate form of Chagas disease and 74.7% (68/91) showed different degrees of cardiac involvement. Among the patients with the indeterminate form of Chagas disease, 34.8% (8/23) were male, with ages ranging from 33 to 70 years (mean of 44±10.3 years). Amongst patients with chronic Chagas cardiomyopathy, 66.2% (45/68) of were male, with ages ranging from 25 to 81 years (mean of 54±10.3 years).
Detection of T. cruzi by blood culture
Sixty-seven (49.6%) of the 135 clinical samples of patients with chronic Chagas disease presented positive BCs. Data concerning blood collection date, age, T. cruzi DTU, BC positivity, parasite load, clinical form of disease for each patient are given in Tables 1 and 2. A total of 63 T. cruzi isolates was obtained by positive blood culture. Of these, sixty-one are associate with discrete typing unit (DTU) II and two isolates from patients with cardiac and indeterminate form of the disease, respectively, were classified as DTU III or IV and DTU V or VI (Tables 1 and 2).
Table 1. Comparison of blood culture, T. cruzi genotype and parasite load in asymptomatic patients with chronic Chagas disease.
| Blood Sample |
Collection Date |
Number of positive tubes | Blood culture | Parasite load ± SD (par. eq.⁄mL) | DTU | Age |
|---|---|---|---|---|---|---|
| 009a | 09⁄23⁄2011 | 1 | POS | 0.79±0.18 | TcII | 58 |
| 0020a | 10⁄21⁄2011 | 0 | NEG | NEG | - | 48 |
| 0020b | 09⁄19⁄2014 | 2 | POS | 0.37±0.26 | TcII | |
| 0024a | 10⁄25⁄2011 | 6 | POS | 36.82±3.50 | TcII | 33 |
| 0024b | 11⁄12⁄2014 | 5 | POS | 13.25±3.03 | TcII | |
| 0025a | 01⁄11⁄2011 | 0 | NEG | NEG | - | 40 |
| 0040a | 11⁄18⁄2011 | 1 | POS | NEG | TcII | 62 |
| 0041a | 11⁄18⁄2011 | 0 | NEG | NEG | - | 56 |
| 0041b | 09⁄26⁄2014 | 0 | NEG | 0.09±0.06 | - | |
| 0048a | 11⁄29⁄2011 | 7 | POS | 85.81±6.22 | TcII | 38 |
| 0052a | 12⁄02⁄2011 | 1 | POS | NEG | ND | 37 |
| 0054a | 12⁄06⁄2011 | 1 | POS | NEG | TcV or VI | 38 |
| 0054b | 11⁄14⁄2014 | 0 | NEG | NEG | - | |
| 0061a | 02⁄13⁄2012 | 0 | NEG | 2.97±0.02 | - | 42 |
| 0061b | 10⁄03⁄2014 | 0 | NEG | 0.32±0.08 | - | |
| 0062a | 02⁄13⁄2012 | 0 | NEG | NEG | - | 36 |
| 0062b | 10⁄03⁄2014 | 0 | NEG | 0.04±0.01 | - | |
| 0063a | 03⁄02⁄2012 | 0 | NEG | 0.01±0.01 | - | 44 |
| 0063b | 09⁄19⁄2014 | 0 | NEG | 0.30±0.16 | - | |
| 0064a | 03⁄02⁄2012 | 1 | POS | NEG | TcII | 52 |
| 0064b | 11⁄28⁄2014 | 2 | POS | 3.15±0.43 | TcII | |
| 0067a | 03⁄09⁄2012 | 2 | POS | NEG | TcII | 70 |
| 0069a | 03⁄20⁄2012 | 0 | NEG | 2.58±1.42 | - | 37 |
| 0069b | 10⁄03⁄2014 | 2 | POS | 0.10±0.03 | TcII | |
| 0072a | 03⁄27⁄2012 | 0 | NEG | 4.81±0.77 | - | 52 |
| 0076a | 04⁄03⁄2012 | 0 | NEG | NEG | - | 36 |
| 0078a | 04⁄17⁄2012 | 0 | NEG | NEG | - | 60 |
| 0078b | 09⁄12⁄2014 | 0 | NEG | 0.27±0.10 | - | |
| 0084a | 04⁄24⁄2012 | 0 | NEG | 0.55±0.15 | - | 37 |
| 0086a | 04⁄24⁄2012 | 0 | NEG | NEG | - | 37 |
| 0086b | 09⁄26⁄2014 | 2 | POS | 0.17±0.03 | ND | |
| 0088a | 04⁄05⁄2012 | 3 | POS | 0.96±0.34 | TcII | 34 |
| 0092a | 05⁄15⁄2012 | 0 | NEG | NEG | - | 43 |
| 0092b | 11⁄18⁄2014 | 0 | NEG | NEG | - | |
| 0094a | 05⁄29⁄2012 | 0 | NEG | 0.31±0.02 | - | 36 |
SD: standard deviation, par. eq./mL: parasite equivalent per milliliter of blood, POS: positive, NEG: negative, ND: not done, a: first sample collected from the patient, b: second sample collected from the patient, DTU: discrete typing units.
Table 2. Comparison of blood culture, T. cruzi genotype and parasite load in patients with different degrees of chronic Chagas disease cardiomyopathy.
| Blood sample | Collection date | CCC | Number of positive tubes | Blood Culture | Parasite load ± SD (par. eq.⁄mL) | DTU | Age |
|---|---|---|---|---|---|---|---|
| 001a | 09⁄09⁄2011 | CCC5 | 7 | POS | 116.10 ±4.37 | TcII | 59 |
| 002a | 09⁄09⁄2011 | CCC3 | 5 | POS | 51.74 ±16.63 | TcII | 41 |
| 003a | 09⁄13⁄2011 | CCC4 | 2 | POS | 2.01±0.76 | TcII | 56 |
| 004a | 09⁄13⁄2011 | CCC3 | 1 | POS | 1.07±0.44 | TcII | 51 |
| 005a | 09⁄13⁄2011 | CCC5 | 5 | POS | 20.86±4.71 | TcII | 71 |
| 006a | 09⁄13⁄2011 | CCC3 | 0 | NEG | NEG | - | 67 |
| 006b | 09⁄26⁄2014 | CCC3 | 0 | NEG | 0.05±0.02 | - | |
| 007a | 09⁄23⁄2011 | CCC5 | 1 | POS | 3.07±0.56 | ND | 45 |
| 008a | 09⁄23⁄2011 | CCC5 | 3 | POS | 0.41±0.35 | TcII | 55 |
| 0010a | 09⁄27⁄2011 | CCC5 | 3 | POS | 4.71±0.06 | TcII | 60 |
| 0012a | 09⁄27⁄2011 | CCC4 | 0 | NEG | NEG | - | 64 |
| 0012b | 11⁄21⁄2014 | CCC4 | 0 | NEG | NEG | - | |
| 0013a | 10⁄04⁄2011 | CCC3 | 4 | POS | 0.47±0.27 | TcII | 48 |
| 0014a | 10⁄04⁄2011 | CCC3 | 0 | NEG | NEG | - | 81 |
| 0015a | 10⁄04⁄2011 | CCC3 | 0 | NEG | 0.41±0.07 | - | 34 |
| 0015b | 11⁄21⁄2014 | CCC3 | 0 | NEG | 2.65±1.88 | - | |
| 0016a | 10⁄21⁄2011 | CCC4 | 1 | POS | 0.29±0.18 | TcII | 46 |
| 0016b | 09⁄19⁄2014 | CCC4 | 0 | NEG | 0.06±0.02 | - | |
| 0017a | 10⁄21⁄2011 | CCC1 | 5 | POS | 24.51±3.23 | TcII | 58 |
| 0017b | 11⁄12⁄2014 | CCC1 | 4 | POS | 3.74±2.78 | TcII | |
| 0018a | 10⁄21⁄2011 | CCC2 | 5 | POS | 12.79±1.49 | TcII | 46 |
| 0018b | 09⁄12⁄2014 | CCC2 | 1 | POS | 0.75±0.54 | TcII | |
| 0019a | 10⁄21⁄2011 | CCC3 | 5 | POS | 36.09±4.53 | TcII | 58 |
| 0019b | 11⁄14⁄2014 | CCC3 | 7 | POS | 5.34±2.22 | TcII | |
| 0021a | 10⁄25⁄2011 | CCC2 | 2 | POS | 0.41±0.35 | TcII | 50 |
| 0022a | 10⁄25⁄2011 | CCC3 | 1 | POS | 14.94±2.86 | ND | 58 |
| 0023a | 10⁄25⁄2011 | CCC5 | 5 | POS | 2.52±0.022 | TcII | 70 |
| 0023b | 11⁄14⁄2014 | CCC5 | 1 | POS | NEG | TcII | |
| 0026a | 11⁄01⁄2011 | CCC1 | 4 | POS | 0.69±0.20 | TcII | 25 |
| 0027a | 11⁄01⁄2011 | CCC5 | 0 | NEG | NEG | - | 56 |
| 0027b | 08⁄29⁄2014 | CCC5 | 0 | NEG | NEG | - | |
| 0028a | 11⁄04⁄2011 | CCC5 | 0 | NEG | NEG | - | 57 |
| 0029a | 11⁄04⁄2011 | CCC5 | 0 | NEG | NEG | - | 59 |
| 0030a | 11⁄04⁄2011 | CCC5 | 0 | NEG | NEG | - | 52 |
| 0031a | 11⁄04⁄2011 | CCC5 | 2 | POS | 4.75±0.11 | TcII | 59 |
| 0032a | 11⁄07⁄2011 | CCC3 | 4 | POS | 9.39±6.24 | TcII | 49 |
| 0033a | 11⁄07⁄2011 | CCC1 | 0 | NEG | NEG | - | 67 |
| 0033b | 09⁄26⁄2014 | CCC1 | 0 | NEG | 0.39±0.25 | - | |
| 0034a | 11⁄07⁄2011 | CCC5 | 5 | POS | 2.79±0.79 | TcII | 48 |
| 0035a | 11⁄07⁄2011 | CCC5 | 1 | POS | NEG | TcII | 68 |
| 0036a | 11⁄07⁄2011 | CCC3 | 0 | NEG | NEG | - | 77 |
| 0037a | 11⁄07⁄2011 | CCC4 | 4 | POS | 1.47±0.02 | TcII | 38 |
| 0038a | 11⁄07⁄2011 | CCC5 | 5 | POS | 1.46±0.76 | TcII | 52 |
| 0038b | 12⁄05⁄2014 | CCC5 | 0 | NEG | 2.06±1.07 | - | |
| 0039a | 11⁄18⁄2011 | CCC3 | 0 | NEG | NEG | - | 44 |
| 0039b | 11⁄12⁄2014 | CCC3 | 0 | NEG | NEG | - | |
| 0042a | 11⁄18⁄2011 | CCC5 | 1 | POS | NEG | Tc II | 55 |
| 0043a | 11⁄22⁄2011 | CCC5 | 0 | NEG | NEG | - | 58 |
| 0043b | 09⁄26⁄2014 | CCC5 | 0 | NEG | 0.07±0.01 | - | |
| 0044a | 11⁄22⁄2011 | CCC5 | 2 | POS | 0.91±0.38 | TcII | 66 |
| 0044b | 11⁄14⁄2014 | CCC5 | 2 | POS | 0.72±0.51 | TcII | |
| 0046a | 11⁄29⁄2011 | CCC5 | 4 | POS | 1.86±0.63 | TcII | 61 |
| 0047a | 11⁄29⁄2011 | CCC3 | 2 | POS | NEG | TcII | 60 |
| 0049a | 12⁄02⁄2011 | CCC5 | 2 | POS | 1.18±0.23 | TcII | 59 |
| 0049b | 12⁄05⁄2014 | CCC5 | 0 | NEG | NEG | - | |
| 0050a | 12⁄02⁄2011 | CCC5 | 1 | POS | 1.71 ±1.02 | TcIII or IV | 58 |
| 0050b | 11⁄14⁄2014 | CCC5 | 0 | NEG | NEG | - | |
| 0053a | 12⁄06⁄2011 | CCC5 | 0 | NEG | NEG | - | 55 |
| 0053b | 11⁄18⁄2014 | CCC5 | 1 | POS | NEG | TcII | |
| 0055a | 12⁄06⁄2011 | CCC5 | 0 | NEG | NEG | - | 69 |
| 0055b | 10⁄03⁄2014 | CCC5 | 0 | NEG | 0.94±0.17 | - | |
| 0056a | 12⁄06⁄2011 | CCC5 | 2 | POS | 4.98±2.12 | TcII | 38 |
| 0056b | 11⁄28⁄2014 | CCC5 | 0 | NEG | 1.17±0.52 | - | |
| 0057a | 12⁄06⁄2011 | CCC5 | 2 | POS | 3.57±2.99 | TcII | 36 |
| 0058a | 02⁄10⁄2012 | CCC5 | 0 | NEG | NEG | - | 58 |
| 0059a | 02⁄10⁄2012 | CCC4 | 1 | POS | NEG | TcII | 41 |
| 0059b | 11⁄28⁄2014 | CCC4 | 1 | POS | NEG | TcII | |
| 0060a | 02⁄10⁄2012 | CCC5 | 0 | NEG | 33.30±0.05 | - | 53 |
| 0065a | 03⁄06⁄2012 | CCC4 | 5 | POS | NEG | Tc II | 53 |
| 0066a | 03⁄06⁄2012 | CCC5 | 0 | NEG | 17.31±1.82 | - | 57 |
| 0066b | 29⁄08⁄2014 | CCC5 | 0 | NEG | 0.22±0.07 | - | |
| 0068a | 03⁄20⁄2012 | CCC5 | 2 | POS | NEG | TcII | 67 |
| 0068b | 12⁄05⁄2014 | CCC5 | 4 | POS | 22.33±0.01 | TcII | |
| 0070a | 03⁄23⁄2012 | CCC5 | 0 | NEG | NEG | - | 56 |
| 0070b | 10⁄03⁄2014 | CCC5 | 0 | NEG | 0.24±0.17 | - | |
| 0071a | 03⁄27⁄2012 | CCC5 | 0 | NEG | NEG | - | 44 |
| 0071b | 11⁄14⁄2014 | CCC5 | 0 | NEG | NEG | - | |
| 0073a | 03⁄27⁄2012 | CCC5 | 2 | POS | 13.89±4.46 | TcII | 54 |
| 0074a | 03⁄27⁄2012 | CCC5 | 1 | POS | 2.04±1.11 | TcII | 54 |
| 0075a | 03⁄27⁄2012 | CCC5 | 0 | NEG | NEG | - | 56 |
| 0075b | 09⁄26⁄2014 | CCC5 | 0 | NEG | 0.24±0.06 | - | |
| 0077a | 04⁄03⁄2012 | CCC5 | 0 | NEG | NEG | - | 59 |
| 0079a | 04⁄17⁄2012 | CCC3 | 0 | NEG | NEG | - | 53 |
| 0079b | 09⁄12⁄2014 | CCC3 | 1 | POS | 0.68±0.61 | TcII | |
| 0080a | 04⁄17⁄2012 | CCC2 | 1 | POS | 1.98±1.01 | TcII | 55 |
| 0081a | 04⁄20⁄2012 | CCC5 | 0 | NEG | NEG | - | 66 |
| 0081b | 08⁄29⁄2014 | CCC5 | 0 | NEG | NEG | - | |
| 0083a | 04⁄20⁄2012 | CCC3 | 0 | NEG | NEG | - | 35 |
| 0083b | 11⁄21⁄2014 | CCC3 | 2 | POS | 2.59±0.98 | TcII | |
| 0087a | 04⁄24⁄2012 | CCC5 | 1 | POS | 0.78±0.56 | TcII | 35 |
| 0089a | 05⁄08⁄2012 | CCC1 | 4 | POS | 3.65±1.18 | TcII | 53 |
| 0089b | 09⁄12⁄2014 | CCC1 | 2 | POS | NEG | TcII | |
| 0090a | 05⁄15⁄2012 | CCC5 | 0 | NEG | NEG | - | 54 |
| 0090b | 09⁄12⁄2014 | CCC5 | 1 | POS | 1.74±0.36 | TcII | |
| 0091a | 05⁄15⁄2012 | CCC3 | 0 | NEG | NEG | - | 59 |
| 0091b | 11⁄18⁄2014 | CCC3 | 0 | NEG | NEG | - | |
| 0093a | 05⁄15⁄2012 | CCC5 | 1 | POS | 0.99±0.27 | TcII | 58 |
| 0093b | 09⁄05⁄2014 | CCC5 | 0 | NEG | 0.07±0.04 | - | |
| 0095a | 05⁄29⁄2012 | CCC4 | 3 | POS | 0.51±0.38 | TcII | 46 |
| 0096a | 05⁄29⁄2012 | CCC2 | 0 | NEG | 0.09±0.02 | - | 49 |
CCC1 to 5: chronic Chagas cardiomyopathy in different degrees of cardiac involvement, SD: standard deviation, par. eq./mL: parasite equivalent per milliliter of blood, POS: positive, NEG: negative, ND: not done, a: first sample of the patient, b: second sample of the patient, DTU: discrete typing units.
Among the 44 patients with two collected blood samples, 22.7% (10⁄44) showed positive BC in the two blood harvesting and 45.5% (20/44) showed negative BC in both samples. On the other hand, 15.9% (7/44) presented positive BC in the first and negative in the second sample. The same amount of samples 15.9% (7⁄44) presented negative BC in the first and positive in the second sample (Table 3).The analysis of BC results showed that the positivity observed in the first and second samples were the same [38.64% (17⁄44)], statistically demonstrating an equality in the first and second sample (p-value = 0.500) (Table 3).
Table 3. Percentage of concordance, point and interval estimates of kappa coefficient according to qPCR and blood culture methods in 44 Chagas disease patients with two blood harvesting.
| Method | 1st sample | 2nd sample | Percentage(Number of patients/total) | Agreement Coefficient |
Type of agreement |
|---|---|---|---|---|---|
| qPCR | Positive | Positive | 31.8 (14/44) | 0.083 [-0.212; 0.379] | Slight |
| Positive | Negative | 9.1 (4/44) | |||
| Negative | Positive | 36.4 (16/44) | |||
| Negative | Negative | 22.7 (10/44) | |||
|
Blood Culture |
Positive | Positive | 22.7 (10/44) | 0.329 [0.034; 0.624] | Fair |
| Positive | Negative | 15.9 (7/44) | |||
| Negative | Positive | 15.9 (7/44) | |||
| Negative | Negative | 45.5 (20/44) |
Trypanosoma cruzi parasite load in chronic Chagas disease patients
Parasite loads were determined by qPCR absolute quantification in a TaqMan multiplex assay targeting T. cruzi satellite DNA and the internal control, IAC. It was possible to observe the dynamic range from 104 to 0.5 parasite equivalents /mL, as previously reported [25,28], with efficiency of 89.5% and coefficient of linearity (r2) of 0.99 (Fig 1).
Fig 1. Dynamic range for Trypanosoma cruzi quantification by Real Time qPCR.
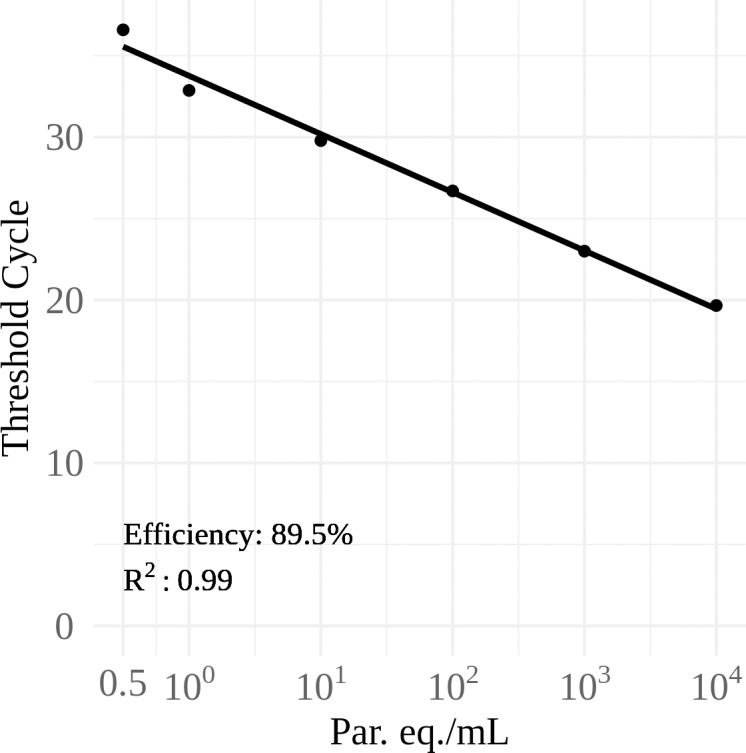
TaqMan qPCR was carried out with serial diluted DNA extracted from blood spiked with T. cruzi [Y strain], ranging from 104 to 0.5 par. eq.⁄mL (parasite equivalent per milliliter of blood).
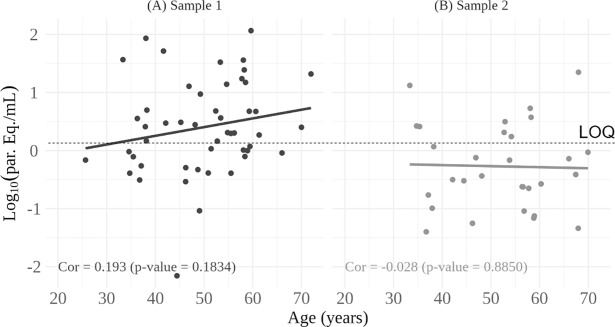
Total qPCR positivity in clinical samples was 58.5% (79/135). Data on collection date, age, DTU, parasite loads and clinical form of the disease for each patient can be seen in Tables 1 and 2.The median parasite load of all positive samples was 1.18 par. eq.⁄mL, varying between 0.01 and 116.10 par. eq.⁄mL. The median parasite load of patients with indeterminate clinical form was 0.46 [0.24–3.02] par. eq.⁄mL, varying between 0.01 and 85.81 par. eq.⁄mL, and 1.74 [0.60–4.74] par. eq.⁄mL for the cardiac patients ranging from 0.05 to 116.10 par. eq.⁄mL (Tables 1 and 2). Analyzing the data from Figs 2 and 3, we found no correlation between T. cruzi loads and the age or clinical manifestation of the disease.
Fig 2. Relationship between parasite load and age of patients with chronic Chagas disease.
The number of positive qPCR results for the first and second clinical samples was respectively, 49 (A) and 30 (B). LOQ: Limit of Quantification [25].
Fig 3. Boxplots of parasite load of chronic Chagas disease patients.
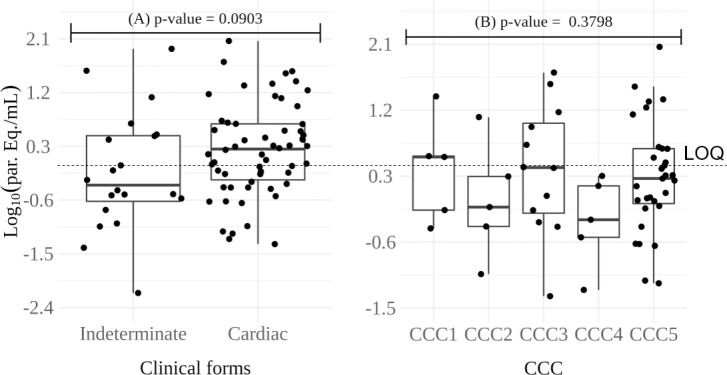
(A) Patients with clinical forms, indeterminate or cardiac, and p-value of the Mann-Whitney-Wilcoxon test. (B) Patients presenting chronic Chagas’ cardiomyopathy (CCC to 5) in different degrees of cardiac involvement and p-value of the Kruskal-Wallis test. Par. eq./mL: parasite equivalent per milliliter of blood, CCC1 to 5: different degrees of cardiac involvement. LOQ: Limit of Quantification [25].
Clinical samples of 44 patients with two blood harvesting were evaluated and compared, and presented parasite loads with approximated values. Only clinical samples from patients 17, 18, 19, 24 and 66 showed differences in parasite load when the second sample was evaluated (Tables 1 and 2). The qPCR was positive in 31.8% (14/44) samples from patients with two blood harvesting and 22.7% (10/44) were negative in both samples. We observed that 9.1% (4/44) presented positive qPCR in the first and were negative in the second sample. In contrast, 36.4% (16/44) presented negative qPCR in the first and positive in the second sample (Table 3). The qPCR positivity increased from 40.9% (18/44) to 68.2% (30/44) (p = 0.005) with the inclusion of a second blood collection.
Association between qPCR assay and blood culture in chronic Chagas disease patients
Of the 135 screened samples, 38.5% (52⁄135) were tested positive for both qPCR and BC, 20.0% (27/135) were only positive for qPCR, 11.1% (15/135) were positive for BC but qPCR negative, and 30.4% (41⁄135) were negative for both assays (Table 4).
Table 4. Percentage of agreement, point and interval estimates of kappa coefficient of 135 blood samples obtained from 91 Chagas disease patients from different endemic regions of the state of Minas Gerais (southern Brazil).
| qPCR | Blood culture | Percentage (Number of patients/total) | Agreement Coefficient |
Type of agreement |
|---|---|---|---|---|
| Positive | Positive | 38.5 (52/135) | 0.374 [0.205; 0.542] | Fair |
| Negative | Positive | 11.1 (15/135) | ||
| Positive | Negative | 20.0 (27/135) | ||
| Negative | Negative | 30.4 (41/135) |
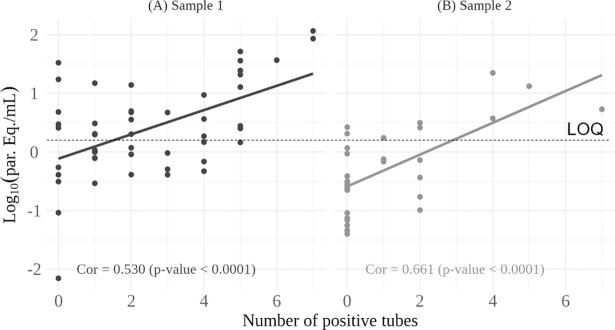
The parasitemia of Chagas disease patients was also evaluated by the number of positive tubes for BC and comparing with the parasite load obtained in qPCR of all positive clinical samples. Fig 4 shows a significant correlation between the number of positive tubes in BC and the parasite load of T. cruzi in clinical samples (p-value<0.0001). We also observed significant correlation between the number of BC positive tubes and the parasite load in the individual analysis of the first and second samples in patients with two blood harvesting (p-value<0.0001) (Fig 4A and 4B).
Fig 4. Correlation between parasite load and number of blood culture positive tubes in patients with chronic Chagas disease.
The number of positive qPCR results for the first and second clinical samples was respectively, 49 (A) and 30 (B). LOQ: Limit of Quantification [25].
Discussion
Due to the sub-patent and transient parasitemia, the direct detection of T. cruzi in the chronic phase of Chagas disease requires biological amplification methods such as blood culture and xenodiagnosis. These methods are more complex, expensive, time-consuming and require special biosecurity conditions in the laboratory [46,47]. Previous reports have shown that multiplex real-time qPCR assay allowed detection and quantification of parasite DNA from clinical samples with variable levels of reliability, complexity, selectivity and analytical sensitivity [21,23–25,28,31,34], also permitting the T. cruzi genotyping in clinical samples [33, 48].
In this study, blood samples from chronic Chagas disease patients with well-defined clinical forms were evaluated, using blood culture (BC) and multiplex quantitative real-time PCR (qPCR) to the detection and quantification of T. cruzi DNA in human blood. All patients presented positive conventional serology for T. cruzi and had not received any specific etiological treatment. For 44 patients, two blood samples were collected, in an interval of two to three years, in order to evaluate the parasite load of chronic patients in the period of 2011–2014.
Herein, BC was positive in 49.6% (67⁄135) of the clinical samples. In a previous study from our group, we detected 54.9% (50/91) of positive BCs, corresponding to first samples collected from the 91 patients [49]. The vast majority of data in the literature have reported BC positivity ranging from 40 to 70% [6,15,41,49–52]. In the patients with two blood samples, blood culture positivity rate was the same (38.6%) for the first and second blood samples. However, the degree of agreement between the two samples was fair, indicating that a patient with positive BC in the first sample can present positive or negative BC in the analysis of the second blood sample. To the patient 0054, for example, it was observed a positive blood culture at the first sample (0054a) but negative at the second (0054b). Our results confirm previous findings and indicate that at least two blood samples should be collected from chronic Chagas disease patients in order to detect circulating T. cruzi [15,16,41,53].
The qPCR method has shown higher potential to diagnose and estimate the parasite load, despite the subpatent and transient parasitemia that occurs in the chronic phase of Chagas disease. According to this methodology, the Limit of quantification was reported as 1.53 parasite equivalents/mL [25], which means that samples with parasite load below this limit can be considered detectable but not quantifiable. Nevertheless, as the majority of samples of patients from Brazil are in this condition, we decided to report the parasite load for all the positive samples. Thus, T. cruzi DNA was detected in 58.5% (79⁄153) of blood samples by qPCR and the median parasite load was 1.18 [0.39–4.23] par. eq.⁄mL, varying between 0.01 and 116.10 par. eq.⁄mL. On the other hand, T. cruzi k-DNA was detected by conventional PCR in 98.9% (90/91) of the first samples collected from these patients [49], demonstrating more efficiency in detecting the parasite in the peripheral blood of infected patients when compared to BC and qPCR. However, conventional PCR does not allow monitoring parasite load in peripheral blood of chronic Chagas disease patients and as a criteria for the isolation of T. cruzi, emphasizing the importance of BC and qPCR for new biological, molecular, biochemical, immunological, genetic studies of parasitic populations and parasite load monitoring.
Our findings corroborate with other studies using qPCR to infer parasite load from blood of Brazilian, Argentines, Bolivians, Colombians and Mexicans chronic Chagas disease patients, where the median parasite load ranged from 1.23 to 4.0 par. eq./mL[25,27,28,31, 34]. Data with chronic Chilean Chagas disease patients have demonstrated parasite loads from <0.1 to 78 par. eq.⁄mL [33], higher than previously described for the same group of patients, fluctuating between <0.1 and 7.9 par. eq.⁄mL [30]. Studies have shown that qPCR has been used for the detection and quantification of T. cruzi load in blood, serum, heart tissue, cord blood, fecal samples of Triatoma infestans (xenodiagnosis), skin tissue samples from Chagas disease patients in the acute and chronic phases and also to differentiate the parasite DTUs [21–34,48], suggesting that genetic differences between parasite strains can influence the parasitic load and PCR positivity [20,22,26,27].
In this study, the detection rate for T. cruzi by qPCR increased from 40.9% to 68.2% in patients with two blood samples collected at different time points; however, the concordance analysis indicated a slight correlation between the samples, with qPCR results from the first sample not often matching the results observed in the second sample.
We performed a comparative analysis of qPCR positivity and blood culture. The two techniques were positive in 38.5% of the samples. Discordant results were observed in 31.1% of the samples, being 11.1% of them positive by BC and qPCR negative, while in 20.0% only the qPCR gave positive results. In contrast, 30.4% of the samples were negative by both techniques. This finding confirms the occurrence of intermittent parasite levels and depends on the number of circulating parasite at the time of blood collection and the number of samples analyzed from the same patient, since in the life cycle of T. cruzi, the release of trypomastigote forms does not occur in a synchronized way. So, the presence of the parasite in peripheral blood at a given time depends on the parasite’s biological cycle, as well as on the immunological equilibrium among parasite and host [54]. Differences in positivity between qPCR and BC can be explained by low parasitemia, probably below the detection limit of the two techniques.
Understanding the structure of T. cruzi population is essential due to the links between parasite transmission cycles and the infection⁄disease. T. cruzi isolates were analyzed by rDNA, COII and SL-IR molecular markers aimed at detecting the six DTUs of T. cruzi. Most isolates from the patients were associated with DTU II. Two isolates from patients with cardiac and indeterminate clinical form, respectively, were also identified associated with DTU III or IV and DTU V or VI [49]. These data were consistent with previous studies showing that DTU II was associated with human infection in the state of Minas Gerais, Brazil [16,42,55].
Consistent with previous studies, we did not find a correlation between neither T. cruzi parasite load nor age and clinical presentation of Chagas disease [21,24,26,27,31,33]. This lack of correlation was also observed in another Brazilian cohort comprising 40 patients with chronic Chagas disease [27]. In our recent study, we did not observe significant difference between BC results, age of patients and clinical form [49]. We believe that the lack of association between T. cruzi parasite load and forms of the disease might be related to parasite tropism for specific organs or host tissues. After their penetration into human tissues, some T. cruzi populations could disappear, while others could invade different tissues, which would be responsible for the various clinical manifestations in Chagas disease. Thus, parasite obtained in the peripheral blood may not represent the populations of T. cruzi present in other tissues and/or organs of the patients. Furthermore, it is more important to analyze parasite present in the bloodstream at different time periods, increasing the chance of recovery of different T. cruzi subpopulations and making possible the analysis of its importance in the pathogenesis of Chagas disease [56,57].
Finally, our results showed a positive correlation between T. cruzi parasite load estimated by qPCR and number of positive BC tubes, demonstrating a high potential of qPCR for diagnosis and monitoring parasite load in peripheral blood of chronic Chagas disease patients. In another work, the parasitic loads of 15 GEB samples from Brazilian chagasic patients were compared with hemoculture. Despite the small number of samples, these authors demonstrated a good correlation between the parasitic load of T. cruzi detected by qPCR and the positivity of blood culture. [28].
Our results suggest that qPCR has diagnostic advantages for T. cruzi detection compared to BC, as it requires low blood volume and shorter processing time, allowing analysis of several samples at the same time. In addition, this tool presents high sensitivity for T. cruzi detection and quantification with lower risk of sample contamination when compared to BC. Another advantage in the use of the multiplex TaqMan assay is the possibility of checking the quality of patients’ blood processing and DNA extraction, especially to avoid false negative results [21,24–27,31,34]. On the other hand, BC has been frequently used for the isolation of T. cruzi, a necessary procedure for studies on biological, biochemical, immunological and some genetic aspects of parasite populations. Thus, BC is the most efficient technique for T. cruzi isolation and its amplification using LIT culture medium [15,16,41].
Taken together, our data suggest that qPCR can be an auxiliary tool for studies that require the isolation of T. cruzi parasite from the bloodstream of chronic Chagas disease patients, after establishing a cut-off for parasite load assuring a relative success rate for their isolation using blood culture technique.
Acknowledgments
We thank Professor Manoel Otávio da Costa Rocha for his contribution and support in the clinical analysis of patients. We thank Afonso da Costa Viana and Orlando Carlos Magno from Departamento de Parasitologia for their technical assistance.
Data Availability
All relevant data are within the manuscript.
Funding Statement
This work was supported by grants from FAPEMIG (Fundação de Amparo à Pesquisa do Estado de Minas Gerais, grant nº 23041), FAPERJ (Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro), Fiocruz (Fundação Oswaldo Cruz) and CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico, grant MCTI/CNPq N° 14/2013 (475572/2013-0)) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) - Finance Code 001. C Britto and OC Moreira are researcher fellows of FAPERJ (CNE - Cientista do nosso Estado, JCNE - Jovem Cientista do Nosso Estado) and CNPq. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Ávila HA, Sigman DS, Cohen LM, Millikan RC, Simpson L. Polymerase chain reaction amplification of Trypanosoma cruzi kinetoplast minicircle DNA isolation from whole blood lysates: diagnosis of chronic Chagas disease. MolBiochemParasitol.1991;40:211–222. [DOI] [PubMed] [Google Scholar]
- 2.Araujo FG. Analysis of Trypanosoma cruzi antigens bound by specific antibodies and by antibodies to related trypanosomatid. Infect Immun. 1986;53:179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antas PRZ, Azevedo EN, Luz MRMP, Medrano-Mercado N, Chaves ACL, Vidigal PG, et al. A reliable and specific enzyme-linked immunosorbent assay for the capture of IgM from human chagasic sera using fixed epimastigotes of Trypanosoma cruzi.Parasitol Res. 2000;86: 813–820. [DOI] [PubMed] [Google Scholar]
- 4.Caballero Z, Sousa OE, Marques WP, Saez-Alquezar A, Umezawa ES. Evaluation of serological tests to identify Trypanosoma cruzi infection in humans and determine cross-reactivity with Trypanosoma rangeli and Leishmania spp. Clin. Vaccine Immunol. 2007;14: 1045–1049. 10.1128/CVI.00127-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krettli AU,Cançado JR Brener Z. Criterion of cure of human Chagas' disease after specific chemotherapy: recent advances. Mem Inst Oswaldo Cruz. 1984;79: 157–164. [Google Scholar]
- 6.Galvão LMC, Nunes RMB, Cançado JR, Brener Z, Krettli AR. Lytic antibody titre as a means of assessing cure after treatment of Chagas disease: a 10 years follow-up study. Trans R Soc Trop Med Hyg. 1993;87: 220–223. [DOI] [PubMed] [Google Scholar]
- 7.Cançado JR. Criteria of Chagas disease cure. Mem Inst Oswaldo Cruz. 1999;94: 331–335. [DOI] [PubMed] [Google Scholar]
- 8.Flores-Chaves M, Fuentes I, Gárate T, Cañavate C. Diagnóstico de laboratorio de la enfermedad de Chagas importada. Enferm Infecc Microbiol Clin. 2006;3: 29–37. [DOI] [PubMed] [Google Scholar]
- 9.Krettli AU. The utility of anti-trypomastigote lytic antibodies for determinig cure of Trypanosoma cruzi infections in treated patients: an overview and perspectives. Mem Inst Oswaldo Cruz. 2009;104: 142–151. [DOI] [PubMed] [Google Scholar]
- 10.Gomes YM, Lorena VMB, Luquetti AO. Diagnosis of Chagas disease: what has been achieved? What remains to be done with regard to diagnosis and follow up studies? Mem Inst Oswaldo Cruz. 2009;104: 115–121. [DOI] [PubMed] [Google Scholar]
- 11.Brumpt E. Le xénodiagnostic. Application au diagnostic de quelques infections parasitaires et en particulier de la trypanosomose de Chagas. Bull Soc Pathol Exot.1914;10: 706–710. [Google Scholar]
- 12.Chiari E, Dias JCP, Lana M, Chiari CA. Hemocultures for the parasitological diagnostic of human chronic Chagas disease. Rev Soc Bras Med Trop. 1989;22: 19–23. [DOI] [PubMed] [Google Scholar]
- 13.Junqueira ACV,Chiari E,Wincker P. Comparison of the polymerase chain reaction with two classical parasitological methods for the diagnosis of Chagas disease in an endemic region of northeastern Brazil. Trans R Soc Trop Med Hyg.1996;90: 129–132. [DOI] [PubMed] [Google Scholar]
- 14.Luquetti AO,Dias JCP,Prata A. Diagnosis and treatment of congenital infection caused by Trypanosoma cruzi in Brazil. Rev Soc Bras Med Trop. 2005;38(Supl. 2): 27–28. [PubMed] [Google Scholar]
- 15.Castro AM, Luquetti AO, Rassi A, Rassi GG, Chiari E, Galvão LMC. Blood culture and polymerase chain reaction for the diagnosis of the chronic phase of human infection with Trypanosoma cruzi. Parasitol Res. 2002;88(10): 894–900. 10.1007/s00436-002-0679-3 [DOI] [PubMed] [Google Scholar]
- 16.D’Ávila DA, Macedo AM, Valadares HMS, Gontijo ED, Castro AM, Machado CR, et al. Probing population dynamics of Trypanosoma cruzi during progression of the chronic phase in chagasic patients. J Clin Microbiol. 2009;47(6): 1718–25. 10.1128/JCM.01658-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schijman AG, Vigliano C, Burgos J, Favaloro R, Perrone S, Laguens R, et al. Early diagnosis of recurrence of Trypanosoma cruzi infection by polymerase chain reaction after heart transplantation of a chronic Chagas’ heart disease patient. J Heart Lung Transplant. 2000;19: 1114–17. [DOI] [PubMed] [Google Scholar]
- 18.Cummings K. L and Tarleton, R. L.2003. Rapid quantitation of Trypanosoma cruzi in host tissue by real-time PCR. Mol. Biochem Parasitol. 2003;29: 53–59. [DOI] [PubMed] [Google Scholar]
- 19.Schijman AG, Altcheh J, Burgos JM, Biancardi M, Bisio M, Levin MJ, et al. Aetiological treatment of congenital Chagas' disease diagnosed and monitored by the polymerase chain reaction. J Antimicrob Chemother. 2003;52:441–49. 10.1093/jac/dkg338 [DOI] [PubMed] [Google Scholar]
- 20.Schijman AG, Bisio M, Orellana L, Sued M, Duffy T, Mejia Jaramillo AM, et al. International study to evaluate PCR methods for detection of Trypanosoma cruzi DNA in blood samples from Chagas disease patients. PLoS Negl Trop Dis. 2011;5(1):e931 10.1371/journal.pntd.0000931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Piron M, Fisa R, Casamitjana N, López-Chejade P, Puig L, Vergés M, et al. Development of a real-time PCR assay for Trypanosoma cruzi detection in blood samples. Acta Trop. 2007;103(3):195–200. 10.1016/j.actatropica.2007.05.019 [DOI] [PubMed] [Google Scholar]
- 22.Britto CC. Usefulness of PCR-based assays to assess drug efficacy in Chagas disease chemotherapy: value and limitations. Mem Inst Oswaldo Cruz. 2009;104: 122–135. [DOI] [PubMed] [Google Scholar]
- 23.Duffy T,Bisio M,Altcheh J, Burgos JM,Diez M, Levin MJ,et al. Accurate real-time PCR strategy for monitoring bloodstream parasite loads in Chagas disease patients. PLoS Negl Trop Dis. 2009;3: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qvarnstrom Y,Schijman AG,Veron V, Aznar C,Steurer F. Sensitive and Specific Detection of Trypanosoma cruzi DNA in Clinical Specimens Using a Multi-Target Real-Time PCR Approach. PLoS Negl Trop Dis. 2012;6: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duffy T, Cura CI, Ramirez JC, Abate T, Cayo NM, Parrado R, et al. Analytical Performance of a Multiplex Real-Time PCR Assay Using TaqMan Probes for Quantification of Trypanosoma cruzi Satellite DNA in Blood Samples. PLoSNegl Trop Dis. 2013;7: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moreira OC, Ramírez JD, Velázquez E, Melo MFAD, Lima-Ferreira C, Guhl F, et al. Towards the establishment of a consensus real-time qPCR to monitor Trypanosoma cruzi parasitemia in patients with chronic Chagas disease cardiomyopathy: a sbstudy from the BENEFIT trial. Acta Trop.2013;125: 23–31. 10.1016/j.actatropica.2012.08.020 [DOI] [PubMed] [Google Scholar]
- 27.Melo MF, Moreira OC, Tenório P, Lorena V, Lorena-Rezende I, Júnior WO, et al. Usefulness of real time PCR to quantify parasite load in serum samples from chronic Chagas disease patients. Parasit Vectors. 2015;8:154 10.1186/s13071-015-0770-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramírez JC, Cura CI, Moreira OC, Lages-Sila E, Juiz N, Velázquez E, et al. Analytical Validation of Quatitative Real-Time PCR Methods for Quantification of Trypanosoma cruzi DNA in Blood Samples from Chagas Disease Patients. J MolDiagn. 2015;17: 605–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saavedra M, Zulantay I, Apt W, Castillo J, Araya E, Martínez G, et al. Quantification by real-time PCR of Trypanosoma cruzi DNA in samples of Triatoma infestans used in xenodiagnosis of chronic Chagas disease patients. 2016;Parasit Vectors. 2016;9(1):382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Apt W, Arribada A, Zulantay I, Saavedra M, Muñoz C, Toro B, et al. Chronic Chagascardiopathy in Chile. Importance of Trypanosoma cruzi burden and clinical evaluation. ActaTrop. 2016;162: 155–166. [DOI] [PubMed] [Google Scholar]
- 31.Hernández C, Cucunubá Z, Flórez C, Olivera M, Valencia C, Zambrano P, et al. Molecular Diagnosis of Chagas Disease inColombia: Parasite loads and Discrete Typing Units in Patients from Acute and Chronic Phases. PLoS Negl Trop Dis. 2016;10(10):e0005112 10.1371/journal.pntd.0005112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramírez JC, Parrado R, Sulleiro E, Barra A, Rodríguez M, Villarroel S, et al. First external quality assurance program for bloodstream Real-Time PCR monitoring of treatment response in clinical trials of Chagas disease. PLoS One. 2017;12(11): 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muñoz C, Apt W, Zulantay I. Real-Time PCR strategy for the identification of Trypanosoma cruzi discrete typing units directly in chronically infected human blood. Infect Genet Evol. 2017;49: 300–308. 10.1016/j.meegid.2017.02.006 [DOI] [PubMed] [Google Scholar]
- 34.Hernández C, Teherán A, Flórez C, Ramírez JD. Comparison of parasite loads in serum and blood samples from patients in acute and chronic phases of Chagas disease. Parasitology. 2018;17:1–7. [DOI] [PubMed] [Google Scholar]
- 35.Rocha MO, Ribeiro AL, Teixeira MM. Clinical management of chronic Chagas cardiomyopathy. Front Biosci. 2003;8: e44–54. [DOI] [PubMed] [Google Scholar]
- 36.Souto RP, Zingales B. Sensitive detection and strain classification of Trypanosoma cruzi by amplification of a ribosomal RNA sequence. Mol Biochem Parasitol. 1993;62(1):45–52. [DOI] [PubMed] [Google Scholar]
- 37.Freitas JM, Augusto-Pinto L, Pimenta JR, Bastos-Rodrigues L, Gonçalves VF, Teixeira SMR, et al. Ancestral genomes, sex, and the population structure of Trypanosoma cruzi. PloS Pathog. 2006;2(3):e24 10.1371/journal.ppat.0020024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burgos JM, Altcheh J, Bisio M, Duffy T, Valadares HMS, Seidenstein ME, et al. Direct molecular profiling of minicircle signatures and lineages of Trypanosoma cruzi bloodstream populations causing congenital Chagas disease. Int J Parasitol. 2007;37(12):1319–27. 10.1016/j.ijpara.2007.04.015 [DOI] [PubMed] [Google Scholar]
- 39.Zingales B, Andrade SG, Briones MRS, Campbell DA, Chiari E, Fernandes O, et al. A new consensus for Trypanosoma cruzi intraspecific nomenclature: second revision meeting recommends TcI to TcVI. Mem Inst Oswaldo Cruz. 2009;104(7):1051–4. [DOI] [PubMed] [Google Scholar]
- 40.Britto C, Cardoso MA, Wincker P, Morel CM. A simple protocol for the physical cleavage of Trypanosoma cruzi kinetoplast DNA present in blood samples and its use in polymerase chain reaction (PCR)-based diagnosis of chronic Chagas' disease. Mem Inst Oswaldo Cruz. 1993;88(1):171–2. [DOI] [PubMed] [Google Scholar]
- 41.Lages-Silva E, Crema E, Ramirez LE, Macedo AM, Pena SDJ, Chiari E. Relationship between Trypanosoma cruzi and human chagasic megaesophagus: blood and tissue parasitism. Am J Trop Med Hyg. 2001;65(5):435–41. [DOI] [PubMed] [Google Scholar]
- 42.Freitas JM, Lages-Silva E, Crema E, Pena SDJ, Macedo AM. Real time PCR strategy for the identification of major lineages of Trypanosoma cruzi directly in chronically infected human tissues. Int J Parasitol. 2005;35(4):411–7. 10.1016/j.ijpara.2004.10.023 [DOI] [PubMed] [Google Scholar]
- 43.Hollander M, Wolfe DA, Chicken E. Nonparametric Statistical Methods. 3rd edition Hoboken New Jersey, USA: John Wiley & Sons; 2014. [Google Scholar]
- 44.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics; 1977. [PubMed] [Google Scholar]
- 45.Fleiss JL, Levin B, Paik MC. Statistical Methods for Rates and Proportions. 3rd edition Hoboken New Jersey, USA: John Wiley & Sons; 2003. [Google Scholar]
- 46.Brener Z. Therapeutic activity and criterion of cure on mice experimentally infected with Trypanosoma cruzi. Rev Inst Med Trop Sao Paulo. 1962;4: 389–396. [PubMed] [Google Scholar]
- 47.Balouz V,Agüero F,Buscaglia C.A. Chagas Disease Diagnostic Applications: Present Knowledge and Future Steps. Adv Parasitol. 2017;97:1–45. 10.1016/bs.apar.2016.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cura CI, Duffy T, Lucero RH, Bisio M, Peneau J, Jimenez-Coelho M, et al. Multiplex real-time PCR assay using TaqMan probes for the identification of Trypanosoma cruzi DTUs in biological and clinical samples. PLos Negl Trop Dis. 2015;9(5):e0003765 10.1371/journal.pntd.0003765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Volpato FCZ, Sousa GR,D’Ávila DA, Galvão LMC, Chiari E. Combined parasitological and molecular-based diagnostic tools improve the detection of Trypanosoma cruzi in single peripheral blood samples from patients with Chagas disease Rev Soc Bras Med Trop. 2017;50(4): 506–515. 10.1590/0037-8682-0046-2017 [DOI] [PubMed] [Google Scholar]
- 50.Chiari E, Brener Z. Contribution to the parasitological diagnosis of human Chagas' disease in its chronic phase. Rev Inst Med Trop São Paulo. 1966;8(3):134–8. [PubMed] [Google Scholar]
- 51.Chiari E, Dias JCP. Notas sobre uma nova técnica de hemocultura para diagnóstico na doença de Chagas a sua fase crônica. Rev Soc Bras Med Trop. 1975;9: 133–136. [Google Scholar]
- 52.Meira WSF, Galvão LMC, Gontijo ED, Machado-Coelho GL, Norris KA, Chiari E. Trypanosoma cruzi recombinant complement regulatory protein: a novel antigen for use in an enzyme-linked immunosorbent assay for diagnosis of Chagas disease. J ClinMicrobiol. 2002;40:3735–3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gomes ML, Macedo AM, Vago AR, Pena SDJ, Galvão LMC, Chiari E. Trypanosoma cruzi: optimization of polymerase chain reaction for detection in human blood. Exp Parasitol. 1998;88(1):28–33. 10.1006/expr.1998.4191 [DOI] [PubMed] [Google Scholar]
- 54.Castro C, Prata A. Absence of both circadian rhythm and Trypanosoma cruzi periodicity with xenodiagnosis in chronic chagasic individuals. Rev Soc Bras Med Trop 2000;33:427–430. [DOI] [PubMed] [Google Scholar]
- 55.Lages-Silva E, Ramirez LE, Pedrosa AL, Crema E, Galvão LMC, Pena SDJ. Variability of kinetoplast DNA gene signatures of Trypanosoma cruzi II strains from patients with different clinical forms of Chagas disease in Brazil. J Clin Microbiol. 2006;44:2167–2171. 10.1128/JCM.02124-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Melo R., Brener Z. Tissue tropism of different Trypanosoma cruzi strains. J Parasitol. 1978;64: 475–482. [PubMed] [Google Scholar]
- 57.Andrade S. G. Morphological and behavioral characterization of Trypanosoma cruzistrains. Rev Soc Bras Med Trop. 1985;18:39–46. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the manuscript.