Abstract
Background:
Several reviews have recently detailed the beneficial effects of weight loss surgery for kidney function. However, these studies have a number of limitations, including small sample size, few done in chronic kidney disease (CKD) stages 3 and 4, and many not including the main bariatric surgery procedures used in the United States today.
Study Design:
This was an observational retrospective cohort study comparing propensity score–matched bariatric surgery patients and nonsurgery control patients who were referred for, but did not have, surgery. Roux-en-Y gastric bypass (RYGB) and sleeve gastrectomy were also compared using propensity matching.
Setting & Participants:
Patients (714 surgery patients; 714 controls) were from a large integrated health care system, a mean of 58 ± 8 (SD) years old, and mostly women (77%) and non-Hispanic whites (56%) and had diabetes mellitus (66%) and/or hypertension (91%).
Predictor:
Predictors at the time of surgery or referral to surgery were age, sex, race/ethnicity, weight, and presence of diabetes and/or hypertension.
Outcomes:
The primary outcome for this study was change in estimated glomerular filtration rate (eGFR) from serum creatinine level over a median 3-year follow-up period.
Measurements:
Serum creatinine was used to calculate eGFR using the CKD-EPI (CKD Epidemiology Collaboration) creatinine equation.
Results:
Surgery patients had 9.84 (95% CI, 8.05–11.62) mL/min/1.73 m2 greater eGFRs than controls at a median 3 years’ follow-up and RYGB patients had 6.60 (95% CI, 3.42–9.78) mL/min/1.73 m2 greater eGFRs than sleeve gastrectomy patients during the same period.
Limitations:
This study is limited by its nonrandomized observational study design, estimation of GFR, and large changes in muscle mass, which may affect serum creatinine level independent of changes in kidney function.
Conclusions:
Bariatric surgery, especially the RYGB procedure, results in significant improvements for up to 3 years in eGFRs for patients with CKD stages 3 and 4.
Keywords: Estimated glomerular filtration rate (eGFR), bariatric surgery, chronic kidney disease (CKD), obesity, eGFR trajectory, CKD stages 3–4, sleeve gastrectomy (SG), Roux-en-Y gastric bypass (RYGB), renal function, CKD progression
Obesity-related mortality has surpassed that from tobacco, accounting for 6 million deaths annually.1,2 It is estimated that part of the cost of severe obesity and its impact on life expectancy is due to severe illnesses (eg, chronic kidney disease [CKD], including end-stage renal disease [ESRD]). Patients with body mass index ≥ 40 kg/m2 are more than 7 times more likely to develop ESRD than patients who are of normal weight.3 In 2010, total Medicare expenditures for ESRD increased 8% to $32.9 billion.4
There is evidence that weight loss may prevent the progression of earlier stages of CKD to ESRD in some individuals.5–7 Unfortunately, even with intensive multicomponent lifestyle interventions, only 50% of studies show a 5% weight loss (considered clinically meaningful) and most participants gain back at least half this lost weight over 18 to 30 months.8 These outcomes have resulted in the development of surgical treatments, referred to as bariatric surgery, for severe obesity. A recent meta-analysis found that bariatric surgery resulted in significantly greater weight loss and higher rates of type 2 diabetes remission when compared with conventional weight loss methods.9
There has been growing interest in the effects of bariatric surgery on kidney function. Several reviews have recently been published10–15 detailing the beneficial effects that weight loss surgery has on kidney function. However, there are a number of limitations in the literature to date. Most studies have 200 to 300 patients who are primarily non-Hispanic white, with only bypass and band operations, limited follow-up, and in primarily research academic medical settings. Three large population-based studies have been published: Swedish Obese Subjects (SOS),16 patients from an existing statewide claims database,17 and a recent study from an integrated health care system in the United States.18 None of these studies included sleeve gastrectomy (SG), the most common procedure performed in the United States.19 In addition, Johnson et al17 studied only patients with type 2 diabetes for the development of microvascular complications (of which CKD was one), and Chang et al18 studied all bariatric patients regardless of stage of kidney disease. No research has been published to date focusing solely on the impact of current United States bariatric procedures on advanced kidney disease.
To address these limitations, we conducted a large observational retrospective cohort study in a real-world clinical setting of 3-year outcomes for a diverse group of severely obese patients with CKD stages 3 and 4. Our primary hypothesis was that bariatric surgery would be associated with significant improvements in kidney function (operationalized by increased estimated glomerular filtration rate [eGFR]) when compared to nonsurgery controls. In addition, based on our previous work with metabolic syndrome and bariatric surgery,20 we hypothesized that Roux-en-Y gastric bypass (RYGB) would be associated with significantly greater increases in eGFR than SG.
METHODS
Setting
Kaiser Permanente Southern California (KPSC) has 14 hospitals and nearly 200 other medical offices with a partnership of more than 5,700 physicians delivering care to more than 4 million members. Details of the bariatric surgery program at KPSC have been published elsewhere.21 Briefly, more than 3,000 weight loss procedures are performed annually by 23 surgeons in 9 hospital facilities. Data about bariatric surgery patients at KPSC are maintained in a registry that contains electronic information from a number of sources (described in Measures section). Bariatric surgery patients at KPSC are similar to patients reported in national published findings from a variety of settings, with the exception that there is a much higher proportion of ethnic/racial minorities (55%) than in other published work.22,23 All procedures were approved by the Institutional Review Board for Human Subjects at KPSC (study #10548). A waiver of consent was approved due to the minimal risk of the study.
Participants
Bariatric surgery patients
Bariatric surgery patients were eligible for the study if they had: (1) an RYGB or SG procedure from January 1, 2008, through December 31, 2012, without a history of a previous procedure or subsequent revisions of their initial procedure throughout the follow-up period (up to May 30, 2015); (2) body mass index ≥ 30 kg/m2 at the time of surgery; and (3) eGFR of 11 to 59 mL/min/1.73 m2 in the 12 months before the date of surgery (the eGFR value closest before the date of surgery meeting this criterion was used for baseline). We chose only RYGB and SG procedures because there were too few of the other primary procedures to study in the KPSC registry (ie, banding). We also chose not to study patients with eGFRs < 11 mL/min/1.73 m2 due to the risk for imminent dialysis, complicating any analysis of the effects of surgery on eGFR. Very few patients with this severity of CKD receive surgery at KPSC.
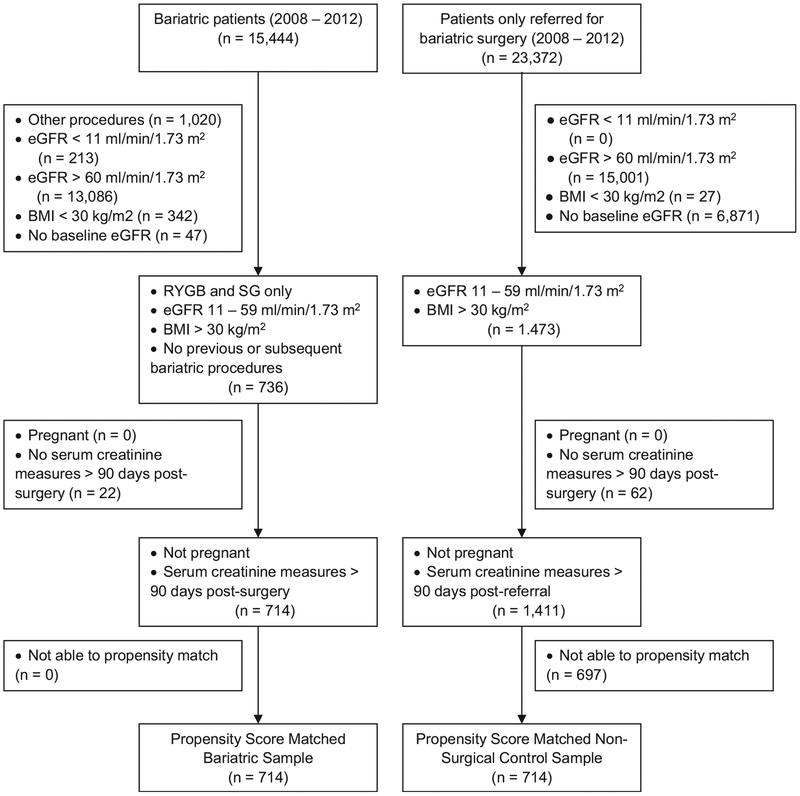
Of these surgery patients (n = 736), we eliminated those who were pregnant at the time of surgery and those who did not have serum creatinine measurements after 90 days following surgery when follow-up began (n = 22). The follow-up period did not begin until 90 days after surgery to avoid the direct effect of the surgical procedure on kidney and metabolic function. No patients were lost in the propensity matching (explained in the analysis section), so we had 714 surgery patients for the final analytic sample. The selection process for patients is shown in Fig 1.
Figure 1.
Participant selection for propensity-matched bariatric and nonsurgery control patients. Abbreviations: BMI, body mass index; eGFR, estimated glomerular filtration rate; RYGB, Roux-en-Y gastric bypass; SG, sleeve gastrectomy.
Nonsurgery Control Patients
Initially, nonsurgery controls were selected based on the following criteria: (1) they had been referred for surgery January 1, 2008 to December 31, 2012, without a history of a bariatric procedure and did not go on to have a bariatric procedure at any time during the follow-up period (ending May 30, 2015); (2) had body mass index ≥ 30 kg/m2 at the time of referral; and (3) had eGFR of 11 to 59 mL/min/1.73 m2 in the 12 months before their referral date (the eGFR value closest in time before the referral date meeting this criterion was used for baseline).
From these control patients (n = 1,473), we eliminated those who were pregnant at the time of referral and those who did not have serum creatinine measurements after 90 days following their referral date when follow-up began (n = 62). After propensity matching, we had 714 nonsurgery control patients for the analyses. The selection process for patients is shown in Fig 1.
Measurements
All data for the study were abstracted from electronic medical records and outside claims processing databases. Data were collected from patients and entered into the electronic medical record by clinical staff as part of routine care. Date of birth, sex, and race/ethnicity were self-reported by patients. In general, height was self-reported by patients and weight and blood pressure were measured by clinical staff. Height and weight were used to calculate body mass index. Comprehensive prescription data were available for each drug sold at system pharmacies, as well as outpatient and inpatient laboratory results. Diagnoses were available for all types of health care use, including outpatient, inpatient, and emergency.
Analyses
Outcome Definition
Serum creatinine level was used to calculate eGFR. There are several equations that estimate GFR and can be applied retrospectively to readily obtainable data.24 Although the most commonly used one is the NKF-KDOQI (National Kidney Foundation–Kidney Disease Outcomes Quality Initiative) – recommended 4-variable MDRD (Modification of Diet in Renal Disease) Study equation,24–26 there are a number of limitations with its development, including that it does not predict with accuracy when eGFR is 60 to 90 mL/min/1.73 m2.25,27–31
An alternative to the 4-variable MDRD Study equation is the CKD-EPI (CKD Epidemiology Collaboration) creatinine equation.32,33 A recent study by Friedman et al,34 specifically testing GFR estimating equations for bariatric surgery patients, found that the most accurate estimates of GFR were obtained by using the CKD-EPI creatinine–cystatin C equation. However, if cystatin C level was not available (as was the case for our study), the CKD- EPI creatinine equation performed adequately. Friedman et al34 recommended its use over the MDRD Study equation. We used the CKD-EPI creatinine equation.35
Propensity Matching
To balance our study populations with respect to baseline characteristics, we used 1:1 caliper propensity score matching. The first hypothesis used propensity scores to match bariatric surgery patients with nonsurgery controls and the second hypothesis used propensity scores to match RYGB patients to SG patients. The date of referral to surgery was used as the index date for matching surgery patients to controls. All patients had CKD stage 3 or 4. The predicted probability of having bariatric surgery (first hypothesis) or RYGB (second hypothesis) was calculated via separate logistic regressions adjusting for the following covariates at the time of surgery or referral: age, weight, sex, race/ethnicity, and presence of hypertension and/or type 2 diabetes. A greedy matching algorithm36 was used to match either the surgery and control groups or the 2 surgery types at a 1:1 match ratio with a caliper equal to 0.2 times the standard deviation of the propensity scores for each regression. Standardized differences were used to evaluate the propensity-matched data. For categorical baseline variables with more than 2 levels, we use a multivariate Mahala-nobis distance method to generalize the standardized difference metric to handle a multinomial sample.37
Statistical Models
The propensity score–matched populations were examined using linear mixed models with random intercept and slopes for each individual to capture the heterogeneity between patients over time.38 Using separate cubic B-spline functions, eGFR trajectories were modeled smoothly over time by study effect: surgery patients compared with nonsurgery controls and RYGB compared to SG. We also included all variables previously listed for the propensity model as covariates in these analyses to obtain “doubly robust” estimates of the differences in eGFR between study groups at each follow-up time point.39,40 Unadjusted and adjusted estimates are presented for comparison.
We also examined weight loss over time for both populations using these same models to better understand how the trajectories between eGFR and weight loss were related. Plots of the trajectories of weight loss, eGFR, and differences between groups in eGFR were then created. For the graphs showing weight loss and eGFR trajectories, model covariates were set to the median values for continuous variables and the mode for categorical variables. All statistical analyses were conducted using R, version 3.2.3 (R Foundation for Statistical Computing).
RESULTS
Participants
Participant characteristics pre- and post–propensity matching are shown in Table 1 for bariatric surgery patients and nonsurgery control patients and Table 2 for RYGB and SG patients. Standardized differences for the propensity-matched populations were all ≤ to 0.1 (see Austin36) and diagnostics of propensity score distributions revealed excellent overlap (plots shown in Fig S1, provided as online supplementary material). On average, patients were about 58 years old, 77% were women, 56% were non-Hispanic whites, 67% had diabetes mellitus, 91% had hypertension, and 63% to 71% of patients were using angiotensin-converting enzyme inhibitor/angiotensin receptor blocker medications at the time of surgery or referral to surgery.
Table 1.
Baseline Characteristics of Bariatric Surgery Patients and Nonsurgery Controls Pre- and Post-Propensity Score Matching
| Before Matching | After Matching | |||||
|---|---|---|---|---|---|---|
| Surgery (n = 714) | Controls (n = 1,411) | P | Surgery (n = 714) | Controls (n = 714) | Absolute Std Diff | |
| Age, y | 58.1 ± 8.46 | 60.1 ± 8.86 | <0.001 | 58.1 ± 8.46 | 58.4 ± 8.90 | 0.03 |
| Weight, lb | 269.8 ± 49.76 | 276.0 ± 53.11 | 0.009 | 269.8 ± 49.76 | 269.9 ± 51.39 | 0.003 |
| BMI, kg/m2 | 44.3 ± 6.60 | 44.2 ± 7.19 | 0.6 | 44.3 ± 6.60 | 44.0 ± 7.16 | 0.03 |
| Female sex | 551 (77.2%) | 914 (64.8%) | <0.001 | 551 (77.2%) | 543 (76.1%) | 0.03 |
| Race/ethnicity | 0.6 | 0.05 | ||||
| Non-Hispanic white | 399 (55.9%) | 757 (53.6%) | 399 (55.9%) | 404 (56.6%) | ||
| Non-Hispanic black | 131 (18.3%) | 264 (18.7%) | 131 (18.3%) | 131 (18.3%) | ||
| Hispanic | 160 (22.4%) | 328 (23.2%) | 160 (22.4%) | 161 (22.5%) | ||
| Other/missing/unknown | 24 (3.4%) | 62 (4.5%) | 24 (3.4%) | 18 (2.6%) | ||
| Hypertension | 648 (90.8%) | 1317 (93.3%) | 0.03 | 648 (90.8%) | 645 (90.3%) | 0.01 |
| Diabetes | 470 (65.8%) | 933 (66.1%) | 0.9 | 470 (65.8%) | 487 (68.2%) | 0.05 |
| Serum creatinine, mg/dL | 1.4 ± 0.50 | 1.5 ± 0.57 | <0.001 | 1.4 ± 0.50 | 1.5 ± 0.56 | 0.09 |
| eGFR, mL/min/1.73 m2 | 48.2 ± 10.12 | 46.9 ± 10.97 | 0.01 | 48.2 ± 10.12 | 47.1 ± 11.09 | 0.1 |
Note: Values for categorical variables are given as count (proportion); for continuous variables, as mean ± standard deviation. Matching was done with age, weight, sex, race/ethnicity, and presence of hypertension and/or type 2 diabetes. Conversion factor for serum creatinine in mg/dL to μmol/L, ×88.4.
Abbreviations: BMI, body mass index; eGFR, estimated glomerular filtration rate; Std Diff, standardized difference.
Table 2.
Baseline Characteristics of RYGB and SG Pre- and Post-Propensity Score Matching
| Before Matching | After Matching | |||||
|---|---|---|---|---|---|---|
| RYGB (n = 414) | SG (n = 300) | P | RYGB (n = 234) | SG (n = 234) | Absolute Std Diff | |
| Age, y | 57.5 ± 8.06 | 59.0 ± 8.93 | 0.006 | 58.2 ± 8.19 | 58.7 ± 9.08 | 0.07 |
| Weight, lb | 274.9 ± 52.83 | 262.8 ± 44.32 | 0.01 | 268.0 ± 51.29 | 265.1 ± 45.50 | 0.06 |
| BMI, kg/m2 | 44.7 ± 6.97 | 43.7 ± 6.03 | 0.1 | 44.1 ± 6.68 | 43.7 ± 6.19 | 0.07 |
| Female sex | 304 (73.4%) | 247 (82.3%) | 0.005 | 187 (79.9%) | 186 (79.5%) | 0.01 |
| Race/ethnicity | 0.4 | 0.05 | ||||
| Non-Hispanic white | 224 (54.1%) | 175 (58.3%) | 134 (57.3%) | 140 (59.8%) | ||
| Non-Hispanic black | 75 (18.1%) | 56 (18.7%) | 42 (17.9%) | 40 (17.1%) | ||
| Hispanic | 102 (24.6%) | 58 (19.3%) | 48 (20.5%) | 45 (19.2%) | ||
| Other/missing/unknown | 13 (3.2%) | 11 (3.7%) | 10 (4.3%) | 9 (3.9%) | ||
| Hypertension | 382 (92.3%) | 266 (88.7%) | 0.1 | 206 (88%) | 213 (91%) | 0.1 |
| Diabetes | 324 (78.3%) | 146 (48.7%) | <0.001 | 146 (62.4%) | 146 (62.4%) | 0 |
| Serum creatinine, mg/dL | 1.4 ± 0.43 | 1.4 ± 0.59 | <0.001 | 1.4 ± 0.36 | 1.4 ± 0.63 | 0.1 |
| eGFR, mL/min/1.73 m2 | 47.7 ± 9.67 | 48.9 ± 10.69 | 0.006 | 48.4 ± 9.33 | 48.5 ± 10.95 | 0.01 |
Note: Values for categorical variables are given as frequency (percentage); for continuous variables, as mean ± standard deviation. Matching was done with age, weight, sex, race/ethnicity, and presence of hypertension and/or type 2 diabetes. Conversion factor for serum creatinine in mg/dL to μmol/L, ×88.4.
Abbreviations: BMI, body mass index; eGFR, estimated glomerular filtration rate; RYGB, Roux-en-Y gastric bypass; SG, sleeve gastrectomy; Std Diff, standardized difference.
Median follow-up times were 3.0 years and 3.5 years for the surgery patients and nonsurgery controls, respectively, with 82% and 87% having at least one serum creatinine measurement in the third year following surgery or referral to surgery. Readmission rates in 30 days after surgery for RYGB (8%) and SG (7%) patients were similar to those for nonsurgery controls (7.5%) within 30 days of referral to surgery.
Median follow-up times for RYGB and SG patients were 3.1 and 2.6 years, respectively, with 84% and 79% having at least one serum creatinine measurement in the third year following surgery. Follow-up time for SG was shorter because it was not performed until 2010. Nonsurgery control patients had a significantly greater number of serum creatinine measurements per year than surgery patients (3.2 and 2.6, respectively; P < 0.001). This was not a clinically meaningful difference.
Bariatric Surgery Patients Compared With Controls
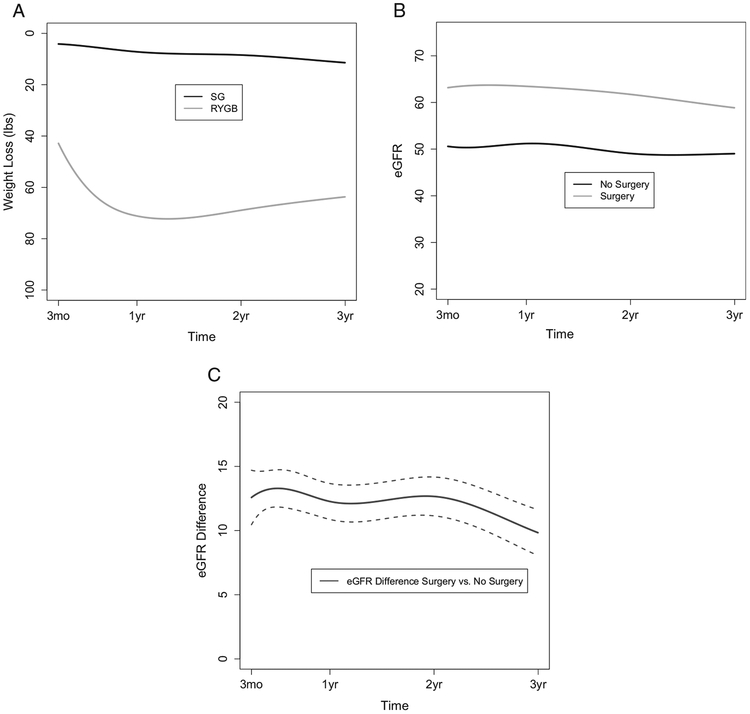
Trajectories for weight loss, eGFR, and the differences between surgery patients and nonsurgery controls in eGFR are shown in Fig 2A to C. Weight loss was negligible for control patients and peak weight loss for surgery patients occurred 12.6 months after surgery. Figure 2B shows eGFR trajectories over time for the 2 groups of patients. At 3 months postsurgery, the mean eGFR of surgery patients increased from 48.2 mL/min/1.73 m2 at baseline (Table 1) to 63.2 mL/min/1.73 m2, which was maintained through year 2 and decreased slightly to 58.9 mL/min/1.73 m2 in the third year of follow-up. Meanwhile, the mean eGFR of nonsurgery control patients changed from 47.1 to 49.0 mL/min/1.73 m2 during this same period.
Figure 2.
Three-year outcomes for (A) weight loss, (B) estimated glomerular filtration rate (eGFR), and (C) eGFR difference in bariatric surgery patients and nonsurgery control patients. Dotted lines in (C) indicate 95% confidence intervals. Abbreviations: RYGB, Roux-en-Y gastric bypass; SG, sleeve gastrectomy.
Figure 2C and Table 3 provide results for statistical comparisons between surgery patients and nonsurgery controls in mean eGFR difference at each follow-up time. At 3 months, the difference in average eGFRs between surgery patients and controls was significant (12.58 [95% CI, 10.46–4.70] mL/min/1.73 m2). This difference remained relatively constant with a difference of 12.66 (95% CI, 11.15–14.17) mL/min/1.73 m2 at 2 years, at which point average eGFR began to decrease at a slightly faster rate for surgery patients when compared with nonsurgery controls into the third year of follow-up. Thus the difference between surgery patients and controls at 3 years was still significant but somewhat attenuated (9.84 [95% CI, 8.05–11.62] mL/min/1.73 m2).
Table 3.
Statistical Comparisons for Differences in Mean eGFR at Various Time Points After Bariatric Surgery/Referral to Bariatric Surgery
| Time Postsurgery | Surgery vs Control | RYGB vs SG | ||||||
|---|---|---|---|---|---|---|---|---|
| Unadj Estimate (95% CI) | P | Adj Estimate (95% CI) | P | Unadj Estimate (95% CI) | P | Adj Estimate (95% CI) | P | |
| 3 mo | 13.92 (11.42–16.41) | <0.001 | 12.58 (10.46–14.7) | <0.001 | 3.65 (-0.74–8.04) | 0.1 | 4.22 (0.49–7.95) | 0.03 |
| 6 mo | 14.54 (12.59–16.49) | <0.001 | 13.29 (11.84–14.74) | <0.001 | 4.47 (1.05–7.89) | 0.01 | 4.75 (2.21–7.29) | <0.001 |
| 12 mo | 13.48 (11.56–15.39) | <0.001 | 12.27 (10.87–13.67) | <0.001 | 4.68 (1.28–8.08) | 0.007 | 5.03 (2.53–7.54) | <0.001 |
| 24 mo | 13.86 (11.86–15.87) | <0.001 | 12.66 (11.15–14.17) | <0.001 | 7.21 (3.67–10.75) | <0.001 | 7.52 (4.84–10.2) | <0.001 |
| 36 mo | 11.00 (8.78–13.23) | <0.001 | 9.84 (8.05–11.62) | <0.001 | 6.36 (2.41–10.3) | 0.002 | 6.6 (3.42–9.78) | <0.001 |
Note: Groups that are compared are propensity-matched bariatric surgery patients and nonsurgery controls and propensity-matched RYGB and SG patients. Results are presented as unadjusted first and then adjusted for the following covariates: baseline eGFR, age, weight, sex, race/ethnicity, and presence of hypertension and/or type 2 diabetes.
Abbreviations: Adj, adjusted; CI, confidence interval: eGFR, estimated glomerular filtration rate; RYGB, Roux-en-Y gastric bypass; SG, sleeve gastrectomy; Unadj, unadjusted.
Differences Between Procedures
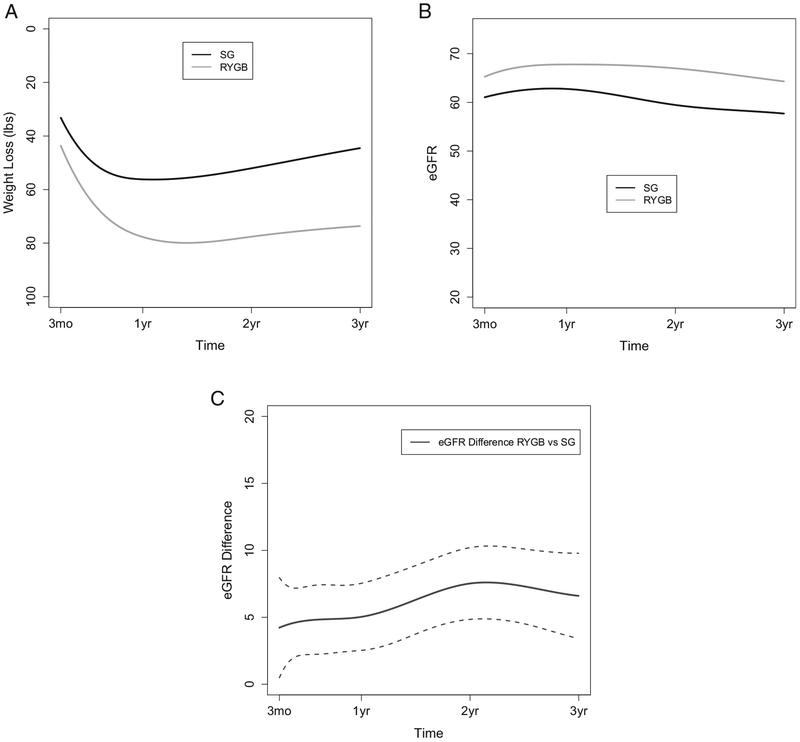
Trajectories for weight loss, eGFR, and the differences between surgical groups in eGFRs during the 3-year follow-up period are shown in Fig 3A to C. Weight loss was greater throughout the follow-up period for RYGB as compared to SG, with peak weight loss occurring at 13.6 and 10.2 months, respectively. Figure 3B shows eGFR trajectories over time for RYGB and SG patients. At 3 months postsurgery, RYGB patients’ mean eGFR had increased from 48.4 mL/min/1.73 m2 at baseline (Table 2) to 65.3 mL/min/1.73 m2, which was maintained throughout the 3-year follow-up period (64.3 mL/min/1.73m2 at 3 years). Although SG patients also had a large change in mean eGFR from baseline (48.5 mL/min/1.73 m2; Table 2) to 3 months postsurgery (61.0 mL/min/1.73 m2), they had a decline in eGFR by the end of the follow-up period (57.7 mL/min/1.73 m2 at 3 years).
Figure 3.
Three-year outcomes for (A) weight loss, (B) estimated glomerular filtration rate (eGFR), and (C) eGFR difference in Roux-en-Y gastric bypass (RYGB) and sleeve gastrectomy (SG) patients. Dotted lines in (C) indicate 95% confidence intervals.
Figure 2C and Table 3 provide results for statistical comparisons between RYGB and SG patients in eGFR mean difference at each follow-up time. At 3 months, there was a significant difference in eGFRs between the RYGB and SG groups (4.22 [95% CI, 0.49–7.95] mL/min/1.73 m2). This difference remained relatively constant for the first year following surgery and then began to increase over the following 2 years, with a significant difference of 6.60 (95% CI, 3.42–9.78) mL/min/1.73 m2 between RYGB and SG patients at 3 years.
DISCUSSION
In one of the largest studies to date regarding the effect of bariatric surgery specific to patients with CKD stages 3 and 4, we found that patients undergoing bariatric surgery experienced subsequent increases in eGFRs when compared with those not having bariatric surgery over a 3-year period. We also found that RYGB was associated with a greater effect on kidney function as measured by eGFR when compared to SG, the fastest growing procedure in the United States.18 The decline in eGFR seen over the long term in all groups (Fig 2) is close to the expected age-related decline in all adults of ~ 1 mL/min/1.73 m2 per year. For every 10-lb weight loss, there was a corresponding increase in eGFR of 0.21 mL/min/1.73 m2, which was greatest in RYGB patients (0.44-unit improvement in eGFR with every 10-lb weight loss).
It is difficult to compare our findings with those of the 2 large population-based studies from the United States that were recently published.17,18 Both studies found significant improvements in kidney-related outcomes following bariatric surgery. However, only the study by Chang et al18 used change in eGFR as an outcome. Although the trajectory of eGFR changes was similar between our bariatric surgery patients and theirs, the rate of change was much greater in our surgery patients than theirs in the first year (15 mL/min/1.73 m2 increase compared to 4.6 mL/min/1.73 m2 increase). This difference is likely due to study populations. Only 4.6% of their study population had CKD stage 3 or higher (n = 91 patients total). When they examined eGFR changes for these patients with more advanced kidney disease, it was similar to ours, at 13.8 mL/min/1.73 m2. In addition, their patients were primarily non-Hispanic white (97%) and had much lower rates of type 2 diabetes at baseline (37.8%) when compared with our bariatric surgery population (65.8%). The findings of our study can be applied to patients with a higher burden of disease (type 2 diabetes, hypertension, and CKD stages 3 and 4) at the time of surgery and to ethnic/racial minority patients (>40% Hispanic or non-Hispanic black).
There was a large difference in eGFRs between bariatric surgery patients and nonsurgery control patients in the period of the first year after surgery (due to increases in eGFRs for surgery patients and decreases in controls), which began to diminish after the first year, primarily due to a more rapid decline in eGFRs for the surgery patients during the third year of follow-up (Fig 3A–C). Chang et al18 saw a similar pattern for their surgery and nonsurgery control patients. The larger difference in the period of the first year after surgery between surgery patients and controls may be due to a number of acute factors resulting from massive weight loss (peak weight loss for RYGB and GS was at ~ 1 year after surgery), none of which we could measure in this study. These might include a decrease in serum creatinine level due to the muscle loss that accompanies massive weight loss (and thus eGFR would appear to greatly increase),14 rapid improvement in levels of inflammatory markers,14,41 and changes in glomerular he-modynamics.42 To avoid the confounding nature of profound changes in body composition during the initial period of weight loss following surgery (12 months) in the interpretation of changes in serum creatinine levels, it may be best to begin the study of bariatric surgery and CKD after the first year of weight loss.
There are a number of other limitations with our study. One of the largest limitations is use of an observational study design. Although we used sophisticated analyses to match bariatric surgery patients to nonsurgery control patients, this does not take the place of random assignment in accounting for all measured and unmeasured differences between patients who choose to have or not have surgery or who choose to have one procedure versus another. Systematic randomized trials, such as those done for diabetes,43 should be done for kidney function to conclude that bariatric surgery caused the improvements in eGFRs that we saw with our study. Ideally, GFR would be directly measured in these studies as well as estimated to clarify the use of estimating equations in future research.
Another limitation of our study was that we examined the impact of surgery on eGFR and not other important indicators of kidney function such as proteinuria or progression to ESRD. In a recent study by Friedman et al,34 in which GFR estimating equations were specifically tested for bariatric surgery patients, the most accurate estimates were obtained by using a modified CKD-EPI equation with serum creatinine and cystatin C levels. Cystatin C level is not routinely collected in our health care system and thus our estimating equation was not the most accurate estimate of “true” GFR in this population.
Finally, our patients were members of an integrated health care system, with insurance coverage for bariatric surgery and access to comprehensive health care. This limits the generalizability of our findings to patients without this access. However, to this point, it could be argued that KPSC may represent the future of health care as systems move toward the integrated medical care and electronic medical and billing record systems that are required by the Affordable Care Act. In addition, KPSC already has the bariatric surgery patient profile we will see in the next 5 to 10 years as the United States becomes more racially/ethnically diverse and bariatric practice shifts strongly toward SG. Racial/ethnic minorities are disproportionately affected by severe obesity and CKD41 and might benefit most from bariatric surgery.
The burden of obesity-related kidney disease is likely to increase in the coming decades, creating an epidemic. It has been estimated that 24.2% of kidney disease cases in the United States among men and 33.9% among women could be prevented if overweight and obesity were eliminated.5 In this study, bariatric surgery, particularly RYGB, was associated with considerable improvements in eGFRs during a median 3 years for patients with severe obesity and CKD stages 3 and 4. In cases in which lifestyle modification and medical management fails, consideration should be given to bariatric surgery to slow the progression of kidney damage for patients with a high comorbid condition burden and more advanced CKD.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Ms Grace Johnston, MBA, for library support.
Support: This research was supported with a grant from the Regional Research Committee of KPSC. The funder did not have any role in study design; collection, analysis, and interpretation of data; writing the report; or the decision to submit the report for publication.
Financial Disclosures: The authors declare that they have no other relevant financial interests.
Contributions: Research area and study design: THI, KJC, SH, SFD; analyses and reporting of results: RB, BJ, HF. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. THI and KJC take responsibility that this study has been reported honestly, accurately, and transparently; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Peer Review: Evaluated by 2 external peer reviewers, a Statistical Editor, a Co-Editor, and Editor-in-Chief Levey.
Footnotes
SUPPLEMENTARY MATERIAL
Figure S1: Distribution of propensity scores across matched and unmatched groups.
Note: The supplementary material accompanying this article (http://dx.doi.org/10.1053/j.ajkd.2016.09.020) is available at www.ajkd.org
REFERENCES
- 1.Division of Nutrition, Physical Activity, and Obesity, Centers for Disease Control and Prevention (CDC). Adult obesity facts. http://www.cdc.gov/obesity/data/adult.html. Accessed January 5, 2015.
- 2.World Health Organization. Mortality and burden of disease attributable to selected major risks. http://www.who.int/healthinfo/global_burden_disease/GlobalHealthRisks_report_full.pdf?ua=1&ua=1. Accessed January 5, 2015.
- 3.Wang Y, Chen X, Song Y, et al. Association between obesity and kidney disease: a systematic review and meta-analysis. Kidney Int. 2008;73(1):19–33. [DOI] [PubMed] [Google Scholar]
- 4.US Renal Data System. Chapter 11: costs of ESRD. http://www.usrds.org/2012/view/v2_11.aspx. Accessed January 5, 2015 [Google Scholar]
- 5.Saiki A, Nagayama D, Ohhira M, et al. Effect of weight loss using formula diet on renal function in obese patients with diabetic nephropathy. Int J Obes (Lond). 2005;29(9):1115–1120. [DOI] [PubMed] [Google Scholar]
- 6.Friedman AN, Chambers M, Kamendulis LM, Temmerman J. Short-term changes after a weight reduction intervention in advanced diabetic nephropathy. Clin J Am Soc Nephrol. 2013;8(11):1892–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morales E, Valero MA, Leon M, Hernandez E, Praga M. Beneficial effects of weight loss in overweight patients with chronic proteinuric nephropathies. Am J Kidney Dis. 2003;41(2): 319–327. [DOI] [PubMed] [Google Scholar]
- 8.Loveman E, Frampton GK, Shepherd J, et al. The clinical effectiveness and cost-effectiveness of long-term weight management schemes for adults: a systematic review. Health Technol Assess. 2011;15(2):1–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ribaric G, Buchwald JN, McGlennon TW. Diabetes and weight in comparative studies of bariatric surgery vs conventional medical therapy: a systematic review and meta-analysis. Obes Surg. 2014;24(3):437–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abou-Mrad RM, Abu-Alfa AK, Ziyadeh FN. Effects of weight reduction regimens and bariatric surgery on chronic kidney disease in obese patients. Am J Physiol Renal Physiol. 2013;305(5):F613–F617. [DOI] [PubMed] [Google Scholar]
- 11.Bolignano D, Zoccali C. Effects of weight loss on renal function in obese CKD patients: a systematic review. Nephrol Dial Transplant. 2013;28(suppl 4):iv82–iv98. [DOI] [PubMed] [Google Scholar]
- 12.Cohen R, Pechy F, Petry T, Correa JL, Caravatto PP, Tzanno-Martins C. Bariatric and metabolic surgery and micro-vascular complications of type 2 diabetes mellitus. J Bras Nefrol. 2015;37(3):399–409. [DOI] [PubMed] [Google Scholar]
- 13.Friedman AN, Wolfe B. Is bariatric surgery an effective treatment for type II diabetic kidney disease? Clin J Am Soc Nephrol. 2016;11(3):528–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neff KJ, Frankel AH, Tam FWK, Sadlier DM, Godson C, le Roux CW. The effect of bariatric surgery on renal function and disease: a focus on outcomes and inflammation. Nephrol Dial Transplant. 2013;28(suppl 4):iv73–iv82. [DOI] [PubMed] [Google Scholar]
- 15.Zhou X, Ling L, Kwong JSW, Yu J, Li Y, Sun X. Impact of bariatric surgery on renal functions in patients with type 2 diabetes: systematic review of randomized trials and observational studies [published online ahead of print May 5, 2016]. Surg Obes Relat Disord. http://dx.doi.org/10.1016/j.soard.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 16.Carlsson LM, Romeo S, Jacobson P, et al. The incidence of albuminuria after bariatric surgery and usual care in Swedish Obese Subjects (SOS): a prospective controlled intervention trial. Int J Obes (Lond). 2015;39(1):169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson BL, Blackhurst DW, Latham BB, et al. Bariatric surgery is associated with a reduction in major macrovascular and microvascular complications in moderately to severely obese patients with type 2 diabetes mellitus. J Am Coll Surg. 2013;216(4): 545–556. [DOI] [PubMed] [Google Scholar]
- 18.Chang AR, Chen Y, Still C, et al. Bariatric surgery is associated with improvement in kidney outcomes. Kidney Int. 2016;90(1):164–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ponce J, Nguyen NT, Hutter M, Sudan R, Morton JM. American Society for Metabolic and Bariatric Surgery estimation of bariatric surgery procedures in the United States, 2011–2014. Surg Obes Relat Dis. 2015;11(6):1199–1200. [DOI] [PubMed] [Google Scholar]
- 20.Coleman KJ, Huang YC, Koebnick C, et al. Metabolic syndrome is less likely to resolve in Hispanics and non-Hispanic blacks after bariatric surgery. Ann Surg. 2014;259(2):279–285. [DOI] [PubMed] [Google Scholar]
- 21.Coleman KJ, Huang YC, Hendee F, Watson HL, Casillas RA, Brookey J. Three-year weight outcomes from a bariatric surgery registry in a large integrated healthcare system. Surg Obes Relat Dis. 2014;10(3):396–403. [DOI] [PubMed] [Google Scholar]
- 22.DeMaria EJ, Pate V, Warthen M, Winegar DA. Baseline data from American Society for Metabolic and Bariatric Surgery-designated Bariatric Surgery Centers of Excellence using the Bariatric Outcomes Longitudinal Database. Surg Obes Relat Dis. 2010;6(4):347–355. [DOI] [PubMed] [Google Scholar]
- 23.Hutter MM, Schirmer BD, Jones DB, et al. First report from the American College of Surgeons Bariatric Surgery Center Network: laparoscopic sleeve gastrectomy has morbidity and effectiveness positioned between the band and the bypass. Ann Surg. 2011;254(3):410–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification and stratification. Kidney Disease Outcome Quality Initiative. Am J Kidney Dis. 2002;39(2)(suppl 1):S1–S246. [PubMed] [Google Scholar]
- 25.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–470. [DOI] [PubMed] [Google Scholar]
- 26.Levey AS, Greene T, Kusek JW, et al. A simplified equation to predict glomerular filtration rate from serum creatinine [abstract]. J Am Soc Nephrol. 2000;11:155A. [Google Scholar]
- 27.Bostom AG, Kronenberg F, Ritz E. Predictive performance of renal function equations for patients with chronic kidney disease and normal serum creatinine levels. J Am Soc Nephrol. 2002;13(8):2140–2144. [DOI] [PubMed] [Google Scholar]
- 28.Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139(2):137–147. [DOI] [PubMed] [Google Scholar]
- 29.Rule AD, Larson TS, Bergstralh EJ, Slezak JM, Jacobsen SJ, Cosio FG. Using serum creatinine to estimate glomerular filtration rate: accuracy in good health and in chronic kidney disease. Ann Intern Med. 2004;141(12):929–937. [DOI] [PubMed] [Google Scholar]
- 30.Rule AD, Rodeheffer RJ, Larson TS, et al. Limitations of estimating glomerular filtration rate from serum creatinine in the general population. Mayo Clin Proc. 2006;81(11):1427–1434. [DOI] [PubMed] [Google Scholar]
- 31.Rule AD, Jacobsen SJ, Schwartz GL, et al. A comparison of serum creatinine-based methods for identifying chronic kidney disease in hypertensive individuals and their siblings. Am J Hypertens. 2006;19(6):608–614. [DOI] [PubMed] [Google Scholar]
- 32.Stevens LA, Claybon MA, Schmid CH, et al. Evaluation of the Chronic Kidney Disease Epidemiology Collaboration equation for estimating the glomerular filtration rate in multiple ethnicities. Kidney Int. 2011;79(5):555–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsushita K, Mahmoodi BK, Woodward M, et al. Comparison of risk prediction using the CKD-EPI equation and the MDRD Study equation for estimated glomerular filtration rate. JAMA. 2012;307(18):1941–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Friedman AN, Moe S, Fadel WF, et al. Predicting the glomerular filtration rate in bariatric surgery patients. Am J Nephrol. 2014;39:8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang D Dalton JE. A unified approach to measuring the effect size between two groups using SAS. In: Proceedings of the SAS® Global Forum Conference Cary, NC: SAS Institute Inc. 2012:335–2012. [Google Scholar]
- 38.Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis. Hoboken, NJ: John Wiley & Sons, Inc; 2011. [Google Scholar]
- 39.Huppler-Hullsiek K, Louis T. Propensity score modeling strategies for the causal analysis of observational data. Biostatistics. 2002;3:179–193. [DOI] [PubMed] [Google Scholar]
- 40.Bang H, Robins J. Doubly robust estimation in missing data and causal inference models. Biometrics. 2005;61:692–972. [DOI] [PubMed] [Google Scholar]
- 41.Fenske WK, Dubb S, Bueter M, et al. Effect of bariatric surgery-induced weight loss on renal and systematic inflammation and blood pressure: a 12-month prospective study. Surg Obes Relat Dis 2013;9(4):559–568. [DOI] [PubMed] [Google Scholar]
- 42.Navaneethan SD, Yehnert H. Bariatric surgery and progression of chronic kidney disease. Surg Obes Relat Dis. 2009;5(6):662–665. [DOI] [PubMed] [Google Scholar]
- 43.Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes-3-year outcomes. N Engl J Med. 2014;370(21):2002–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.