Abstract
Inhibitors that target specific kinases or oncoproteins have become popular additions to or replacements for cytotoxic chemotherapies to treat many different types of cancer. However, many tumors lack a discernable target kinase and an amplified oncoprotein and/or rely on several cooperating mechanisms for progression. Thus, combinations of targeted therapies are essential for treating many cancers to avoid the rapid emergence of resistance. In this issue of the JCI, Ren et al. use an elegant kinase activity–profiling method and identify activity of the oncogene polo-like kinase-1 (PLK1) as an important driver of double-hit lymphoma (DHL), an aggressive subgroup of B cell lymphoma characterized by chromosomal translocations involving c-MYC and BCL2 or BCL6. Moreover, PLK1 activity was associated with MYC expression and poor prognosis in DHL patients. PLK1 inhibition with volasertib, alone and in combination with the BCL-2 inhibitor venetoclax, was efficacious in multiple DHL models, including mice harboring DHL patient–derived xenografts. Together, these data support PLK1 as a promising prognostic marker and therapeutic target for DHL.
A treatment-refractory disease
Double-hit lymphoma (DHL) is a very aggressive subgroup of B cell lymphoma that possesses translocations in MYC and BCL2 or BCL6. Similarly, double-protein–expression lymphoma (DEL) refers to the subgroup of B cell lymphoma that results from overexpression of MYC and BCL-2 through other means, such as gene amplification. Approximately 20% to 30% of patients with diffuse large B cell lymphoma (DLBCL) fall into the expanded DHL category, which includes both DHL and DEL, and these patients have a worse prognosis than those without alterations in both MYC and BCL-2 when treated with standard therapy for non-Hodgkin lymphoma (1, 2). Unlike the more manageable DLBCL, which is curable in about 60% of patients treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP), patients with DHL on R-CHOP have a median overall survival ranging between 5 and 24 months (3, 4). The combination of increased cell proliferation and survival through dysregulated MYC and decreased apoptosis through enhanced BCL-2 signaling is hypothesized to underlie aggressiveness and treatment resistance in DHL (Figure 1).
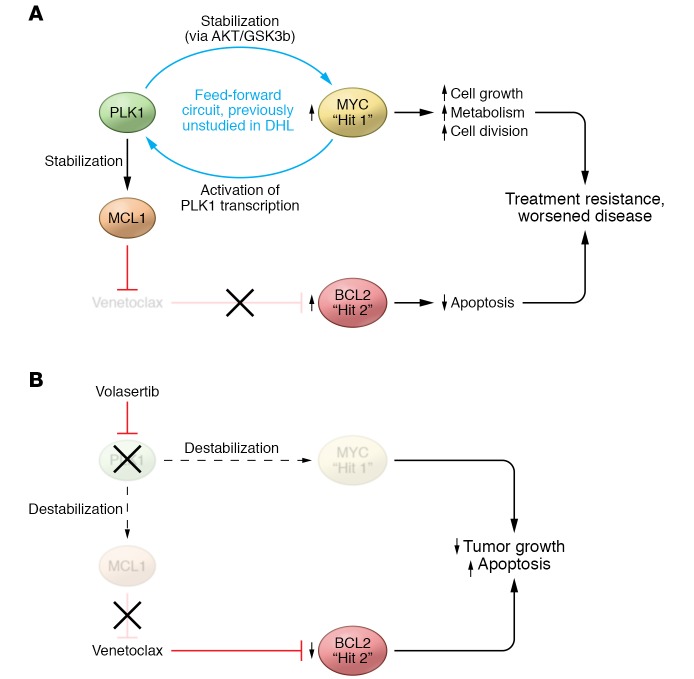
Figure 1. PLK1 activity contributes to disease pathogenesis in DHL.
DHL is characterized by overactivity of both MYC and BCL-2 oncoproteins. MYC overactivity contributes to the development of cancer by increasing cell proliferation. Aberrant BCL-2 activity helps prevent apoptosis, and while not sufficient to cause cancer alone, can support metabolism and cell-cycle changes by other oncogenes. (A) In this issue, Ren et al. identify PLK1 as an important regulator of DHL. Specifically, PLK1 was shown to stabilize MCL1, a BCL-2 family member that is known to mediate resistance to the BCL-2 inhibitor venetoclax. Moreover, PLK1 interacts with MYC to form a feed-forward circuit in which PLK1 helps stabilize MYC and MYC activates further PLK1 transcription. (B) Ren et al. show that targeting PLK1 with volasertib destabilizes MYC and MCL1 in DHL and may be a way to indirectly target MYC, which is currently considered “undruggable.”
A new hallmark of DHL
Polo-like kinase-1 (PLK1) overexpression has been observed in several cancers and is associated with a worse prognosis (5). In this issue, Ren and colleagues reveal a previously undescribed role for this kinase in DHL (6). Using activity-based protein profiling of isogeneic doxycycline-repressible MYC B cell lines with high or low BCL2 expression, a gene expression profiling data set with 484 primary B cell lymphoma samples, and a tissue microarray comprising primary DLBCL patient samples, the authors showed that increased PLK1 expression and activity correlate with MYC expression and result in poorer outcomes in the DHL subset of patient samples, illustrating elevated PLK1 activity as another signature of DHL. Pharmacological inhibition or CRISPR-mediated genetic deletion of PLK1 in cell lines resulted in time- and dose-dependent decreases in MYC levels, showing that PLK1 expression and activity are required to maintain MYC expression in DHL. This integrated and comprehensive analysis is unique and applicable to other cancer types for future investigation.
Even more important than the identification of PLK1 overexpression and activity in DHL is the elucidation of the mechanism of a feed-forward circuit between PLK1 and MYC. MYC stabilization is well known to be regulated at the highly conserved amino acids threonine 58 and serine 62. Phosphorylation at serine 62, which is mediated by a number of kinases, including PLK1, stabilizes MYC, and subsequent phosphorylation at threonine 58, which is mediated by GSK3β, promotes MYC ubiquitination by the E3 ubiquitin ligase FBW7 and consequent proteasome degradation (7). Ren et al. have now demonstrated that PLK1 stabilizes MYC in DHL by activating an AKT/GSK3β-signaling axis (6). Active GSK3β phosphorylates MYC at threonine 58; however, phosphorylation of GSK3β by AKT prevents MYC phosphorylation. Inhibition of PLK1 via CRISPR knockout or pharmacologically with volasertib decreased AKT activity in DHL, resulting in MYC destabilization. Interestingly, this mechanism of increased MYC degradation differs from that shown in previous work in neuroblastoma, where PLK1 inhibition stabilizes N-MYC by increasing levels of FBW7, which directly increases MYC ubiquitination (8). Additionally, by using ChIP assays, Ren et al. showed a significant increase in MYC recruitment to the PLK1 promoter–proximal E-box motif in several MYC-driven lymphoma lines, suggesting that MYC directly activates PLK1 expression in DHL (6). Thus, PLK1-mediated stabilization of MYC drives PLK1 expression, thereby forming a previously undescribed loop that contributes to the potential differential biology and clinical behavior of DHL (Figure 1). Furthermore, PLK1 activation was shown to enhance tumor dependence upon MCL1, a common mediator of resistance to selective BCL-2 inhibitors such as venetoclax, for survival (9).
A promising therapeutic target
When evaluating preclinical models of cancer therapies, there very often are substantial differences in efficacy between in vitro and in vivo experiments. Several factors, including interaction of tumor with microenvironment, altered bioavailability, metabolic changes, and distribution of potential therapies, have emerged during studies of animal models. Yet there is still room to critique the relevance of some in vivo models. Xenografts of cell lines into immunocompromised mice are commonly used to model disease development and response to treatment, and while these cell lines were once derived from a patient’s actual tumor, changes that occur over time may cause unknown but meaningful differences in response to treatment. Ren et al. improve upon commonly used methods for testing cancer therapies by using ex vivo patient samples and patient-derived xenograft (PDX) models of DHL to show efficacy of PLK1 and BCL-2 inhibition alone and in combination (6).
Ren and colleagues employed an ex vivo model, previously published by the Tao group (10), that allowed reconstruction of the lymphoma microenvironment by seeding primary DHL cells in plates coated with human-derived stroma cells and collagen. Consideration of the increased complexities of how cells communicate with each other, the extracellular matrix, and with various secreted factors has had a profound influence on therapy and clinical outcomes (11, 12). The PDX model, while still a human xenograft, differs from cell-line xenografts because primary tissue more closely recapitulates DHL disease. General weaknesses of a PDX approach include difficulty obtaining samples, a diminished ability to replicate or validate findings, and an inability to study interactions of disease or treatments with the immune system. However, PDX models have several potential strengths, including the ability to study the mechanisms of disease and response to treatment for any patient’s individual tumor. PDX models consequently show promise as being clinically relevant for investigation of disease pathogenesis and may serve as a means to design and deliver truly personalized health care. The in vitro, ex vivo, and PDX in vivo models used by Ren et al. determined that PLK1 and BCL-2 inhibition have efficacy as single agents and that efficacy is further increased by a combination strategy (6). Together, these results support the development of clinical trials targeting PLK1 in DHL.
Future directions
Ren and colleagues have revealed PLK1 as a promising therapeutic target in DHL by showing expression and increased activity of PLK1, identifying a PLK1/MYC feed-forward loop, and showing efficacy of PLK1 inhibition along with selective BCL-2 inhibition in several specific DHL models (6). The data provided in this study indicate that dual inhibition of PLK1 and BCL-2 have a greater effect than single inhibition of either target; however, Ren et al. did not fully explore the degree of synergy between the PLK1 inhibitor volasertib and the BCL-2 inhibitor venetoclax for treating DHL. Combination therapies are popular across many medical specialties because of the potential to obtain a greater effect with less treatment. With rising healthcare costs and the potential for side effects, a deeper investigation into the doses of each drug required when used in combination may prove useful for improving patient outcomes. PLK1 inhibition has also been shown to be effective in neuroblastoma (8) and, recently, anaplastic thyroid cancer (13) and glioma (14). The effectiveness of PLK1 inhibition in other cancers coupled with the fact that both the PLK1 and the BCL-2 inhibitors used by Ren et al. have defined safe doses for administration and have relatively nonoverlapping toxicity profiles provides justification for a clinical trial to assess the safety and activity of these or similar agents in DHL. Furthermore, the establishment of the previously unknown role of PLK1 in DHL provides a novel path for exploring more detailed mechanisms of disease development in DHL and other cancers. Finally, the successful indirect targeting of MYC through PLK1 in DHL suggests that similar approaches may be feasible for reducing MYC or other oncogenes currently considered “undruggable” in other diseases.
Acknowledgments
We would like to acknowledge Katie Williams for helpful comments and suggestions on this commentary.
Version 1. 11/05/2018
Electronic publication
Version 2. 12/03/2018
Print issue publication
Footnotes
Conflict of interest: JCB receives research dollars from the Leukemia & Lymphoma Society, Celgene, Acerta Pharma, and Pharmacyclics for support of the performance of clinical trials. In addition, JCB has several issued and pending patents against targets different than the ones identified in this paper.
Reference information: J Clin Invest. 2018;128(12):5206–5208. https://doi.org/10.1172/JCI124919.
See the related article at PLK1 stabilizes a MYC-dependent kinase network in aggressive B cell lymphomas.
Contributor Information
Lapo Alinari, Email: lapo.alinari@osumc.edu.
John C. Byrd, Email: john.byrd@osumc.edu.
References
- 1.Horn H, et al. MYC status in concert with BCL2 and BCL6 expression predicts outcome in diffuse large B-cell lymphoma. Blood. 2013;121(12):2253–2263. doi: 10.1182/blood-2012-06-435842. [DOI] [PubMed] [Google Scholar]
- 2.Johnson NA, et al. Concurrent expression of MYC and BCL2 in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol. 2012;30(28):3452–3459. doi: 10.1200/JCO.2011.41.0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrans S, et al. Rearrangement of MYC is associated with poor prognosis in patients with diffuse large B-cell lymphoma treated in the era of rituximab. J Clin Oncol. 2010;28(20):3360–3365. doi: 10.1200/JCO.2009.26.3947. [DOI] [PubMed] [Google Scholar]
- 4.Savage KJ, et al. MYC gene rearrangements are associated with a poor prognosis in diffuse large B-cell lymphoma patients treated with R-CHOP chemotherapy. Blood. 2009;114(17):3533–3537. doi: 10.1182/blood-2009-05-220095. [DOI] [PubMed] [Google Scholar]
- 5.Liu Z, Sun Q, Wang X. PLK1, A potential target for cancer therapy. Transl Oncol. 2017;10(1):22–32. doi: 10.1016/j.tranon.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ren Y, et al. PLK1 stabilizes a MYC-dependent kinase network in aggressive B cell lymphomas. J Clin Invest. 2018;128(12):5517–5530. doi: 10.1172/JCI122533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sears RC. The life cycle of C-myc: from synthesis to degradation. Cell Cycle. 2004;3(9):1133–1137. [PubMed] [Google Scholar]
- 8.Xiao D, et al. Polo-like kinase-1 regulates Myc stabilization and activates a feedforward circuit promoting tumor cell survival. Mol Cell. 2016;64(3):493–506. doi: 10.1016/j.molcel.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 9.Teh TC, et al. Enhancing venetoclax activity in acute myeloid leukemia by co-targeting MCL1. Leukemia. 2018;32(2):303–312. doi: 10.1038/leu.2017.243. [DOI] [PubMed] [Google Scholar]
- 10.Zhao X, et al. Unification of de novo and acquired ibrutinib resistance in mantle cell lymphoma. Nat Commun. 2017;8:14920. doi: 10.1038/ncomms14920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu T, Dai Y. Tumor microenvironment and therapeutic response. Cancer Lett. 2017;387:61–68. doi: 10.1016/j.canlet.2016.01.043. [DOI] [PubMed] [Google Scholar]
- 12.Hui L, Chen Y. Tumor microenvironment: Sanctuary of the devil. Cancer Lett. 2015;368(1):7–13. doi: 10.1016/j.canlet.2015.07.039. [DOI] [PubMed] [Google Scholar]
- 13.De Martino D, Yilmaz E, Orlacchio A, Ranieri M, Zhao K, Di Cristofano A. PI3K blockage synergizes with PLK1 inhibition preventing endoreduplication and enhancing apoptosis in anaplastic thyroid cancer. Cancer Lett. 2018;439:56–65. doi: 10.1016/j.canlet.2018.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higuchi F, et al. PLK1 inhibition targets Myc-activated malignant glioma cells irrespective of mismatch repair deficiency-mediated acquired resistance to temozolomide. Mol Cancer Ther. doi: 10.1158/1535-7163. https://doi.org/10.1158/1535-7163.MCT-18-0177 [published online ahead of print September 14, 2018]. [DOI] [PMC free article] [PubMed] [Google Scholar]