Abstract
A screening of several Chinese medicinal herbs for nematicidal properties showed that Arisaema erubescens (Wall.) Schott tubers possessed significant nematicidal activity against the root-knot nematode (Meloidogyne incognita). From the ethanol extract, two nematicidal flavone-C-glycosides were isolated by bioassay-guided fractionation. The compounds were identified as schaftoside and isoschaftoside on the basis of their phytochemical and spectral data. Schaftoside and isoschaftoside possessed strong nematicidal activity against M. incognita (LC50 = 114.66 μg/mL and 323.09 μg/mL, respectively) while the crude extract of A. erubescens exhibited nematicidal activity against the root-knot nematode with a LC50 value of 258.11 μg/mL.
Keywords: nematicidal activity, Arisaema erubescens, Meloidogyne incognita, schaftoside, isoschaftoside
1. Introduction
Plant-parasitic nematodes are responsible for substantial economic losses to agricultural crops. Nematode management is generally based upon chemical treatments (soil fumigation), but environmental concerns and governmental regulations [1] are now resulting in a strong interest in nematicides of natural origin [2,3]. One alternative is to screen naturally occurring plant secondary compounds for appropriate activity. Many plant constituents and metabolites have been investigated for activity against plant-parasitic nematodes [4,5,6,7,8,9,10]. Varied nematicidal substances of plant origin such as triglycerides, sesquiterpenes, alkaloids, steroids, diterpenes and flavonoids have been identified in this way [2]. During our screening program for new agrochemicals from local wild plants and Chinese medicinal herbs, an ethanol extract of Chinese Arisaema erubescens (Wall.) Schott (Chinese cobra lily, Family: Araceae) tubers was found to possess strong nematicidal activity against the root-knot nematode, Meloidogyne incognita (Kofoid and White) Chitwood. Meloidogyne incognita is the most economically important and widely distributed nematode throughout China and a cause of considerable crop losses.
A. erubescens is widely distributed in China [11] and its tubers are used as a Traditional Chinese Medicine. The actions of the medicinal herb are to remove damp-phlegm, to dispel wind and arrest convulsions, and to promote the subsidence of induration and swelling [12]. Its pharmacological anticonvulsant and anti-cancer effects have been reproduced in a modern pharmacological study [13]. Due to the fact that it is a common Chinese herb used in medicine, the chemical constituents and bioactivities of A. erubescens tubers have been extensively studied and the known chemical constituents of this medicinal herb include calcium oxalate, paeonol, aurantiamide acetate, monoterpenoids, fatty acids, flavonoids, and alkaloids [14,15,16,17,18,19,20]. Ethanol extracts of A. erubescens tubers were found to possess insecticidal activity against the house flies (Musca domestica) [20]. The n-butanol extracts of A. erubescens tuber exhibited a quick knockdown molluscicidal activity against Oncomlania hupensis [21,22]. However, no bioactive compounds against nematodes have been isolated and identified from this Chinese medicinal herb. In this paper, we report the isolation and characterization of two natural compounds flavone-C-glycosides obtained from A. erubescens tubers by bioassay-guided fractionation and their nematocidal assessment against M. incognita.
2. Results and Discussion
2.1. Isolated Bioactive Compounds
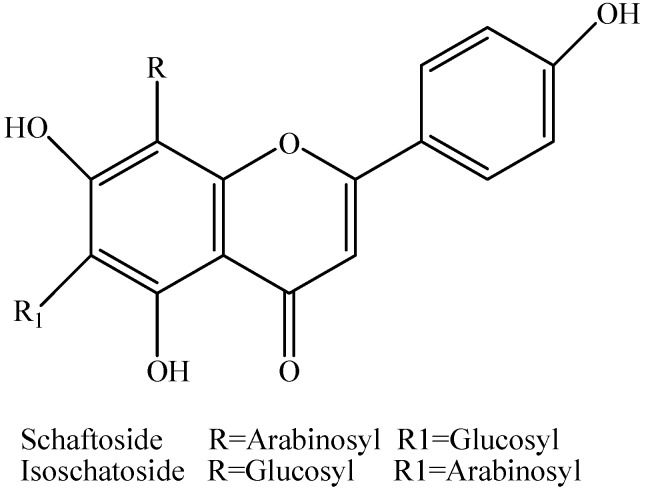
Two bioactive compounds were isolated and based on bioassay-guided fractionation and identified based on their spectroscopic data and comparison with literature vales. Their chemical structures are given in Figure 1.
Figure 1.
Structures of nematicidal flavone-C-glycosides isolated from Arisaema erubescens tubers.
2.2. Nematocidal Activity
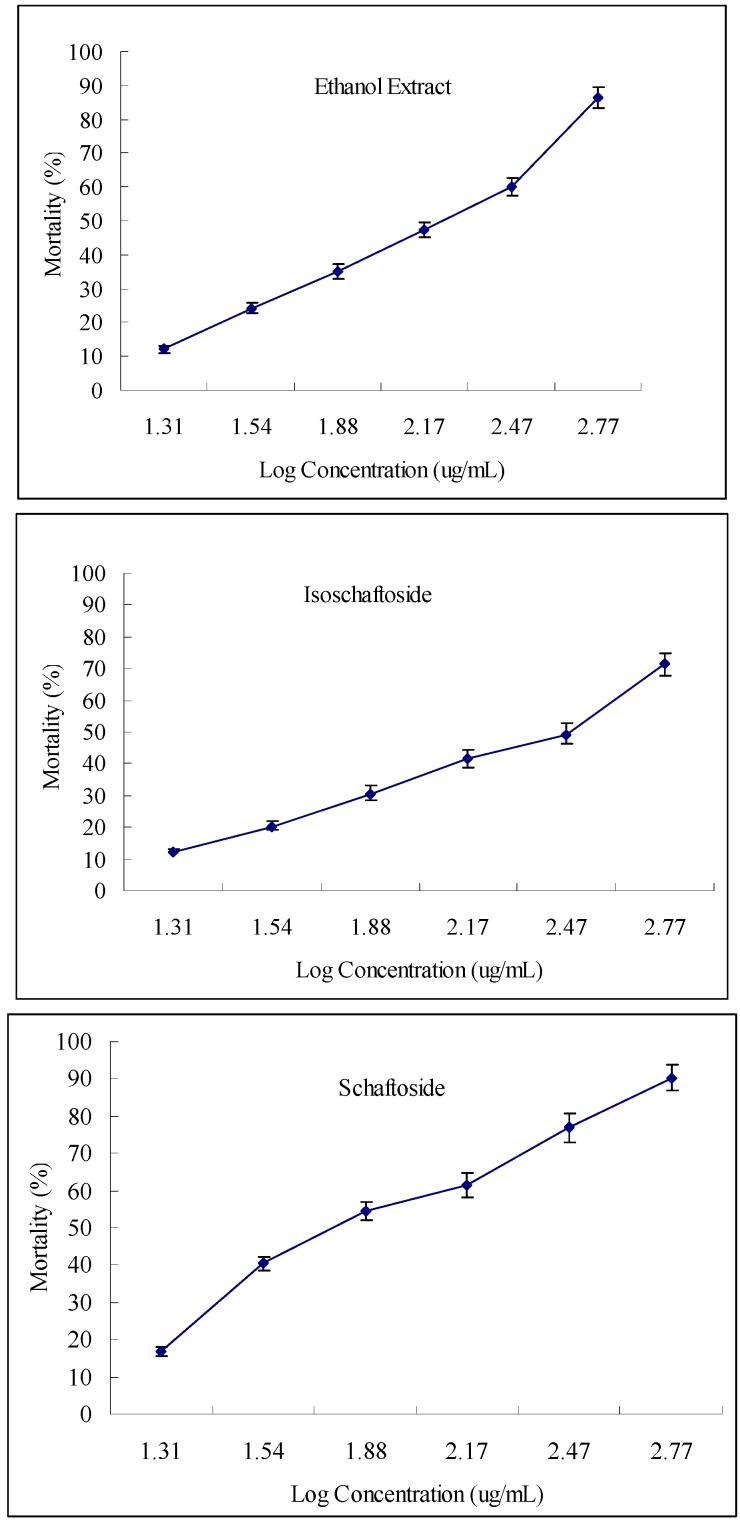
The ethanol extract of A. erubescens tubers exhibited toxicity against the root-knot nematode with a LC50 value of 258.11 μg/mL (Table 1). Compared with the positive control, carbofuran (LC50 = 72.29 μg/mL), the crude extract of A. erubescens tubers was 3.5 times less active against the root-knot nematode. However, considering carbofuran is a synthetic pesticide, this nematocidal activity of the crude extract of A. erubescens tubers is quite promising. Of the two identified active substances, schaftoside (LC50 = 114.66 μg/mL) was more toxic than isoschafoside (LC50 = 323.09 μg/mL) against the root-knot nematodes (Table 1). Schaftoside was two times more toxic against M. incognita compared with the crude extract and exhibits the same level of toxicity as the positive control carbofuran (Table 1). A literature survey shows that only one flavone-C-glycoside, lantanoside, exhibited toxicity against M. incognita and 90% mortality (24 h) was observed at a concentration of 1% (10 mg/mL approximately) [29]. Flavonoid glycosides have been reported to stimulate the probing of rice plant hoppers [30,31,32], whereas apigenin-C-glycosides such as schaftoside deterred feeding of Nilaparvata lugen [33]. Moreover, isoschaftoside from Desmodium uncinatum root exudates was found to inhibit growth of germinated Striga hermonthica radicles [34].
Table 1.
Nematicidal activity of ethanol extract of Arisaema erubescens and isolated flavone-C-glycosides against Meloidogyne incognita.
| Treatments | Concentrations (μg/mL) | LC50 (μg/mL) | 95% Fiducial limits | Chi-Square Tests (χ2) |
|---|---|---|---|---|
| Ethanol extract | 20.0-600.0 | 258.11 | 189.42-379.64 | 6.45 |
| Schaftoside | 20.0-600.0 | 114.66 | 52.27-160.64 | 6.98 |
| Isoschaftoside | 20.0-600.0 | 323.09 | 176.45-517.66 | 4.74 |
| Carbofuran | 25.0-400.0 | 72.29 | 37.86-117.97 | 13.57 |
3. Experimental
3.1. General
Melting points were measured on a Buchi 535 melting point apparatus and are uncorrected. FTIR spectra were recorded on a Magna IRTM spectrometer 750. 1H- and 13C-NMR spectra were recorded on a JEOL JNM-AL300 spectrometer (300 MHz) using DMSO-d6 as solvent with TMS as internal standard. FAB-MS was determined on an APEX II (Bruker Daltonic Inc) spectrometer and UV was carried out on a Shimadzu UV-2450 spectrometer.
3.2. Plant Material, Extraction and Isolation of Active Ingredients
Dried tubers (4 kg) of A. erubescens were purchased from the Anguo Chinese Medicinal Herbs Market (Anguo 071200, Hebei Province, China). The species was identified, and the voucher specimens (BNU-CMH-Dushuahan-2009-08-25-006) were deposited at the Herbarium (BNU) of College of Life Sciences, Beijing Normal University. The tubers were ground to a powder using a grinding mill (Retsch Muhle, Germany), and the powder was extracted with 80% ethanol (50 L) at room temperature over a period of three weeks. The extract was evaporated to 1,200 mL under reduced pressure using a vacuum rotary evaporator, water (800 mL) was added and the extract was partitioned between ethanol-water and petroleum ether (3 × 2,000 mL). The petroleum ether extracts were evaporated to give a residue (8.0 g). The aqueous layer was re-partitioned with ether (3 × 2,000 mL) to provide a residue (8.0 g) after evaporation of ether. The aqueous layer was applied to a polyamide column (100-200 mesh, Jiangsu Changfeng Chemical Co., Ltd.), eluting with H2O and ethanol. The water-eluting fraction was further applied to a Macroreticular absorbing resin AB-8 (Tianjin Nanda Resin Technology Co., Ltd.), eluting with 10% ethanol, 30% ethanol and 50% ethanol to obtain fractions D1 (7.1 g), D2 (13.4 g) and D3 (1.3 g), respectively. D2 was further fractioned by polyamide column chromatography, Sephadex LH-20 (Pharmacia, Sweden), Toyopearl HW-40F (TOSOH (Shanghai) CO., Ltd.) and HPLC (Waters Delta Prep 4000) to obtain two bioactive compounds which was determined to be schaftoside (2.7 g) and isoschaftoside (51.0 mg). The structures of the compounds were elucidated based on mass spectrometry and nuclear magnetic resonance.
3.3. Compound Characterization
Schaftoside. Amorphous yellow powder (EtOH), m.p. 222-224 °C. UV (λMeOH nm): 270, 330; IR (νKBr cm−1): 3423, 2926, 1649, 1576, 1356, 1217, 1053; FAB-MS (m/z): 565 (M+ + 1), 509 (M+-H+-3 × 18), 467 (M+-H+-2 × 18-60), 429 (M+-H+-134), 345 (M+-2H+-2 × 18-60-121), 307, 257, 219. 1H-NMR δ ppm:13.79 (s, 1H, 5-OH), 7.97 (d, J = 6.0 Hz, 2H), 6.92 (d, J = 6.0 Hz, 2H), 6.73 (s, 1H), 4.96 (d, J = 9.3 Hz, 1H, arabinose anomeric-H), 4.53 (d, J = 8.7 Hz, 1H, glucose anomeric-H), 4.00-3.00 (m, sugar-H). 13C-NMR δ ppm: 182.3 (C-4), 163.6 (C-2), 161.2 (C-7), 161.2 (C-5), 160.8 (C-4’), 153.4 (C-9), 128.8 (C-2’, 6’), 121.3 (C-1’), 116.0 (3’, 5’), 109.3 (C-6), 103.7 (C-8), 103.0 (C-10), 102.5 (C-3), 79.4 (G-3, 5), 74.9 (A-1), 74.3 (G-1), 73.7 (A-3), 70.3 (G-4, A-5), 70.0 (A-4), 69.8 (A-2), 68.4 (G-2), 60.8 (G-6). The MS, 1H- and 13C-NMR data were in agreement with the reported data [24,25,26].
Isoschaftoside. Yellowish powder (EtOH), m.p. 239-240 °C. UV (λMeOH nm): 270, 330. FAB-MS (m/z): 565 (M+ + 1), 547 (M+ + 1-18), 511 (M+ + 1-3 × 18), 475 (M+ + 1-90), 427 (M+ + 1-18-120). 1H-NMR δ ppm:13.82 (1H, s, 5-OH), 10.29 (1H, s, 7-OH), 9.19 (H, s, 4’-OH), 8.32 (2H, d, J = 7.9 Hz, 2’, 6’-H), 6.92 (2H, d, J = 7.9 Hz, 3’, 5’-H), 6.87 (1H, s, 3-H), 4.77 (anomeric-H), 4.72 (anomeric-H), 4.70-3.00 (m, sugar-H). 13C-NMR δ ppm: 183.2 (C-4), 165.2 (C-2), 162.1 (C-7), 161.9 (C-4), 159.1 (C-4’), 156.1 (C-9), 130.6 (C-2’, 6’), 121.9 (C-1’), 116.8 (C-3’, 5’), 108.9 (C-6), 106.0 (C-8), 104.7 (C-10), 102.9 (C-3), 81.5 (G-5), 76.6 (G-3), 75.1 (A-1), 74.7 (G-1, A-3), 71.0 (A-5), 70.5 (G-4), 70.1 (A-4), 69.3 (A-2), 69.1 (G-2), 62.2 (G-6). The MS, 1H- and 13C-NMR data were in agreement with the reported data [24,25,26,27,28].
3.4. Nematicidal Assay
Second stage juveniles (J2) of M. incognita were obtained from a pure culture that was previously initiated by egg masses and propagated on tomato (Solanum lycopersicum) in the glasshouse. Egg masses were hand picked using sterilized forceps from heavily infected roots (40 days after incubation). These egg masses were washed in distilled water, placed in 15 mesh sieves (8 cm in diameter) containing crossed layers of tissue paper in Petri-dishes with water just deep enough to contact the egg masses and incubated 25-26 °C to obtain freshly hatched second stage juveniles (J2). Only juveniles collected within 48 h were used. Range-finding studies were run to determine the appropriate testing concentrations. A serial dilution of ethanol extract of A. erubescens (five concentrations, dissolved first in 10 μL ethanol) and pure compounds (five concentrations) was prepared in H2O solution with 2% DMSO. Aliquots of H2O (20 µL) containing ca. 30 juveniles (J2) were transferred to vials to which 980 µL of the solution containing ethanol extract or pure compounds was added. The vials were kept on a hood at 25 °C. The inactive nematodes were counted every 24 h for 72 h. After the last count, the inactive juveniles were maintained in distilled H2O for 24 h to observe their revival. Six repetitions for each treatment were performed using H2O and a 2% DMSO in H2O solution as well as a 2% DMSO in H2O solution containing 10 μL ethanol H2O as control. The experiments were repeated in three times. Results from all replicates for the pure compounds and ethanol extract were subjected to probit analysis using the PriProbit Program V1.6.3 to determine LC50 (median lethal concentration) values and their 95% confidence intervals (CI 95%) [23].
Figure 2.
Dose-response curves of Meloidogyne incognita (J2) treated with ethanol extract of A. erubescens and the two flavone-C-glycosides.
4. Conclusions
Based on mass screening of medicinal herbs, the ethanol extract of A. erubescens tubers was found to possess strong toxicity against the root-knot nematode species M. incognita. Two nematicidal flavone-C-glycosides were isolated and identified from the extract by bioactivity-guided fractionation. Schaftoside exhibits the same level of toxicity against M. incognita as the positive control (the commercial pesticide carbofuran). Moreover, the compound was two times more toxic against M. incognita than the crude extract. These findings suggest that the ethanol extract of A. erubescens tubers and two isolated compound show potential for development as natural pesticides for the control of nematodes.
Acknowledgements
This work was funded by National New-drug Innovation Project 2009ZX09501-014 and the Hi-Tech Research and Development of China 2011AA10A202. We thank Liu Q.R. from the College of Life Sciences, Beijing Normal University, Beijing 100875, for the identification of the investigated medicinal herb.
Footnotes
Sample Availability: Samples of the crude extracts and pure compounds are available from the authors.
References and Notes
- 1.United Nations Environment Programme. The Montreal Protocol on Substances that Deplete the Ozone Layer. WHO; Geneva, Swizerland: 2000. [accessed on 20 June 2011]. Available online: http://ozone.unep.org/pdfs/Montreal-Protocol2000.pdf. [Google Scholar]
- 2.Chitwood D.J. Phytochemical based strategies for nematode control. Ann. Rev. Phytopathol. 2002;40:221–249. doi: 10.1146/annurev.phyto.40.032602.130045. [DOI] [PubMed] [Google Scholar]
- 3.Rich J.R., Dunn R.A., Noling J.W. Nematicides: Past and Present Uses. In: Chen Z.X., Chen S.Y., Dickson D.W., editors. Nematology: Nematode Management and Utilization. Vol. 2. CABI Publishing; Wallingford, UK: 2004. pp. 1179–1200. [Google Scholar]
- 4.Ntalli N.G., Ferrari F., Giannakou I., Menkissoglu-Spiroudi U. Phytochemistry and nematicidal activity of the essential oils from 8 Greek Lamiaceae aromatic plants and 13 terpene components. J. Agric. Food Chem. 2010;58:7856–7863. doi: 10.1021/jf100797m. [DOI] [PubMed] [Google Scholar]
- 5.Ntalli N.G., Vargiu S., Menkissoglu-Spiroudi U., Caboni P. Nematicidal carboxylic acids and aldehydes from Melia azedarach fruits. J. Agric. Food Chem. 2010;58:11390–11394. doi: 10.1021/jf1025345. [DOI] [PubMed] [Google Scholar]
- 6.Sultana N., Akhter M., Khan R.A., Afza N., Tareen R.B., Malik A. Nematicidal natural products from the aerial parts of Buddleja crispa. Nat. Prod. Res. 2010;24:783–788. doi: 10.1080/14786410802496846. [DOI] [PubMed] [Google Scholar]
- 7.Echeverrigaray S., Zacaria J., Beltrao R. Nematicidal activity of monoterpenoids against the root-knot nematode Meloidogyne incognita. Phytopathology. 2010;100:199–203. doi: 10.1094/PHYTO-100-2-0199. [DOI] [PubMed] [Google Scholar]
- 8.Thoden T.C., Boppre M., Hallmann J. Effects of pyrrolizidine alkaloids on the performance of plant-parasitic and free-living nematodes. Pest Manage. Sci. 2009;65:823–830. doi: 10.1002/ps.1764. [DOI] [PubMed] [Google Scholar]
- 9.Shakil N.A., Pankaj, Kumar J., Pandey R.K., Saxena D.B. Nematicidal prenylated flavanones from Phyllanthus niruri. Phytochemistry. 2008;69:759–764. doi: 10.1016/j.phytochem.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 10.Kim J., Seo S.M., Lee S.G., Shin S.C., Park I.K. Nematicidal activity of plant essential oils and components from coriander (Coriandrum sativum), Oriental sweetgum (Liquidambar orientalis) and valerian (Valeriana wallichii) essential oils against pine wood nematode (Bursaphelenchus xylophilus) J. Agric. Food Chem. 2008;56:7316–7320. doi: 10.1021/jf800780f. [DOI] [PubMed] [Google Scholar]
- 11.Wu C.Y., Li H. Flora Reipublicae Popularis Sinica. 13(2) Science Press; Beijing, China: 1995. pp. 188–191. [Google Scholar]
- 12.Jiangsu New Medical College. Encyclopedia of Chinese Medicinal Substances. Shanghai People’s Publisher; Shanghai, China: 1986. pp. 329–333. [Google Scholar]
- 13.Yang Z.H., Yi J.Y., Wei Z.R., Yan W.Q. Effect of extract of Rhizoma Arisaematis on languish and mechanism of SMMC-7721 cell of human liver cancer lines. Chin. Med. Old People. 2007;27:142–144. (in Chinese with English abstract) [Google Scholar]
- 14.Ducki S., Hadfield J.A., Lawrence N.J., Zhang X.G., McGown A.T. Isolation of paeonol from Arisaema erubescens. Planta Med. 1995;61:586–587. doi: 10.1055/s-2006-959390. [DOI] [PubMed] [Google Scholar]
- 15.Ducki S., Hadfield J.A., Zhang X.G., Lawrence N.J., McGown A.T. Isolation of aurantiamide acetate from Arisaema erubescens. Planta Med. 1996;62:277–278. doi: 10.1055/s-2006-957878. [DOI] [PubMed] [Google Scholar]
- 16.Du S.S., Xu Y.C., Wei L.X. Analysis of the fatty acids in the rhizome of Arisaema erubescens. J. Beijing Univ. TCM. 2003;26:44–46. (in Chinese with English abstract) [Google Scholar]
- 17.Du S.S., Xu Y.C., Wei L.X. Chemical constituents of Arisaema erubescens. Zhong Cao Yao. 2003;34:310–311. (in Chinese with English abstract) [Google Scholar]
- 18.Du S.S., Lei N., Xu Y.C., Wei L.X. Study on flavonoids of Arisaema erubescens. Chin. Pharmacol. J. 2005;40:1457–1459. (in Chinese with English abstract) [Google Scholar]
- 19.Xu H.H., Zhang N.J., Casida J.E. Insecticides in Chinese medicinal plants: Survey leading to jacaranone, a neurotoxicant and glutathione-reactive quinol. J. Agric. Food Chem. 2003;51:2544–2547. doi: 10.1021/jf021164x. [DOI] [PubMed] [Google Scholar]
- 20.Yang N.J., Liu W.W., Huo X., Gao Y.Q., Liu J.H. Determination of chemical constituents of the volatile oil from Arisaema erubescens (Wall.) Schott. Biotechnology. 2007;17:52–54. (in Chinese with English abstract) [Google Scholar]
- 21.Zhang Y., Ke W.S., Yang J.L., Ma A.N., Yu Z.S. The toxic activities of Arisaema erubescens and Nerium indicum mixed with streptomycete against snails. Environ. Toxicol. Pharmacol. 2009;27:283–286. doi: 10.1016/j.etap.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 22.Ke W.S., Yang J.L., Meng Z., Ma A.N. Evaluation of molluscicidal activities of Arisaema tubers extracts on the snail Oncomelania hupensis. Pestic. Biochem. Physiol. 2008;92:129–132. doi: 10.1016/j.pestbp.2008.07.003. [DOI] [Google Scholar]
- 23.Sakuma M. Probit analysis of preference data. Appl. Entomol. Zool. 1998;33:339–347. [Google Scholar]
- 24.Li Q.M., van den Heuvel H., Delorenzo O., Corthout J., Pieters L.A., Vlietinck A.J., Claeys M. Mass spectral characterization of C-glycosidic flavonoids isolated from a medicinal plant (Passiflora incarnata) J. Chromatogr. 1991;562:435–446. doi: 10.1016/0378-4347(91)80597-6. [DOI] [PubMed] [Google Scholar]
- 25.Raffaelli A., Moneti G., Mercati V., Toja E. Mass spectrometric characterization of flavonoids in extracts from Passiflora incarnate. J. Chromatogr. 1997;777:223–231. doi: 10.1016/S0021-9673(97)00260-4. [DOI] [Google Scholar]
- 26.Wagner H., Obermeier G., Chari V.M., Galle K. Flavonoid-C-glycosides from Triticum aestivum L. J. Nat. Prod. 1980;43:583–587. doi: 10.1021/np50011a009. [DOI] [Google Scholar]
- 27.Hussein S.A.M., Barakat H.H., Nawar M.N.M., Willuhn G. Flavonoids from Ephedra aphylla. Phytochemistry. 1997;45:1529–1532. [Google Scholar]
- 28.Yasukawa K., Kaneko T., Yamanouchi S. Studies on the constituents in the water extracts of crude drugs on the leaves of Desmodium stryacifolium Merr. (I) Yakugaku Zasshi. 1986;106:517–519. [Google Scholar]
- 29.Begum A., Wahab A., Siddiqui B.S., Qamar F. Nematicidal constituents of the aerial parts of Lantana camara. J. Nat. Prod. 2000;63:765–767. doi: 10.1021/np9903548. [DOI] [PubMed] [Google Scholar]
- 30.Adjei-Afriyie F., Kim C.S., Takemura M., Ishikawa M., Horiike M. Isolation and identification of the probing stimulants in the rice plant for the white-back planthopper, Sogatella furcifera (Homoptera: Delohacidae) Biosci. Biotechnol. Biochem. 2000;64:443–446. doi: 10.1271/bbb.64.443. [DOI] [PubMed] [Google Scholar]
- 31.Adjei-Afriyie F., Kim C.S., Takemura M., Ishikawa M., Tebayashi S., Horiike M. Probing stimulants from the rice plant towards the smaller brown planthopper, Laodelphax striatellus (Fallen) (Homoptera: Delohacidae) Z. Naturforsch. 2000;55:1038–1043. doi: 10.1515/znc-2000-11-1232. [DOI] [PubMed] [Google Scholar]
- 32.Kim M., Koh H.K., Fukami H. Isolation of C-glycosylflavones as probing stimulant of planthoppers in rice plant. J. Chem. Ecol. 1998;11:441–452. doi: 10.1007/BF00989555. [DOI] [PubMed] [Google Scholar]
- 33.Grayer R.J., Harborne J.B., Kimmins F.M., Stevenson P.C., Wijayagunasekera H.N.P. Phenolics in rice phloem sap as sucking deterrents to the brown plant hopper Nilaparvata lugens. Acta Hort. 1994;381:691–694. [Google Scholar]
- 34.Hooper A.M., Tsanuo M.K., Chamberlain K., Tittcomb K., Scholes J., Hassanali A., Khan Z.R., Pickett J.A. Isoschaftoside, a C-glycosylflavonoid from Desmodium uncinatum root exudate, is an allelochemical against the development of Striga. Phytochemistry. 2010;71:904–908. doi: 10.1016/j.phytochem.2010.02.015. [DOI] [PubMed] [Google Scholar]