Abstract
Two new 9,19-cycloartane triterpene glycosides 1-2, together with four known compounds—26-deoxyactein (3), actein (4), 7,8-didehydro-26-deoxyactein (5) and cimiaceroside B (6)—were isolated from the rhizome of Cimicifuga foetida. The new triterpene glycosides were identified as 23-O-methyl-24-deoxy-2'-O-(3''-methylmalonyl)-cimiaceroside B (1) and 2'-O-(3''-methylmalonyl)actein (2) based on analysis of their spectral data and chemical reactions.
Keywords: Cimicifuga foetida, cycloartane triterpenoid, cimiaceroside, cimifoside
1. Introduction
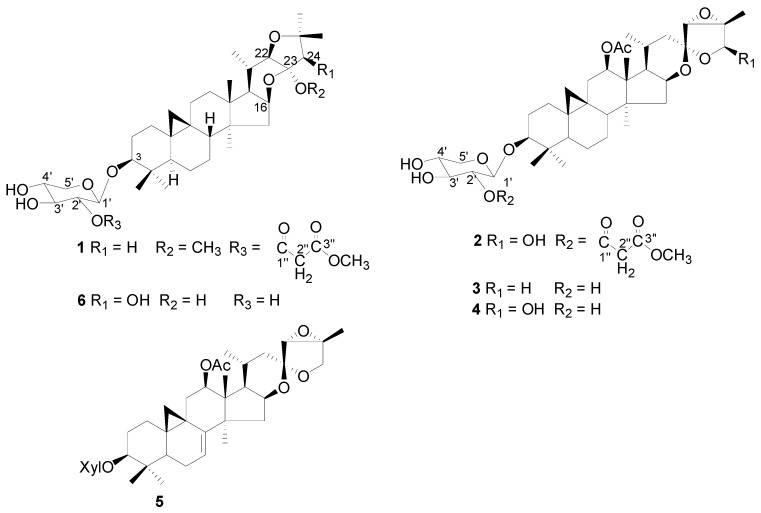
Cimicifuga species are popular Chinese Traditional Medicine, and they are also widely used as a herbal dietary supplement for the relief of symptoms related to menopause [1,2] with a clinical history going back over forty years [3] in the United States and the European Union. Previous investigations revealed that triterpene glycosides were the main active components and could be used as marker compounds to standardize the extracts [4]. In our continuing to search on C. foetida from different geographic regions, we have separated more than 40 triterpene glycosides [5,6,7,8,9,10,11]. In the present investigation on C. foetida collected in Lijiang county, Yunnan Province, two new glycosides 1-2, besides the known compounds 26-deoxyactein (3) [12], actein (4) [13], 7,8-didehydro-26-deoxyactein (5) [14] and cimiaceroside B (6) [15] were isolated from it (Figure 1). This report describes the isolation and structure elucidation of the new compounds.
Figure 1.
Chemical structures of triterpenoid glycoside from the C. foetida.
2. Results and Discussion
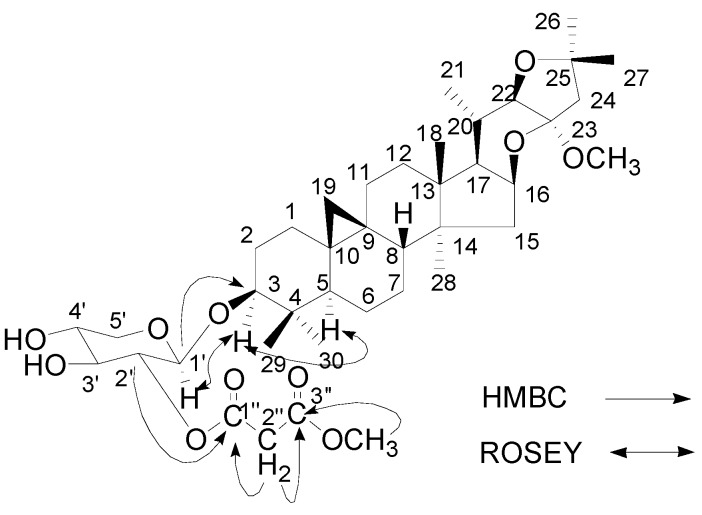
Compound 1 was isolated as a white powder. Its molecular formula was determined as C40H62O11 deduced from the negative HRFABMS (m/z 717.3146 [M-H]-, calcd. 717.3158 for C40H61O11) and the 1H- and 13C-NMR spectra. The IR spectrum of 1 showed absorptions at 3,454, 1,728 and 1,211 cm−1 due to hydroxyl and carbonyl groups. The overall physical properties and NMR spectral profile suggested a cycloartane type of triterpene glycoside, a characteristic and distinguishable chemical marker of Cimicifuga plants [12]. The characteristic cyclopropane methylene signals at δH 0.13 and 0.39 (each 1H, d, J = 2.9 Hz); six tertiary methyl groups at δH 1.14 (Me-18), 1.51 (Me-26), 1.61 (Me-27), 0.83 (Me-28), 1.30 (Me-29), 0.97 (Me-30) (each 3H, s), and one secondary methyl group at δH 1.15 (3H, d, J = 6.4 Hz, Me-21); one methylene signal at δH 3.78 (2H, s, H-2''); and two methoxyl groups at δH 3.36 (3H, s) and δH 3.65 (3H, s); and an anomeric proton at δH 4.83(d, J = 6.4 Hz) were observed in the 1H-NMR spectrum (see Table 1). The 13C-NMR spectrum of 1 displayed 40 carbons, of which 30 carbons were ascribable to the triterpene aglycone, five carbons to a pentose residue at δC 104.2 (C-1'), 76.8 (C-2'), 76.6 (C-3'), 71.3 (C-4'), 67.1 (C-5'), four carbons to one monomethyl malonate group at δC 166.5 (C-1''), 42.0 (C-2''), 167.2 (C-3'') and 52.3 (3''-OCH3), and one carbons to one methoxyl at δC 49.6 (24-OCH3) (see Table 1). The NMR spectroscopic data of 1 showed great resemblance with those of 2'-O-malonylcimiaceroside B [16], except for the changes of C-3'', C-23, C-24, and the location of the monomethyl malonate group was also assigned to C-2' of the xylose unit in the following elucidation. First, all δC and δH of 1 were assigned by a detailed analysis of the HSQC spectrum. Then, in the HMBC spectrum, an informative correlation was observed between H-1' of the anomeric signal at δH 4.89 and a CH signal at δC 88.5 (C-3), suggesting that the sugar moiety was linked to C-3. After acid hydrolysis, only xylose was identified in the aqueous fraction by TLC comparison with an authentic sample, indicating the sugar unit in 1 was xylose. Moreover, the proton coupling constants of H-1' (J = 6.4 Hz) suggested 1 had a β-D-xylopyranoside moiety, which was further supported by an obvious correlation between H-1' (δH 4.89) of the xylose unit and H-3 (δH 3.60) of the aglycon in the ROESY spectrum (Figure 2). A significant ROESY correlation between H-3 and H-5 suggested a β-orientation of the substituent group at C-3. Furthermore, the location of the monomethyl malonate group could be unambiguously assigned to C-2' of the xylose unit by HMBC, as a correlation was observed between H-2' (δH 5.54, t, J = 6.9 Hz) and the carbonyl signal at δC 166.5. In the HMBC, two conspicuous correlations could also be observed between the methoxyl (δH 3.65) and the carbonyl (C-3'', δC 167.2), and between the methoxyl (δH 3.36) and the hemiacetal (C-23, δC 109.7), which suggested that the one methoxyl located the malonyl group to form a monomethyl malonate group, and another methoxyl was linked to C-23. There was a notable difference at C-24 between compound 1 and 2'-O-malonylcimiaceroside B, namely a methine signal at C-24 in 2'-O-malonylcimiaceroside B, while a methylene signal was observed at C-24 (δC 30.0, t) in compound 1, which was confirmed by HMBC correlations from δH 1.54 (m, H-24) to δC 109.7 (s, C-23) and to 83.4 (s, C-25). Thus, compound 1 was determined to be 23-O-methyl-24-deoxy-2'-O-(3''-methylmalonyl)-cimiaceroside B, named cimiaceroside E.
Table 1.
The 1H and 13C-NMR data of 1 and 2 in C5D5N (δin ppm).
| No | 1 a | 2 b | No | 1 a | 2 b | ||||
|---|---|---|---|---|---|---|---|---|---|
| 13C | 1H | 13C | 1H | 13C | 1H | 13C | 1H | ||
| 1 | 32.0 t | 1.20 m; 1.52 m | 31.8 t | 1.15 m; 1.52 m | 20 | 34.3 d | 2.15 m | 26.0 d | 1.79 m |
| 2 | 29.8 t | 1.85 m; 2.20 m | 29.7 t | 1.88 m; 2.28 m | 21 | 17.5 q | 1.15 d 6.4 | 21.0 q | 0.94 d 6.3 |
| 3 | 88.5 d | 3.39 d 3.5 | 88.2 d | 3.31 dd 4.5, 11.5 | 22 | 86.3 d | 3.67 m | 37.6 t | 1.65 m; 2.20 m |
| 4 | 41.0 s | / | 40.9 s | / | 23 | 109.7 s | / | 105.8 s | / |
| 5 | 47.3 d | 1.33 m | 46.9 d | 1.26 m | 24 | 30.0 t | 1.54 m | 63.5 d | 3.90 s |
| 6 | 20.9 t | 0.73 m; 1.31 m | 20.1 t | 0.65 m; 1.45 m | 25 | 83.4 s | / | 65.6 s | / |
| 7 | 26.1 t | 1.61 m | 25.7 t | 0.95 m; 1.30 m | 26 | 27.1 q | 1.51 s | 98.4 d | 5.70 s |
| 8 | 47.5 d | 1.49 m | 45.8 d | 1.52 m | 27 | 24.6 q | 1.61 s | 13.1 q | 1.74 s |
| 9 | 19.7 s | / | 20.4 s | / | 28 | 19.7 q | 0.83 s | 19.5 q | 0.75 s |
| 10 | 26.4 s | / | 26.7 s | / | 29 | 25.6 q | 1.30 s | 25.6 q | 1.08 s |
| 11 | 26.5 t | 1.79 m | 36.7 t | 1.16 m; 2.68 dd 8.3, 15.9 | 30 | 15.2 q | 0.97 s | 15.1 q | 0.91 s |
| 12 | 33.4 d | 1.50 m | 77.1 d | 5.05 dd 3.7, 8.5 | 1' | 104.2 d | 4.83 d 7.4 | 104.1 d | 4.79 d 7.8 |
| 13 | 46.9 s | / | 48.7 s | / | 2' | 76.8 d | 5.54 t 6.9 | 76.7 d | 5.50 t 8.4 |
| 14 | 45.3 s | / | 47.8 s | / | 3' | 76.6 d | 4.15 m | 76.1 d | 4.11 m |
| 15 | 42.8 t | 1.58 m; 1.87 m | 43.6 t | 1.89 dd 8.1, 12.6; 1.71 m | 4' | 71.3 d | 4.18 m | 71.3 d | 4.15 m |
| 16 | 72.5 d | 4.40 dd 6.0, 12.5 | 73.0 d | 4.58 dd 7.1, 14.2 | 5' | 67.1 t | 3.64 m; 4.29 dd 3.5, 8.7 | 67.1 t | 3.60 m; 4.25 dd 7.1, 11.9 |
| 17 | 51.6 d | 1.50 m | 56.4 d | 1.77 m | 1'' | 166.5 s | / | 166.5 s | / |
| 18 | 20.6 q | 1.14 s | 13.6 q | 1.33 s | 2'' | 42.0 t | 3.78 s | 42.0 t | 3.74 s |
| 19 | 30.2 t | 0.13 d 2.9; 0.39 d 2.9 | 29.6 t | 0.17 d 4.0; 0.50 d 4.0 | 3'' | 167.2 s | / | 167.2 s | / |
Note: a 23-OCH3 (δC 49.6, q; δH 3.36, s); 3''-CH3O (δC 52.3, q; δH 3.65, s); b 12-COCH3 (δC 170.6, s), 12-COCH3 (δC 21.7, q; δH 2.12, s); 3''-CH3O (δC 52.3, q; δH 3.63, s).
Figure 2.
The key HMBC and ROSEY correlations of 1.
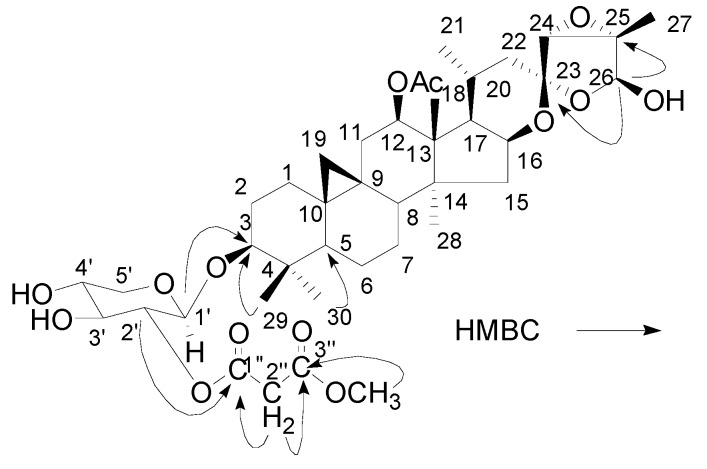
Compound 2 was isolated as a white powder. The negative HR-FAB-MS of 2 showed a quasimolecular ion at m/z 775.4365, corresponding to the molecular formula of C41H60O14. The IR spectrum of 2 showed absorptions at 3,448, 2,500–3,000, 1,737 and 1,244 cm−1 due to hydroxyl and carbonyl groups. Its 1H-NMR spectrum (Table 1) exhibited characteristic cyclopropane methylene signals at δH 0.17 and 0.50 (each 1H, d, J = 4.0 Hz); seven methyls at δH 1.33 (3H, s, Me-18), 0.94 (3H, d, J = 6.3 Hz, Me-21), 5.70 (3H, s, Me-26), 1.70 (3H, s, Me-27), 0.75 (3H, s, Me-28), 1.08 (3H, s, Me-29) and 0.91 (3H, s, Me-30); Additionally, other signals for an anomeric proton at δH 4.79 (1H, d, J = 7.8 Hz, H-1'), for an acetyl group at δH 2.12 (3H, s), and for a monomethyl malonate group at δH 3.74 (2H, s, H-2'') and δH 3.63 (3H, s) were all observed. The 13C-NMR and DEPT spectra showed a total of 41 carbon signals, among which 30 carbons were ascribable to the triterpene aglycone, five carbons to a pentose residue, four carbons to a monomethyl malonate group, and two carbons to an acetyl group (see Table 1). A comparison of the 1H- and 13C-NMR spectra of 2 with those of actein [13] revealed that 2 has an additional monomethyl malonate unit. Long-range correlations between δH 4.79 (H-1') and 88.6 (C-3), and between δH 5.05 (dd, J = 3.7, 8.4 Hz, H-12) and 170.6 (12-COCH3) were observed in the HMBC spectrum of 2, which assigned the xylose linked at C-3 and the acetyl connected to C-12, respectively. The xylose was also detected in the aqueous fraction of the acid hydrolysis products of 2. The additional monomethyl malonate unit was connected at C-2' of xylose, which could be confirmed by HMBC correlation between H-2' (δH 5.50, t, J = 8.4 Hz) and the carbonyl signal at δC 166.5 (s, C-1'') (see Figure 3). On the basis of the above evidence, the chemical structure of 2 was assigned as 2'-O-(3''-methylmalonyl)-actein, named cimifoside E.
Figure 3.
The key HMBC correlations of 2.
3. Experimental
3.1. General
Melting points were determined on a Mel-Temp II; Optical rotations were recorded on a Horiba SEPA-300 polarimeter; IR spectra were recorded on a Shimadzu IR-450 instrument, and are reported in cm−1; UV spectra were obtained in MeOH with a Shimadzu UV-2401A spectrometer, and absorption maxima are given in nm; 1H-, 13C-, and 2D-NMR spectra (all in C5D5N) were recorded with Bruker AV400 or DRX500 instruments, using TMS as an internal standard. Mass spectral data were recorded on a VG Autospec 3000 spectrometer. Silica gel (200–300 mesh, Qingdao Marine Chemical, P.R. China), Lichroprep RP-18 (40–63 um, Merck, Darmstadt, Germany) and Sephadex LH-20 (Pharmacia Fine Chemical Co., Ltd.) were used for column chromatography (CC). Fractions were monitored by TLC, and spots were visualized by heating TLC sprayed with 10% H2SO4.
3.2. Plant Material
The rhizomes of C. foetida were collected in Lijiang, Yunnan Province, China, in June, 2003 and authenticated by Prof. Zong-Yu Wang (Kunming Institute of Botany, CAS). A voucher specimen (KUN No. 200308025) of the collection has been deposited at State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, the Chinese Academy of Sciences.
3.3. Extraction and Isolation
The dried, milled rhizomes of C. foetida (23.4 kg) were exhaustively extracted with 90% MeOH (75 L) under reflux (4 times). The extract was evaporated under reduced pressure to yield a syrup-like residue (about 3.9 kg). The syrup was suspended in H2O-MeOH (9:1), and extracted successively with EtOAc (15 L, 3 times, room temperature). The organic layer was dried (about 1.7 kg), and then absorbed on silica gel (2 kg), and subjected to column chromatography eluting with a gradient system of CHCl3-MeOH from 0 to 100%. The fractions were monitored by TLC, and combined to give seven fractions (Fr.1-7). Fr.2 (55 g), Fr.3 (152 g) contained abundant triterpenoids by TLC examination, indicating the two fractions were worthy of further investigation. Fr. 2 was rechromatographed on a column (silica gel, CHCl3-MeOH 100:1, 80:1, 65:1, 50:1) to obtain subfraction Fr.2.1, containing compounds 3 (15 mg) and 4 (79 mg). The subfraction 2.1 was subjected to RP-C18 by using MeOH-H2O (80:20) as mobile phase to give compounds 1 and 2. Fr. 3 was subjected to CC (silica gel, CHCl3-MeOH 30:1, 20:1, 10:1 gradient ) and further purified by Sephadex LH-20 with eluting MeOH to give 5 (76 mg) and 6 (43 mg).
Cimiaceroside E (1). white powder; m.p. 172 °C; [α] 27D -25.0° (c = 0.80, CHCl3: EtOH (1:1)); negative FABMS m/z (%) 717 [M-H]– (8), 689 (25), 118 (43), 601 [M-117]– (4) and 117 [C3H7O4]– (44); negative HRFABMS (m/z 717.3146 [M-H]–, calcd. 717.3158 for C40H61O11);IR (KBr) νmax 3454, 2957, 2927, 2870, 1728, 1631, 1442, 1381, 1366, 1339, 1211, 1172, 1072, 1052, 971, 897, 820, 633, 583, 537 cm−1. 1H-NMR (500 MHz, C5D5N) and 13C-NMR data (100 MHz, C5D5N) see Table 1.
Cimifoside E (2). white powder; m.p. 168–179 °C; [α] 27D -45.5° (c = 1.10, CHCl3 : EtOH (1:1)); negative FABMS m/z 775 [M-H]–, 662 [M-103]– and 104 [C3H4O4]–; negative HRFABMS (m/z 775.4365 [M-H]−, calcd. 775.4382 for C41H59O14); IR (KBr) νmax 3455, 2953, 1760, 1737, 1457, 1440, 1369, 1348, 1244, 1204, 1163, 1075, 1042, 1024, 984, 831, 726, 633, 604, 540 cm–1. 1H-NMR (500MHz, C5D5N) and 13C-NMR data (100 MHz, C5D5N) see Table 1.
3.4. Acid Hydrolysis of Compounds 1, 2
Compounds 1 and 2 (6 mg of each) were refluxed with 5% HCl in MeOH (7 mL) for 8 h. Each mixture was diluted with H2O and neutralized with NaHCO3. The neutral hydrolysate revealed the presence of only xylose by TLC (n-BuOH-AcOH-H2O, 4:1:1, Rf = 0.4) upon comparison with the authentic sample.
4. Conclusions
Due to their medicinal uses, chemical constituents of Cimicifuga species have been extensively studied by several groups [7,12,17]. To date, more than 200 triterpenes have been isolated from the genus [4,18]. In our continuing studies, new compounds had still been found. In this paper, new triterpene glycosides with a monomethyl malonate group rarely found in the genus Cimicifuga were identified.
Acknowledgements
The project was financially supported by the Knowledge Innovation Program of the CAS (Grant No. KSCX2-YW-G-038, KSCX2-EW-R-15, KSCX2-YW-R-194, KSCX2-YW-R-29 and KZCX2-XB2-15-03), Foundation of State Key Laboratory of Phytochemistry and Plant Resources in West China (P2008-ZZ05 and P2010-ZZ14).
Footnotes
Sample Availability: Samples of the compounds 1–6 are available from the authors.
References
- 1.Lieberman S.J. A review of the effectiveness of Cimicifuga racemosa (black cohosh) for the symptoms of menopause. Women’s Health. 1998;7:525–529. doi: 10.1089/jwh.1998.7.525. [DOI] [PubMed] [Google Scholar]
- 2.Mckenna D.J., Jones K., Humphrey S., Hughes K. Black cohosh: Efficacy, safety, and use in clinical and preclinical applications. Altern. Ther. 2001;7:93–100. [PubMed] [Google Scholar]
- 3.Liske E., Wustenberg P. Therapy of climacteric complaints with Cimicifuga racemosa: herbal medicine with clinically proven evidence. Menopause. 1998;5:250–256. [Google Scholar]
- 4.Lin Y.P., Qiu M.H., Li Z.R. Studies on the chemical constituents and biologic activities of Cimicifuga. Nat. Prod. Res. Dev. 2002;14:58–76. [Google Scholar]
- 5.Qiu M.H., Kim J.H., Lee H.K., Min B.S. Anticomplement activity of cycloartane glycosides from the rhizome of Cimicifuga foetida. Phytother. Res. 2006;20:945–948. doi: 10.1002/ptr.1982. [DOI] [PubMed] [Google Scholar]
- 6.Sun L.R., Yan J., Pei S.J., Qiu M.H. A new cycloartane triterpenoid from the rhizome of Cimicifuga foetida collected in Dali. Acta Bot. Yunnan. 2005;27:331–336. [Google Scholar]
- 7.Sun L.R., Qing C., Zhang Y.L., Jia S.Y., Li Z.R., Pei S.J., Qiu M.H., Michael L.G., Qiu S.X. Cimicifoetisides A and B, two cytotoxic cycloartane triterpenoid glycosides from the rhizomes of Cimicifuga foetida, inhibit proliferation of cancer cells. Beilstein J. Org. Chem. 2007;3:3. doi: 10.1186/1860-5397-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun L.R., Yan J., Lu L., Pei S.J., Li Z.R., Zhou L., Zhang X.M., Qiu M.H. Cimicifine A: A novel triterpene alkaloid from the rhizomes of Cimicifuga foetida. Helv. Chim. Acta. 2007;7:1313–1318. [Google Scholar]
- 9.Sun L.R., Yan J., Nian Y., Zhou L., Zhang H.J., Qiu M.H. New triterpene diglycosides from the rhizome of Cimicifuga foetida. Molecules. 2008;13:1712–1721. doi: 10.3390/molecules13081712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu L., Chen J.C., Nian Y., Sun Y., Qiu M.H. Trinor-cycloartane glycosides from the rhizomes of Cimicifuga foetida. Molecules. 2009;14:1578–1584. doi: 10.3390/molecules14041578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nian Y., Zhang Y.L., Chen J.C., Lu L., Qiu M.H., Qing C. Cytotoxic chemical constituents from the roots of Cimicifuga fetida. J. Nat. Prod. 2010;73:93–98. doi: 10.1021/np9003855. [DOI] [PubMed] [Google Scholar]
- 12.Chen S.N., Li W.K., Fabricant D.S., Santarsiero B.D., Mesecar A., Fitzloff J.F., Fong H.H.S., Farnsworth N.R. Isolation, structure elucidation, and absolute configuration of 26-deoxyactein from Cimicifuga racemosa and clarification of nomenclature associated with 27-deoxyactein. J. Nat. Prod. 2002;65:601–605. doi: 10.1021/np010494t. [DOI] [PubMed] [Google Scholar]
- 13.Li C.J., Li Y.H., Chen S.F., Xiao P.G. Triterpenoids from Cimicifuga foetida L. Acta Pharm. Sin. 1994;29:449–453. [Google Scholar]
- 14.Kusano A., Takahira M., Shibano M., Miyase T., Kusano G. Studies on the constituents of Cimicifuga species. XXVI. Twelve new cyclolanostanol glycosides from the underground parts of Cimicifuga simplex Wormsk. Chem. Pharm. Bull. 1999;47:511–516. doi: 10.1248/cpb.47.511. [DOI] [Google Scholar]
- 15.Kusan A., Yakahira M., Shibano M., Miyase T., Okuyama T., Kusano G. Structures of two new cyclolanostanol xylosides: cimiacerosides A and B. Heterocycles. 1998;48:1003–1014. doi: 10.3987/COM-98-8121. [DOI] [Google Scholar]
- 16.Kusano A., Shibano M., Kusano G. Studies on the constituents of Cimicifuga species. XXVII. Malonyl cyclolanostanol glycosides from the underground parts of Cimicifuga simplex Wormsk. Chem. Pharm. Bull. 1999;47:1175–1179. doi: 10.1248/cpb.47.1175. [DOI] [Google Scholar]
- 17.Liu Y., Chen D.H., Si J.Y., Tu G.Z., An D.G. Two new cyclolanostanol xylosides from the aerial parts of Cimicifuga dahurica. J. Nat. Prod. 2002;65:1486–1488. doi: 10.1021/np020130g. [DOI] [PubMed] [Google Scholar]
- 18.Li J.X., Yu Z.Y. Cimicifugae rhizoma: from origins, bioactive constituents to clinical outcomes. Curr. Med. Chem. 2006;13:2927–2951. doi: 10.2174/092986706778521869. [DOI] [PubMed] [Google Scholar]