Abstract
Nutrient pollution is of worldwide environmental and health concerns due to extensive use of nitrogen fertilizers and release of livestock waste, which induces nitrite compounds in aquatic systems. Here-in a surface-enhanced Raman scattering (SERS) sensor is developed for nitrite detection based on coupling between the plasmonic gold nanostars and the silver nanopyramid array. When nitrite is present in the assay, an azo group is formed between the 1-naphthylamine-functionalized silver nanopyramids and the 4-aminothiophenol-functionalized gold nanostars. This not only generates the SERS spectral finger-print for selective detection, but also creates “hot spots” at the gap between the Au nanostars and the Ag nanopyramids where the azo group is located, amplifying SERS signals remarkably. Finite-difference time-domain (FDTD) simulation shows a SERS enhancement factor of 4×1010 at the “hot spots”. As a result, the SERS sensor achieves a limit of detection of 0.6 pg/mL toward nitrite in water, and enables nitrite detection in real-world river water samples. In addition, this sensor eliminates the use of any Raman reporter and any expensive molecular recognition probe such as antibody and aptamer. This highly sensitive, selective and inexpensive SERS sensor has unique advantages over colorimetric, electrochemical and fluorescent devices for small molecule detection.
Keywords: nitrite, sensor, surface-enhanced Raman scattering, silver nanopyramid array, surface plasmon resonance
Graphical Abstract

Nutrient pollution, which primarily originates from extensive usage of fertilizers and livestock waste, pose a threat on the ecological system.1–3 It was estimated that 1×1011 kg of reactive nitrogen were released annually from nitrogen fertilizers around the world in the last decade, and ended up in aquatic systems in the form of nitrogen-containing compounds such as nitrite.4–6 Nitrite is accountable for the rampant growth of eutrophication and algae in water bodies.7–8 It can enter the food chain, and is taken by human. It is found to be correlated with the cause of methemoglobinemia in infants. Therefore, it is imperative to monitor nitrite in aquatic systems.
A common method for nitrite screening is Griess Test, which is based on the formation of a red pink color after the reaction between Griess reagents and the nitrite sample.9–11 However, the colorimetric nature makes Griess Test vulnerable to interferences from colored sample matrices, and suffer from poor sensitivity. Nitrite can also be measured quantitatively using chromatography methods,12–13 which act as the golden standard and is sensitive to part-per-billion level in drinking water. But considering the so-phisticated operational procedure, high cost and immobility of ion chromatography, it is not suitable for routine monitoring of nitrite in field. Nowadays, efforts have been devoted to the development of inex-pensive, rapid, portable and user-friendly sensors based on electrochemistry and fluorescence.14–21 How-ever, the reported sensors show high noise levels and limited sensitivity.
It is well known that combination of the sensing signal labels/reporters with molecular recognition probes is required for most types of prevailing sensors in order to capture specific analytes and to transduce the sensing signal. For example, for detection of antigens or small molecule analytes, antibodies or aptamers are usually used as the molecular recognition probes to capture antigens or small molecule analytes; and fluorescent labels or redox probes are typically used to transduce the sensing signal. Compared to colorimetric, electrochemical and fluorescent sensors, the SERS devices can recognize the molecular spectral fingerprints of analytes and/or transduce the sensing signal by directly acquiring the SERS spectrum of analytes.22–26 This unique feature of SERS not only enables high selectivity and strong resistance to the interference in the complex sample matrix, but also may eliminate the use of the sensing signal labels/reporters or/and the molecular recognition probes in devices, which simplifies the design of sensors, and saves the cost. SERS sensors have been widely used for biomarker detection, environmental pollutant monitoring, drug and explosive measurement, and etc.27–29 Currently, SERS sensors build on the plasmonic nanostructures as the SERS substrates, such as Au and Ag in the form of colloidal nanoparticles or two-dimensional (2D) nanoarray pattern-based chips.30–31 The 2D plasmonic nanoarray pattern-based chips are of particular interest because they are able to generate “hot spots” in a large space, amplifying the SERS signal.32–35 However, it still remains a significant challenge in massive production of reproducible 2D plasmonic nanoarray pattern-based chips as the SERS substrate.36–37
In the present work, a facile SERS sensor has been developed for nitrite detection by coupling gold nanostars onto an ordered silver nanopyramid array pattern. The sensor transduces the SERS spectral fingerprints of an azo group, which is formed only in the presence of nitrite in the assay, allowing detection of nitrite without the aid of any antibody or aptamer. In the meanwhile, the plasmonic coupling between the Au nanostars and the Ag nanopyramid array pattern generates the intense “hot spots”, enormously amplifying the SERS intensity of the newly formed azo group. Furthermore, the long-range ordered array of Ag nanopyramid makes it possible to amplify the SERS signals reproducibly. All these combined features of the SERS sensor not only improve the sensitivity toward nitrite detection, but also enable selectivity and reproducibility.
RESULTS AND DISCUSSIONS
Optical Properties of Ag Nanopyramid Array and Au Nanostars.
Fabricated by nanosphere lithography38–39 with the procedure shown in Figure S1, the Ag nanopyramid arrays exhibited a tetrahedron shape with three sharp edges and a needle-like tip at the apex, as shown in Figure 1(a) and 1(b). 67 objectives were measured for the Ag nano pyramids based on the SEM images. The average size of Ag nano pyramids was ~207 nm in height and ~120 nm in base length with the standard deviations of 17 nm and 18 nm. Based on Mie theory, the overall extinction of a metal particle includes the contributions of both light absorption and scattering. A plasmonic particle can absorb or scatter light when localized surface plasmon resonance (LSPR) is excited by incident light, which is dependent on the particle size. When the particle is small (typically less than 50 nm), light absorption is dominant while light scattering is negligible. When the particle is large (for example, 100 nm in a diameter), light scattering is strong while absorption is weak. Given that the Ag nanopyramid was quite large (~200 nm in height and ~120 nm in base length), light scattering was dominant in the present work.
Figure 1.
(a) and (b) SEM images of the Ag nanopyramid array pattern; (c) TEM image of the Au nanostars (inset shows a single Au nanostar); (d) Measured and simulated back reflection spectra for the Ag nanopyramid array pattern and absorption spectra for the Au nanostars. The back reflection and absorption intensities in (d) have been normalized for comparision.
25 objectives were measured for the Au nanostars based on the SEM and TEM images. The average size of Au nanostars was 80 nm with a standard deviation of 6 nm. The Au nanostars showed an average size of 80 nm, as shown in Figure 1(c). Despite the difference in shape, the Au nanostars and the Ag nanopyramid array pattern exhibited similar surface plasmon resonance spectral features with both the peaks centered at around 785 nm, which was also confirmed by the calculated UV-Visible spectra, as shown in Figure 1(d). The consistency between the simulated and the measured spectra implied predict-ability of optical properties as well as controllability of the obtained geometries, allowing design of the optimal SERS substrates with aid of finite-difference time-domain (FDTD) simulation. Furthermore, the spectral overlap suggested an intense electron wave function interaction, leading to plasmonic coupling between the Ag nanopyramid array and the Au nanostar under a 785 nm laser excitation.
Prior to sensor construction, FDTD simulation was implemented to study plasmon modes as well as plasmonic coupling, which could give an insight of the origin of the electromagnetic enhancement. We modelled the scenario of an Au nanostar sitting on one face of an Ag nanopyramid (Figure 2), which is also the most probable coupling case. The separation distance between the Au nanostar and the Ag nanopyramid was set as 1 nm, which is the approximate length of the newly formed azo compound bridging the Ag nanopyramid and the Au nanostar, as shown in Figure 3. The input plane wave was polarized in a way to enable the strongest plasmonic coupling. The simulated electromagnetic field distribution suggested that the Ag nanopyramid array predominately displayed vertex and edge plasmon modes, whereas the Au nanostar exhibited a tip plasmon mode. Upon plasmonic coupling, intense gap plasmon modes were generated at the 1 nm gap between an Au nanostar and an Ag nanopyramid, which was exactly the origin of the electromagnetic field enhancement. The SERS enhancement factor consisting of an excitation enhancement (|E785|/|E7850|)2 and the emission enhancement (|E862|/|E8620|)2 was also calculated, where E785 and E862 are the EM field at the excitation wavelength (785 nm) and the Stokes-shifted wavelength (862 nm) for the coupling normalized by the EM field at incident wavelengths E7850 and E8620, respectively. It was found that an SERS enhancement factor (|E785|/|E7850|)2·(|E862|/|E8620|)2 of ~4×1010 was achieved when an Au nanostar sitting on the face of an Ag nanopyramid. That is, the Raman intensity of the molecules at the gap would be amplified ~4×1010 times. As a comparison, a factor of ~5×106 was achieved for either an individual Au nanostar or an individual Ag nanopyramid.
Figure 2.
Simulated electromagnetic field distribution and SERS enhancement factor for various coupling scenarios between a silver nanopyramid and a gold nanostar with an excitation wavelength of 785 nm. The gap distance was set as 1 nm for the Au nanostar coupled with the silver nanopyramid, or with a silver film, with a SiO2 nanopyramid array. The arrows in the simulated electric field distribution indicate the polarization directions.
Figure 3.
Mechanism of nitrite detection with the SERS sensor. (a) Reaction: 1-naphthylamine (1-NA) and 4-aminothiophenol (4-ATP) reacts in the presence of nitrite ions under acidic condition, generating an azo compound with a SERS fingerprint (marked using a red color); (b) Detection scheme: the 1-NA functionalized Ag nanopyramid array captures the 4-ATP functionalized Au nanostar via formation of the azo compound.
Operating Mechanism of Sensor.
As shown in Figure 3(a), when the nitrite ions are present in the assay, the diazotization reaction between 1-naphthylamine (1-NA) and 4-aminothiophenol (4-ATP) happens,9–10 producing an azo compound which exhibited the SERS fingerprints. This eliminates the use of any Raman reporter in the SERS sensor design for detection of nitrite. The SERS sensor consisted of the Ag nanopyramid array pattern and the Au nanostars, which are initially functionalized with 1-NA and 4-ATP, respectively, as shown in Figure 3(b). The nitrite ions first react with the amine group of 4-ATP under an acidic condition, leading to the formation of diazonium compounds. The diazonium compounds further react with 1-NA, producing the azo compounds. As a result, the Au nanostars are covalently connected to the Ag nanopyramid array via the formation of the azo group. These newly formed azo group not only displays a SERS spectral fingerprint, but also benefits from the intense EM field enhancement thanks to the “hot spots” between the Ag nanopyramid and the Au nanostars. The mechanism was verified by the SERS spectra in Figure 4(a). The newly formed azo groups exhibited at least three new SERS peaks at 1140 cm−1, 1389 cm−1, and 1434 cm−1 as compared to the 4-ATP functionalized Au nanostars and the 1-NA functionalized Ag nanopyramid array. The uniformity of the substrate was also studied by detecting the SERS signal at 15 random locations across the 1-NA functionalized Ag nano-pyramid array. The relative standard deviation (RSD) was found to be 6.6%. Furthermore, the SERS sensor was tested separately against the possibly interfering inorganic compounds such as NaNO3, NaCl, Na2SO4, KCl, and K3PO4. As shown in Figure 4(b), the SERS sensor barely responded to any of the above inorganic compounds. Since the new SERS peaks were solely related to the azo group formed by the diazotization reaction in the presence of nitrite. This unique feature can significantly improve the signal-to-noise ratio, allowing the SERS sensor to specifically detect nitrite with minimal interference. In addition, unlike other SERS-based immunoassays or DNA-based SERS biosensors which heavily rely on expensive biological reagents such as antibodies and DNA, this SERS sensor employed only two small molecules (1-NA and 4-ATP). This makes the sensor cost-effective without compromising the performance.
Figure 4.
(a) SERS spectra comparison: the new azo compound produces three characteristic SERS peaks from the azo group at 1140 cm−1, 1389 cm−1, and 1434 cm−1 as compared to the 1-NA functionalized Ag nanopyramid array and the 4-ATP functionalized Au nanostar; (b) Selectivity test: possibly interfering compounds K3PO4, KCl, Na2SO4, NaCl, and NaNO3 were tested separately but resulted in barely detectable SERS peak at 1140 cm-1.
Calibration of SERS Sensor.
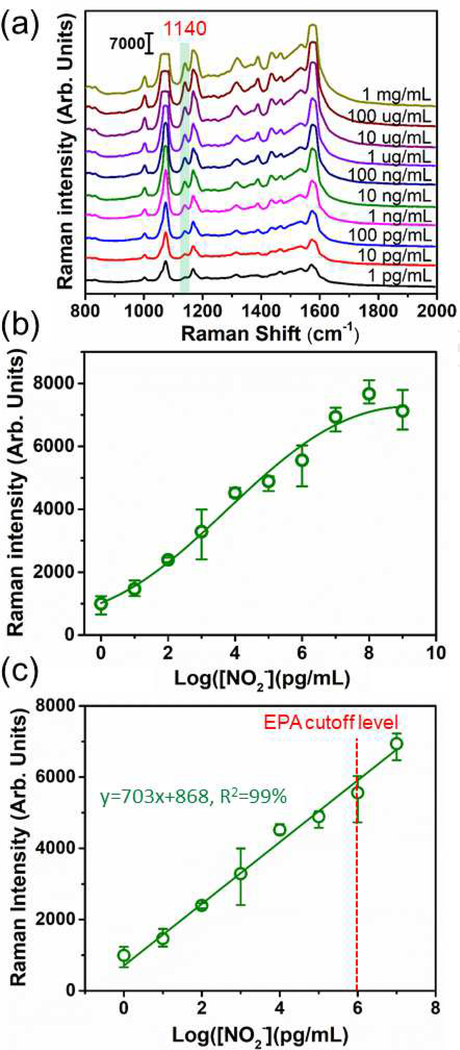
The SERS sensor was calibrated using the standard nitrite solutions. The concentration ranged from 1 pg/mL to 1 mg/mL with an interval of 1 order of magnitude. It is noted that the SERS spectra in Figure 5(a) were intentionally offset for a better visualization of the spectra evolution. After the addition of a nitrite solution, three new SERS peaks at 1140 cm−1, 1389 cm−1, and 1434 cm−1 showed up as expected (Figure 5(a)). The SERS intensity became intensified with an increase in the concentration of nitrite. All these three peaks can be used for quantification of the nitrite concentration based on the statistical analysis. Because the 1140 cm−1 peak responded to nitrite most sensitively although the difference among the three characteristic SERS peaks is marginal as shown in Figure S2, it was selected to build the calibration curve for nitrite detection. The SERS intensity of the 1140 cm−1 peak was plotted as a function of the logarithmic concentration of nitrite (Figure 5(b)), which showed a continuous SERS intensity increase till saturation with increasing the nitrite concentration. In the linear response region, the calibration curve was fitted with an equation of y=703x+868, R2=99%, where y is the measured intensity of the SERS peak at 1140 cm−1, x is the logarithmic concentration of nitrite, as shown in Figure 5(c). The limit of detection (LOD) was calculated to be 0.6 pg/mL based on three times signal-to-noise ratio. The SERS sensor showed lower LOD than the common techniques used for nitrite detection, such as chromatography and Griess test. The LODs are typically 1.6~75 ppb and 23~115 ppb for chromatography42,43 and Griess test44, respectively. Such a low LOD for the SERS sensor resulted from the SERS enhancement factor of ~1010, demonstrating high sensitivity of the SERS sensor. The SERS sensor also displayed an extended linear detection range spanning from 1 pg/mL to 10 ug/ml. This will endow the sensor with a wide range of applications for detection of either trace amount of nitrite or heavily accumulated nitrite pollutant in river, lake, pond, and etc. It is noteworthy that U.S. Environmental Protection Agency (EPA) regulates that the maximum contaminant level (MCL) for nitrite in drinking water is 1.0 μg/mL, which falls into the linear detection range of the SERS sensor. In addition, the selection of a single characteristic SERS peak at 1140 cm−1 to build the calibration curve was further justified by comparing with the full spectrum analysis using principle component analysis (PCA), as shown in Figure S3, where the response of the SERS sensor to nitrite concentration variation is very similar. Comparison of the response of the SERS sensor to varying nitrite concentrations between using the single SERS peak at 1140 cm−1 and using the full spectrum analysis by PCA was also shown in Figure S4, where the difference is marginal.
Figure 5.
SERS sensor calibration. (a) SERS spectra obtained at various concentrations of nitrite; (b) Plot of the intensity at 1140 cm−1 versus the logarithmic concentration of nitrite; (c) Fitting of the linear region in (b); The red dashed line shows the Maximum Contaminant Level of nitrite in drinking water regulated by EPA.
Detection of Nitrite in River Water.
The SERS sensor was used to test the water samples taken from Monongahela River near the Evansdale campus of West Virginia University in Morgantown, West Virginia. It was treated by natural sedimentation and filtration prior to testing by the SERS sensor. After acquisition of 19 SERS spectra from the sample, the nitrite level in river water sample was calculated according to the equation of the calibration curve: y=703x+868. It was found to be 105.94 ± 0.58 pg/mL (a mean value of 0.87 μg/mL with an upper limit of 3.3 μg/mL and a lower limit of 0.23 μg/mL) with a relative standard deviation (RSD) of 8.4%. In comparison, the sample was also tested by EPA 300.0 Ion Chromatography (Exova Inc), where the nitrite concentration was found to be 1.00 μg/mL, which was consistent with the result obtained by the SERS sensor.
In short, a SERS sensor developed in the present work is highly sensitive and selective. The detection scheme developed in this work can be adapted for measuring other ions and small molecules. For rapid and convenient measurement of nitrite and small molecules, it is desirable to incorporate a sensor into a microfluidic chip to achieve automation, sample handling and friendly user interface. For promoting the SERS sensor development, optical readers of SERS sensor need to be further improved in terms of size and cost.
CONCLUSIONS
In summary, an ordered silver nanopyramid array pattern was successfully fabricated with nanosphere lithography. By coupling the gold nanostars to the silver nanopyramid array pattern, a SERS sensor was built to detection nitrite. The presence of nitrite not only created three unique SERS peaks at 1140 cm−1, 1389 cm−1, and 1434 cm−1 from the azo group, but also resulted in the formation of “hot spots” where the azo group were located at the gap between the gold nanostar and the silver nanopyramid. This unique sensing mechanism eliminated the use of any aptamer or antibody, and enabled high sensitivity of the SERS sensor. As a result, the SERS sensor exhibited a LOD of 0.6 pg/mL toward nitrite detection in deionized water. The capability of the SERS sensor was demonstrated for nitrite detection in river water. The result showed that the SERS sensor was highly sensitive, selective and inexpensive; and it possessed unique advantages over the colorimetric, electrochemical and fluorescent devices for small molecule detection.
METHODS
Chemicals and Materials.
Polystyrene microspheres (diameters of 500, 600, and 1000 nm) were purchased from Thermo Scientific. Sodium hydrobromide (99%), polyvinylpyrrolidone (average molecular weight 10,000), N,N-dimethylformamide (ACS reagent, ≥99.8%), 4-aminothiophenol (97%), 1naphthylamine (≥99.0%), sodium nitrite (ACS reagent, ≥97.0%), and hydrochloric acid (36.5–38.0%) were purchased from Sigma-Aldrich. Chloroauric acid trihydrate (ACS, 99.99%) and trisodium citrate dehydrate (ACS, 90.0+%) were purchased from Alfa Aesar. Quartz slides were purchased from AdValue Technology. River water sample was collected from Monongahela River near Evansdale Campus of West Virginia University in Morgantown, West Virginia. DI water was produced by Milli-Q Millipore system (18.2 MΩ cm, Millipore Corp., Billerica, MA) and was used for washing and reactions. All chemicals were directly obtained from commercial vendors and used without further purification.
Ag Nanopyramid Array Fabrication.
The Ag nanopyramid array patterns were fabricated on the quartz slides (roughly 1 cm × 1 cm) in the cleanroom by nanosphere lithography38–39, as shown in Figure S1. Specifically, the quartz slides were first cleaned by immersing into acid piranha (the ratio of sulfuric acid to hydrogen peroxide is 3:1) at 90 °C for 1 hour. Caution: acid piranha is very dangerous and needs to be handled with extreme care! The quartz slides were then rinsed thoroughly using deionized (D.I.) water, and further cleaned three times by consecutive sonication in ethanol and D.I. water, respectively. Afterwards, a monolayer of polystyrene microsphere (PS) was dip-coated on the quartz slides in a hexagonal pattern. The diameter of PS was varied from 500 nm and 600 to 1000 nm to tune the optical response of the resulting pyramid array. After that, a 5 nm thick titanium layer and a 200 nm thick silver film were deposited onto the quartz slides by e-beam evaporator. The PS beads were removed by sonication in methanol for five minutes and blown to dry using compressed air, resulting in the hexagonally ordered Ag nanopyramid array patterns.
Au Nanostar Synthesis.
The Au nanostars were synthesized using a two-step approach reported previously.40 To begin with, the Au nanoseeds were first synthesized. 1 mL of 1 wt% chloroauric acid was first added to 90 mL of D.I. water, followed by 2 mL of 38.8 mM trisodium citrate solution. Afterwards, 1 mL of 0.075 wt% sodium hydrobromide solution was added into the above solution and kept reacting overnight. Subsequently, polyvinylpyrrolidone was dissolved into a 50 mL of Au nanoseed solution; and the mixture was stirred for 24 hours. In order to synthesize the Au nanostars, 82 μL of 50 mM chloroauric acid was added into a 15 mL of 10 mM polyvinylpyrrolidone solution, which was followed by the addition of 43 μL of Au nanoseeds. The mixture was stirred overnight before being centrifuged and washed using ethanol and water. The washing and centrifugation steps were repeated three times before the obtained Au nanostars were finally dissolved into ethanol.
Instruments and Characterization.
Titanium and silver were deposited using an e-beam evaporator (Kurt J Lesker, Model#LAB18). Ag nanopyramid arrays were characterized under a JEOL JSM-7600F scanning electron microscope (SEM). The Au nanostars were characterized using a JEOL JEM-2100F transmission electron microscope (TEM). An Ocean Optics USB 4000 spectrometer was used to acquire the reflection spectra of the fabricated Ag nanopyramid arrays. Raman spectra were acquired using the iRaman plus (Model# BWS465, B&W Tek) with an excitation wavelength of 785 nm.
FDTD Simulation.
Optical properties of the Ag nanopyramid array patterns, the Au nanostars were studied using finite different time domain (FDTD) simulation. FDTD software is commercially available from Optiwave Systems Inc. A grid size of 1 nm was used to construct the simulation cell. A plane wave with a center wavelength of 600 nm was used as the input light source. The wavelength-dependent refractive index of silver was taken from Palik.41 The refractive index of quartz slides was modeled as a constant of 1.53. Periodic boundary conditions were applied for all simulations.
SERS Measurement.
The Au nanostars were first functionalized with 4-aminothiophenol (4-ATP). Specifically, 10 uL of 10 mM 4-ATP in ethanol was added into 10 mL of Au nanostars. The mixture was stirred overnight before being centrifuged and washed three times to remove excessive 4-ATP molecules using D.I. water. It was then dissolved in D.I. water, resulting in the Au star@ATP nanoparticles. The pH of Au star@ATP nanoparticle solution was adjusted to ~3.5 using 2 M hydrochloric acid.
The Ag nanopyramid array patterns were functionalized with 1-naphthylamine (1-NA). Specifically, an Ag nanopyramid array was incubated into 10 mL of 10 mM 1-NA ethanolic solution overnight, forming Ag nanopyramid@NA. It was then rinsed using ethanol to remove excessive 1-NA molecules and dried using compressed air.
Standard nitrite solutions with concentrations of 1 pg/mL, 10 pg/mL, 100 pg/mL, 1 ng/mL, 10 ng/mL, 100 ng/mL, 1 μg/mL, 10 μg/mL, 100 μg/mL, and 1 mg/mL were prepared by dissolving sodium nitrite into D.I. water, respectively.
In order to calibrate the sensor for nitrite detection, 5 μL of standard nitrite solutions was first mixed with 50 μL of Au nanostar@ATP solution. The mixed solution was immediately dispensed onto the surface of a Ag nanopyramid@NA substrate. After 10 minutes of incubation, the Ag nanopyramid@NA was washed using D.I. water and dried using compressed air. SERS spectra were then acquired from the surface of the Ag nanopyramid array substrate. In this process, the laser power was set at 10% of the full power (>320 mW at laser port, 420 mW Max from the vendor) with an integration exposure time of 10 s for each measurement.
To detect nitrite in river water, the water sample was first treated by natural sedimentation for a week, and then further purified by filtration using a filter paper prior to measurement. The procedure for SERS measurement was the same as above.
Supplementary Material
Highlights:
Label-free: no any sensing signal label/reporter is used to construct the surface-enhanced Raman scattering (SERS) sensor.
High specificity: the newly formed azo group in the presence of the analyte (nitrite) generates unique SERS spectral fingerprints for nitrite detection.
High sensitivity: the SERS enhancement factor owing to the coupling between Au nanostars and Ag nanopyramid array reaches ~4×1010 at the hot spots, allowing the sensor to achieve a limit of detection of 0.6 pg/mL toward nitrite in water.
Low cost: the sensor design has eliminated the use of a Raman label and expensive reagents such as aptamers and antibodies, making the sensor cost-effective.
Acknowledgment
This work was supported by NIH (R15NS087515) and NSF (CBET-1336205). The use of WVU Shared Research Facilities was acknowledged. M. L. acknowledges financial support by the National Thousand Young Talents Program of China, Innovation-Driven Project of Central South University (No. 2018CX002) and Hunan Provincial Science & Technology Program (No. 2017XK2027).
Footnotes
The authors declare no competing financial interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Socolow RH Nitrogen management and the future of food: Lessons from the management of energy and carbon. P. Natl. Acad. Sci. USA 1999, 96, 6001–6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woodward G; Gessner MO; Giller PS; Gulis V; Hladyz S; Lecerf A; Malmqvist B; McKie BG; Tiegs SD; Cariss H; Dobson M; Elosegi A; Ferreira V; Graca MAS; Fleituch T; Lacoursiere JO; Nistorescu M; Pozo J; Risnoveanu G; Schindler M; Vadineanu A; Vought LBM; Chauvet E Continental-Scale Effects of Nutrient Pollution on Stream Ecosystem Functioning. Science 2012, 336, 1438–1440. [DOI] [PubMed] [Google Scholar]
- 3.Shibata H; Branquinho C; McDowell WH; Mitchell MJ; Monteith DT; Tang JW; Arvola L; Cruz C; Cusack DF; Halada L; Kopacek J; Maguas C; Sajidu S; Schubert H; Tokuchi N; Zahora J Consequence of altered nitrogen cycles in the coupled human and ecological system under changing climate: The need for long-term and site-based research. Ambio 2015, 44, 178–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen JH; Pang S; He LL; Nugen SR Highly sensitive and selective detection of nitrite ions using Fe3O4@SiO2/Au magnetic nanoparticles by surface-enhanced Raman spectroscopy. Biosens. Bioelectron. 2016, 85, 726–733. [DOI] [PubMed] [Google Scholar]
- 5.Strokal M; Ma L; Bai ZH; Luan SJ; Kroeze C; Oenema O; Velthof G; Zhang FS Alarming nutrient pollution of Chinese rivers as a result of agricultural transitions. Environ. Res. Lett. 2016, 11, 024014. [Google Scholar]
- 6.Fields S Global nitrogen - Cycling out of control. Environ Health Persp 2004, 112, A556–A563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luo YH; Wen GQ; Dong JC; Liu QY; Liang AH; Jiang ZL SERS detection of trace nitrite ion in aqueous solution based on the nitrosation reaction of rhodamine 6G molecular probe. Sensor Actuat. B-Chem. 2014, 201, 336–342. [Google Scholar]
- 8.Tang IH; Sundari R; Lintang HO; Yuliati L Detection of nitrite and nitrate ions in water by graphene oxide as a potential fluorescence sensor. IOP Conf. Ser.-Mat. Sci 2016, 107, 012027. [Google Scholar]
- 9.Daniel WL; Han MS; Lee JS; Mirkin CA Colorimetric Nitrite and Nitrate Detection with Gold Nanoparticle Probes and Kinetic End Points. J. Am. Chem. Soc. 2009, 131, 6362–6363. [DOI] [PubMed] [Google Scholar]
- 10.Correa-Duarte MA; Perez NP; Guerrini L; Giannini V; Alvarez-Puebla RA Boosting the Quantitative Inorganic Surface-Enhanced Raman Scattering Sensing to the Limit: The Case of Nitrite/Nitrate Detection. J. Phys. Chem. Lett 2015, 6, 868–874. [DOI] [PubMed] [Google Scholar]
- 11.Giustarini D; Rossi R; Milzani A; Dalle-Donne I Nitrite and nitrate measurement by Griess reagent in human plasma: Evaluation of interferences and standardization. Method Enzymol 2008, 440, 361–380. [DOI] [PubMed] [Google Scholar]
- 12.Kissner R; Koppenol WH Qualitative and quantitative determination of nitrite and nitrate with ion chromatography. Method Enzymol. 2005, 396, 61–68. [DOI] [PubMed] [Google Scholar]
- 13.Di Matteo V; Esposito E Methods for the determination of nitrite by high-performance liquid chromatography with electrochemical detection. J Chromatogr A 1997, 789, 213–219. [DOI] [PubMed] [Google Scholar]
- 14.Zou CE; Yang BB; Bin D; Wang J; Li SM; Yang P; Wang CQ; Shiraishi Y; Du YK Electrochemical synthesis of gold nanoparticles decorated flower-like graphene for high sensitivity detection of nitrite. J Colloid Interf Sci 2017, 488, 135–141. [DOI] [PubMed] [Google Scholar]
- 15.Cai MY; Chai XY; Wang XD; Wang T An Acid-Inert Fluorescent Probe for the Detection of Nitrite. J Fluoresc 2017, 27, 1365–1371. [DOI] [PubMed] [Google Scholar]
- 16.Chen J; Ma Q; Wang CH; Hu XY; Gao YJ; Wang H; Qin DD; Lu XQ A simple fluorescence sensor for the detection of nitrite (NO2-) in real samples using water-dispersible graphitelike carbon nitride (w-g-C3N4) nanomaterials. New J Chem 2017, 41, 7171–7176. [Google Scholar]
- 17.Li LB; Liu D; Wang K; Mao HP; You TY Quantitative detection of nitrite with N-doped graphene quantum dots decorated N-doped carbon nanofibers composite-based electrochemical sensor. Sensor Actuat. B-Chem. 2017, 252, 17–23. [Google Scholar]
- 18.Wang P; Wang MY; Zhou FY; Yang GH; Qu LL; Miao XM Development of a paperbased, inexpensive, and disposable electrochemical sensing platform for nitrite detection. Electrochem Commun 2017, 81, 74–78. [Google Scholar]
- 19.Su CH; Sun CL; Liao YC Printed Combinatorial Sensors for Simultaneous Detection of Ascorbic Acid, Uric Acid, Dopamine, and Nitrite. Acs Omega 2017, 2, 4245–4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davis J; Moorcroft MJ; Wilkins SJ; Compton RG; Cardosi MF Electrochemical detection of nitrate and nitrite at a copper modified electrode. Analyst 2000, 125, 737–741. [Google Scholar]
- 21.Wang P; Mai ZB; Dai Z; Li YX; Zou XY Construction of Au nanoparticles on choline chloride modified glassy carbon electrode for sensitive detection of nitrite. Biosens. Bioelectron. 2009, 24, 3242–3247. [DOI] [PubMed] [Google Scholar]
- 22.Sharma B; Frontiera RR; Henry AI; Ringe E; Van Duyne RP SERS: Materials, applications, and the future. Mater. Today 2012, 15, 16–25. [Google Scholar]
- 23.Sigle DO; Kasera S; Herrmann LO; Palma A; de Nijs B; Benz F; Mahajan S; Baumberg JJ; Scherman OA Observing Single Molecules Complexing with Cucurbit[7]uril through Nanogap Surface-Enhanced Raman Spectroscopy. J. Phys. Chem. Lett 2016, 7, 704–710. [DOI] [PubMed] [Google Scholar]
- 24.Zrimsek AB; Wong NL; Van Duyne RP Single Molecule Surface-Enhanced Raman Spectroscopy: A Critical Analysis of the Bianalyte versus Isotopologue Proof. J. Phys. Chem. C 2016, 120, 5133–5142. [Google Scholar]
- 25.Wang F; Cao SY; Yan RX; Wang ZW; Wang D; Yang HF Selectivity/Specificity Improvement Strategies in Surface-Enhanced Raman Spectroscopy Analysis. Sensors-Basel 2017, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petti L; Rippa M; Capasso R; Zhou J; Maglione MG; Pannico M; La Manna P; Musto P Plasmonic octagonal quasicrystals for surface enhanced Raman sensing. Advanced Device Materials 2015, 1, 47–51. [Google Scholar]
- 27.Schlucker S Surface-Enhanced Raman Spectroscopy: Concepts and Chemical Applications. Angew. Chem. Int. Edit. 2014, 53, 4756–4795. [DOI] [PubMed] [Google Scholar]
- 28.Muehlethaler C; Leona M; Lombardi JR Review of Surface Enhanced Raman Scattering Applications in Forensic Science. Anal. Chem. 2016, 88, 152–169. [DOI] [PubMed] [Google Scholar]
- 29.Park SG; Ahn MS; Oh YJ; Kang M; Jeong Y; Jeong KH Nanoplasmonic Biopatch for in vivo Surface Enhanced Raman Spectroscopy. Biochip J. 2014, 8, 289–294. [Google Scholar]
- 30.Li JF; Huang YF; Ding Y; Yang ZL; Li SB; Zhou XS; Fan FR; Zhang W; Zhou ZY; Wu DY; Ren B; Wang ZL; Tian ZQ Shell-isolated nanoparticle-enhanced Raman spectroscopy. Nature 2010, 464, 392–395. [DOI] [PubMed] [Google Scholar]
- 31.Xie W; Schlucker S Rationally designed multifunctional plasmonic nanostructures for surfaceenhanced Raman spectroscopy: a review. Rep. Prog. Phys. 2014, 77, 116502. [DOI] [PubMed] [Google Scholar]
- 32.Lin TH; Linn NC; Tarajano L; Jiang B; Jiang P Electrochemical SERS at Periodic Metallic Nanopyramid Arrays. J Phys Chem C 2009, 113, 1367–1372. [Google Scholar]
- 33.Jin ML; Pully V; Otto C; van den Berg A; Carlen ET High-Density Periodic Arrays of Self-Aligned Subwavelength Nanopyramids for Surface-Enhanced Raman Spectroscopy. J Phys Chem C 2010, 114, 21953–21959. [Google Scholar]
- 34.Tabatabaei M; Sangar A; Kazemi-Zanjani N; Torchio P; Merlen A; Lagugne-Labarthet F Optical Properties of Silver and Gold Tetrahedral Nanopyramid Arrays Prepared by Nanosphere Lithography. J Phys Chem C 2013, 117, 14778–14786. [Google Scholar]
- 35.Linn NC; Sun CH; Arya A; Jiang P; Jiang B Surface-enhanced Raman scattering on periodic metal nanotips with tunable sharpness. Nanotechnology 2009, 20. [DOI] [PubMed] [Google Scholar]
- 36.Alonso-Gonzalez P; Albella P; Schnell M; Chen J; Huth F; Garcia-Etxarri A; Casanova F; Golmar F; Arzubiaga L; Hueso LE; Aizpurua J; Hillenbrand R Resolving the electromagnetic mechanism of surface-enhanced light scattering at single hot spots. Nat. Commun 2012, 3, 684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ding SY; You EM; Tian ZQ; Moskovits M Electromagnetic theories of surface-enhanced Raman spectroscopy. Chem. Soc. Rev. 2017, 46, 4042–4076. [DOI] [PubMed] [Google Scholar]
- 38.Haynes CL; Van Duyne RP Nanosphere Lithography: A Versatile Nanofabrication Tool for Studies of Size-Dependent Nanoparticle Optics. The Journal of Physical Chemistry B 2001, 105, 5599–5611. [Google Scholar]
- 39.Masson J-F; Murray-Methot M-P; Live LS Nanohole arrays in chemical analysis: manufacturing methods and applications. Analyst 2010, 135, 1483–1489. [DOI] [PubMed] [Google Scholar]
- 40.Zheng P; Li M; Jurevic R; Cushing SK; Liu YX; Wu NQ A gold nanohole array based surface-enhanced Raman scattering biosensor for detection of silver(I) and mercury(II) in human saliva. Nanoscale 2015, 7, 11005–11012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Palik ED, Handbook of optical constants of solids. Academic press: Orlando, 1985. [Google Scholar]
- 42.Miura Y; Hamada H Ion chromatography of nitrite at the ppb level with photometric measurement of iodine formed by post-column reaction of nitrite with iodide, J Chromatogr A. 1999, 850, 153–160. [DOI] [PubMed] [Google Scholar]
- 43., Ito K; Takayama Y; Makabe N; Mitsui R; Hirokawa T Ion chromatography for determination of nitrite and nitrate in seawater using monolithic ODS columns., J Chromatogr A. 2005, 1083, 63–67. [DOI] [PubMed] [Google Scholar]
- 44.Shen Y; Zhang Q; Qian X; Yang Y Practical assay for nitrite and nitrosothiol as an alternative to the griess assay or the 2,3-diaminonaphthalene assay, Anal. Chem. 2015, 87, 1274–1280. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.