Abstract
The screening of several Chinese medicinal plants for insecticidal principles showed that essential oil of Rhododendron anthopogonoides flowering aerial parts possessed significant toxicity against maize weevils, Sitophilus zeamais. A total of 37 components were identified in the essential oil and the main constituents of the essential oil were 4-phenyl-2-butanone (27.22%), nerolidol (8.08%), 1,4-cineole (7.85%), caryophyllene (7.63%) and γ-elemene (6.10%), followed by α-farnesene (4.40%) and spathulenol (4.19%). Repeated bioactivity-directed chromatographic separation on silica gel columns led us to isolate three compounds, namely 4-phenyl-2-butanone, 1,4-cineole, and nerolidol. 4-Phenyl-2-butanone shows pronounced contact toxicity against S. zeamais (LD50 = 6.98 μg/adult) and was more toxic than either 1,4-cineole or nerolidol (LD50 = 50.86 μg/adult and 29.30 μg/adult, respectively) against the maize weevils, while the crude essential oil had a LD50 value of 11.67 μg/adult. 4-Phenyl-2-butanone and 1,4-cineole also possessed strong fumigant toxicity against the adults of S. zeamais (LC50 = 3.80 mg/L and 21.43 mg/L) while the crude essential oil had a LC50 value of 9.66 mg/L.
Keywords: Rhododendron anthopogonoides, Sitophiluszeamais, contact toxicity, fumigant, 4-phenyl-2-butanone, essential oil composition
1. Introduction
Currently, control of stored product insects relies heavily on the use of synthetic insecticides and fumigants, which has led to problems such as disturbance of the environment, increasing application costs, pest resurgence, pest resistance to pesticides and lethal effects on non-target organisms in addition to direct toxicity to the users [1]. Thus, there is a considerable interest in developing natural products that are relatively less damaging to mammalian health and the environment than existing conventional pesticides, as alternatives to non-selective synthetic pesticides to control the pests of medical and economic importance [2,3]. In recent years, various workers have been concentrating their efforts on the search for natural products as an alternative to conventional insecticides and fumigants, as well as the re-evaluation of traditional botanical pest control agents [4,5,6,7,8,9,10,11].
Botanical pesticides have the advantage of providing novel modes of action against insects that can reduce the risk of cross-resistance, as well as offering new leads for the design of target-specific molecules [2]. During a screening program for new agrochemicals from Chinese medicinal herbs and local wild plants, the essential oil derived from flowering aerial parts of Rhododendron anthopogonoides Maxim. (Family: Ericaceae) was found to possess strong insecticidal activity against the maize weevil, Sitophilus zeamais Motsch. R. anthopogonoides is a shrub, growing on the damp sides of mountains and widely distributed in northwest China, especially in Sichuan, Qinghai, and Gansu provinces [12]. Its flowers, leaves, and twigs are used as traditional Chinese folk medicine for treating chronic bronchitis and coronary heart disease [13]. Investigations have shown that the crude drug contains monterpenoids, sesquiterpenoids, triterpenoids, flavonoids, steroids, coumarins, lignans, cerebrosides, tetracyclic chromane derivatives, tannins, and alkaloids [14,15,16,17,18,19,20,21]. The essential oil of this medicinal herb has also been investigated in the previous studies [22,23,24,25,26]. The essential oil of R. anthopogonoides aerial parts was demonstrated to inhibit growth of bacteria (Bacillus subtilis, Escherichia coli, Proteus vulgaris, and Staphyloccocus aureus) [23]. Moreover, the essential oil of R. anthopogonoides exhibited strong antifeedant, stomach poison, contact toxicity, and growth inhibitory effects to the larvae of grassland caterpillar (Gynaephora menyuanenis) [27]. However, no compounds active against stored product insects were isolated from the essential oil of R. anthopogonoides so far. In this paper, we report the isolation of three components derived from this essential oil active against the maize weevil and the chemical composition and insecticidal activities of the essential oil of R. anthopogonoides were also determined.
2. Results and Discussion
2.1. Isolated Bioactive Compounds
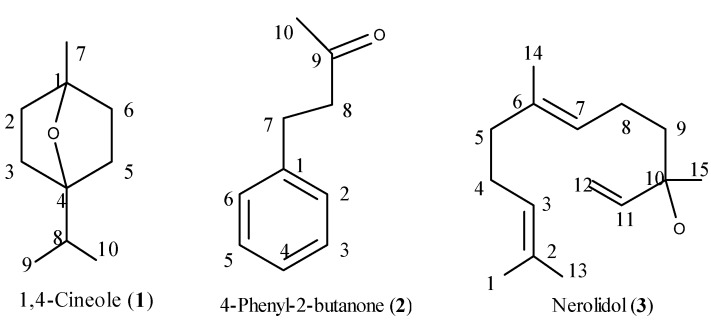
Three bioactive compounds were isolated and based on bioassay-guided fractionation and identified based on their spectroscopic data and comparison with literature values. Their chemical structures are given in Figure 1.
Figure 1.
Structures of active compounds isolated from R. anthopogonoides flowering aerial parts.
Among the five major components of R. anthopogonoides essential oil, only three of them have been isolated. Caryophyllene and γ-elemene were not isolated in the present study by using bioactivity-guided fractionation. It maybe the concentrations used to test fractionation was too low.
2.2. Chemical Constituents of the Essential Oil
The oil yield of R. anthopogonoides flowering aerial parts was 0.86% v/w and the density of the concentrated extract was determined to be 0.92 g/mL. The GC-MS results for R. anthopogonoides essential oil are presented in Table 1. A total of 37 components were identified in the essential oil, accounting for 95.03% of the total oil (Table 1) and the main constituents of the essential oil were 4-phenyl-2-butanone (27.22%), nerolidol (8.08%), 1,4-cineole (7.85%), caryophyllene (7.63%) and γ-elemene (6.10%), followed by α-farnesene (4.40%) and spathulenol (4.19%). The chemical composition of the essential oil was different from that reported in other studies. For example, 4-phenyl-2-butanone (50%) was the main constituent of R. anthopogonoides (leaves and young twigs, collected from Datong, Qinghai Province, 36.92° N latitude and 101.67° E longitude) essential oil, followed by selina-3,7(11)-diene (20%) and neofuranodiene (10%) [24], while Li et al. [25] found that the essential oil of R. anthopogonoides (also harvested from Datong, Qinghai Province) contained4-phenyl-2-butanone (52.16%), D-limonene (4.81%), eudesma-3,7(13)-diene (4.34%), and β-myrcene (4.19%). However, methyl palmitate (17.08%), dibutyl phthalate (15.77%) and γ-cadinene (5.33%) were the main constituents of R. anthopogonoides young aerial parts, harvested from Guide, Qinghai Province (36.04° N latitude and 101.43° E longitude) essential oil [23]. The essential oil of R. anthopogonoides (aerial parts, collected from Tianzhu, Gansu Province, 37.24° N latitude and 102.84° E longitude) contained 3,7-cyclodecane-1-one (15.53%), 4-phenyl-2-butanone (13.11%), 1-propoxy-1,3-dimethoxylbene (10.80%), O-(O-methoxyphenoxy) phenol (6.65%) and 4α-methyldecahydro-naphthalene (5.94%). The above results show the wide variation in chemical composition and yield of R. anthopogonoides essential oil, which may be related to the herbal source (climate, soil conditions and geographical location), herbal parts used, collection time (seasonal factors), chemotype of the plant species, and/or the analytical methods used. However, 4-phenyl-2-butanone was demonstrated to be one of major constituents of the essential oil in all the previous reports, as well as in the present experiments. Moreover, the above findings suggest that further studies on plant cultivation and essential oil standardization are needed because the chemical composition of the essential oil of R. anthopogonoides varies greatly with the plant population.
Table 1.
Chemical composition of the essential oil of R. anthopogonoides flowering aerial parts.
| Compounds | RI * | Relative content (%) |
|---|---|---|
| β-Thujene | 920 | 2.38 |
| α-Pinene | 939 | 0.78 |
| Camphene | 954 | 0.69 |
| β-Pinene | 974 | 0.22 |
| β-Myrcene | 991 | 0.54 |
| 1,4-Cineole | 1018 | 7.85 |
| (+)-Limonene | 1029 | 1.17 |
| 1,8-Cineole | 1031 | 0.98 |
| (E)-β-Ocimene | 1068 | 0.24 |
| Linalool | 1097 | 0.31 |
| 4-Terpineol | 1177 | 1.48 |
| α-Terpineol | 1188 | 0.97 |
| 4-Phenyl-2-butanone | 1218 | 27.22 |
| γ-Pyronene | 1338 | 0.12 |
| Longipinene | 1350 | 0.14 |
| α-Ylangene | 1370 | 1.41 |
| α-Copaene | 1375 | 1.59 |
| β-Elemene | 1389 | 0.27 |
| (Z)-Caryophyllene | 1409 | 1.79 |
| α-Gurjunene | 1411 | 0.81 |
| α-Santalene | 1420 | 0.57 |
| Caryophyllene | 1423 | 7.63 |
| α-Bergamotene | 1433 | 0.97 |
| γ-Elemene | 1437 | 6.10 |
| 2,3-Dimethylnaphthalene | 1443 | 0.29 |
| β-Farnesene | 1453 | 0.23 |
| 1,4,7,-Cycloundecatriene, 1,5,9,9-tetramethyl-, Z,Z,Z- | 1456 | 1.82 |
| Curcumene | 1481 | 0.63 |
| α-Farnesene | 1505 | 4.40 |
| β-Sesquiphellandrene | 1523 | 3.09 |
| Nerolidol | 1567 | 8.08 |
| Dendrolasin | 1571 | 1.13 |
| Spathulenol | 1578 | 4.19 |
| (+)-Viridiflorol | 1588 | 0.55 |
| trans-β-Elemenone | 1597 | 1.83 |
| α-Santalol | 1681 | 2.32 |
| Total | 95.03 |
* RI, retention index as determined on a HP-5MS column using the homologous series of n-hydrocarbons as reference.
2.3. Contact and Fumigant Toxicity
4-Phenyl-2-butanone shows pronounced contact toxicity against S. zeamais (LD50 = 6.98 μg/adult) while the crude essential oil had a LD50 value of 11.67 μg/adult (Table 2). 1,4-Cineole and nerolidol also exhibited contact toxicity against S. zeamais (LD50 = 50.86 μg/adult, and 29.30 μg/adult, respectively). Only 4-phenyl-2-butanone exhibits stronger contact toxicity than the crude essential oil against the maize weevils. 1,4-Cineole and nerolidol exhibit 4- and 2-times less activity against S. zeamais, respectively. The above results indicated that constituent compounds may have synergistic action or compound(s) with stronger toxicity in the essential oil were not isolated. However, when compared with the famous botanical insecticide, pyrethrum extract (25% pyrethrine I and pyrethrine II), 4-phenyl-2-butanone exhibits the same level of contact toxicity against the maize weevils and the essential oils were almost 2 times less active against S. zeamais adults because pyrethrum extract displayed a LD50 value of 4.87 μg/adult (Table 2). Compared with pyrethrum extract, 1,4-cineole and nerolidol were 10- and 6-times less active against S. zeamais, respectively.
Table 2.
Toxicity of compounds isolated from R. anthopogonoides flowering aerial partsagainst S. zeamais adults.
| Compounds | Contact Toxicity | Fumigant Toxicity | ||||
|---|---|---|---|---|---|---|
| 7 d LD50 (μg/adult) (95% FL) | Slope ± SE | Chi square (χ2 ) | 7 d LC50 (mg/L) (95% FL) | Slope ± SE | Chi Square (χ2 ) | |
| 4-Phenyl-2-butanone | 6.98 (6.63–7.36) | 8.22 ± 0.92 | 13.12 | 3.80 (3.48–4.20) | 6.67 ± 0.49 | 14.95 |
| 1,4-Cineole | 50.86 (46.14–56.62) | 4.01 ± 0.41 | 19.32 | 21.43 (20.64–25.04) | 4.14 ± 0.47 | 16.52 |
| Nerolidol | 29.30 (26.54–31.86) | 4.52 ± 0.49 | 22.68 | >353.00 | - | - |
| Crude oil | 11.67 (10.98–12.84) | 5.99 ± 0.61 | 11.96 | 9.66 (8.79–10.64) | 4.43 ± 0.50 | 13.80 |
| Pyrethrum extract | 4.87 (4.36–5.32) | 0.73 ± 0.021 | 13.51 | - | - | - |
| MeBr * | - | 0.67 | - | - | ||
* data from Liu and Ho [28].
4-Phenyl-2-butanone and 1,4-cineole also possess fumigant toxicity against S. zeamais (LC50 = 3.80 mg/L, and 21.43 mg/L, respectively), while the crude essential oil showed a LC50 value of 9.66 mg/L (Table 2) and nerolidol did not exhibit any fumigant toxicity against S. zeamais at the concentrations tested. 4-Phenyl-2-butanone was a bioactive (fumigant) constituent in the essential oil because it was 2.5 times more toxic to the maize weevils than the crude essential oil. The commercial grain fumigant, methyl bromide (MeBr) was reported to have fumigant activity against S. zeamais adults with a LC50 value of 0.67 mg/L [28], thus the isolated constituent 4-phenyl-2-butanone and the essential oil were 6- or 14-times less toxic to S. zeamais adults compared with MeBr. However, compared with other essential oils reported in the literature, the essential oil of R. anthopogonoides flowering aerial parts exhibited stronger than or the same level of fumigant toxicity against the maize weevils, e.g., essential oils of Murraya exotica (LC50 = 8.29 mg/L) [31], Artemisia lavandulaefolia (LC50 = 11.2 mg/L) [30], A. vestita (LC50 = 13.42 mg/L) [32], Illicium simonsii (LC50 = 14.95 mg/L) [32], A. sieversiana (LC50 = 15.0 mg/L) [30], and Ostericum sieboldii (20.92 mg/L) [34].
Considering the currently used fumigants are synthetic insecticides, the fumigant activity of the crude essential oil and the two isolated compounds are quite promising and they show potential for development as possible natural fumigants for the control of stored product insects. For the practical use of R. anthopogonoides essential oil and their constituents as novel fumigants or insecticides to proceed, further research is needed to establish their human safety and to evaluate the toxicity against other species. Additionally, their fumigant and insecticide modes of action need to be established and formulations for improving insecticidal potency and stability, thereby reducing costs, need to be developed.
3. Experimental
3.1. General
1H-NMR spectra were recorded on Bruker (Fallanden, Switzerland) ACF300 [300 MHz (1H)] and AMX500 [300 MHz (1H)] instruments using deuterochloroform (CDCl3) as the solvent with tetramethylsilane (TMS) as the internal standard. Electron impact mass spectra (EIMS) were determined on a ThermoQuest Trace 2000 mass spectrometer at 70 eV (probe). Silica gel (160–200 mesh) and pre-coated GF254 plates were purchased from Qingdao Marine Chemical Plant (Shandong Province, China). Fluon was purchased from ICI America Inc (Bridgewater, NJ, USA). C8–C24 n-alkanes were purchased from Sigma-Aldrich (USA). All other chemicals and reagents were of analytical grade.
3.2. Plant Material
Fresh flowering aerial parts (10 kg of leaves, stems and flowers) of R. anthopogonoides were harvested in July 2009 from Guide (36.04° N latitude and 101.43° E longitude), Qinghai Province, China. The aerial parts were air-dried for one week and ground to a powder. The plant species was identified and the voucher specimens (CAU-liuzhilong-2009-07-09-011) were deposited at the Department of Entomology, China Agricultural University. The ground powder of R. anthopogonoides was subjected to hydrodistillation using a modified Clevenger-type apparatus for 6 h and extracted with n-hexane. Anhydrous sodium sulphate was used to remove water after extraction. Essential oil was stored in an airtight container in a refrigerator at 4 °C.
3.3. Insects
The maize weevils (S. zeamais) were obtained from laboratory cultures maintained in the dark in incubators at 28–30 °C and 70%–80% relative humidity. The insects were reared on whole wheat at 12%–13% moisture content. Unsexed adult weevils used in all the experiments were about 2 weeks old.
3.4. Gas Chromatography and Mass Spectrometry
Gas chromatographic analysis was performed on an Agilent 6890N instrument (Agilent Technologies, Santa Clara, CA, USA) equipped with a flame ionization detector and an HP-5MS (30 m × 0.25 mm × 0.25 μm) capillary column, while the essential oil components were identified on an Agilent Technologies 5973N mass spectrometer. The GC settings were as follows: The initial oven temperature was held at 60 °C for 1 min and ramped at 10 °C min−1 to 180 °C for 1 min, and then ramped at 20 °C min−1 to 280 °C for 15 min. The injector temperature was maintained at 270 °C. The samples (1 μL) were injected neat, with a split ratio of 1:10. The carrier gas was helium at flow rate of 1.0 mL min−1. Spectra were scanned from 20 to 550 m/z at 2 scans s−1. Most constituents were identified by gas chromatography by comparison of their retention indices with those of the literature [22,23,24,25,26] or with those of authentic compounds available in our laboratories. The retention indices were determined in relation to a homologous series of n-alkanes (C8–C24) under the same operating conditions. Further identification was made by comparison of their mass spectra with those stored in NIST 05 and Wiley 275 libraries or with mass spectra from the literature [35]. Component relative percentages were calculated based on GC peak areas without using correction factors.
3.5. Contact Toxicity Using Topical Application
The contact toxicity of the essential oil and isolated compounds against S. zeamais adults was measured as described by Liu and Ho [28]. Range-finding studies were run to determine the appropriate testing concentrations. A serial dilution of the essential oil/compounds (15.0%–1.0% for S. zeamias, v/w, five concentrations) was prepared in n-hexane. Initial experiments were conducted to determine appropriate ranges of testing concentrations. Aliquots (0.5 μL) of the dilutions were applied topically to the dorsal thorax of the insects. Controls were determined using n-hexane. Both treated and control insects were then transferred to glass vials (10 insects/vial) with culture media and kept in incubators at 29–30 °C, 70%–80% r.h. Mortality of insects was observed daily until end-point mortality was reached one week after treatment. The experiments were repeated in three times. The LD50 values were calculated by using Probit analysis [29]. The positive control, pyrethrum extract (25% pyrethrine I and pyrethrine II), was purchased from Fluka Chemie.
3.6. Fumigant Toxicity
The fumigant activity of the essential oil and the pure compounds against S. zeamais adults was tested as described by Liu and Ho [28]. A serial dilution of the essential oil and isolated compounds was prepared in n-hexane. Filter papers (Whatman Cat No. 1001020, diameter 2.0 cm) were each impregnated with 20 L of an appropriate concentration (25.0%–1.0% for S. zeamias, v/w, five concentrations) of the essential oil/compounds, and placed on the underside of the screw cap of a glass vial (diameter 2.5 cm, height 5.5 cm, 25 mL). Preliminary range finding studies were performed with each of the compounds/essential oil to determine the appropriate testing concentrations. Fluon was used inside glass vial to prevent insects from the treated filter paper. The solvent was allowed to evaporate for 30 s before the cap was screwed tightly on the glass vial containing 10 insects.Preliminary experiments demonstrated that 30 s was sufficient for the evaporation of solvents. n-Hexane was used as a control. Five replicates were carried out for all treatments and controls, and they were incubated for 24 h. The insects were then transferred to clean vials with some culture media and returned to the incubator and observed daily for determination of end-point mortality, which was reached after one week. The experiments were repeated in three times. The LC50 values were calculated using Probit analysis [29].
3.7. Isolation of Active Ingredients
The crude essential oil (25 mL) was chromatographed on a silica gel (Merck 9,385, 1,000g, Merck Chemicals Co., Ltd., Shanghai, China) column (85 mm i.d., 850 mm length) by gradient elution with a mixture of solvents (n-hexane, n-hexane-ethyl acetate, and acetone). Fractions of 500 mL were collected and concentrated at 40 °C, and similar fractions according to TLC profiles were combined to yield 28 fractions. Fractions (7, 11 and 19) that possessed contact/fumigant toxicity, with similar TLC profiles, were pooled and further purified by preparative silica gel column chromatography (PTLC) until to obtain three pure compounds for determining structure as 1,4-cineole (1, 1.2 g), 4-phenyl-2-butanone (2, 1.7 g) and nerolidol (3, 1.9 g). The structures of the compounds were elucidated based on high-resolution electron impact mass spectrometry and nuclear magnetic resonance.
3.8. Compound Characterization
1,4-Cineole (1), colorless oil. EI-MS m/z (%): 154 (26), 125 (29), 111 (73), 71 (60), 69 (35), 55 (41), 43 (100), 41 (44), 27 (22). C10H18O. 1H-NMR (500 MHz, CDCl3) δ (ppm): 0.93 (3H, s, 9-CH3), 0.94 (3H, s, 10-CH3), 1.42 (3H, s, 7-CH3), 1.48-1.67 (8H, m, 2-CH2, 3-CH2, 5-CH2, 6-CH2), 2.04 (1H, m, J = 13.7, 6.8 Hz, H-8). 13C-NMR (125 MHz, CDCl3) δ (ppm): 18.12 (C-9), 18.18 (C-10), 21.22 (C-7), 32.92 (C-3, C-5), 33.06 (C-2, C-6), 37.22 (C-8), 82.96 (C-1), 89.68 (C-4). The data matched with the previous reports [36,37].
4-Phenyl-2-butanone (2), colorless oil, EI-MS m/z (%): 149 (8), 148 (73), 133 (14), 105 (81), 104 (11), 91 (60), 79 (13), 78 (11), 77 (17), 43 (100), 51 (11). C10H12O. 1H-NMR (500 MHz, CDCl3) δ (ppm): 2.11 (3H, s, 9-CH3), 2.73 (2H, t, J = 8.0 Hz, 7-CH2), 2.90 (2H, t, J = 8.0 Hz, 8-CH2), 7.19 (1H, dd, J = 8.0 Hz, 4-H), 7.21 (2H, d, J = 12.0, 8.0 Hz, H-2, H-6), 7.30 (2H, dd, J = 12.0, 8.0 Hz, H-3, H-5). 13C-NMR (125 MHz, CDCl3) δ (ppm): 29.74 (C-10), 29.95 (C-7), 45.02 (C-8), 126.12 (C-4), 128.35 (C-3, C-5), 128.51 (C-2, C-6), 141.10 (C-1), 207.62 (C-9). The 1H and 13C-NMR data were in agreement with the reported data [38,39].
Nerolidol (3), colorless oil. EI-MS m/z (%): 204 (3), 161 (11), 136 (15), 107 (24), 93 (50), 81 (27), 71 (37), 69 (100), 55 (26), 43 (28), 41 (65). C15H26O. 1H-NMR (500 MHz, CDCl3) δ (ppm): 1.29 (3H, s, 15-CH3), 1.56 (2H, m, J = 9.8, 6.1 Hz, 9-CH2), 1.59 (3H, s, 1-CH3), 1.60 (3H, s, 13-CH3), 1.69 (3H, s, 14-CH3), 1.76 (1H, br, 10-OH), 1.97–2.09 (6H, 4-CH2, 5-CH2, 8-CH2), 5.04–5.12 (2H, m, 12-CH2), 5.15 (1H, t, 3-H), 5.22 (1H, dd, J = 17.3, 1.2 Hz, 7-H), 5.92 (1H, m, J = 17.3, 10.7 Hz, 11-H). 13C-NMR (125 MHz, CDCl3) δ (ppm): 16.01 (C-14), 17.69 (C-8), 22.72 (C-1), 25.71 (C-4), 26.63 (C-13), 27.86 (C-15), 39.70 (C-5), 42.04 (C-9), 73.49 (C-10), 111.67 (C-12), 124.22 (C-3), 124.24 (C-7), 131.41 (C-2), 135.52 (C-6), 145.05 (C-11). The data matched with the previous reports [40].
4. Conclusions
Based on mass screening of medicinal herbs, the essential oil of R. anthopogonoides flowering aerial parts was found to possess toxicity against the maize weevils (S. zeamais). Three active constituents were isolated from the oil by bioactivity-guided fractionation and identified. 4-Phenyl-2-butanone exhibits the same level of contact toxicity against the maize weevils and the essential oils were 2-times less active against S. zeamais adults when compared with pyrethrum extract. Moreover, the isolated constituent 4-phenyl-2-butanone and the essential oil exhibit strong fumigant toxicity against S. zeamais adults, although they were 6- or 14-times less toxic when compared with MeBr. These findings suggest that essential oil of R. anthopogonoides and the three compounds may have potential to be developed as new natural fumigants/insecticides for the control stored product insects.
Acknowledgements
This work was funded by the Hi-Tech Research and Development of China 2011AA10A202 and 2006AA10A209 and National New-drug Innovation Project 2009ZX09501-014. We thank Liu Q.R. from the College of Life Sciences, Beijing Normal University, Beijing 100875, for the identification of the investigated medicinal herb.
Footnotes
Samples Availability: Samples of the crude extracts and pure compounds are available from the authors.
References and Notes
- 1.Zettler J.L., Arthur F.H. Chemical control of stored product insects with fumigants and residual treatments. Crop Prot. 2000;19:577–582. doi: 10.1016/S0261-2194(00)00075-2. [DOI] [Google Scholar]
- 2.Isman M.B. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Ann. Rev. Entomol. 2006;51:45–66. doi: 10.1146/annurev.ento.51.110104.151146. [DOI] [PubMed] [Google Scholar]
- 3.Rajendran S., Srianjini V. Plant products as fumigants for stored-product insects control. J. Stored Prod. Res. 2008;44:126–135. doi: 10.1016/j.jspr.2007.08.003. [DOI] [Google Scholar]
- 4.Wu H., Zhang G.A., Zeng S.Y., Lin K.C. Extraction of allylisothiocyanate from horseradish (Armoracia rusticana) and its fumigant insecticidal activity on four stored-product pests of paddy. Pest Manag. Sci. 2009;65:1003–1008. doi: 10.1002/ps.1786. [DOI] [PubMed] [Google Scholar]
- 5.Wang J.L., Li Y., Lei C.L. Evaluation of monoterpenes for the control of Tribolium castaneum (Herbst) and Sitophilus zeamais Motsch. Nat. Prod. Res. 2009;23B:1080–1088. doi: 10.1080/14786410802267759. [DOI] [PubMed] [Google Scholar]
- 6.Liu Z.L., Chu S.S., Jiang G.H. Feeding deterrents from Zanthoxylum schinifolium against two stored-product insects. J. Agric. Food Chem. 2009;57:10130–10133. doi: 10.1021/jf9012983. [DOI] [PubMed] [Google Scholar]
- 7.Chu S.S., Hu J.F., Liu Z.L. Composition of essential oil of Chinese Chenopodium ambrosioides and insecticidal activity against maize weevil, Sitophilus zeamais. Pest Manag. Sci. 2011;67:714–718. doi: 10.1002/ps.2112. [DOI] [PubMed] [Google Scholar]
- 8.Liu Z.L., Chu S.S., Jiang G.H. Toxicity of Schizonepeta multifida essential oil and its constituent compounds towards two grain storage insects. J. Sci. Food Agric. 2011;91:905–909. doi: 10.1002/jsfa.4263. [DOI] [PubMed] [Google Scholar]
- 9.Chu S.S., Jiang G.H., Liu Z.L. Insecticidal components from the essential oil of Chinese medicinal herb, Ligusticum chuanxiong Hort. E-J. Chem. 2011;8:300–304. doi: 10.1155/2011/163760. [DOI] [Google Scholar]
- 10.Mossi A.J., Astolfi V., Kubiak G., Lerin L., Zanella C., Toniazzo G., Oliveira D., Treichel H., Devilla I.A., Cansian R., Restello R. Insecticidal and repellency activity of essential oil of Eucalyptus sp. against Sitophilus zeamais Motschulsky (Coleoptera, Curculionidae) J. Sci. Food Agric. 2011;91:273–277. doi: 10.1002/jsfa.4181. [DOI] [PubMed] [Google Scholar]
- 11.Suthisut D., Fields P.G., Chandrapatya A. Fumigant toxicity of essential oils from three Thai plants (Zingiberaceae) and their major compounds against Sitophilus zeamais, Tribolium castaneum and two parasitoids. J. Stored Prod. Res. 2011;47:222–230. doi: 10.1016/j.jspr.2011.03.002. [DOI] [Google Scholar]
- 12.Committee of Flora of China. Flora of China. Vol. 57. Science Press; Beijing, China: 1999. p. 180. [Google Scholar]
- 13.Jiangsu New Medical College. Dictionary of Chinese Herbal Medicine. Shanghai Science and Technology Press; Shanghai, China: 1977. pp. 264–265. [Google Scholar]
- 14.Iwata N., Kitanaka S. Tetracyclic chromane derivatives from Rhododendron anthopogonoides. J. Nat. Prod. 2010;73:1203–1206. doi: 10.1021/np900543r. [DOI] [PubMed] [Google Scholar]
- 15.Zhao L., Ge J., Qiao C., Zhang H., Jiang S. Separation and quantification of flavonoid compounds in Rhododendron anthopogonoides Maxim by high-performance liquid chromatography. ActaChromatogr. 2008;20:135–146. [Google Scholar]
- 16.Zhang Z.L., Chuan F.Y., Liu Y.L. Studies on the chemical constituents of Rhododendron anthopogonoides. Chin. Tradit. Herb. Drugs. 1980;11:393–394. (in Chinese with English abstract) [Google Scholar]
- 17.Zheng S.Z., Ma X.M., Sheng Q., Yang H.P., Shen X.W. Chemical constituents of Rhododendron anthopogonoides Maxim. Nat. Prod. Res. Develop. 2003;15:387–389. [Google Scholar]
- 18.Dai S.J., Yu D.Q. Triterpenoids of Rhododendron anthopogonoides. Chin. J. Nat. Med. 2005;3:347–349. (in Chinese with English abstract) [Google Scholar]
- 19.Dai S.J., Chen R.Y., Zhang P.C., Yu D.Q. A new compound from Rhododendron anthopogonoides Maxim. J. Asian Nat. Prod. Res. 2005;7:681–685. doi: 10.1080/1028602032000169541. [DOI] [PubMed] [Google Scholar]
- 20.Dai S.J., Chen R.Y., Yu D.Q. Studies on the flavonoid compounds of Rhododendron anthopogonoides. China J. Chin. Materia Med. 2004;29:44–47. (in Chinese with English abstract) [PubMed] [Google Scholar]
- 21.Dai S.J., Yu D.Q. Studies on flavonoids in stem of Rhododendron anthopogonoides II. China J. Chin. Materia Med. 2005;30:1830–1833. (in Chinese with English abstract) [PubMed] [Google Scholar]
- 22.Shi Z.X. Gas-liquid chromatographic analysis of essential oils from four species of Rhododendron on Qinghai plateau. Chin. Tradit. Herb. Drugs. 1981;12:15–17. (in Chinese with English abstract) [Google Scholar]
- 23.Liu B. Chemical components and antimicrobial effect of volatile oil from Rhododendron anthopogonoides. Pratacultural Sci. 2007;24:61–63. (in Chinese with English abstract) [Google Scholar]
- 24.Lu Y.C., Wang Y.L., Bai Y.F. Study on the chemical composition of the essential oil of Rhododendron anthopogonoides Maxim. Acta Chim. Sin. 1980;38:140–148. (in Chinese with English abstract) [Google Scholar]
- 25.Li W.W., Hu F.Z., Shi Z.X. Study on the chemical compounds in the volatile oils of the Tibetan medicine, Rhododendron anthopogonoides. J. Yunnan Univ. 2004;26:48–51. (in Chinese with English abstract) [Google Scholar]
- 26.Zhang J., Ma J.Y., Yang Y.L., Yao J., Huang A.L., Gao L.M., Zhao W.J. GC-MS analysis of volatile constituents from Rhododendron anthopogonoides. Chin. Tradit. Herb. Drugs. 2003;34:304–305. (in Chinese with English abstract) [Google Scholar]
- 27.Yan L., Hu F.Z., Wu J., Han F.L. The biological activity of essential oil in Rhododendron anthopogonoides Maxim and Artemisia dubia Wall. ex Bess. to grassland caterpillar, Gynaephora menyuanenis (Lepidoptera: Lymantriidae) Acta Agric. Boreali-Occidentalis Sin. 2009;18:58–63. (in Chinese with English abstract) [Google Scholar]
- 28.Liu Z.L., Ho S.H. Bioactivity of the essential oil extracted from Evodia rutaecarpa Hook f. et Thomas against the grain storage insects, Sitophilus zeamais Motsch. and Tribolium castaneum (Herbst) J. Stored Prod. Res. 1999;35:317–328. doi: 10.1016/S0022-474X(99)00015-6. [DOI] [Google Scholar]
- 29.Sakuma M. Probit analysis of preference data. Appl. Entomol. Zool. 1998;33:339–347. [Google Scholar]
- 30.Liu Z.L., Liu Q.R., Chu S.S., Jiang G.H. Insecticidal activity and chemical composition of the essential oils of Artemisia lavandulaefolia and Artemisia sieversiana from China. Chem. Biodiv. 2010;7:2040–2045. doi: 10.1002/cbdv.200900410. [DOI] [PubMed] [Google Scholar]
- 31.Li W.Q., Jiang C.H., Chu S.S., Zuo M.X., Liu Z.L. Chemical composition and toxicity against Sitophilus zeamais and Tribolium castaneum of the essential oil of Murraya exotica aerial parts. Molecules. 2010;15:5831–5839. doi: 10.3390/molecules15085831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chu S.S., Liu Q.R., Liu Z.L. Insecticidal activity and chemical composition of the essential oil of Artemisia vestita from China against Sitophilus zeamais. Biochem.Syst. Ecol. 2010;38:489–492. doi: 10.1016/j.bse.2010.04.011. [DOI] [Google Scholar]
- 33.Chu S.S., Liu S.L., Jiang G.H., Liu Z.L. Composition and toxicity of essential oil of Illicium simonsii Maxim (Illiciaceae) fruit against the maize weevils. Rec. Nat. Prod. 2010;4:205–210. [Google Scholar]
- 34.Liu Z.L., Chu S.S., Jiang G.H. Insecticidal activity and composition of essential oil of Ostericum sieboldii (Apiaceae) against Sitophilus zeamais and Tribolium castaneum. Rec. Nat. Prod. 2011;5:74–81. [Google Scholar]
- 35.Adams R.P. Identification of essential oil components by gas chromatography/quadrupole mass spectroscopy. Allured; Carol Stream, IL, USA: 2001. [Google Scholar]
- 36.Carr G., Dean C., Whittaker D. Terpenoid ether formation in superacids. J. Chem. Soc. Perkin Trans. 2. 1988;3:351–354. [Google Scholar]
- 37.Asakawa Y., Matsuda R., Tori M., Hashimoto T. Preparation of biologically active substances and animal and microbial metabolites from menthols, cineoles and kauranes. Phytochemistry. 1988;27:3861–3869. doi: 10.1016/0031-9422(88)83033-4. [DOI] [Google Scholar]
- 38.Fox D.J., Pedersen D.S., Warren S. Diphenylphosphinoyl-mediated synthesis of ketones. Org. Biomol. Chem. 2006;4:3102–3107. doi: 10.1039/b606873a. [DOI] [PubMed] [Google Scholar]
- 39.Black P.J., Edwards M.G., Williams J.M.J. Borrowing hydrogen: Indirect “Wittig” olefination for the formation of C–C bonds from alcohols. Eur. J. Org. Chem. 2006;19:4367–4378. doi: 10.1039/b511053j. [DOI] [PubMed] [Google Scholar]
- 40.Suarez L.E.C., Menichini F., Monache F.D. Tetranortriterpenoids and dihydrocinnamic acid derivatives from Hortia colombiana. J. Braz. Chem. Soc. 2002;13:339–344. doi: 10.1590/S0103-50532002000300008. [DOI] [Google Scholar]