Abstract
The synthesis and aromatase inhibitory activity of androst-4-en-, androst-5-en-, 1β,2β-epoxy- and/or androsta-4,6-dien-, 4β,5β-epoxyandrostane-, and 4-substituted androst-4-en-17-oxime derivatives are described. Inhibition activity of synthesized compounds was assessed using aromatase enzyme and [1β-3H]androstenedione as substrate. Most of the compounds displayed similar to or more aromatase inhibitory activity than formestane (74.2%). 4-Chloro-3β-hydroxy-4-androsten-17-one oxime (14, 93.8%) showed the highest activity, while 4-azido-3β-hydroxy-4-androsten-17-one oxime (17, 32.8%) showed the lowest inhibitory activity for aromatase.
Keywords: aromatase inhibitor, breast cancer, conjugated androstadiene-17-oxime, 4-substituted androstene-17-oxime
1. Introduction
Breast cancer is the most common cause of death from cancer in women. Estrogens are involved in numerous physiological processes including the development and maintenance of the female sexual organs, the reproductive cycle, reproduction, and various neuroendocrine functions. On the other hand, estrogens enhance growth and proliferation of certain target cells, such as breast epithelial cells and estrogen-dependent mammary carcinoma cells [1]. Most cases (around 80%) of breast cancer occur in postmenopausal women, and the majority of the tumor is found to be hormone-dependent, where estrone (E1) and estradiol (E2) play an important role in the development and evolution of the disease [2,3,4,5]. The conversion of E1 and E2 from androst-4-ene-3,17-dione (androstenedione, AD) and testosterone is catalyzed by aromatase. Compounds that inhibit enzyme aromatase have applications in the treatment of advanced estrogen-dependent breast cancer [6,7]. Over the past two decades, highly potent and specific aromatase inhibitors have been studied as a logical treatment strategy and some have already been approved for clinical use. These include two classes of compounds: steroids, exemestane (Aromasin®), formestane (Lentaron®) and nonsteroids, anastrozole (Arimidex®) and letrozole (Femara®) (Figure 1).
Figure 1.
Chemical structures of known steroidal and nonsteroidal aromatase inhibitors.
Brodie and colleagues [8] showed that extra unsaturation in the A and/or B rings of 4-androstene-dione leads to compounds that are effective inhibitors of aromatase. Numerous aromatase inhibitors, analogs of 4-androstenedione, have been described, including 4-hydroxy- [9], 4-amino- [10], 4-mercapto- [11], 4-(O-alkyl)-, 4-(O-aryl)-, 4-(alkyl)- and 4-(aryl) [12] derivatives, which have been evaluated clinically.
Hydroxyimino steroids represent a distinct class of antineoplastic agents [13,14] and varied placement of the hydroxyimino group on the parental steroid skeleton results in remarkable changes in the antineoplastic activity profile of the compounds [15,16].
Some steroidal 6-hydroxyimino-4-en-3-ones have shown a high affinity for human placental aromatase and function as competitive inhibitors of this enzyme [17]. 3- and 17-Hydroxyimino-2-alkylaminoethyl steroid derivatives were synthesized and evaluated for antineoplastic activity and aromatase inhibitory activity [18].
In the present study, to explore the effect of extended linear conjugation in the rings A and/or B and the effect on the position of the epoxy ring and effect of substituent at 4-position of 17-hydroxyimino androstane skeleton structure on aromatase inhibition, we synthesized 17-hydroxyimino derivatives having additional double bonds at C-1-C-2, at C-4-C-5, at C-6-C-7, or both positions and 17-hydroxyimino derivatives of 1,2- or 4,5-epoxyandrostene and/or -diene and of 4-substituted 4-androstene. Synthesized compounds primarily evaluated their aromatase inhibitory activity by the radiometric method in vitro.
2. Results and Discussion
2.1. Synthesis
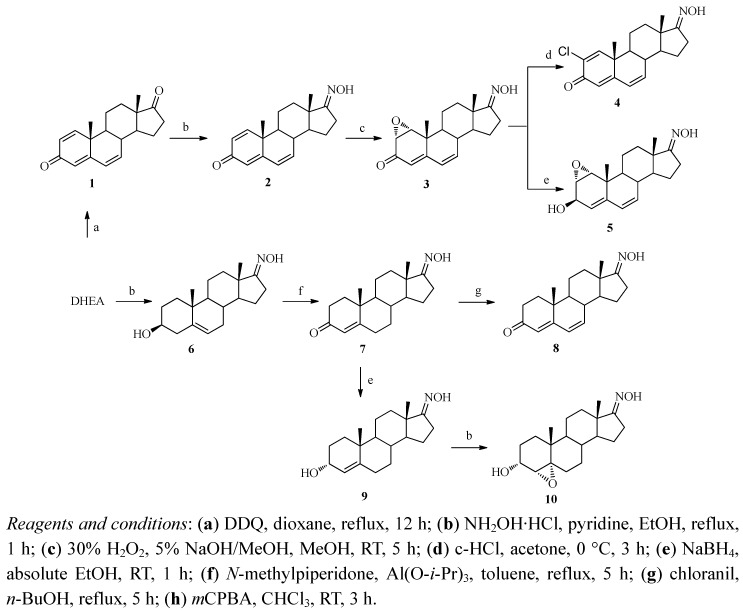
The syntheses of androst-4-en-, androst-5-en- and androsta-4,6-diene-17-oxime are shown in Scheme 1. Oxidation of dehydroepiandrosterone (DHEA) with 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) in dioxane afforded compound 1 [19]. Further reaction of compound 1 with hydroxylamine hydrochloride in ethanol afforded 1,4,6-androstatriene-3,17-dione 17-oxime (2). The structure of 2 was confirmed from a new broad singlet peak at δ 8.34 ppm (C=NOH) in the 1H-NMR spectrum and the absence of C-17 carbonyl peak in the 13C-NMR spectrum. The epoxidation of compound 2 with 30% hydrogen peroxide in methanol in the presence of sodium hydroxide stereoselectively and regioselectively afforded 1α,2α-epoxy compound 3 which was confirmed by 1D-NOESY, as there was a correlation between the 1β-H and the 19β-CH3 group. Two epoxy proton peaks at δ 3.60 ppm (H-2) and δ 3.47 ppm (H-1) in the 1H-NMR and two epoxy carbon peaks at δ 54.8 ppm (C-1) and δ 59.5 ppm (C-2) and disappearance of two double bond carbon peaks in 13C-NMR spectrum confirmed the structure.
Scheme 1.
Synthetic procedures of androst-4-en-, androst-5-en- and androsta-4,6-dien-17-oxime derivatives.
The chlorination reaction of compound 3 under acidic conditions (conc. HCl) in acetone afforded 2-chloro-1,4,6-androstatriene-3,17-dione 17-oxime (4). The disappearance of the epoxy peak and a new peak at δ 7.47 ppm (H-1) in the 1H-NMR spectrum and new two double bond carbon peaks at δ 139.8 ppm (C-2) and δ 150 ppm (C-1) in its 13C-NMR spectrum and the [M]+ mass spectral peak at m/z 331 confirmed the structure. Further reduction of compound 3 with sodium borohydride in absolute ethanol afforded the 3β-hydroxy compound 5. The presence of peaks at δ 3.18 ppm (H-1), 3.29 ppm (H-2) and 4.42 ppm (H-3) in the 1H-NMR spectrum and a peak at δ 67.1 ppm (C-3) in the 13C-NMR spectrum and the mass spectral fragment peak at m/z 299 [M-H2O]+ confirmed the structure. The 3β-OH configuration was determined by 1D-NOESY as there was no correlation between 2β-H and the 3α-H.
DHEA was reacted with hydroxylamine hydrochloride in the presence of pyridine to afford 3-hydroxy-5-androstene-17-oxime (6). The presence of one broad singlet oxime peak at δ 10.02 ppm in the 1H-NMR spectrum and the disappearance of the 17-carbonyl carbon peak and the appearance of a new peak at δ 167.8 ppm (C=NOH) in the corresponding 13C-NMR spectrum and the mass spectral peak at m/z 303 ([M]+) confirmed the structure.
Compound 6 was subjected to Oppenauer oxidation in the presence of N-methylpiperidone and aluminium iso-propoxide to yield the 3-carbonyl compound, 4-androstene-3,17-dione-17-oxime (7). Oxidation of 7 with chloranil instead of DDQ afforded the diene analogue 4,6-androstadiene-3,17-dione-17-oxime (8). The structure of 7 was identified by two peaks at δ 6.15 (H-7) and 6.14 (H-6) in the 1H-NMR spectrum and new double bond carbon peaks at δ 139.6 ppm (C-7) and δ 128.7 ppm (C-6) in the 13C-NMR spectrum. Further reduction of compound 7 with sodium borohydride in absolute ethanol afforded the 3α-hydroxy compound 9 whose structure was confirmed by 1D-NOESY as there was a correlation between 3β-H and 19β-CH3 group. A new multiplet peak at δ 4.14 ppm (H-3) in the 1H-NMR spectrum and the disappearance of the carbonyl peak from the 13C-NMR and a new peak at δ 67.9 ppm (C-3) confirmed this structure.
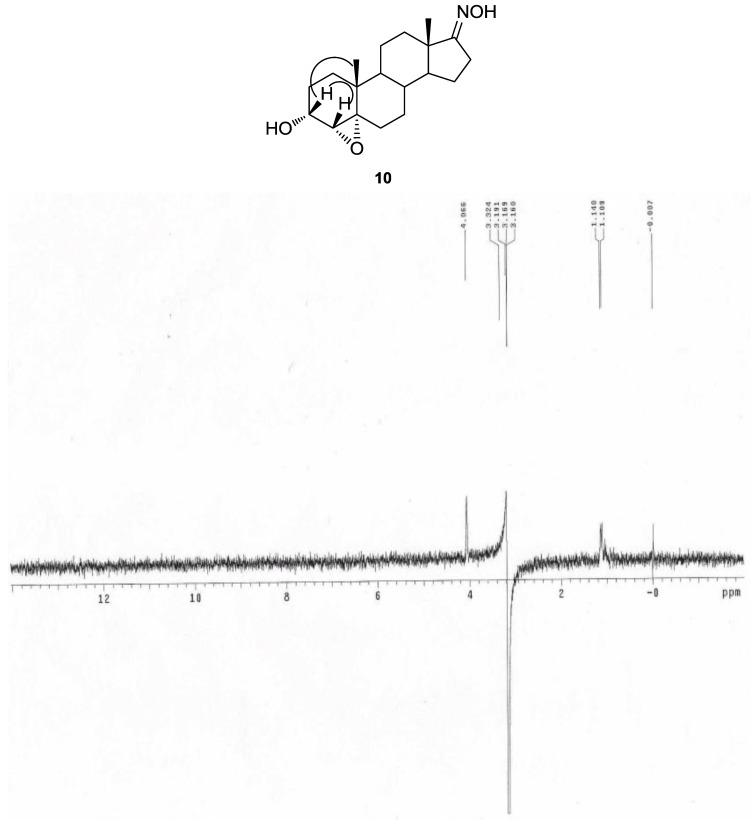
Epoxidation of compound 9 with m-CPBA in chloroform afforded 4α,5α-epoxy compound 10, which was confirmed by a new epoxy peak at δ 3.21 ppm in the 1H-NMR spectrum and disappearance of two double bond carbon peaks and two epoxy carbon peaks at δ 68.8 ppm (C-5) and δ 64.4 ppm (C-4) in the 13C-NMR spectrum.
The α-configuration of the epoxy ring was identified by the 1D-NOESY spectrum as there was correlation between 3β-H (δ 4.11 ppm) and 4β-H (δ 3.21 ppm) with the 19β-CH3 group (δ 1.08 ppm) (Figure 2).
Figure 2.
1D NOESY spectrum of spectrum of 3β-H and 4β-H with 19β-CH3 of 10.
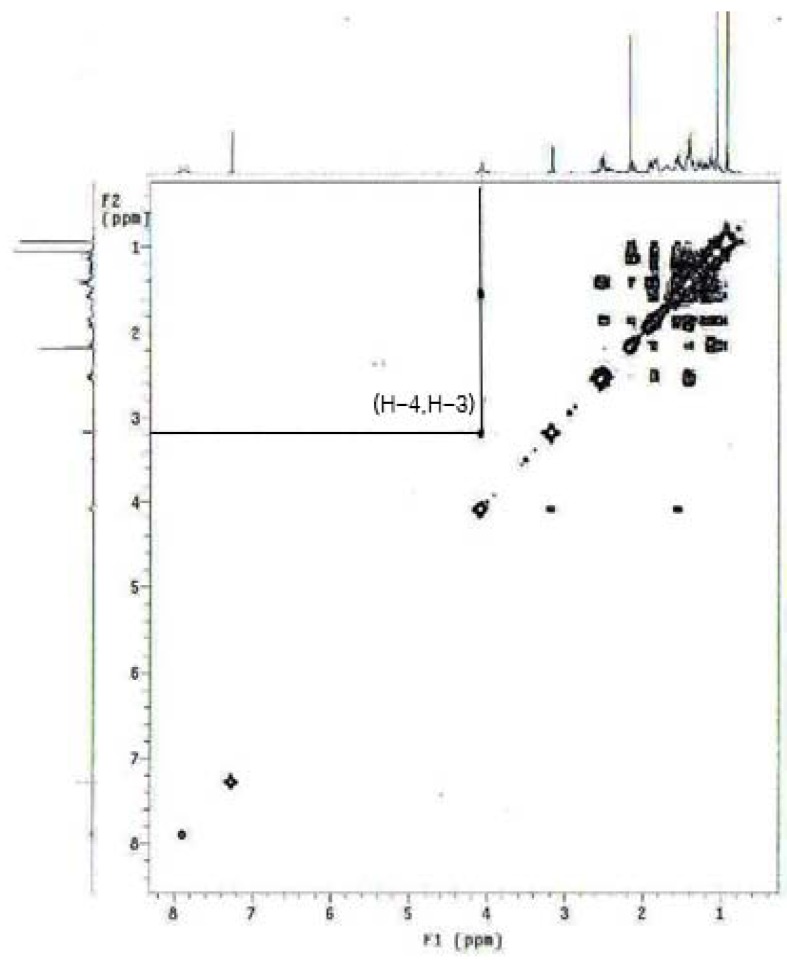
A correlation between 3β-H and 4β-H was observed in the 1H-1H COSY spectrum of 10 (Figure 3). Furthermore, it was reported that the presence of the 3α-hydroxy group of the allylic alcohol directed the epoxidation exclusively to the α-face of the double bond of the compound [20,21]. The presence of one new singlet peak at δ 5.74 ppm (H-4) in the 1H-NMR spectrum and the appearance of new 3-carbonyl peak (C-3) at δ 199.6 ppm in 13C-NMR spectrum confirmed the structure.
Figure 3.
1H-1H COSY spectrum of 10.
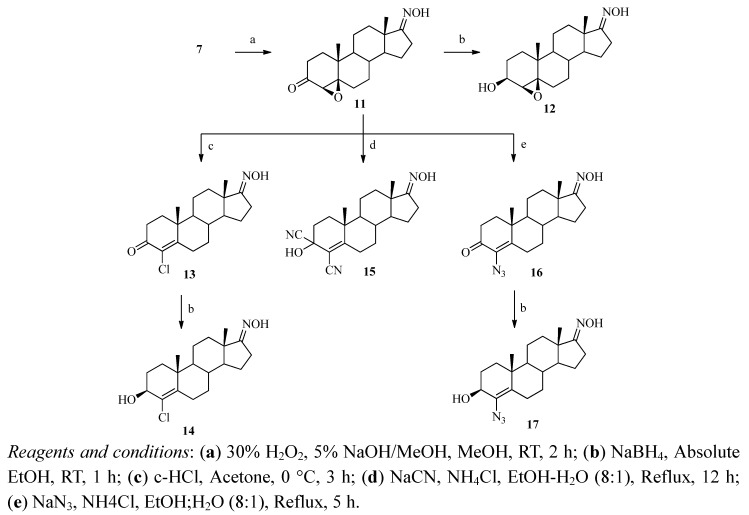
The syntheses of 4-substituted androst-4-en-17-oximes are shown in Scheme 2. Epoxidation of 7 was carried out with 30% hydrogen peroxide in the presence of base to afford 4β,5β-epoxy-androstane-3,17-dione-17-oxime (11). The presence of a new epoxy peak at δ 2.99 ppm (H-4) in the 1H-NMR spectrum and the appearance of two new epoxy carbon peaks at δ 70.2 and 62.8 ppm in the 13C-NMR spectrum confirmed the structure. The 4β,5β-epoxy stereochemistry was determined from the 1D-NOESY (Figure 4) and 1H-1H COSY (Figure 5) spectra. The β-configuration of the 4,5-epoxy ring was determined by irradiation of the H-4 proton, which did not result in a NOE at H-19 (δ 1.19 ppm) and by the fact that no correlation between any proton and 4α-H was observed in the 1H-1H COSY spectrum of 11.
Scheme 2.
Synthesis of 4-substituted androst-4-en-17-oxime derivatives.
Figure 4.
1D-NOESY spectrum of 4α-H with 19β-CH3 of 11.
Figure 5.
1H-1H COSY spectrum of 11.
Reduction of compound 11 with sodium borohydride in absolute ethanol afforded 3β-hydroxy compound 12, as confirmed by 1D-NOESY, the presence of a new peak at δ 3.69 ppm (H-3) in the 1H-NMR spectrum and a new peak at δ 65.7 ppm (C-3) and absence of C-3 carbonyl peak in the 13C-NMR spectrum.
Chlorination of 11 to cleave the epoxy group under acidic conditions (concentrated HCl in acetone) afforded 4-chloro-4-androstene-3,17-dione-17-oxime (13). Disappearance of the epoxy peak from the 1H-NMR spectrum and appearance of two new quaternary carbon peaks of the C-4 and C-5 carbons at δ 164.2 and 127.2 ppm in the 13C-NMR spectrum verified the structure. Reduction of compound 13 with sodium borohydride in absolute ethanol afforded 4-chloro-3β-hydroxy-4-androsten-17-one oxime (14), which was confirmed by 1D-NOESY. The β-configuration of the OH group at the 4 position was determined by irradiation of the H-3 proton which did not show a NOE at H-19. A new multiplet peak at δ 4.14 ppm (H-3) in the 1H-NMR spectrum and disappearance of the carbonyl peak from the 13C-NMR spectrum and a new peak for C-3 at δ 69.7 ppm confirmed the proposed structure of compound 14. Reaction of 11 with sodium cyanide in the presence of ammonium chloride in ethanol yielded the compound 3,4-dicyano-3-hydroxy-4-androsten-17-one oxime (15). Disappearance of the epoxy peak from the 1H-NMR spectrum and the C-3 carbonyl peak and new peaks at δ 171.1 ppm (C-5), δ 122.2 ppm (C-3 CN and C-4 CN) and δ 116.3 ppm (C-4) in the 13C-NMR spectrum and the peak at m/z 326 [M-HCN]+ in its mass spectrum confirmed the structure. Reaction of compound 11 with sodium azide in ethanol and in the presence of ammonium chloride afforded 4-azido-4-androstene-3,17-dione-17-oxime (16). Disappearance of the epoxy peak from the 1H-NMR spectrum, two new peaks at δ 154.5 ppm (C-4) and δ 128.9 ppm (C-5) in the 13C-NMR spectrum confirmed this structure. Further reduction of compound 16 with sodium borohydride in absolute ethanol afforded the 3β-hydroxy compound 17, which was confirmed by 1D-NOESY. A new multiplet peak at δ 4.13 ppm in the 1H-NMR spectrum and disappearance of the carbonyl peak from the 13C-NMR spectrum and a new peak at δ 67.5 ppm of C-3 and the MS peak at m/z 316 [M-N2]+ confirmed the structure.
2.2. Aromatase Inhibitory Activity
The enzyme used in this study was obtained from twice-washed human placental microsomes, as described previously by Ryan [22], and lyophilized to minimize loss of enzyme activity. Aromatase inhibitory activity was determined in vitro by the radiometric method developed by Thompson and Siiteri [23] as modified by Reed and Ohno [24]. This assay quantifies the production of [3H2O] released from [1β-3H]androstenedione after aromatization and the substrate was measured at 0.25 μM concentration. Aromatization was measured at an inhibitor concentration of 100 nM.
Table 1 summarizes the results of the initial screening assay for the androst-4-en-, androst-5-en-, 1β,2β-epoxy- and/or androsta-4,6-dien-, 4β,5β-epoxyandrostane-, and 4-substituted androstene 17-oxime derivatives synthesized in the study. The results showed that most of compounds displayed similar to or more activity than 4-hydroxy-4-androstene-3,17-dione (Formestane, 74.2%) for aromatase inhibition, except 4-azido-3β-hydroxy-4-androsten-17-one oxime (17, 32.8%).
Table 1.
Screening results of aromatase inhibitory activity by synthesized compounds.
| Compounds | % Inhibition * | Compounds | % Inhibition |
|---|---|---|---|
| 1 | 92.9 ± 5.4 | 10 | 79.9 ± 4.1 |
| 2 | 71.1 ± 4.8 | 11 | 81.2 ± 3.8 |
| 3 | 72.2 ± 4.1 | 12 | 88.1 ± 2.4 |
| 4 | 70.1 ± 3.9 | 13 | 75.4 ± 3.0 |
| 5 | 84.6 ± 2.8 | 14 | 93.8 ± 1.1 |
| 6 | 82.3 ± 3.5 | 15 | 85.2 ± 2.6 |
| 7 | 79.6 ± 4.6 | 16 | 76.7 ± 3.6 |
| 8 | 76.7 ± 2.7 | 17 | 32.8 ± 5.1 |
| 9 | 74.2 ± 3.3 | Formestane | 74.2 ± 2.2 |
* All incubations were carried out with 100 nM inhibitor and 0.25 μM [1β-3H]androstenedione for 15 min. Results were normalized against a control treatment with vehicle and are the mean of at least three independent experiments done in triplicate.
1,4,6-Androstatriene-3,17-dione (1) showed high aromatase inhibitory activity (92.9%), but compound 2, the 17-oxime analog of 1, showed lower activity (71.1 ± 4.8%). The 1α,2α-epoxy analog 3 (72.2 ± 4.1%) and 2-chloro-1,4,6-androstatriene-3,17-dione-17-oxime (4, 70.1 ± 3.9) exhibited similar inhibitory activity to 2. According to the previous observation by Numazawa et al., double bonds and epoxy groups confer the planarity of the A-ring required for anti-aromatase activity [25,26]. Therefore, the 17-hydoximino group and 1,2-epoxy or 2-chloro groups of androsta-diene and –triene skeleton rather seemed to decrease the activity. 1α,2α-Epoxy-3β-hydroxy-4,6-androstadien-17-one oxime (5, 84.6 ± 2.8%) displayed more activity than the 3-carbonyl derivative 3.
DHEA-17-oxime (6, 82.3 ± 3.5%) and 4β,5β-epoxyandrostane-3,17-dione-17-oxime (11, 81.2 ± 3.8%) displayed similar results. 4α,5α-Epoxy-3α-hydroxyandrostan-17-one oxime (10, 79.9 ± 4.1%) was similar to 3α-hydroxy-4-androstene-17-one oxime (9, 74.2 ± 3.3%) and less active than the 4β,5β-epoxy-3β-hydroxy compound (12, 88.1 ± 2.4%), which was more active than the 3-carbonyl compound 11. An epoxy ring and the β-configuration of the 4,5-epoxy ring appeared to be beneficial for activity compared with a double bond.
4-Chloro-3β-hydroxy-4-androsten-17-one oxime (14) showed the highest aromatase inhibitory activity (93.8 ± 1.1%) of all synthesized compounds, but the 3-carbonyl compound (13, 75.4 ± 3.0%) of 14 was less active than 14 for aromatase inhibition. The presence of a 3β-hydroxy group appeared to be necessary for achieving higher aromatase inhibitory activity. 3,4-Dicyano-3-hydroxy-4-androsten-17-one oxime (15, 85.2 ± 2.6%) showed less activity than 14. Unexpectedly, 4-azido-3β-hydroxy-4-androsten-17-one oxime (17, 32.8 ± 5.1%) showed the lowest aromatase inhibition activity. The 3-carbonyl compound of 17, 4-azido-4-androstene-3,17-dione 17-oxime (16, 76.7 ± 3.6%) showed better activity than the 3β-hydroxy compound, contrary to the inhibitory activity result of the corresponding 4-chloro analog. It was reported that aromatase might have a tightly fitted hydrophobic binding pocket in the active site around C-4 region of 4-androstenedione [27]. On the basis of this report, we thought that chloro group of 14 probably might bind more effectively than the azido group of 17 in the hydrophobic pocket. Compounds exhibiting effective inhibition in the initial screening assay will be further evaluated in order to characterize the nature of their interaction with the catalytic site.
3. Experimental
3.1. General
All non-aqueous reactions were performed under a dry atmosphere of nitrogen. The commercial reagents were purchased from Aldrich, Fluka, or Sigma Chemical Company. Solvents were purified and dried prior to use. Melting points were measured on Thomas-Hoover melting point apparatus and not corrected. 1H-, 13C-NMR, HSQC, HMQC and NOESY spectra were taken on Varian 400 MHz spectrometer in CDCl3, and DMSO-d6. Chemical shifts (δ) are in parts per million (ppm) relative to tetramethylsilane, and coupling constants (J) are in Hertz. IR spectra were determined on a FT-IR JASCO 4100 spectrometer. GC/MS spectra were obtained on a Shimadzu QP 5050 and JEOL GC Mate 2 mass spectrometers. Elemental analysis was performed on a Yanaco CHN Corder MF-3 automatic elemental analyser. Analytical TLC was performed on pre-coated silica gel 60 F254 plates (Merck). Solvent systems for TLC were ethyl acetate/n-hexane mixtures and 10% MeOH in dichloromethane. Column chromatography was carried out on Merck silica gel 9385 (230–400 mesh) eluting with ethyl acetate/n-hexane mixtures.
3.2. Synthesis
3.2.1. 1,4,6-Androstatriene-3,17-dione 17-oxime (2)
To a solution of 1,4,6-androstatriene-3,17-dione [28] (1, 1 g, 3.54 mmol) in ethanol (10 mL) hydroxylamine hydrochloride (386 mg, 5.6 mmol) and pyridine (0.5 mL) were added and the mixture was allowed to reflux for 1 h. After cooling to room temperature, the ethanol was evaporated and water was added and the mixture extracted with ethyl acetate (3 × 15 mL). The organic solution was dried with anhydrous MgSO4, filtered and concentrated to yield a crude oily product which was further purified by silica gel column chromatography (ethyl acetate-n-hexane = 1:2) to afford compound 2 (716 mg, 70%,) as a whitish solid. Mp 200–201 °C; FT-IR: 3565 (OH), 1735 (17C=O), 1649 (3C=O), 1454 (C=C), 949 (N-O) cm−1; 1H-NMR (CDCl3) δ: 8.34 (1H, br s, =N-OH), 7.27 (1H, d, J = 10.0 Hz, H-1), 6.29 (1H, dd, J = 2.4, 8.0 Hz, H-7), 6.26 (1H, dd, J = 2.4, 8.0 Hz, H-6), 6.06 (1H, d, J = 10.0 Hz, H-2), 6.03 (1H, s, H-4), 1.22 (3H, s, H-19), 1.04 (3H, s, H-18); 13C-NMR (CDCl3) δ: 186.4 (C-3), 169.9 (C-3), 162.2 (C-5), 152.8 (C-1), 136.9 (C-7), 128.4 (C-2), 124.3 (C-6), 124.3 (C-4), 51.3, 48.6, 44.4, 41.3, 37.6, 33.8, 25.1, 22.9, 21.6, 20.9, 17.3; EI-MS (m/z): 297 [M]+; Anal. Calcd. for C19H23NO2 (297.17): C, 76.73; H, 7.80; N, 4.71%. Found: C, 76.82; H, 7.56%.
3.2.2. 1α,2α-Epoxy-4,6-androstadiene-3,17-dione 17-oxime (3)
To a solution of 2 (761 mg, 2.5 mmol) in methanol (40 mL) was added 5% NaOH (1.5 mL) and 30% H2O2 (8.5 mL) and the mixture was allowed to stir at room temperature for 5 h. After completion of the reaction, the reaction mixture was concentrated under reduced pressure to remove the methanol and hydrogen peroxide, water was added to the residue and the mixture was extracted with dichloromethane (3× 20 mL). The organic solvent was dried with anhydrous MgSO4, filtered and concentrated to yield a crude oily product which was purified by silica gel column chromatography (ethyl acetate-n-hexane = 1:3) to afford compound 3 (350 mg, 44%) as a white solid. Mp 149–150 °C; FT-IR: 3580 (OH), 1661 (C=O), 1617 (C=N), 1454 (C=C), 1032 (C-O), 950 (N-O) cm−1; 1H-NMR (CDCl3) δ: 9.01 (1H, br s, =N-OH), 6.19 (1H, dd, J = 1.6, 10.0 Hz, H-7), 6.13 (1H, d, J = 10.0 Hz, H-6), 5.68 (1H, s, H-4), 3.60 (1H, d, J = 6.8 Hz, H-2), 3.48 (1H, d, J = 6.4 Hz, H-1), 1.21 (3H, s, H-19), 1.03 (3H, s, H-18); 13C-NMR (CDCl3) δ: 194.9 (C-3), 169.8 (C-17), 158.4 (C-5), 138.8 (C-7), 128.6 (C-6), 120.1 (C-4), 59.5 (C-2), 54.8 (C-1), 51.1, 46.3, 44.0, 39.1, 36.9, 33.6, 25.2, 22.9, 20.9, 18.6, 17.2; EI-MS (m/z): 313 [M]+; Anal. Calcd. for C19H23NO3 (313.17): C, 72.82; H, 7.40; N, 4.47%. Found: C, 72.34; H, 7.54; N, 4.32%.
3.2.3. 2-Chloro-1,4,6-androstatriene-3,17-dione 17-oxime (4)
To a ice cooled solution of 4 (100 mg, 0.31 mmol) in acetone (4 mL) in an ice bath was added concentrated HCl (1 mL) and the mixture was allowed to stir for 12 h. Then it was neutralized with aqueous 10% NaOH. The solid that appeared was filtered and dried to give a yellowish-white solid which was further purified by recrystallization with methanol to afford compound 4 (50 mg, 32%). Mp 265–266 °C; FT-IR: 3560 (OH), 1732 (C=O), 1454 (C=C), 950 (N-O), 676 (C-Cl) cm−1; 1H-NMR (DMSO-d6) δ: 10.16 (1H, br s, =N-OH), 7.47 (1H, s, H-1), 6.39 (1H, d, J = 9.2 Hz, H-7), 6.21 (1H, d, J = 10.0 Hz, H-6), 6.14 (1H, s, H-4), 1.22 (3H, s, H-19), 0.93 (3H, s, H-18); 13C-NMR (DMSO-d6) δ: 178.6 (C-3), 167.7 (C-17), 163.8 (C-5), 150.2 (C-1), 139.8 (C-2), 131.5 (C-7), 127.4 (C-6), 122.5 (C-4), 51.2, 48.8, 44.2, 44.1, 37.5, 34.2, 25.4, 22.8, 21.7, 21.2, 17.8; EI-MS (m/z): 331 [M]+; Anal. Calcd. for C19H22ClNO2 (331.13): C, 68.77; H, 6.68; N, 4.22%. Found: C, 68.25; H, 6.47; N, 4.30%.
3.2.4. 1α,2α-Epoxy-3β-hydroxy-2,4-androstadien-17-one Oxime (5)
To a solution of 4 (200 mg, 0.63 mmol) in absolute ethanol (10 mL) was added sodium borohydride (48 mg, 1.27 mmol) and the mixture was allowed to stir at room temperature for 2 h. Then ethanol was removed by evaporation followed by addition of water and extraction with ethyl acetate (3 × 10 mL). The combined organic layers were dried with anhydrous MgSO4, and concentrated to give a crude oily product which was further purified by silica gel column chromatography (ethyl acetate-n-hexane = 1:1) to afford compound 5 (49%, 100 mg) as a white solid. Mp 230–232 °C; FT-IR: 3353 (C-OH), 1652 (C=N), 1455 (C=C), 1032 (C-O) cm−1; 1H-NMR (CDCl3) δ: 10.1 (1H, br s, =N-OH), 5.87 (1H, d, J = 10.0 Hz, H-7), 5.61 (1H, d, J = 10.0 Hz, H-6), 5.05 (1H, s, H-4), 4.42 (1H, br s, H-3), 3.29 (1H, d, J = 4.0 Hz, H-2), 3.18 (1H, d, J = 4.8 Hz, H-1), 0.87 (3H, s, H-19), 0.84 (3H, s, H-18); 13C-NMR (CDCl3) δ: 170.5 (C-17), 140.9 (C-5), 132.2 (C-7), 131.5 (C-6), 126.3 (C-4), 67.1 (C-3), 59.6 (C-2), 58.7 (C-1), 54.3, 48.3, 46.1, 39.2, 38.4, 36.9, 27.8, 25.4, 23.4, 20.1, 19.7; EI-MS (m/z): 299 [M-H2O]+; Anal. Calcd. for C19H25NO3 (315.18): C, 72.35; H, 7.99; N, 4.44%. Found: C, 72.01; H, 7.67; N, 4.57%.
3.2.5. 3β-Hydroxy-5-androsten-17-one Oxime (6)
To a solution of DHEA (3.0 g, 10.41 mmol) in ethanol (50 mL) was added hydroxylamine hydrochloride (792 mg, 25.0 mmol) and pyridine (1.6 mL) and the mixture allowed to reflux for 3 h. Then, after cooling to room temperature, the ethanol was evaporated and the white precipitate formed upon addition of water was filtered to afford a crude precipitate which was recrystallized from methanol and H2O to give 6 (3 g, 95%) as a pure white product . Mp 201–203 °C (205–206 °C [20]); Anal. Calcd. for C19H29NO2 (303.22): C, 75.21; H, 9.63; N, 4.62%. Found: C, 75.43; H, 9.12; N, 4.21%.
3.2.6. 4-Androstene-3,17-dione 17-oxime (7)
To a solution of 6 (3 g, 9.9 mmol) in toluene (250 mL) was added N-methylpiperidone (14 mL, mmol) and aluminiun isopropoxide (1.10 g, 5.4 mmol) and the mixture was was allowed to reflux for 5 h under nitrogen. After cooling the toluene was evaporated and water (80 mL) and ethyl acetate (50 mL) were added and left to stir at room temperature overnight and the aqueous layer was then extracted with additional ethyl acetate (3 × 50 mL). The organic layer was further washed with 5% HCl followed by aqueous 10% NaOH and water and finally dried with anhydrous MgSO4 and concentrated in vacuo to afford the desired product 7 (2.97 g, 93%) as a white solid. Mp 172–173 °C; FT-IR: 3580 (OH), 1737 (C=O), 1688 (C=N), 1454 (C=C), 955 (N-O) cm−1; 1H-NMR (CDCl3) δ: 8.85 (1H, s, =N-OH), 5.74 (1H, s, H-4), 1.21 (3H, s, H-19), 0.96 (3H, s, H-18); 13C-NMR (CDCl3) δ: 199.6 (C-3), 170.8 (C-17), 170.6 (C-5), 124.2 (C-4), 54.0, 53.4, 43.9, 38.8, 35.8, 35.1, 34.1, 33.9, 32.8, 31.5, 25.2, 23.3, 20.7, 17.5, 17.2; EI-MS (m/z): 301 [M]+; Anal. Calcd. for C19H27NO2 (301.20): C, 75.71; H, 9.03; N, 4.65%. Found: C, 75.11; H, 8.96; N, 4.56%.
3.2.7. 2,4-Androstadiene-3,17-dione 17-oxime (8)
To a solution of 7 (100 mg, 0.33 mmol) in n-butyl alcohol was added chloranil (86 mg, 0.35 mmol) and the mixture allowed to reflux for 5 h. Then it was allowed to cool and concentrated to remove the n-butyl alcohol, water was added and the mixture extracted with ethyl acetate (3 × 10 mL). The organic solvent was dried with anhydrous MgSO4, filtered and concentrated to yield a crude oily product which was further purified by silica gel column chromatography (ethyl acetate-n-hexane = 1:3) to afford the desired product 8 (10 mg, 10%) as an yellowish-white solid. Mp 192–193 °C; FT-IR: 3591 (OH), 1688 (C=O), 1643 (C=N), 1454 (C=C), 957 (N-O) cm−1; 1H-NMR (CDCl3) δ: 8.40 (1H, br s, =N-OH), 6.15 (2H, dd, J = 1.8, 9.8 Hz, H-7), 6.14 (1H, d, J = 9.6 Hz, H-6), 5.70 (1H, s, H-4), 1.09 (H, s, H-19), 1.01 (3H, s, H-18); 13C-NMR (CDCl3) δ: 199.6 (C-3), 170.4 (C-17), 163.4 (C-5), 139.6 (C-7), 128.7 (C-6), 124.2 (C-4), 51.2, 51.0, 44.8, 37.1, 36.3, 34.1, 34.0, 33.8, 52.1, 22.9, 20.4, 17.2, 16.5; EI-MS (m/z): 299 [M]+; Anal. Calcd. for C19H25NO2 (299.19): C, 76.22; H, 8.42; N, 4.68%. Found: C, 76.87; H, 8.13; N, 4.87%.
3.2.8. 3α-Hydroxy-4-androsten-17-one Oxime (9)
To a solution of 7 (1 g, 3.3 mmol) in absolute ethanol (50 mL) was added sodium borohydride (250 mg, 6.6 mmol) and the mixture was allowed to stir at room temperature for 3 h. Absolute ethanol was removed by evaporation followed by addition of water afterwards to obtain a white solid which was filtered to afford compound 9 (800 mg, 78%,) as a white solid. Mp 170–171 °C; FT-IR: 3576 (N-OH), 3451 (C-OH), 1668 (C=N), 1454 (C=C), 958 (N-O) cm−1; 1H-NMR (CDCl3) δ: 8.34 (1H, br s, =N-OH), 5.30 (1H, s, H-4), 4.14 (1H, m, H-3), 1.03 (3H, s, H-19), 0.97 (3H, s, H-18); 13C-NMR (CDCl3) δ: 171.2 (C-17), 147.0 (C-4), 124.0 (C-5), 67.9 (C-3), 54.7, 53.7, 44.1, 37.5, 35.5, 35.4, 34.1, 32.6, 32.1, 29.5, 25.3, 23.3, 20.7, 19.0, 17.2; EI-MS (m/z): 285 [M_H2O]+; Anal. Calcd. for C19H29NO2 (303.22): C, 75.21; H, 9.63; N, 4.62%. Found: C, 75.87; H, 9.54; N, 4.29%.
3.2.9. 4α,5α-Epoxy-3α-hydroxyandrostan-17-one Oxime (10)
To a solution of 9 (750 mg, 2.47 mmol) in chloroform was added m-CPBA (673 mg, 3.9 mmol) and the mixture was allowed to stir at room temperature for 3 h. Water was added water and the mixture was extracted with dichloromethane (3 × 50 mL). The organic solvent was dried with anhydrous MgSO4, filtered and concentrated to give a crude pale yellow precipitate which was further purified by silica gel column chromatography (ethyl acetate-n-hexane = 1:1) to afford compound 10 (300 mg, 38%) as a white solid. Mp 164–165 °C; FT-IR: 3577 (N-OH), 3397 (C-OH), 1616 (C=N), 1032 (C-O), 952 (N-O) cm−1; 1H-NMR (CDCl3) δ: 8.07 (1H, br s, =N-OH ), 4.11 (1H, m, H-3), 3.21 (1H, d, J = 4.0 Hz, H-4), 1.08 (3H, s, H-19), 0.93 (3H, s, H-18); 13C-NMR (CDCl3) δ: 171.0 (C-17), 68.8 (C-5), 64.4 (C-4), 64.1 (C-3), 53.6, 47.7, 44.1, 36.4, 34.6, 34.0, 31.1, 29.9, 26.6, 26.1, 25.2, 23.4, 21.0, 19.1, 17.2; EI-MS (m/z): 301 [M-H2O]+; Anal. Calcd. for C19H29NO3 (319.21): C, 71.44; H, 9.15; N, 4.38%. Found: C, 71.01; H, 9.02; N, 4.10%.
3.2.10. 4β,5β-Epoxyandrostane-3,17-dione 17-oxime (11)
To a solution of 7 (1 g, 3.31 mmol) in methanol was added 5% NaOH in methanol (2.24 mL) and 30% H2O2 (12 mL) and the mixture was allowed to stir at room temperature for 2 h. Then it was concentrated to remove the methanol and 30% H2O2 and further purified by silica gel column chromatography (ethyl acetate-n-hexane = 1:3) to afford the desired product 11 (500 mg, 45%) as a white solid. Mp 190–191 °C; FT-IR: 3598 (OH), 1713 (C=O), 1651 (C=N), 1032 (C-O), 957 (N-O) cm−1; 1H-NMR (CDCl3) δ: 8.65 (1H, s, =N-OH), 2.99 (1H, s, H-4), 1.19 (3H, s, H-19), 0.94 (3H, s, H-18); 13C-NMR (CDCl3) δ: 206.7 (C-3), 170.5 (C-17), 70.2 (C-4), 62.8 (C-5), 53.4, 46.9, 44.1, 37.5, 34.6, 33.7, 32.6, 30.0, 29.8, 26.3, 25.1, 23.3, 21.2, 19.1, 17.3; EI-MS (m/z): 317 [M]+; Anal. Calcd. for C19H27NO3 (317.20): C, 71.89; H, 8.57; N, 4.41%. Found: C, 71.45; H, 8.34; N, 4.54%.
3.2.11. 4β,5β-Epoxy-3β-hydroxyandrostan-17-one oxime (12)
To a solution of 11 (266 mg, 0.83 mmol) in absolute ethanol (7 mL) was added sodium borohydride (63 mg, 1.66 mmol) and the mixture stirred at room temperature for 2 h. Then ethanol was removed and water was added and the reaction mixture was extracted with dichloromethane (3 × 10 mL) to afford the crude compound which was purified by column chromatography (ethyl acetate-n-hexane = 1:1) to afford 12 (222 mg, 83%) as a white solid. Mp 245–246 °C; FT-IR: 3578 (NOH), 3440 (C-OH), 1660 (C=N), 1032 (C-O), 956 (N-O) cm−1; 1H-NMR (DMSO-d6) δ: 10.12 (1H, s, =N-OH), 3.69 (1H, m, H-3), 2.76 (1H, s, H-4), 0.99 (3H, s, H-19), 0.89 (3H, s, H-18); 13C-NMR (DMSO-d6) δ: 167.6 (C-17), 65.8 (C-5), 65.7 (C-4), 65.7 (C-3), 53.2, 45.6, 43.1, 36.1, 34.1, 33.9, 30.4, 29.4, 26.1, 25.4, 24.8, 22.8, 20.3, 18.6, 17.1; EI-MS (m/z): 302 [M-OH]+; Anal. Calcd. for C19H29NO3 (319.21): C, 71.44; H, 9.15; N, 4.38%. Found: C, 71.21; H, 9.01; N, 4.07%.
3.2.12. 4-Chloro-4-androstene-3,17-dione 17-oxime (13)
To a solution of 11 (100 mg, 0.31 mmol) in acetone (2 mL) in an ice bath was added conc. HCl (0.46 mL) and the mixture was allowed to stir for 4 h. Then it was neutralized with aq. 10% NaOH. A solid appeared that was filtered off and further purified by silica gel column chromatography (ethyl acetate-n-hexane = 1:3) to afford the desired product 13 (80 mg, 74%) as a white solid. Mp 223–224 °C; FT-IR: 3588 (N-OH), 1684 (C=O), 1615 (C=N), 1454 (C=C), 953 (N-O), 745 (C-Cl) cm−1; 1H- NMR (CDCl3) δ: 7.99 (1H, s, =N-OH), 1.26 (3H, s, H-19), 0.97 (3H, s, H-18); 13C-NMR (CDCl3) δ: 190.7 (C-3), 170.7 (C-17), 164.2 (C-4), 127.7 (C-5), 54.2, 53.3, 43.9, 41.6, 34.7, 34.6, 34.1, 33.9, 30.7, 29.0, 25.2, 23.2, 20.8, 17.9, 17.2; EI-MS (m/z): 335 [M]+; Anal. Calcd. for C19H26ClNO2(335.17): C, 67.94; H, 7.80; N, 4.17%. Found: C, 67.23; H, 7.85; N, 4.23%.
3.2.13. 4-Chloro-3β-hydroxy-4-androsten-17-one Oxime (14)
To a solution of 13 (50 mg, 0.14 mmol) in absolute ethanol (5 mL) was added NaBH4 (10.5 mg, 0.28 mmol) and the mixture was stirred at room temperature for 1 h. After completion of the reaction the absolute ethanol was removed in vacuo and the product further purified by silica gel column chromatography (ethyl acetate-n-hexane = 1: 3) to afford compound 14 (20 mg, 35%) as a white solid. Mp 165–166 °C; FT-IR: 3578 (N-OH), 3281 (C-OH), 1650 (C=N), 1454 (C=C), 958 (N-O), 732 (C-Cl) cm−1; 1H-NMR (CDCl3) δ: 8.18 (1H, s, =N-OH), 4.14 (1H, m, H-3), 1.14 (3H, s, H-19), 0.94 (3H, s, H-18); 13C-NMR (CDCl3) δ: 171.0 (C-17), 142.4 (C-4), 128.8 (C-5), 69.7 (C-3), 54.4, 53.6, 44.1, 40.6, 35.0, 34.0, 33.8, 31.2, 28.1, 27.1, 25.2, 23.2, 20.9, 19.4, 17.2; EI-MS (m/z): 337 [M]+; Anal. Calcd. for C19H28NO2 (337.18): C, 67.54; H, 8.35; N, 4.15%. Found: C, 67.10; H, 8.09; N, 4.20%.
3.2.14. 3,4-Dicyano-3-hydroxy-4-androsten-17-one Oxime (15)
To a solution of 11 (100 mg, 0.52 mmol) in ethanol-water (8:1, 9 mL) was added sodium cyanide (120 mg, 2.45 mmol) and ammonium chloride (75 mg, 1.4 mmol) and the mixture was allowed to reflux for 12 h. Then it was cooled down and neutralized with 10% HCl, followed by removal of ethanol and the mixture was further extracted with ethyl acetate (3 × 15 mL) and dried with anhydrous MgSO4 to afford the the desired product 15 (80 mg, 64%) as a creamy white solid. Mp 232–233 °C; FT-IR: 3353 (C-OH), 2173 (C≡N), 1661 (C=N), 1454 (C=C), cm−1; 1H-NMR (CDCl3) δ: 1.23 (3H, s, H-19), 0.88 (3H, s, H-18); 13C-NMR (CDCl3) δ: 172.3 (C-17), 171.1 (C-5), 122.2 (4-CN, 3-CN), 116.3 (C-4), 82.2 (C-3), 53.2, 43.9, 43.7, 40.6, 36.7, 33.8, 33.8, 30.2, 29.7, 25.5, 25.4, 22.9, 21.2, 19.2, 17.0; EI-MS (m/z): 326 [M-HCN]+, 309 [M-HCNOH]+; Anal. Calcd. for C19H27N3O2 (353.21): C, 71.36; H, 7.70; N, 11.89%. Found: C, 71.61; H, 7.54; N, 12.03%.
3.2.15. 4-Azido-4-androstene-3,17-dione 17-oxime (16)
To a solution of 11 (200 mg, 0.63 mmol) in ethanol-water (8:1, 9 mL) was added sodium azide (240 mg, 3.7 mmol) and ammonium chloride (101 mg, 1.89 mmol) and the mixture was allowed to reflux for 5 h. Then it was neutralized with 10% HCl and allowed to cool overnight whereupon yellowish white crystals were obtained, which were filtered off to afford the desired product 16 (77 mg, 34%) as a pale yellow solid. Mp 233–234 °C; FT-IR: 3589 (OH), 1684 (C=O), 1616 (C=N), 1454 (C=C), 1350 (C-N), 958 (N-O) cm−1; 1H-NMR (CDCl3) δ: 8.37 (1H, s, =N-OH), 1.21 (3H, s, H-19), 0.96 (3H, s, H-18); 13C-NMR (CDCl3) δ: 193.3 (C-3), 170.9 (C-17), 154.5 (C-4), 128.9 (C-5), 54.4, 53.4, 43.9, 39.3, 34.7, 34.6, 33.9, 30.9, 26.2, 25.2, 23.2, 20.7, 18.8, 17.7, 17.27; EI-MS (m/z): 341 [M-1]+; Anal. Calcd. for C19H26N4O2(342.21): C, 66.64; H, 7.65; N, 16.36%. Found: C, 66.27; H, 7.38; N, 16.18%.
3.2.16. 4-Azido-3β-hydroxy-4-androsten-17-one Oxime (17)
To a solution of 16 (50 mg, 0.14 mmol) in absolute ethanol (5 mL) was added sodium borohydride (11 mg, 0.29 mmol) and the mixture was left to react at room temperature for 1 h. After completion of the reaction the ethanol was concentrated, followed by addition of water. The aqueous layer was further extracted with dichloromethane (3 × 5 mL) followed by further purification by silica gel column chromatography (ethyl acetate-n-hexane = 1:3) to afford the desired product 17 (63%, 45 mg) as a white solid. Mp 218–219 °C; FT-IR: 3577 (N-OH), 3476 (C-OH), 1686 (C=N), 1454 (C=C), 1350 (C-N), 949 (N-O) cm−1; 1H-NMR (CDCl3) δ: 8.37 (1H, s, =N-OH), 4.13 (1H, m, H-3), 1.03 (3H, s, H-19), 0.92 (3H, s, H-18); 13C-NMR (CDCl3) δ: 171.2 (C-17), 135.1 (C-4), 128.6 (C-5), 67.5 (C-3), 60.0, 54.0, 53.7, 44.1, 38.5, 35.1, 34.1, 33.1, 31.8, 29.6, 25.2, 23.2, 21.0, 19.7, 17.3; EI-MS (m/z): 316 [M-N2]+; Anal. Calcd. for C19H28N4O2 (344.22): C, 66.25; H, 8.19; N, 16.27%. Found: C, 66.67; H, 8.05; N, 16.48%.
3.3. Aromatase Inhibition Assay
3.3.1. Enzyme Preparation
Human placental microsomes (sedimenting after 60 min at 105,000 g) was obtained as described by Ryan [22]. They were washed once with 0.05 mM dithiothreitol (DTT) solution, lyophilized, and stored at −20 °C. No significant loss of activity occurred over the two month duration of the study.
3.3.2. Inhibition Study
The method of Thompson and Siiteri [23] as modified by Reed and Ohno [24], was used in our studies. This assay quantitates the production of [3H]H2O released from [1β-3H]androstenedione after aromatization. [1β-3H]androstenedione was purchased from PerkinElmer Life Science (Boston, MA, USA). All tested compounds were dissolved in DMSO and enzymatic studies were performed in 0.1 M phosphate buffer, pH 7.4, at a final incubation volume of 3.0 mL. The incubation mixture contained 2.5 mΜ glucose-6-phosphate, 0.5 mM NADP, 7 unit glucose-6-phosphate dehydrogenase, 100 nM concentration of inhibitors, 0.25 μM (0.25 μCi) [1β-3H]androstenedione, 1.0 mM EDTA, 10 nM phosphate buffer, and 20 μg of lyophilized human aromatase. Incubations were carried out for 15 min at 37 °C in the air and were terminated by addition of 5 mL CHCl3, followed by vortexing for 40 s. After centrifugation at 15,000 g for 5 min, a 0.5 mL aliquot was removed and added to scintillation mixture for determination of 3H2O production. All experiments were carried out imtriplicate.
4. Conclusions
Most of synthesized 17-hydroximino steroidal compounds showed good aromatase inhibitory activity. 4-Chloro-3β-hydroxy-4-androsten-17-one oxime (14) showed the highest aromatase inhibitory activity of all the synthesized compounds. On the contrary, the 4-azido derivative 17 showed the lowest activity. From the viewpoint that the 3-carbonyl compounds (4, 11 and 13) showed lower activity than the corresponding 3β-hydroxy compounds (5, 12 and 14), the 3β-hydroxy group seems to be required for activity. A 1,2- or 4,5-epoxy ring did not influence the aromatase inhibitory activity and the 17-hydroximino group seemed to decrease the activity in some compounds.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (KRF-2007-531-E00111).
Supplementary Materials
Footnotes
Sample Availability: Samples of the compounds are available from the authors.
References and Notes
- 1.Kellis J., Jr., Vickery L.E. Purification and characterization of human placental aromatase cytochrome P-450. J. Biol. Chem. 1987;262:4413–4420. [PubMed] [Google Scholar]
- 2.Clarke L.H., Olivio S., Kerr L., Bouker K.B., Clarke R. Do estrogens always increase breast cancer risk? J. Steroid Biochem. Mol. Biol. 2002;80:163–174. doi: 10.1016/S0960-0760(01)00184-4. [DOI] [PubMed] [Google Scholar]
- 3.Woo P.M., Woo L.W.L., Humphreys A., Chander S.K. A letrozole-based dual aromatase-sulphatase inhibitor with in vivo activity. J. Steroid Biochem. Molec. Biol. 2005;94:123–130. doi: 10.1016/j.jsbmb.2004.12.028. [DOI] [PubMed] [Google Scholar]
- 4.Chetrite G.S., Prieto J.C.C., Philippe J.C., Pasqualini J.R. Estradiol inhibits the estrone sulfatase activity in normal and cancerous human breast tissues. J. Steroid Biochem. Mol. Biol. 2007;104:289–292. doi: 10.1016/j.jsbmb.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 5.Kendall A., Folkerd E.J., Dowsett M. Influences on circulating oestrogens in postmenopausal women: Relationship with breast cancer. J. Steroid Biochem. Mol. Biol. 2007;103:99–109. doi: 10.1016/j.jsbmb.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 6.Brueggemeier R.W., Hackett J.C., Diaz-Cruz E.S. Aromatase inhibitors in the treatment of breast cancer. Endocr. Rev. 2005;26:331–345. doi: 10.1210/er.2004-0015. [DOI] [PubMed] [Google Scholar]
- 7.Sikora M.J., Condero K.E., Larios J.M., Johnson M.D., Lippman M.E., Rae J.M. The androgen metabolic 5-androstane-3,17-diol (3-Adiol) induces breast cancer growth via estrogen receptor; implication for aromatase inhibitor resistance. Breast Cancer Res. Treat. 2009;115:289–296. doi: 10.1007/s10549-008-0080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwarzel W.C., Kruggel W.G., Brodie H.J. Studies on the mechanism of estrogen biosynthesis. VII. The development of inhibitors of the enzyme system in human placenta. Endocrinology. 1973;92:866–880. doi: 10.1210/endo-92-3-866. [DOI] [PubMed] [Google Scholar]
- 9.Brodie A.M.H., Garrett W.M., Hendrickson J.R., Marcotte P.A., Robinson C.H. Inactivation of aromatase activity in placental and ovarian microsomes by 4-hydroxyandrostene-3,17-dione and 4-acetoxyandrostenedione-3,17-dione. Steroids. 1981;38:693–702. doi: 10.1016/0039-128X(81)90087-8. [DOI] [PubMed] [Google Scholar]
- 10.Disalle E., Giudici D., Ornati G., Briatico G., D’Alessio R., Villa V., Lombardi P. 4-Aminoandrostenedione derivatives: A novel class of irreversible aromatase inhibitors. Comparison with FCE24304 and 4-hydroxyandrostenedione. J. Steroid Biochem. Mol. Biol. 1990;37:369–374. doi: 10.1016/0960-0760(90)90486-5. [DOI] [PubMed] [Google Scholar]
- 11.Lesuisse D., Gourvest J.F., Hartman C., Tric B., Benslimane O., Philibert D., Vevert J.P. Synthesis and evaluation of a new series of mechanism-based aromatase inhibitors. J. Med. Chem. 1992;35:1588–1597. doi: 10.1021/jm00087a013. [DOI] [PubMed] [Google Scholar]
- 12.Abul-Hajj Y.J., Liu X.-P., Hedge M. Synthesis and evaluation of substituted-4-androstene-3,17-dione derivatives as aromatase inhibitors. J. Steroid Biochem. Mol. Chem. 1995;54:111–119. doi: 10.1016/0960-0760(95)00130-R. [DOI] [PubMed] [Google Scholar]
- 13.Rodríguez J., Nunez L., Peixinbo S., Jimenez C. Isolation and synthesis of the first natural 6-hydroxyimino-4-en-3-one steroids from the sponges Cinachyrella spp. Tetrahedron Lett. 1997;38:1833–1836. doi: 10.1016/S0040-4039(97)00163-9. [DOI] [Google Scholar]
- 14.Deive N., Rodriguez J., Jimenez C. Synthesis of cytotoxic 6E-hydroxyimino-4-ene steroids: Structure/activity studies. J. Med. Chem. 2001;44:2612–2618. doi: 10.1021/jm010867n. [DOI] [PubMed] [Google Scholar]
- 15.Cui J.G., Fan I., Huang L.I., Liu H.L., Zhou A.M. Synthesis and evaluation of some steroid oximes as cytotoxic agents: Structure/activity studies (I) Steroids. 2009;74:62–72. doi: 10.1016/j.steroids.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 16.Poza J., Rega M., Paz V., Alonso B., Rodriguez J., Salvador N. Synthesis and evaluation of new 6-hydroxyiminosteroid analogues as cytotoxic agents. Bioorg. Med. Chem. 2007;15:4722–4740. doi: 10.1016/j.bmc.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 17.Hollands H.L., Kumaresan S., Tan L., Nzar V.C.O. Synthesis of 6-hydroximino-3-oxo steroids, a new class of aromatase inhibitor. J. Chem. Soc. Perkin Trans. 1. 1992;13:585–587. [Google Scholar]
- 18.Jindal D.P., Chattopadhaya R., Guleria S., Gupta R. Synthesis and antineoplastic activity of 2-alkylaminoethyl derivatives of various steroidal oximes. Eur. J. Med. Chem. 2003;38:1025–1034. doi: 10.1016/j.ejmech.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 19.Covey D.F., Hood H.F. Enzyme-generated intermediates derived from 4-androstene-3,6,17-trione and 1,4,6-androstatriene-3,17-dione. Endocrinology. 1981;108:1597–1599. doi: 10.1210/endo-108-4-1597. [DOI] [PubMed] [Google Scholar]
- 20.Gamoh K., Hirayama M., Ikekawa N. Stereocontrolled synthesis of Withanolide D and related compounds. J. Chem. Soc. Perkin Trans. 1. 1984:440–454. [Google Scholar]
- 21.Sharpless K.B., Verhoeven T.R. Metal-catalysed, highly selective oxygenations of olefins and acetylenes with tert-butyl hydroperoxide. Practical considerations and mechanisms. Aldrichim. Acta. 1979;12:63–75. [Google Scholar]
- 22.Ryan K.J. Biological aromatization of steroids. J. Biol. Chem. 1959;234:268–272. [PubMed] [Google Scholar]
- 23.Thompson A.E., Jr., Siiteri P.K. The involvement of human microsomal cytochrome P-450 in aromatization. J. Biol. Chem. 1975;249:5373–5378. [PubMed] [Google Scholar]
- 24.Reed K.C., Ohno S. Kinetic properties of human placental aromatase. J. Biol. Chem. 1976;251:1625–1631. [PubMed] [Google Scholar]
- 25.Numazawa M., Mutsumi A., Tachibana M., Hoshi K. Synthesis of androst-5-en-7-ones and androst-3,5-diene-7-ones and their related 7-deoxyanalogues as conformational and catalytic probes for the active site on aromatase. J. Med. Chem. 1994;37:2198–2205. doi: 10.1021/jm00040a012. [DOI] [PubMed] [Google Scholar]
- 26.Numazawa M., Yamaguchi S. Synthesis and structure-activity relationships of 6-phenylaliphatic-substituted C19 steroids having a 1,4-diene, 4,6-diene or 1,4,6-triene structure as aromatase inhibitors. Steroids. 2001;64:187–196. doi: 10.1016/S0039-128X(98)00088-9. [DOI] [PubMed] [Google Scholar]
- 27.Abul-Hajj Y.J. Aromatase inhibition by 4-thiosubstituted-4-androstene-3,17-dione derivatives. J. Steroid Biochem. 1990;35:139–143. doi: 10.1016/0022-4731(90)90158-O. [DOI] [PubMed] [Google Scholar]
- 28.Ma E., Kim E. Epoxidation and reduction of DHEA, 1,4,6-androstatrien-3-one and 4,6-androstadien-3β,17β-diol. Molecules. 2005;10:572–582. doi: 10.3390/10030572. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.