Abstract
Seven 3′,8′′-linked bioflavonoids, including one new compound, (2′′S)-2′′, 3′′-dihydroamentoflavone-4′-methyl ether (1) and six known compounds: (2S)-2,3- dihydroamentoflavone-4′-methyl ether (2), (2S,2′′S)-2,3,2′′,3′′-tetrahydroamento- flavone-4′-methyl ether (3), (2S,2′′S)-tetrahydroamentoflavone (4), (2S)-2,3-dihydro- amentoflavone (5) and (2′′S)-2′′,3′′-dihydroamentoflavone (6) and amentoflavone (7), were isolated from the 60% ethanolic extract of Selaginella uncinata (Desv.) Spring. The structures of these compounds were elucidated mainly by analysis of their 1D and 2D NMR spectroscopic data, and their absolute configurations were determined by circular-dichroism (CD) spectroscopy. All the seven compounds showed protective effect against anoxia in the anoxic PC12 cells assay, in which compound 6 displayed particularly potent activity.
Keywords: Selaginella uncinata (Desv.) Spring; 3′,8′′-linked biflavonoids; protective effect against anoxia; (2′′S) 2′′,3′′-dihydroamentoflavone-4′-methyl ether
1. Introduction
Selaginella uncinata (Desv.) Spring, which is a Chinese herbal medicine known as “Cui Yun Cao”, is widely distributed in the southwest China and used to treat jaundice, dysentery, edema and rheumatism diseases. [1] Earlier phytochemical investigation into this plant led to the isolation of several biflavonoids, flavonoids, chromone glycosides and phenolic constituents [2,3,4].
Hypoxia is a common environmental stress in high altitude. It can influence signaling pathways and cell functions, so several cell types including neuroendocrine chromaffin cells have evolved to sense oxygen levels and initiate specific adaptive responses to hypoxia. As a rat pheochromocytoma cell line derived from a tumor of adrenal medulla chromaffin tissue, PC12 is an oxygen-sensitive cell type and a useful system to study the effects of hypoxia [5].
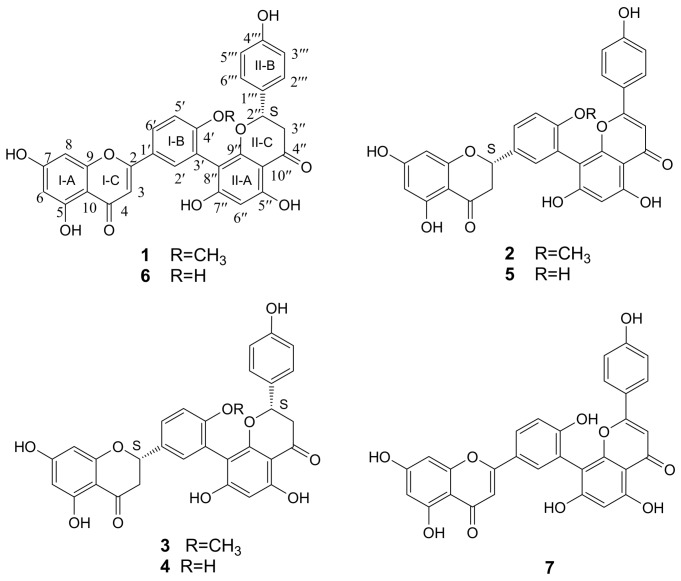
In a previous search for compounds displaying protective effects against anoxia, the 60% ethanolic extract of S. unicinata displayed potent effects in the anoxic PC12 cells assay. Recently, we have reported 3′,6′′-linked biflavonoids isolated from the EtOAc-soluble fraction of the 60% EtOH extract of the whole plant [6]. In continuation of the research on the EtOAc-soluble fraction, seven amentoflavone-type biflavonoids have now been isolated, including one new compound (2′′S)-2′′,3′′-dihydroamentoflavone- 4′-methyl ether (1) and six known compounds 2-7 (Figure 1).
Figure 1.
Chemical structures of compounds 1-7.
2. Results and Discussion
The EtOAc soluble fraction was subjected to silica gel, Sephadex LH-20 and ODS column chromatography, and finally purified by preparative reverse-phase HPLC to afford seven compounds. The seven compounds showed positive reaction with Mg/HCl, which indicated that they were flavonoids.
Compound 1 was isolated as an amorphous yellow powder. The UV spectrum of the compound was typical of biflavanones, with a maximum at 288 nm (log ε 4.92), followed by a shoulder at 327 nm (log ε 4.79) [11]. The IR spectrum showed absorption bands at 3351, 1632, 1498, 1452 cm−1, suggesting the presence of hydroxyl, chelated carbonyl, and aromatic ring functionalities. The positive and negative ESI-MS of 1 gave peaks at m/z 577 [M+Na]+ and 553 [M-H]−, corresponding to a molecular formula of C31H22O10, which was confirmed by HR-TOF-MS (found m/z 577.1099, calcd. 577.1111), consistent with the compound being a biflavonoid.
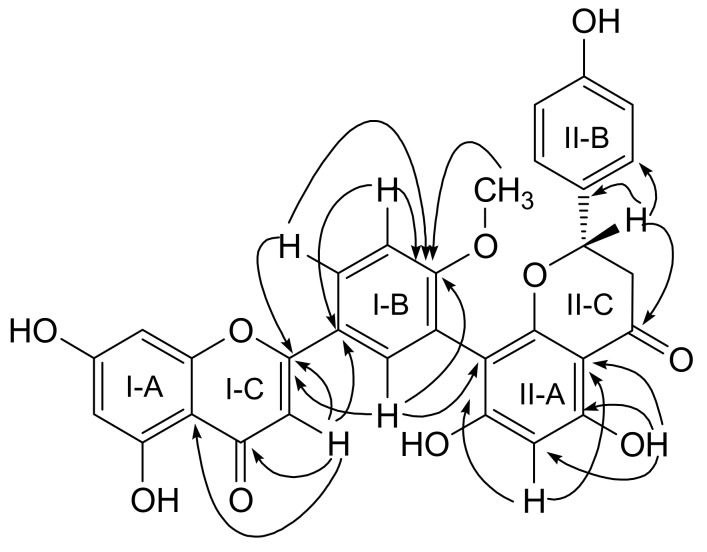
In the 1H-NMR spectrum of 1, one sharp singlet at δ 12.55 was attributed to the H-bonded OH-C (5) or OH-C (5′′), and the broad resonance at δ 9.51 resulted from one of the remaining nonchelated OH group (table 1). The 1H-NMR spectrum of 1 exhibited a one-proton singlet at δ 6.76 and three double doublets at δ 5.51 (1H, dd, J = 12.7, 2.9 Hz, H-2′′), 3.31 (1H, dd, J = 17.1, 12.7 Hz, H-3′′α), and 2.60 (1H, dd, J = 17.1, 2.9 Hz, H-3′′β), characteristic of a flavone and flavanone unit, respectively. The signals at δ 7.96 (1H, dd, J = 8.7, 2.2 Hz, H-6′), 7.90(1H, d, J = 2.2 Hz, H-2′), and 7.15(1H, d, J = 8.7 Hz, H-5′) revealed an AMX coupling system in the 3′, 4′-bisubstituted I-B ring of 1 indicating that C-3′was the position of linkage of the two flavonoid units [7]. The doublets at δ 7.15 (2H, d, J = 8.7 Hz, H-2′′′, 6′′′) and 6.60 (2H, d, J = 8.7 Hz, H-3′′′, 5′′′) could be assigned to the AA′XX′ spin system of the symmetric para-substituted II-B ring of 1. Two meta-coupled proton signals at H-6 and H-8 in I-A ring of 1 appeared at δ 6.22 (1H, d, J = 2.0 Hz, H-6) and 6.54 (1H, d, J = 2.0 Hz, H-8). Further, one proton signal at H-6′′ in II-A ring of 1 appeared at δ 6.05 (1H, s, H-6′′), which was confirmed by an HMBC experiment (Figure 2). The remaining signal at δ 3.72 (3H, s) showed a cross-peak with δ 7.15 (H-5′) in the NOESY spectrum, which confirmed that the OCH3 group is attached to C-4′.
Table 1.
1H- and 13C-NMR spectral data of compound 1 (recorded at 400/100 MHz in DMSO-d6; δ in ppm, J in Hz) a.
| NO. | δH (J, Hz ) a | δc | NO. | δH (J, Hz ) a | δc |
|---|---|---|---|---|---|
| 2 | 164.0 | 2′′ | 5.51 (1H, dd, J = 13.1, 2.8 Hz) | 78.0 | |
| 3 | 6.76 (1H, s) | 102.7 | 3′′ | 3.31 (1H, dd, J = 17.1, 13.1 Hz) 2.60 (1H, dd, J = 17.1, 2.8 Hz) |
41.2 |
| 4 | 181.9 | 4′′ | 196.2 | ||
| 5 | 161.3 | 5′′ | 160.5 | ||
| 6 | 6.22(1H, d, J = 2.0 Hz) | 98.8 | 6′′ | 6.05 (1H, s) | 95.4 |
| 7 | 164.1 | 7′′ | 164.2 | ||
| 8 | 6.54 (1H, d, J = 2.0 Hz) | 94.0 | 8′′ | 104.6 | |
| 9 | 157.3 | 9′′ | 162.0 | ||
| 10 | 103.8 | 10′′ | 101.1 | ||
| 1′ | 122.0 | 1′′′ | 129.6 | ||
| 2′ | 7.90 (1H, d, J = 2.2 Hz) | 131.1 | 2′′′/6′′′ | 7.15 (2H, d, J = 8.7 Hz) | 127.5 |
| 3′ | 122.5 | 4′′′(-OH) | 9.51 (1H, br s) | 157.1 | |
| 4′ | 161.3 | 3′′′/5′′′ | 6.60 (2H, d, J = 8.7 Hz) | 114.6 | |
| 5′ | 7.15 (1H, d, J = 8.7 Hz) | 111.2 | |||
| 6′ | 7.96 (1H, dd, J = 8.7, 2.2 Hz) | 127.3 | |||
| OMe-4′ | 3.72 (3H, s) | 55.6 |
a br s, broad singlet; d, doublet; dd, double doublet; s, singlet.
Figure 2.
Key HMBC of compounds 1.
The 13C-NMR spectrum (Table 1) showed signals for 31 carbons, including two carbonyl groups (δ 196.2 and 181.9) and one methoxyl group (δ 55.6). All the chemical shifts of carbons connected with protons were confirmed using the HSQC experiment. In the HMBC spectrum (Figure 2), the OH-5′′ signal at δ 12.55 was correlated with resonance at δ 95.4 (C-6′′) and δ 101.1 (C-10′′); the H-2′ signal at δ 7.90 correlated with C-8′′ signal (δ104.6) and the H-6′′signal at δ 6.05 with resonance at C-5′′ (δ160.5), C-7′′ (δ164.2), C-8′′ (δ104.6) and C-10′′ (δ101.1), indicating that 1 was a biflavonoid with a C-3′′-C-8′′interflavonoid linkage corresponding to the amentoflavone series [8]. The 1H- and 13C-NMR signal assignments were achieved by combination of 1H-1H COSY, HSQC and HMBC spectral elucidation, and comparison with literature values of 2′′,3′′-dihydroamentoflavone [9]. On comparison of the 13C-NMR spectrum (Table 1) with that of 2′′,3′′-dihydroamentoflavone, it was observed that C-1′ showed a downfield shift of △1.8 ppm and C-5′ showed an upfield shift of △5.2 ppm in 1, respectively.
The CD spectrum of 1 showed a positive Cotton effect at the n→π* absorption band of 327 nm ([Δε]327nm +6.91) and a negative Cotton effect at the π→π* absorption band of 288 nm ([Δε]288 nm −14.59), indicating compound 1 with 2′′S configuration possessing a conformation with P-helicity of the heterocyclic ring and having a C2′′ equatorial aryl group. Furthermore, C-2′′ was assigned the S configuration in accordance with literature values of compound (2′′S)-tetrahydroamentoflavone [10]. Thus, compound 1 is a new compound and was identified as (2′′S)-2′′, 3′′-dihydroamentoflavone-4′- methyl ether.
Along with the new compound, six known biflavonoids including (2S)-2,3-dihydroamento- flavone-4′-methyl ether (2) [11], (2S,2′′S)-2,3,2′′,3′′-tetrahydroamentoflavone-4′-methyl ether (3) [11], (2S,2′′S)-tetrahydroamentoflavone (4) [12], (2S)-2,3-dihydroamentoflavone (5) [13], (2′′S)-2′′, 3′′-dihydroamentoflavone (6) [9], amentoflavone (7) [14] were also isolated.
The protective effect against anoxia of compounds 1-7 was evaluated by the anoxic PC12 cells assay (Table 2). All seven compounds showed protective effect against anoxia, with compound 6 displaying the most potent protective effect, while the other compounds showed moderate effects.
Table 2.
The protective effect against anoxia of compounds 1-7 in the anoxic PC12 cells assay.
| Compounds | Promoted Survival Rate (%) | ||
|---|---|---|---|
| 45 μmol/L | 90 μmol/L | 180 μmol/L | |
| Blank control | 17.81 ± 1.34 | ||
| Negative control | 16.56 ±1.66 | 17.69 ± 1.68 | 15.12 ± 1.87 |
| Baicalin | 21.53 ±1.17 ** | 26.24 ±2.08 ** | 39.30 ± 2.31 ** |
| 1 | 20.88 ±1.41 * | 25.92 ±2.23 ** | 31.53 ± 2.39 ** |
| 2 | 18.07 ± 1.83 | 23.56 ±2.64 ** | 30.52 ± 2.49 ** |
| 3 | 17.27 ±1.61 | 24.15 ± 1.76 ** | 28.02 ± 2.45 ** |
| 4 | 18.24 ±1.09 | 20.25± 1.47 ** | 27.89 ±1.57 * |
| 5 | 16.16 ±0.21 | 20.88 ±1.41 * | 25.67 ± 1.83 ** |
| 6 | 19.35 ± 1.53 | 24.71 ± 2.75 ** | 38.27 ±3.24 ** |
| 7 | 18.82 ± 1.23 | 21.07 ± 2.35 * | 25.76 ± 1.19 ** |
Date were expressed as mean±SD (n = 3). Statistical significance was determined by Student t-test. * p < 0.05, ** p < 0.01 as compared with the control.
3. Experimental
3.1. General
UV spectra were obtained on a Shimadzu UV2401PC spectrophotometer and IR spectra were recorded on a Shimadzu FTIR8900 spectrophotometer using KBr disks. Optical rotations were measured on a Jasco P-1020 digital polarimeter and CD spectra were recorded on a JASCO 810 spectropolarimeter. NMR data were obtained on a Bruker AV-400 spectrometer, with TMS as an internal standard. Mass spectra were determined on a Bruker Esquire 2000 spectrometer, and HR-ESI-MS were acquired using a Micromass Q-TOF mass spectrometer. HPLC analyses were carried out on an Agilent 1100 Series instrument equipped with an PDA detector, using an analytical Shim-pack VP-ODS (4.6 × 250 mm, 5 μm, Shimadzu) or a preparative Shim-pack VP-ODS (20 × 250 mm, 5 μm, Shimadzu) column. Column chromatography (CC) was conducted using silica gel (Qing Dao Hai Yang Chemical Group Co., Qing Dao, China), ODS-A120-S150 (YMC Co., Ltd, Komatsu, Japan), and Sephadex LH-20 (Amersham Biosciences, Uppsala, Sweden).
3.2. Plant material
Herbs of S. unicinata were collected in Guangxi Province, China, in August 2004, and were identified by Professor Sun Qi-shi (Shenyang Pharmaceutical University, Shenyang, China). A voucher specimen (No.Y01156SU) is deposited in the Department of Natural Products Chemistry, Shenyang Pharmaceutical University.
3.3. Extraction and isolation
The air-dried whole herbs (4.2 Kg) of S. uncinata were cut into pieces and refluxed with 60% (v/v) EtOH (126 L, 3 times). The dried extract (856 g) was dissolved in water and successively partitioned with EtOAc and water-saturated n-BuOH to give three parts, the EtOAc (160 g), n-BuOH (90.4 g) and H2O (600 g) soluble parts. The EtOAc soluble part was subjected to silica gel (200–300 mesh) column chromatography and eluted with a CHCl3-MeOH gradient system. Fifteen fractions were obtained. Fraction 7 (18 g), eluted with CHCl3-MeOH (95:5), was separated on a Sephadex LH-20 column (CHCl3-MeOH, 1:1), an ODS column (MeOH-H2O, 7:3), preparative reverse-phase HPLC (Shimadzu, 20 × 250 mm, MeOH- H2O-HAc, 60:40:0.1, flow rate 10 mL/min) to give compounds 1 (15 mg) and 2 (50 mg). Fraction 6 (8.5 g), eluted with CHCl3-MeOH (97: 3), was further separated again on a silica gel (200–300 mesh) column (cyclohexane-acetone, 6:4), a Sephadex LH-20 column (CHCl3-MeOH, 9:1), an ODS column (MeOH-H2O, 6:4), and then passed through preparative reverse-phase HPLC (Shimadzu, 20 × 250 mm, MeOH-H2O-HAc, 60:40:0.1, flow rate 10 mL/min), to give compound 3 (32 mg). Fraction 8 (19.2 g), eluted with CHCl3-MeOH (9:1), was separated on a Sephadex LH-20 column (CHCl3-MeOH, 1:1), an ODS column (MeOH-H2O, 7:3), preparative reverse-phase HPLC (Shimadzu, 20 × 250 mm, MeOH-H2O-HAc, 65:35:0.1, flow rate 10 mL/min) to give compounds 4 (15 mg) and 5 (50 mg). Fraction 9 (45 g), eluted with CHCl3-acetone (8:2-7:3), was separated on a Sephadex LH-20 column (CHCl3-MeOH, 1:1), an ODS column (MeOH-H2O, 7:3), preparative reverse-phase HPLC (Shimadzu, 20 × 250 mm, MeOH-H2O-HAc, 65:35:0.1, flow rate 10 mL/min) to give compounds 6 (17 mg) and 7 (40 mg).
(2′′S)-2′′,3′′-Dihydroamentoflavone-4′-methyl ether (1): Amorphous yellow powder; [α] +3.12° (c 1.0, DMSO); UV λmax (MeOH) (log ε) nm: 327 (log ε 4.79), 288 (log ε 4.92); IRνmax (KBr) cm−1: 3351, 1632, 1498, 1452, 1343, 1254, 1165, 1087, 828; ESI-MS: m/z 577 [M+Na]+, 553 [M-H]−; HR-TOF-MS (positive) m/z: 577.1099 [M+Na]+ (calcd. for C31H22O10Na, 577.1111); CD (MeOH): [Δε]327nm +6.91, [Δε]288nm −14.59. 1H-NMR (DMSO-d6): Table 1; 13C-NMR (DMSO-d6): Table 1.
(2S)-2,3-Dihydroamentoflavone-4′-methyl ether (2): Amorphous yellow powder; [α] +6.30° (c 1.0, DMSO). UV λmax (MeOH) (log ε) nm: 327 (log ε 5.02), 288 (log ε 5.20). IRνmax (KBr) cm−1: 3408, 1640, 1608, 1500, 1462, 1339, 1354, 1281, 1170, 1094, 836 cm−1. ESI-MS: m/z 577 [M+Na]+, 553 [M-H]−. HR-TOF-MS (positive) m/z 577.1099 [M+Na]+ (calcd. for C31H22O10 Na, 577.1111). CD (MeOH): [Δε]327nm +5.76, [Δε]288nm −13.87.
(2S,2′′S)-2,3,2′′,3′′-Tetrahydroamentoflavone-4′-methyl ether (3): Amorphous yellow powder; [α] −3.80° (c 1.0, DMSO). UV λmax (MeOH) (log ε) nm: 289 (log ε 4.66), 203 (log ε 4.91). IRνmax (KBr) cm−1:3357, 1636, 1504, 1454, 1339, 1254, 1246, 1157, 1088, 829 cm−1. ESI-MS: m/z 579 [M+Na]+, 555 [M-H]−. HR-TOF-MS (positive): m/z 579.1240 (calcd. for C31H24O10Na, 579.1267). CD (MeOH): [Δε]327nm +4.57, [Δε]288nm −10.65.
(2S,2′′S)-Tetrahydroamentoflavone (4): Amorphous yellow powder; [α] + 2.96° (c 1.0, DMSO). UV λmax (MeOH) (log ε) nm: 289 (log ε 4.73), 203 (log ε 4.95). IRνmax (KBr) cm−1: 3306, 1625, 1494, 1447, 1352, 1250, 1170, 1085, 825 cm−1. ESI-MS: m/z 543 [M+H]+, 541 [M-H]−. CD (MeOH): [Δε]301nm +7.33, [Δε]276nm −6.65.
(2S)-2,3-Dihydroamentoflavone (5): Amorphous yellow powder; [α] +4.48° (c 1.0, DMSO). UV λmax (MeOH) (log ε) nm: 327 (log ε 4.31), 286 (log ε 4.54). IRνmax (KBr) cm−1: 3090, 1647, 1555, 1497, 1342, 1238, 1157, 1088, 833 cm−1. ESI-MS: m/z 563 [M+Na]+, 539 [M-H]−. CD (MeOH): [Δε]327nm +7.24, [Δε]286nm −10.45.
(2′′S)-2′′,3′′-Dihydroamentoflavone (6): Amorphous yellow powder; [α] +1.04° (c 1.0, DMSO). UV λmax (MeOH) (log ε) nm: 325 (log ε 4.56), 289 (log ε 4.60). IRνmax (KBr) cm−1: 3306, 1625, 1494, 1447, 1352, 1250, 1170, 1085, 825 cm−1. ESI-MS: m/z 541 [M+H]+, 539 [M-H]−. [Δε]325nm +4.06, [Δε]289nm −12.33.
Amentoflavone (7): Amorphous yellow powder; UV λmax (MeOH) (log ε) nm: 333 (log ε 4.79), 270 (log ε 4.82). IRνmax (KBr) cm−1:3082, 1651, 1609, 1574, 1493, 1423, 1358, 1285, 1242, 1165, 1107, 833 cm−1. ESI-MS: m/z 539 [M+H]+, 537 [M-H]−.
3.4. The anoxic PC12 cells assay
The PC12 cells were cultured in a medium that consisted of 85% DMEM (Gibco), 10% heat-inactivated (56 °C for 30 min) horse serum (Hyclone), 5% fetal bovine serum (Hyclone) and glutamine 0.10 g/L for 5 generations before used. Then, The cells were dispersed with pipette and seeded in a 35 mm culture dish at a density of 2 × 105 cells/mL with 2 mL/per dish and incubated in a gas mixture of 90% air and 10% CO2 atmosphere at 37 °C for 8 days. The PC12 cells with samples (in 10% DMSO) were divided into groups with three dishes per group (45 μmol/L, 90 μmol/L and 180 μmol/L), respectively. A blank control group without any supplement was carried out and 10% DMSO (DMSO: normal saline = 1:9) was added to the PC12 cells as a negative control. The result showed that the toxicity of 10 % DMSO in the anoxic cells is not significant. Baicalin which displayed potent protective effect against anoxia in the PC12 cells assay was used as a positive control [15]. 24 hours after that, the cells were moved into a sealed container with 90% N2 and 10% CO2 gas for 12 hours. Cell viability was assessed by trypan blue exclusion. The viable cells were counted under inverted phase contrast microscope (400×) in 10 visual fields randomly [16]. The promoted survival rate (%) of each sample contrasted to the control was calculated. Each experiment repeats 2 times. The data was presented as mean ± SD, n = 10 visual fields and statistics were performed as Student’s T test.
4. Conclusions
By bioassay-guided fractionation using anti-anoxic activity, seven 3′,8′′-linked biflavonoids have been isolated from the 60% ethanol extract of the dried whole herbs of S. unicinata. Among these one, (2′′S)-2′′,3′′-dihydroamentoflavone-4′-methyl ether (1), is a new compound, and other six are known compounds: (2S)-2,3-dihydroamentoflavone-4′-methyl ether (2), (2S,2′′S)-2,3,2′′,3′′-tetra- hydroamentoflavone-4′-methyl ether (3), (2S,2′′S)-tetrahydroamentoflavone (4), (2S)-2,3-dihydro- amentoflavone (5), (2′′S)-2′′,3′′-dihydroamentoflavone (6) and amentoflavone (7). The protective effect against anoxia of the compounds was evaluated in the PC12 cells assay, and all seven compounds showed protective effects, with compound 6 displaying the most potent activity. The present research offers further proof that S. unicinata contains many bioflavonoids which display protective effect against anoxia. This finding may provide a hint for the search of new and bioactive biflavonoids from this plant.
Acknowledgements
This project was financially supported by the National Basic Research Program of China for support of this research (No.2006CB504100) and the research grant from Guangdong Province 211 Project. We are grateful to Q. S. Sun (Shenyang Pharmaceutical University, Shenyang, China) for identifying the plant materials, and to H. Gao, X. Zhang, X. L. Wang and J. H. Huang (Shenzhen Research Center of Traditional Chinese Medicines and Natural Products, Shenzhen, China) for measuring ESI-MS and NMR measurements.
Footnotes
Sample Availability: Samples of the compounds are available from the authors.
References
- 1.Jiangsu New Medical College . Dictionary of Chinese Herb Medicines. Shanghai Scientific and Technologic Press; Shanghai, China: 1986. pp. 1472–1473. [Google Scholar]
- 2.Ma L.Y., Ma S.C., Wei F., Lin R.C., But P.P.-H., Lee S.H.-S., Lee S.F. Uncinoside A and B, two new antiviral chromone glycosides from Selaginella uncinata. Chem. Pharm. Bull. 2003;51:1264–1267. doi: 10.1248/cpb.51.1264. [DOI] [PubMed] [Google Scholar]
- 3.Zheng J.X., Wang N.L., Gao H., Liu H.W., Chen H.F., Fan M., Yao X.S. A new flavonoid with a benzoic acid substituent from Selaginella uncinata. Chin. Chem. Lett. 2008;19:1093–1095. doi: 10.1016/j.cclet.2008.06.024. [DOI] [Google Scholar]
- 4.Zheng J.X., Wang N.L., Cheng H.F., Yao X.S. Isolation and identification of phenolic constituents from Selaginella uncinata (Desv.) Spring. Zhongguo Yaowu Huaxue Zazhi. 2007;17:302–305. [Google Scholar]
- 5.Alvarez-Tejado M., Naranjo-Suárez S., Jiménez C., Carrera A.C., Landázuri M.O., Peso L. Hypoxia induces the activation of the phosphatidylinositol 3-kinase/Akt cell survival pathway in PC12 cells: protective role in apoptosis. J. Biol. Chem. 2001;276:22368–22374. doi: 10.1074/jbc.M011688200. [DOI] [PubMed] [Google Scholar]
- 6.Zheng J.X., Wang N.L., Liu H.W., Chen H.F., Li M.M., Wu L.Y., Fan M., Yao X.S. Four new biflavonoids from Selaginella uncinata and their anti-anoxic effect. J. Asian Nat. Prod. Res. 2008;10:945–952. doi: 10.1080/10286020802181166. [DOI] [PubMed] [Google Scholar]
- 7.He K., Timmermann B.N., Aladesanmi A.J., Zeng L. A biflavonoid from Dysoxylum lenticellare. Phytochemistry. 1996;42:1199–1201. doi: 10.1016/0031-9422(96)00010-6. [DOI] [Google Scholar]
- 8.Markham K.R., Sheppard C., Geiger H. Carbon-13 NMR of flavonoids. Part IV. Carbon-13 NMR studies of some naturally occurring amentoflavone and hinokiflavone biflavonoids. Phytochemistry. 1987;26:3335–3337. [Google Scholar]
- 9.Ohmoto T., Yosida O. Constituents of pollen. XI. Constituents of Cryptomeria japonica D. Don. Chem. Pharmaceut. Bull. 1983;31:919–924. doi: 10.1248/cpb.31.919. [DOI] [Google Scholar]
- 10.Swamy R.C., Kunert O., Schuhly W. Structurally unique biflavonoids from Selaginella chrysocaulos and Selaginella bryopteris. Chem. Biodivers. 2006;3:405–413. doi: 10.1002/cbdv.200690044. [DOI] [PubMed] [Google Scholar]
- 11.Yao X.S., Wang N.L., Fan M., Zheng J.X., Wu L.Y., Liu H.W., Ding A.S., Gao H., Dai Y. Application of flavone derivatives as antioxidation and anti-hypoxia drug or food and their preparation. CN101361733. Chinese Patent. 2009
- 12.Ahmed I., Ishratullah K., Ilyas M., Rahman W., Seligmann O., Wagner H. Tetrahydroamentoflavone from nuts of Semecarpus prainii. Phytochemistry. 1981;20:1169–1170. doi: 10.1016/0031-9422(81)83061-0. [DOI] [Google Scholar]
- 13.Skopp G., Schwenker G. Biflavonoids from Schinus terebinthifolius Raddi (Anacardiaceae) Z. Naturforsch. B. 1986;41B:1479–1482. doi: 10.1515/znb-1986-1125. [DOI] [Google Scholar]
- 14.Silva G.L., Chai H., Gupta M.P., Farnsworth N.R., Cordell G.A., Pezzuto J.M., Beecher C.W.W., Kinghorn A. Cytotoxic biflavonoids from Selaginella willdenowii. Phytochemistry. 1995;40:129–134. doi: 10.1016/0031-9422(95)00212-P. [DOI] [PubMed] [Google Scholar]
- 15.Li C.T., Zhang W.P., Fang S.H., Lu Y.B., Zhang L.H., Qi L.L., Huang X.Q., Huang X.J., Wei E.Q. Baicalin attenuates oxygen-glucose deprivation induced injury by inhibiting oxidative stress-mediated 5-lipoxygenase activation in PC12 cells. Acta Pharmacol. Sin. 2010;31:137–144. doi: 10.1038/aps.2009.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang C.P., Zhu L.L., Zhao T., Zhao H.Q., Huang X., Ma X., Fan M. Characteristics of Neural Stem Cells Expanded in Lowered Oxygen and the Potential Role of Hypoxia-Inducible Factor-1Alpha. NeuroSignals. 2007;15:259–265. doi: 10.1159/000103385. [DOI] [PubMed] [Google Scholar]