Abstract
In this work, we clarified the role of acquisition parameters and quantification methods in myocardial blood flow (MBF) estimability for myocardial perfusion imaging using CT (MPI-CT). We used a physiologic model with a CT simulator to generate time-attenuation curves across a range of imaging conditions, i.e. tube current-time product, imaging duration, and temporal sampling, and physiologic conditions, i.e. MBF and arterial input function width. We assessed MBF estimability by precision (interquartile range of MBF estimates) and bias (difference between median MBF estimate and reference MBF) for multiple quantification methods. Methods included: six existing model-based deconvolution models, such as the plug-flow tissue uptake model (PTU), Fermi function model, and single-compartment model (SCM); two proposed robust physiologic models (RPM1, RPM2); model-independent singular value decomposition with Tikhonov regularization determined by the L-curve criterion (LSVD); and maximum upslope (MUP). Simulations show that MBF estimability is most affected by changes in imaging duration for model-based methods and by changes in tube current-time product and sampling interval for model-independent methods. Models with three parameters, i.e. RPM1, RPM2, and SCM, gave least biased and most precise MBF estimates. The average relative bias (precision) for RPM1, RPM2, and SCM was ≤11% (≤10%) and the models produced high-quality MBF maps in CT simulated phantom data as well as in a porcine model of coronary artery stenosis. In terms of precision, the methods ranked best-to-worst are: RPM1 > RPM2 > Fermi > SCM > LSVD > MUP >> other methods. In terms of bias, the models ranked best-to-worst are: SCM > RPM2 > RPM1 > PTU > LSVD >> other methods. Models with four or more parameters, particularly 5-parameter models, had very poor precision (as much as 310% uncertainty) and/or significant bias (as much as 493%) and were sensitive to parameter initialization, thus suggesting the presence of multiple local minima. For improved estimates of MBF from MPI-CT, it is recommended to use reduced models that incorporate prior knowledge of physiology and contrast agent uptake, such as the proposed RPM1 and RPM2 models.
Keywords: myocardial perfusion, physiologic modeling, cardiac CT, parameter estimation, estimability, image analysis, system identification
Introduction
In the coronary artery disease (CAD) workup, noninvasive tests are recommended to guide diagnosis, risk stratification, and management plans (Fraker and Fihn 2007, Montalescot et al 2013). However, current noninvasive tests show a low positive predictive value for CAD. In a study of patients with stable angina, atypical chest pain, or no symptoms, nearly one-third of patients with a high-risk finding from noninvasive testing showed no obstructive disease in follow-up invasive coronary angiography (ICA) (Patel et al 2014). In the same report, more than half of the patients with low- or intermediate-risk noninvasive test results were found to have no obstructive disease on ICA. These shortcomings highlight the need for a gatekeeper exam that is more effective than existing strategies.
Myocardial perfusion imaging (MPI) is a noninvasive test that can be performed by different modalities, each with advantages and disadvantages, to detect the physiologic evidence of clinically significant ischemia. Nuclear MPI techniques include single photon emission computed tomography (SPECT) and positron emission tomography (PET), with SPECT being more widely available and most commonly used (Duvall et al 2015). MPI-SPECT is often interpreted in terms of relative radiotracer uptake where regions of low signal are suggestive of disease. Despite advancements in SPECT imaging, such as ECG-gating, hybrid SPECT/CT, and improved count sensitivity (Piccinelli and Garcia 2016), PET is generally considered a superior modality for MPI (Ardle et al 2012). PET is currently the preferred modality for noninvasive perfusion quantification with the potential to assess multi-vessel disease (Desiderio et al 2018). However, major disadvantages of PET are cost and limited availability. Another option for MPI is magnetic resonance imaging (MRI) which has better spatial resolution and diagnostic accuracy than SPECT (Chung et al 2010), can provide quantitative perfusion assessment (Schwab et al 2015), and does not expose patients to ionizing radiation. However, lengthy imaging time, operator dependency, limited availability, patient contraindications due to implants or other factors, and cost have prevented MRI from becoming the standard MPI modality (Varga-Szemes et al 2015).
CT has great potential for noninvasive assessment of CAD as it can rapidly and reliably image the coronary arteries, provide measurements of myocardial perfusion (Rochitte et al 2014), and is more widely available than other imaging modalities (Eurostat 2017). Coronary CT angiography can even be used to measure a CT-derived fractional flow reserve (FFRCT, such as from HeartFlow (Min et al 2015)), although this gives no indication of absolute myocardial blood flow. Whereas single-shot MPI-CT provides relative, qualitative perfusion evaluation suitable for assessment of single-vessel disease, dynamic MPI-CT can be used to quantitatively assess perfusion. Quantitative perfusion evaluation using MPI-CT could serve a similar role as MPI-PET in the assessment of single- and multi-vessel CAD and potentially also in the assessment of microvascular disease (MVD), something not assessed by FFRCT. The combination of MPI-CT and CT angiography could make CT an ideal gatekeeper to ICA.
Interest in MPI-CT has grown as scanning-related technical challenges have been addressed. Some scanners now have the coverage to acquire whole-heart 3D images in a single heartbeat (Hubbard et al 2016). Challenges with spatial mis-registration have been addressed via the use of registration and spatio-temporal filtering (Huber et al 2013, Muenzel et al 2013, Isola et al 2010, Fahmi et al 2014). Fast rotation speeds and ECG-gating mitigate reconstruction artifacts (Barrett and Keat 2004, Ritchie et al 1992). Energy-sensitive CT minimizes beam hardening artifacts (So et al 2011, Fahmi et al 2016) which can be misconstrued as perfusion defects and lead to false positives. Iterative model-based reconstructions can significantly reduce image noise and enable low x-ray dose protocols (Beister et al 2012, Eck et al 2015, Gramer et al 2012). The current technical landscape creates the potential for CT to become a standard platform for MPI.
A number of techniques exist to quantify myocardial blood flow (MBF) from dynamic, contrast-enhanced scans (Lee 2002). The three categories are: upslope-based methods, model-independent deconvolution methods, and model-based methods. The maximum-upslope method (Peters et al 1987) assumes no contrast agent efflux and thus typically underestimates MBF (Bindschadler et al 2014). Recently, a first pass analysis (FPA) technique has been reported which sums Hounsfield Unit (HU) values over large myocardial regions and assesses contrast agent flux over a short time scale (Hubbard et al 2016, Ziemer et al 2015), thereby improving MBF estimates compared to maximum-upslope. While promising, FPA requires whole-heart coverage scanners and likely depends upon the two image frames chosen for analysis. These upslope-based methods do not capture other tissue properties likely involved in CAD and MVD (i.e., blood volume and vascular permeability). Deconvolution enables one to obtain the tissue impulse response function (IRF) and derive blood flow, mean transit time, and blood volume (Lee 2002). Model-independent deconvolution, such as Tikhonov-regularized singular value decomposition (SVD), mitigates the need for explicit physiologic assumptions but requires careful selection of regularization parameters (Fieselmann et al 2011). However, IRFs produced by SVD can exhibit non-physiologic properties and can significantly bias MBF (Eck et al 2016). Alternatively, model-based flow estimation methods can incorporate model constraints and prior knowledge to address these problems.
The variability in parameter estimates across different processing methods highlights the need to assess techniques. Different algorithms, different implementations of the same algorithm, different commercial software applications, and even upgrades to the same software can yield significantly different quantitative estimates from the same data (Kudo et al 2013, Goh et al 2011, 2007, Eck et al 2016). When deriving MBF from parametric models, a recurring challenge is that of model selection which must consider a number of factors such as data quality, administered contrast agent, and underlying physiologic processes. Reports have described such considerations from theoretical and practical perspectives (Sourbron and Buckley 2012, Schwab et al 2015, Kershaw and Cheng 2010, Luypaert et al 2011, Jerosch-Herold et al 1998). MBF, and other parameters, are estimable if values can be precisely and accurately estimated in the presence of noise. In MRI, a two-compartment exchange model and a two-compartment uptake model have been ruled out due to poor MBF estimability (Schwab et al 2015). Similar studies have ruled out complex models in PET in favor of robust, reduced models (Coxson et al 1997). For MPI-CT, only recently have flow estimation models been compared for appropriateness. In one report (Bindschadler et al 2014), authors evaluated three models: a two-compartment model, an axially-distributed model, and the adiabatic approximation to the tissue homogeneity model. Authors observed similar precision and bias of MBF estimates between models over a range of dose and temporal sampling conditions. Authors concluded that there was no particular advantage between the three models. However, there were important limitations in this study that influence MBF estimability: a single imaging duration was used (30 s) which can vary in clinical practice, residual beam hardening artifacts may have strongly influenced MBF estimates which can be mitigated by dual-energy imaging (So et al 2011, Fahmi et al 2016), and the tested models had similar, limited parameterizations. Further investigation into MPI-CT acquisition and quantification methods can help to elucidate advantages and pitfalls, thus leading to more robust assessment than previous techniques.
In this study, we examine the role of MPI-CT acquisition conditions and quantification methods in MBF estimability, as assessed by precision and bias. We include new robust physiologic models that incorporate prior knowledge of microvasculature and contrast agent extraction, develop a novel semi-analytic model implementation which mitigates parameter estimation problems that arise due to numerical errors, improve upon a physiologic simulator from the literature, and assess a range of acquisition conditions relevant to MPI-CT. Our principal approach is to use a model-to-models test whereby we generate time-attenuation curves (TACs) from a physiologic model and CT simulator and then determine the ability of multiple computational methods to estimate the known simulated flow. A preliminary report indicated that some methods can significantly overestimate flow values (Eck et al 2016), leading us to this more thorough examination of MBF estimability. As patient x-ray dose is an issue in CT, we investigated relevant acquisition parameters including x-ray tube current-time product, sampling interval, and scan duration. Understanding the role of acquisition parameters and MBF quantification methods can guide the development of low-dose scanning protocols as well as processing for robust and accurate MPI.
Computational Methods for Myocardial Blood Flow Estimation
Model-based MBF estimation
We evaluate eight perfusion models: six from the literature and two newly proposed. We use the term “MBF” to refer to the amount of blood flow to a mass of myocardial tissue, given in units of mL/min-100g. MBF is estimated by iteratively optimizing parameters until an estimated model time-concentration curve best fits the measured tissue time-concentration curve in the least squares sense. The model time-concentration curve, Cm(t) (mg/mL), is calculated by convolution of a measured arterial input function (AIF), Ca(t) (mg/mL), with the unit-less model impulse response function (IRF) scaled by MBF, RMBF(t) (mL/min-100g), and multiplied by a tissue density scaling, ρ (g/mL). Note that HU values in MPI-CT change linearly with contrast agent concentration (Fahmi et al 2016). HU values from measured TACs are used as a surrogate measure for contrast agent concentration after subtraction of a pre-contrast baseline value. Mathematically, the convolution is given by the integral:
| (1) |
where ti is the time of a sampled data point ranging from time index i=1,2,…, N for N scans. We used ρ=1.05 g/mL (Vinnakota and Bassingthwaighte 2004).
We briefly describe the eight tested perfusion models for RMBF(t). Models 1–7 can be described as reduced forms of two-region, intravascular-extravascular contrast agent exchange models (Figure 1) in which both regions contribute to the CT signal. In the adiabatic approximation to the tissue homogeneity model (AATH), contrast agent, also referred to as indicator, enters the intravascular compartment (red) after time delay, td, from the AIF. Assuming plug flow, contrast agent moves through the region in infinitesimally small, discrete increments from left to right with characteristic intravascular transit time (ITT) related to MBF and vb, ITT=vb/MBF, in units of mL/g and s, respectively. Before leaving the intravascular space, a proportion of contrast agent, described by the extraction fraction, E, extravasates into the well-mixed extravascular space. The remaining intravascular contrast agent portion then leaves the observation region. The extravascular contrast agent then exchanges back into the intravascular region and washes out with characteristic decay constant, k=(E∙MBF)/ve in units of s−1, where ve is the extravascular volume in units of mL/g. An alternative two-region model, the two-compartment exchange model (2CXM), consists of two well-mixed regions with exchange governed by the permeability-surface area product (PS, units of mL/min-g). Simplifications of the two-region models lead to the other models tested. These include: (A) Contrast agent does not wash out of the extravascular space, giving the plug-flow tissue uptake model (AATH → PTU) and compartmental tissue uptake model (2CXM → CTU); (B) Physiologic constraints on ITT and E give proposed robust physiologic models (AATH → RPM1, RPM2); (C) The observation region is a single, well-mixed region, giving the single-compartment model (2CXM → SCM). Impulse response models and free parameters are listed in Table 3 and described in the Appendix.
Figure 1.
Conceptual representations of the AATH and 2CXM models (top-left, bottom-left) and their corresponding impulse response functions (top-right, bottom-right). In the AATH model (top-left), contrast agent passes into and through the intravascular space (red), exchanges with the well-mixed extravascular space (blue), and exits the observation region (grey) giving rise to an impulse response function (top-right) with a box-like intravascular portion and exponential decay extravascular portion. In the 2CXM model (bottom-left), the intravascular compartment is well-mixed and exchanges with the extravascular space according to the rate described by PS which gives rise to an impulse response that is the sum of two exponentials (bottom-right). The y-axes of the impulse response function plots denote normalized indicator concentration scaled by MBF which has units of mL/min-100g. This conceptual representation is adapted from (Sourbron and Buckley 2012).
Table 3.
Impulse response models and free parameters.
| Model | Free parameters |
|---|---|
| AATH | td, MBF, ITT, E, k |
| 2CXM | td, A, B, k+, k− |
| PTU | td, MBF, ITT, E |
| CTU | td, MBF, E, k |
| RPM1 | td, MBF, k |
| RPM2 | td, MBF, k |
| SCM | td, MBF, k |
| Fermi | td, MBF, t0, k |
Model-independent MBF estimation
We applied two model-independent techniques from the literature, singular value decomposition (SVD) and maximum-upslope (MUP), giving a total of ten quantitative methods evaluated in this work. The SVD method has been previously applied to MPI-CT and uses Tikhonov regularization where the regularization factor is determined by the L-curve criterion (LSVD) (Fahmi et al 2016). We use an established method to estimate the maximum-upslope which has been applied to MPI-CT (Bindschadler et al 2014). More details are in the Appendix.
Implementing the impulse response models
For all impulse response models, the convolution was implemented semi-analytically. This approach minimizes numerical errors which can appear when using other methods such as discrete convolution. The AIF is expressed as a piecewise polynomial, i.e. a sequence of polynomials defined for adjacent time intervals to pass through the measured data values (De Boor 1978). Model output is a sum of the convolution integrals, one for each polynomial piece. Each convolution integral is calculated by analytically integrating the product of the polynomial and the IRF for a selected model. Parameter estimation was performed using an unweighted sum-of-squared difference objective function and gradient-based optimization algorithm with analytically determined gradients. Implementation details are in the Appendix.
Experimental Methods
Physiologic simulator
The physiologic simulator was adopted from the literature (Bindschadler et al 2014). It simulates the concentration-time dynamics of a contrast agent as it passes through the large blood pools in the heart and eventually through the myocardial tissue. The simulator assumes constant flow and includes dispersion and delay between large blood pools. The input to the simulator is the iodine concentration in the right atrium modeled as a gamma-variate function (see Figure 2). Downstream toward the myocardium, large blood pools, i.e. the right ventricle, left atrium, left ventricle, and coronary artery, exhibit delay and dispersion as contrast agent passes from one blood pool to another. Within the myocardium there are 20 flow-paths, each containing an arteriole, capillary, and venule, and with different flow rates ranging from 0.05 to three times the mean flow to the myocardium, as sampled from a lagged normal density curve. Capillary permeability to the contrast agent is flow-dependent, as previously described (Appendix of Bindschadler et al 2014). This generalized physiologic simulator can generate data with dispersive effects, time delays, varied cardiac output, varied coronary blood flow, and varied MBF.
Figure 2.
Comparison of in vivo porcine TAC measurements to the standard and modified physiologic simulator. (a) CT image with MBF map overlay from RPM1 is shown for a pig with partial balloon-induced LAD coronary artery occlusion (FFR=0.59). Large ROIs are drawn in the LV cavity, normal, and flow-deprived myocardia in (a), giving AIF and myocardial TACs, respectively in (b). With MBF∝MBV2, the modified physiologic simulator generates TACs similar to measured porcine data, as shown in (c), with the exception of recirculation effects. With the standard simulator having fixed MBV, there is little observed change between TACs at significantly different MBF levels (d). MBF is in units of mL/min-100g. TACs are shown after subtraction of baseline HU values.
We modified the simulator above to account for changes in vascular volume at different perfusion levels. It is known that MBF is mediated by changes in vascular tone and resistance, with dilated vessels leading to higher MBF; for example, it has been observed that MBF is proportional to the square of myocardial blood volume (MBV) (Wu et al 1992). We include this relationship to adjust arteriole, capillary, and venule volumes. We changed capillary volume to , arteriole volume to , and venule volume to Proportionality constants were chosen such that at MBF=100 mL/min-100g we obtained the same values as in (Bindschadler et al 2014) i.e., Vart= 0.05 mL/g Vcap=0.016 mL/g and Vven= 0.06mL/g. This provides βart= 0.005, βcap= 0.0016, and βven= 0.006, all with units .
The concentration of the contrast agent in the right ventricle (RV) was driven by a gamma-variate function similar to the observed pig data (see Figure 2), except without recirculation:
| (2) |
with parameters set to ymax=270 HU, tpeak=7 s, and td,RV=0 s (Bindschadler et al 2014). The parameter α determines the width of the RV TAC which is varied in AIF simulation experiments. Larger α corresponds to a narrower TAC.
Digital MPI-CT phantom
We created simulated dynamic perfusion data with realistic CT noise using the physiologic simulator described above and a CT simulator, giving what we call the digital MPI-CT phantom. The numerical CT simulator (Radonis software, Philips Research and Development, Hamburg, Germany) has been applied in previous publications (Riviere and Vargas 2008, Hansis and Shao 2011) and models a realistic CT scanner (Brilliance 64, Philips), with cone beam source, finite width detector grid, x-ray pre-filtration, and Poisson noise sampling. In this work, we used only 70 keV photons in the CT simulation to generate a true mono-energetic MPI-CT data set in order to avoid confounding effects of beam-hardening artifacts. Line integrals are computed based on analytic object geometries with defined mass-attenuation coefficients from NIST (Hubbell and Seltzer 1995). The numerical CT simulator accounts for Poisson noise in the projections but does not include x-ray scatter or electronic noise. The digital MPI-CT phantom is anthropomorphic and contains major structures in the field of view, i.e. the left ventricle, right ventricle, myocardium, ribs, and spine. Homogeneous contrast agent dynamics were generated in the right ventricle, left ventricle, descending aorta, and myocardium using the physiologic simulator described above.
Simulation experiments and analysis
MPI-CT simulation experiments were performed to assess the role of acquisition conditions on MBF estimability. Five simulation experiments, with a total of 11 unique data conditions, were performed with independent variables varied as indicated in Table 4. Nominal simulation conditions used an RV TAC α=2.2 (LV FWHM=13 s), 40 s imaging duration, 1 s sampling interval, 100 mAs tube current-time product, and MBF=100 mL/min-100g. The noise level experiments were selected to evaluate the effect of changes in x-ray dose on MBF estimability. The nominal tube current-time product of 100 mAs was selected based on previous work (Fahmi et al 2016). The 50 mAs and 200 mAs tube current-time products led to image SNR values in the LV myocardium at peak enhancement of 8.6 and 17.1, respectively, within the range evaluated in a previous study (Figure 4 of (Eck et al 2015)). Imaging duration conditions were selected to evaluate the range of possible scan durations given that MPI-CT acquisitions are typically ECG-gated at once-per-heartbeat for 30–50 scans. The sampling interval conditions were selected based on potential dose reduction strategies where data could be acquired every-other heartbeat or every-third heartbeat given a 60 beat-per-minute heart rate. The AIF conditions were selected to assess the effect of AIFs with different widths which can vary in patients due to variability in cardiac output or contrast agent injection rates. The simulated MBF conditions were selected to span a range from ischemic (MBF=50 mL/min-100g), to normal at-rest (MBF=100 mL/min-100g), and normal at-stress (MBF=200 mL/min-100g).
Table 4.
Simulation experiments and conditions.
| Simulation Experiment | Conditions (units) |
|---|---|
| Noise level (Tube current-time product) | [50, 100, 200] mAs |
| Imaging duration | T=[30, 40, 50] s |
| Sampling interval | Δt=[1, 2, 3] s |
| Arterial input function width | α=[1.1, 2.2,
4.4] FWHM=[18, 13, 10] s |
| MBF | MBF=[50, 100, 200] mL/min-100g |
The same simulated data and results were used for the underlined conditions to serve as a reference as individual imaging parameters varied.
The full-width at half-max (FWHM) of the left ventricle enhancement curve corresponds to the α value, e.g. α=1.1 has FWHM=18 s.
Figure 4.
Median and interquartile range of the MBF estimates for quantification methods under different acquisition conditions. Each row indicates the effects of change in a given acquisition condition (first: tube current-time product; second: imaging duration; third: sampling interval; fourth: AIF FWHM). For each quantification method, bar heights and error bars depict the median and interquartile range (25% to 75%), respectively, of MBF estimates. The red dashed horizontal lines indicate the known simulated MBF=100 mL/min-100g. MBF precision generally improves as the tube current-time product increases, imaging duration lengthens, and sampling interval shortens. Quantification methods marked with * indicate median and/or 75% MBF estimate (upper error bar) is greater than 200 mL/min-100g. Aside from the condition of interest, nominal simulation parameters were used. MBF is in units of mL/min-100g.
Simulations and analyses were conducted as follows. Under each condition, a digital MPI-CT phantom data set was generated and subsequently processed by the ten quantification methods. LV myocardium was manually segmented once in the digital phantom and the same mask was used for all analyses. Spatial smoothing was applied to voxels within the mask using a 5×5 pixel averaging kernel (3.4×3.4 mm) to reduce noise prior to MBF estimation. Voxels on the edge of the myocardium were not averaged with non-myocardium voxels and were thus somewhat noisier. No temporal smoothing was performed. Each voxel was then processed as an individual TAC. In total, there were 33,605 TACs for this simulation analysis (11 unique conditions × 3,055 voxels per condition).
Perfusion SNR (SNRP) was used to quantify the overall quality of TACs. SNRP for each condition was calculated from the average TAC over all of the myocardium, Imean(t), and the noise standard deviation at the initial time point, σnoise,
| (3) |
For each measured TAC, MBF was estimated by iteratively optimizing model parameters in order to minimize the unweighted least-squares objective function. The function “fmincon,” a gradient-based, interior-point optimizer from the MATLAB Optimization Toolbox (Mathworks.com, 2016), was used to optimize model parameters. The following stopping criteria for the optimizer were used: default first-order optimality tolerance (“OptimalityTolerance”=1e-6), default constraint tolerance (“ConstraintTolerance”=1e-6), and strict parameter step size tolerance (“StepTolerance”=1e-20). If a maximum number of objective function evaluations or iterations were reached (3,000) the optimization was re-initialized at another randomized set of initial parameters and repeated until the tolerance criteria were met. Parameters were constrained to feasible values, e.g. non-negative MBF, non-negative decay constants, non-negative td and ITT, and k+>k-. Optimal parameters were recorded for each TAC and MBF values were analyzed.
MBF estimability was assessed by the precision and bias of MBF estimates. Precision was assessed by the interquartile range, IQR, calculated by taking the difference between the 75th and the 25th percentile estimates. Bias was assessed by the median estimate minus the known, true simulated MBF. Relative precision was also used to assess variation in MBF estimates and was calculated by dividing the IQR by the median MBF and expressed as a percentage. Robust statistics, such as median and IQR, were used rather than mean and standard deviation to mitigate the effects of outlier flow values which could be similarly rejected in visual and quantitative analysis of perfusion maps. Since the myocardium was uniformly perfused in simulations, any variation in MBF estimates across the myocardium was due to the effects of data acquisition, image reconstruction, and/or quantification method.
Parameter initialization experiment
An experiment was performed to evaluate the presence of local minima and whether final parameter estimates were dependent on initialization. A subset of 50 voxels was randomly selected from the digital phantom myocardium under nominal simulation conditions. The TAC from each voxel was fit using impulse response models with 200 different parameter initializations giving 200 sets of estimated parameters. Parameter initializations for each model were randomly selected and spanned a broad range of physiologically relevant values. Best-fit parameters with the smallest sum-of-squared differences were recorded as the optimum. Estimated parameters from other initializations were compared to the optimum and considered to have converged if they were within a defined tolerance level, i.e. within 0.1 s for time-related parameters, 1 mL/min-100g for MBF, 0.01 for extraction fraction, and 0.01 s−1 for decay parameters. We assessed optimum convergence by the percent of parameter initializations that converged to the optimum, e.g. if 180 parameter initializations converged to the optimum and 20 initializations terminated elsewhere, a value of 90% would be recorded. This process was repeated for all 50 TACs and the average optimum convergence was recorded. Details regarding parameter initialization are provided in Appendix 6 of (Eck 2018).
Arterial input function and model output experiment
The ability to distinguish model tissue curves after convolution with the AIF was assessed. The fast-changing intravascular portion of IRFs may not be excited by the low-frequency content of AIFs present in MPI-CT, prompting this assessment. Two AIFs were used, i.e. a typical AIF (FWHM=13 s, α=2.2) and a hypothetical narrow AIF (FWHM=4 s, α=100). The AATH model was used to generate IRFs and corresponding model output curves for both AIFs. Three sets of parameters were used with MBF values spanning a range from healthy resting MBF to healthy, pharmacologically-induced stress MBF (Prior et al 2012): [td=1 s, MBF=100 mL/min-100g, ITT=2 s, E=0.6, k=0.05 s−1]; [td=1 s, MBF=200 mL/min-100g, ITT=1 s, E=0.3, k=0.05 s−1]; [td=2.5 s, MBF=400 mL/min-100g, ITT=0.5 s, E=0.15, k=0.05 s−1].
In vivo experiment, porcine model of coronary stenosis
A representative porcine MPI-CT data set was used for comparison of the standard and modified physiologic simulator as well as for comparison to MBF maps from the digital phantom. We used a percutaneous, closed-chest porcine model of coronary stenosis guided by fractional flow reserve (FFR) (Fahmi et al 2016). MPI-CT data was acquired using a spectral detector CT scanner (SDCT, prototype of the IQon product, Philips) while the pig had a partial angioplastic, balloon-induced stenosis in the left anterior descending (LAD) coronary artery with FFR=0.59. Acquisition conditions used a tube voltage of 120 kVp, tube current-time product of 100 mAs per scan, 45 scans, 4 cm coverage, 0.27 s rotation speed, ECG-gating at 45% R-R interval (end systole), 360 degree, 120 mm field-of-view, ventilator off to avoid breathing motion, and 22.6 mSv total effective dose. Starting 4 s before initiation of CT scans, 20 mL of iodinated contrast agent (Omnipaque 350, 350 mg/mL Iodine) was intravenously injected at 5 mL/s and followed by 20 mL of saline. Virtual mono-energetic 70 keV images were reconstructed using a projection-based material decomposition (Fahmi et al 2016). Non-rigid registration (Modat et al 2010) was applied to reduce motion between dynamic frames. Myocardium was segmented using commercial research software (QMass research version 25 June, 2013; http://www.medis.nl/). Spatial noise was reduced using a 5×5 pixel (1.2×1.2 mm) averaging kernel on the myocardium. Pig MPI-CT data were used to evaluate whether the modified physiologic simulator with changing blood volume more accurately represents observed data than the standard physiologic simulator. MBF maps were generated using all 10 quantification methods and compared to simulation results with regard to trends between quantification methods.
Results
Standard vs modified physiologic simulator
The modified physiologic simulator matches the pig MPI-CT data more closely than the standard simulator (Figure 2). The separation between ischemic and healthy myocardium in pig is much larger than that observed from the standard physiologic simulator with fixed MBV. Furthermore, the standard simulator provides TACs that saturate above approximately MBF=150 mL/min-100g. Peak enhancement for the standard simulator at MBF=500 mL/min-100g was only 11% greater than peak enhancement at MBF=150 mL/min-100g, whereas the peak enhancement for the modified simulator at MBF=500 mL/min-100g was 78% greater than the value at MBF=150 mL/min-100g. With the flow-dependent MBV relationship in the modified simulator (MBF ∝ MBV2), we observed a larger separation of healthy and ischemic TACs which more closely matched the pig data.
Digital MPI-CT phantom experiments
Figure 3 shows representative fits of models to TACs from three different voxels in the digital phantom at nominal acquisition conditions and simulated MBF=100 mL/min-100g. The average SNRP over the myocardium was 3.3 before 5×5 pixel spatial averaging. TACs shown in Figure 3 are after spatial averaging in which average SNRP=10.3. Although AATH has the best visual fit to data and the lowest sum-of-squared difference value, MBF estimates vary widely, i.e. from 81 to 227 mL/min-100g. PTU fits the data nearly as well as AATH, although MBF estimates also vary widely, i.e. from 80 to 147 mL/min-100g. Of the three models, RPM1 has the poorest visual fit in the regions of rapid upslope and contrast agent wash-out, although MBF estimates tend to be much closer to the actual flow than PTU and AATH, i.e. from 103 to 105 mL/min-100g, thus suggesting an improvement in both accuracy and precision.
Figure 3.
Model fits to different noise realizations and variation of MBF estimates. Best-fit curves of AATH, PTU, and RPM1 to TACs from three different voxels in the digital phantom are shown. The AATH model visually fits each curve very well but has a wide variation in estimated MBF. The PTU model has a slightly poorer fit to data but has less variation in estimated MBF. RPM1 has a narrow range of MBF estimates at the cost of a poorer fit to data. Nominal parameters were used in the simulation. MBF is in units of mL/min-100g.
Figure 4 shows MBF estimates for all 10 quantification methods as a function of noise level (tube current-time product), imaging duration, sampling interval, and AIF width, as obtained from the digital MPI-CT phantom. There are notable differences in bias (median estimate minus actual MBF=100 mL/min-100g) and precision (IQR of estimates). Overall, we observe that 5-parameter models (2CXM, AATH) are severely biased and imprecise across all tested conditions, thus indicating that they are inappropriate. Two of the 4-parameter models (CTU, PTU) are sensitive to acquisition conditions, whereas the other 4-parameter model, Fermi, is more robust. As a result of these observations, 2CXM, AATH, CTU, and PTU are eliminated from further analysis of precision and bias. For other methods, we observe trends in precision and bias across acquisition conditions. As tube-current time product was reduced (top-row), precision degraded but the effect on bias differed between quantification methods. For SCM, RPM1, RPM2, Fermi, and MUP, median MBF was unchanged across the mAs levels, indicating robustness to noise. The median MBF for LSVD increased by 5% from 200 mAs to 50 mAs, indicating its bias is dependent on noise level. Across imaging durations (second row), shorter duration worsened precision and bias compared to longer durations. Among model-based methods, RPM1 had the best relative precision at 30 s imaging duration (10%) and had the least change in median MBF (22%) when the duration was changed from 50 s to 30 s. At the 50 s duration, RPM1 and RPM2 were least biased among the model-based methods (2%). Changes in sampling interval (third row) affected quantification methods to varying degrees; the 3-parameter models (SCM, RPM1, RPM2) had slightly degraded precision and unchanged bias, LSVD had a large change in relative precision from 13% at 1 s to 25% at 3 s, and MUP was robust to sampling interval. Changes in the AIF width (bottom row) did not have significant effects on the MBF bias or precision. Overall, regarding the effect of acquisition conditions on MBF bias and precision, imaging duration had the greatest effect on the model-based methods, sampling interval had the largest effect on LSVD, and noise level (tube current-time product) had the largest effect on MUP.
Figure 5 and 6 show model fits and MBF estimates at different simulated MBFs. In Figure 5, TACs at different simulated MBFs share characteristics with TACs observed in pig experiments; at higher MBF levels, e.g. 200 vs 50 mL/min-100g, TACs have greater peak enhancement and more rapid wash-out (see Figure 2(b)). Similar to Figure 3, a better model fit does not indicate a more accurate and precise MBF estimate. Although RPM1 had a slightly worse fit to data than PTU and AATH, estimates were more precise (Figure 6). With the exception of 2CXM, all quantification methods properly identified increasing MBF although with different amounts of bias. The 3-parameter models (SCM, RPM1, RPM2) were least biased and most precise.
Figure 5.
Model fits to TACs obtained from the same voxel location at multiple simulated MBF levels. TACs are shown after 5×5 pixel spatial averaging. (Left-to-right) Simulated MBF values are 50, 100, and 200 mL/min-100g, and the SNRP values are 7.7, 10.3, and 16.3, respectively. Ranking models by quality of fits, as assessed visually and from sum-of-squared differences, gives AATH>PTU>RPM1. However, RPM1 generally provides more precise MBF estimates than PTU or AATH, as shown in Figure 6. Aside from MBF, nominal simulation parameters were used.
Figure 6.
Median and interquartile range of MBF estimates from quantification methods at different simulated MBF levels. Bar heights for each quantification method indicate the median MBF estimated in the myocardium. The red dashed horizontal lines indicate the known simulated MBF values. With the exception of 2CXM, all methods have median MBF estimates that increase with simulated MBF. Aside from simulated MBF, nominal simulation parameters were used. The * indicates that the median and/or 75% MBF estimate was greater than 400mL/min-100g.
Figure 7 shows the overall bias (median MBF – simulated MBF) and precision (MBF IQR) characteristics of quantification methods across the simulation conditions shown in Figure 4 and Figure 6. In the left panel, all ten quantification methods are shown. Note that CTU, PTU, 2CXM, and AATH, had relative MBF precision >100% of median MBF in at least one condition. In the right panel, six of the better-performing models are shown, all with relative precision <25% (SCM, RPM1, RPM2, Fermi, MUP, LSVD). Of these six methods, model-based methods had better average relative precision than model-independent methods (≤10% vs >15%). Fermi had the poorest bias, over-estimating MBF on average by 67%. Model-independent methods were also significantly biased, underestimating MBF on average by 51% and 18% for MUP and LSVD, respectively. The 3-parameter models were least biased and most precise. Ranked in order of best-to-worst for average relative precision, models were RPM1 (7.2%), RPM2 (8.5%), and SCM (9.8%). Ranked in order of best-to-worst for average magnitude of the relative bias, models were SCM (10.3%), RPM2 (10.6%), and RPM1 (11.0%).
Figure 7.
MBF precision and bias characteristics of quantification methods across all 11 simulation conditions. Units are mL/min-100g. (Left) Some model-based methods, i.e. 2CXM, AATH, CTU, and PTU, have poor absolute precision (>100 mL/min-100g) and relative precision (>100%) in at least one condition. (Right) Over all tested conditions, Fermi, the 3-parameter models (RPM1, RPM2, SCM), and both model-independent methods (MUP, LSVD) had acceptable absolute precision (<25mL/min-100g). RPM1, RPM2, and SCM were overall less biased and more precise than Fermi, MUP, and LSVD.
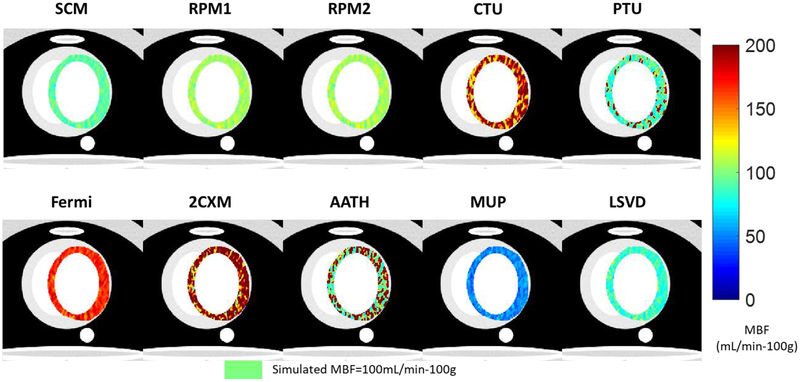
Figure 8 shows MBF maps obtained by the ten quantification methods in digital MPI-CT phantom data at nominal conditions. General trends from the precision and bias assessments (Figure 4, Figure 6, and Figure 7) are observed. The 3-parameter models (SCM, RPM1, RPM2) were closest to the simulated MBF value and maps were least noisy. CTU over-estimated MBF and had an extremely noisy map. PTU had median MBF near the simulated value but with a noisy map. Fermi had little noise in its map but significantly over-estimated MBF. Model-independent methods (MUP, LSVD) had relatively little noise in their maps but under-estimated MBF. The 5-parameter models (2CXM, AATH) produced extremely noisy maps and overestimated MBF. Overall, the 3-parameter models and model-independent LSVD produced high-quality MBF maps.
Figure 8.
MBF maps estimated on digital phantom MPI-CT data. Simulated MBF was uniform in the myocardium with MBF=100 mL/min-100g (light green color) using the physiologic simulator. From left to right: (Top) SCM, RPM1, RPM2, CTU, PTU (Bottom) Fermi, 2CXM, AATH, MUP, LSVD. The same color bar is used for all images. Nominal acquisition parameters were used.
Parameter initialization experiment
Differences between models in terms of convergence to optimal, best-fit parameters were observed. SCM, RPM1, and CTU had optimum convergence percentages of 100%. RPM2 and PTU gave >99% optimum convergence. Other models differed in their tendency terminate in local minima. Optimum convergence percentages were: AATH (98%), Fermi (96%), and 2CXM (61%). Therefore, 2CXM was the most sensitive to parameter initialization. However, even when optimal parameters were obtained they did not necessarily provide accurate MBF; for 2CXM, the median MBF estimate from the best-fit optima was 321 mL/min-100g, much higher than the known, simulated MBF of 100 mL/min-100g.
Arterial input function and model output experiment
Figure 9 demonstrates that typical AIFs blur high-frequency components of IRFs which may contribute to the poor MBF estimability of some models. Three unique IRFs from the AATH model with MBFs ranging from 100 to 400 mL/min-100g give nearly identical model outputs when excited by the typical broad AIF. Models which simultaneously estimate multiple parameters related to the fast-changing intravascular portion of the IRF would thus have poor MBF estimability in this case. A hypothetical narrow AIF led to distinct model outputs; however, this AIF could not clinically be created with a venous injection of contrast.
Figure 9.
AIF properties and MBF estimability. The typical AIF does not excite fast dynamics of IRFs, thus leading to poor MBF estimability in some models. (a) A typical AIF is plotted alongside a hypothetical narrow pulse. (b) IRFs from the AATH model are shown with different MBF, time delay, intravascular transit time, and extraction fraction. The y-axis denotes normalized indicator concentration scaled by MBF. (c) TACs obtained by convolving IRFs in (b) with the typical AIF in (a). (d) TACs after convolution with the narrow AIF in (a) that excites fast dynamics. Model output curves from the three unique impulse responses are nearly identical with the typical “broad” AIF, thus rendering MBF not estimable in the presence of noise. The high-frequency content of the narrow AIF leads to distinguishable TACs.
In vivo experiment, porcine model of coronary stenosis
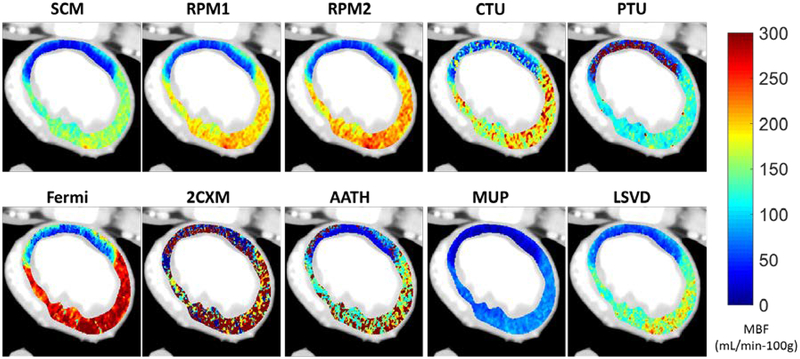
Figure 10 shows MBF maps from the porcine model with partial LAD occlusion (FFR=0.59). Similar overall trends can be observed as compared to the digital phantom perfusion maps shown in Figure 8. Models with more than three free parameters are generally noisier than the 3-parameter models, with the exception of the Fermi model. PTU yields MBF estimates in healthy myocardium with relatively low noise, however the estimates in the ischemic myocardium are noisy. Fermi produced high MBF estimates relative to SCM, RPM1, and RPM2, which was also observed in simulations. MUP and LSVD under-estimated MBF relative to 3-parameter models, also observed in simulations. Overall, the 3-parameter models (SCM, RPM1, RPM2), 4-parameter Fermi model, and model-independent methods (LSVD, MUP) have little noise in their MBF maps and clearly delineate healthy and ischemic flow distributions.
Figure 10.
MBF maps estimated in a porcine ischemic model at FFR=0.59. MBF estimates follow relative trends as observed in simulation experiments. A flow deficit in the large LAD myocardial territory is clear with all methods, except 2CXM, and a transmural perfusion gradient can also be observed with RPM1, RPM2, and Fermi.
Discussion
We found that accurate and precise MBF estimates from MPI-CT data were best obtained using 3-parameter models that include prior information regarding tissue physiology and contrast agent extraction (RPM1, RPM2). Models with more than three free parameters and the model-independent quantification methods had significant bias and/or poor precision across the range of acquisition and physiologic conditions. With a sufficiently long imaging duration, at least 40 s, the 3-parameter models, i.e. SCM, RPM1, and RPM2, were robust to decreases in x-ray exposure, lengthened sampling intervals, and broader arterial input functions, thus providing the lowest overall MBF bias (≤11% on average) and best overall precision (<10% on average). RPM1 provided the best relative precision in every condition which translated to low-noise MBF maps. The 3-parameter models also provided low-noise MBF maps in in vivo pig data with clear delineation of a flow deficit.
MBF estimability was dependent on acquisition condition and quantification method which has implications for protocol design and dose reduction. For model-based methods, imaging durations greater than 30 s were important for estimating parameters related to contrast agent extraction and wash-out. In order to minimize x-ray dose without sacrificing MBF precision and accuracy, dose reduction in model-based methods should retain longer imaging duration and either use fewer image frames or lower exposure per image frame. Regarding the model-independent methods, LSVD and MUP, reductions in imaging duration did not hamper MBF precision or accuracy whereas longer sampling intervals or lower tube current-time product degraded precision. Therefore, imaging duration should be minimized when processing data with LSVD or MUP as long as the baseline myocardium HU and peak myocardium HU are captured.
There is a bias toward underestimation among the best models as the simulated flow is increased (Figure 6). A similar phenomenon has been observed in PET imaging where low order models, e.g. one-tissue compartment model with spillover correction having three parameters, provide uptake parameter estimates which are approximately linearly proportional to MBF at low flows but nonlinear at higher flows (Lortie et al 2007). An accepted solution in PET is to correct uptake values for the flow-dependent extraction fraction via a characterized relationship, an option which could be followed in MPI-CT. In terms of the RPM1 and RPM2 models, the underlying issue in our MPI-CT data is that the lumped parameter extraction fraction does not account for reduced extraction at high flow rates. Simply adding another free parameter would not be advisable as every additional free parameter contributes to imprecision in the presence of noise. Correcting values after the fact might be the preferred option in order to maintain precise MBF estimation.
We observed advantages and disadvantages of model-independent techniques. Maximum-upslope was found to provide relatively precise MBF estimates but significantly underestimated MBF, as seen in a previous report (Bindschadler et al 2014). LSVD also tended to underestimate MBF, possibly due to overly aggressive regularization of the impulse response function. The L-curve criterion in LSVD provides a heuristic for choosing the Tikhonov regularization parameter but does not guarantee selection of a physiologically relevant value. Under-estimation in LSVD may be due to the low frequency content of the AIFs which will influence LSVD to restrain the IRF to smooth forms. This behavior of LSVD differs from our previous preliminary study in pig experiments in which LSVD tended to overestimate MBF (Eck et al 2016). However, CT imaging artifacts, such as motion (Pack et al 2016), were more prevalent in that study. Such artifacts may have introduced high-frequency noise in the AIF which would not have been filtered by LSVD, leading to high-frequency oscillations in the IRF and over-estimation of MBF. Therefore, it may be that LSVD under-estimates MBF under conditions with minimal imaging artifacts but may over-estimate MBF when artifacts are increasingly severe.
The low frequency content of AIFs appears to hamper parameter estimation when modeling the fast intravascular portion of IRFs. It was observed that realistic input functions can lead to model outputs that are nearly indistinguishable across a broad physiologic range of flow values. Although the narrow input function alleviated this effect, it is not possible to produce such an input in practice as venous injections of contrast agent are dispersed before reaching the left ventricle and myocardium. In order to estimate MBF using models that contain fast intravascular kinetics, constraints on model parameters are required, such as those in RPM1 and RPM2.
We observed that models were generally robust to parameter initialization. All models except 2CXM had generally favorable convergence to optimal parameters. Conversely, the poor convergence of 2CXM is suggestive of multiple local minima. Even when analyzing only the best-fit 2CXM parameters, MBF was still biased and imprecise, suggesting fundamental limitations of the model. Furthermore, the use of robust statistics in this work, i.e. median and interquartile range, helps ensure that observed trends in MBF precision and bias across the simulations are due to model behaviors and not due to the parameter optimization procedure.
The use of additional prior information or model constraints could improve parameter estimability and/or enable assessment of other tissue properties. Model parameters could be estimated other means, as has been done with time delay (Cheong et al 2003), and used as constraints. The use of blood pool contrast agents (Hainfeld et al 2006, Kroft and de Roos 1999, Jerosch-Herold et al 1999) would eliminate extraction fraction and wash-out decay constants along with the uncertainty they introduce into parameter estimation, which may significantly improve MBF accuracy and precision. Prior information on parameter values could be used with a Bayesian approach, such as max a posteriori estimation, as has been applied in PET cerebral perfusion (Rizzo et al 2013), acting as a softer constraint than the rigid E=0.6 in RPM1 and RPM2 used in this work. The use of other objective functions, such as weighted sum of squares, could also improve parameter estimates by incorporating knowledge of artifacts or noise magnitude. However, careful selection of weights is important, as improper weights can negatively impact parameter estimates (Muzic and Christian 2006).
We note the limitations of this work. First, the majority of analyses were carried out on simulation data which will differ from in vivo data. While the physiologic simulator accounts for aspects such as myocardial flow heterogeneity, flow-dependent capillary permeability, and now flow-dependent vascular volume, patients may differ. Patients may have different vascular volume or capillary permeability than that which is assumed in the physiologic simulator. In pathologic cases, capillary permeability is likely to change in the presence of prolonged ischemia (Lardo 2006, Zhu et al 2009). In the presence of a significant stenosis, there can be additional time delay and dispersion of the AIF (Calamante et al 2000, 2003). However, it is encouraging that the trends in the MBF map appearance across quantification methods are similar between the porcine data and the simulated data. Another limitation is that the CT simulator used in this work did not include artifacts such as beam hardening, scatter, motion, or partial scan, nor did it have electronic noise. However, this work provides a “best case” baseline for MBF quantification and characterizes the behavior of quantification methods when artifacts are corrected. Further investigation into the effects of specific artifacts on MBF precision and bias is warranted. Despite these limitations, the physiologic simulator and CT simulator enable controlled, reproducible experiments for evaluation of acquisition and quantification methods which may otherwise be confounded by numerous factors in in vivo experiments.
Conclusion
Using numerical simulations and model-to-models tests, we found that reduced, 3-parameter models, i.e. RPM1, RPM2, and SCM, provide the most precise and accurate MBF estimates across a range of MPI-CT acquisition conditions and physiologic conditions. Models with four or more free parameters had poor MBF precision and/or significant bias. Bias was more pronounced at the shortest tested imaging duration, i.e. 30 s, for model-based methods than at the longer imaging durations, i.e. 40 s and 50 s, thus indicating that MPI-CT acquisitions ≥40 s should be preferred. Model-independent LSVD and maximum-upslope provided relatively precise MBF estimates, although tended to greatly underestimate MBF. Considering the precision and bias for MBF estimation using model-based methods, reducing temporal sampling and reducing exposure per image frame seems to be preferable to reducing imaging duration for dose reduction. We recommend reduced, 3-parameter models that incorporate prior information of physiology and contrast agent extraction for typical MPI-CT datasets. The two proposed robust physiologic models, RPM1 and RPM2, and the single-compartment model were found to be best for MBF estimation, with little overall bias, ≤11%, and precise estimation, ≤10%, over the tested conditions. This work provides a foundation for the development and application of quantification methods and acquisition protocols for precise, accurate MBF estimation in MPICT.
Table 1.
List of acronyms and definitions.
| Acronym | Definition |
|---|---|
| AATH | Adiabatic Approximation of the Tissue Homogeneity model |
| AIF | Arterial Input Function |
| CTU | Compartmental Tissue Uptake model |
| Fermi | Fermi model |
| FFR | Fractional Flow Reserve |
| FWHM | Full-Width at Half Maximum |
| IQR | Interquartile Range |
| IRF | Impulse Response Function |
| LAD | Left Anterior Descending coronary artery |
| LSVD | SVD with Tikhonov regularization determined by the L-curve criterion |
| LV | Left Ventricle of the heart |
| MPI-CT | Myocardial Perfusion Imaging with Computed Tomography |
| MUP | Maximum Upslope |
| MVD | Microvascular Disease |
| PTU | Plug-flow Tissue Uptake model |
| RPM1 | Robust Physiologic Model with fixed E, fixed ITT |
| RPM2 | Robust Physiologic Model with fixed E, MBF-dependent ITT |
| SCM | Single-Compartment Model |
| SVD | Singular Value Decomposition |
| TAC | Time-Attenuation Curve |
| 2CXM | Two-Compartment Exchange Model |
Table 2.
List of parameters and definitions.
| Parameter | Definition |
|---|---|
| A | Scaling parameter for the slowly decaying exponential (2CXM) |
| α | Width parameter of gamma-variate function |
| B | Scaling parameter for the quickly decaying exponential (2CXM) |
| β | Proportionality constant for the flow and blood volume relationship |
| Ca(t) | Arterial time-concentration curve |
| Cm(t) | Model tissue time-concentration curve |
| CRV(t) | Right ventricle time-concentration curve from the gamma-variate function |
| Ct(t) | Measured tissue time-concentration curve |
| E | Extraction Fraction |
| ITT | Intravascular Transit Time |
| k | Decay constant for contrast agent wash-out |
| k− | Decay constant of the slowly decaying exponential (2CXM) |
| k+ | Decay constant of the quickly decaying exponential (2CXM) |
| MBF | Myocardial Blood Flow |
| MBV | Myocardial Blood Volume |
| Φ | Unweighted sum-of-squared difference objective function |
| PS | Permeability-Surface Area product |
| ρ | Tissue density |
| RMBF(t) | Flow-scaled tissue impulse response function |
| SNRP | Perfusion Signal-to-Noise Ratio |
| T | Mean voxel transit time |
| Tc | Mean capillary transit time |
| td | Time delay between arterial input and tissue curve |
| td,RV | Time delay parameter of the gamma-variate function |
| Te | Mean extravascular transit time |
| θ | Generalized variable for model parameters |
| tpeak | Peak time parameter of the gamma-variate function |
| t0 | Width parameter in the Fermi model |
| Vart | Arteriole volume |
| vb | Intravascular (blood) volume |
| Vcap | Capillary volume |
| ve | Extravascular volume |
| Vven | Venule volume |
| ymax | Maximum value of the gamma-variate function |
Acknowledgements
The authors acknowledge Steve Schomisch and Cassie Cipriano of the CWRU Animal Resource Center for their assistance with animal experiments; Bonnie Hami of University Hospitals Cleveland Medical Center for editorial assistance; and the support of the Global Advanced Innovation Center collaboration between CWRU, University Hospitals Cleveland Medical Center, and Philips Healthcare. Furthermore, the authors acknowledge funding from NIH R01 EB004070, Ohio Third Frontier research grant Cardiac Perfusion with Computed Tomography, and training grant NIH T32 EB007509. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the state of Ohio.
Appendix
Model-based MBF estimation
The impulse response functions and free parameters for the eight models are described. Further information about these models and their derivation can be found in these references: (Sourbron and Buckley 2012, So et al 2012, Jerosch-Herold et al 1998, Luypaert et al 2011).
-
1)Adiabatic approximation of the tissue homogeneity model (AATH). The observation region is composed of intravascular and extravascular regions. Assumptions include plug flow through the intravascular space and that contrast agent concentrations in the extravascular space change much more slowly than in the intravascular space, thus allowing the aggregate extraction of contrast agent from the intravascular space to be represented by the extraction fraction. AATH is also known as an approximation to the Johnson-Wilson model with five free parameters: td (s), MBF, ITT (s), E (unit-less), and k (s−1). The corresponding IRF is given by:
(4) -
2)Two-compartment exchange model (2CXM). The myocardium consists of well-mixed intravascular and extravascular compartments with exchange between them. This model does not assume plug flow which may not be valid when considering that myocardium has a relatively chaotic spatial arrangement of capillaries, arterioles, and venules with heterogeneous volumes and individual flows (Sourbron and Buckley 2012). The IRF is given by the sum of two exponentials with five free parameters: td, A (mL/min-100g), B (mL/min-100g), k- (s−1), and k+ (s−1):
where A is the scale factor of the slowly decaying exponential with decay constant k- and B is the scale factor of the quickly decaying exponential with decay constant k+ (see Figure 1). These parameters are related to underlying tissue properties such as MBF; intravascular volume (vb, mL/g tissue); extravascular volume (ve, mL/g tissue); and permeability-surface area product (PS, mL/min-g tissue). The relationships are:(5) ,
,
,
,
Tc = vb/MBF, mean capillary transit time,
Te = ve/PS, mean extravascular transit time,
T = (vb+ve)/MBF, mean voxel transit time.
-
3)
Plug-flow tissue uptake model (PTU). Contrast agent remains trapped in the extravascular space after entering, i.e. equivalent to k=0 in AATH (Sourbron and Buckley 2012). The PTU model has four free parameters: td, MBF, ITT, and E.
-
4)Compartmental tissue uptake model (CTU). Myocardium is described as two lumped compartments in which contrast agent extracts from the intravascular compartment to the extravascular compartment where it becomes trapped. The difference between CTU and PTU is that CTU is the sum of an exponential and a step function, whereas PTU is a piecewise step function (Sourbron and Buckley 2012). The CTU model has four free parameters: td, MBF, E, and k.
(6) -
5)
Robust physiologic model with fixed E and ITT (RPM1). This model is a reduced form of AATH with parameters fixed to E=0.6 and ITT=2 s. These values were selected when considering CT contrast agent extraction in the literature which can range from E=0.3 to E=0.8 (Idée et al 2002), CT voxel size and capillary flow velocity (Kiyooka et al 2005), goodness-of-fit to preclinical data, and parameter estimability across imaging conditions. The RPM1 model has three free parameters: td, MBF, and k.
-
6)
Robust physiologic model with fixed E and flow-dependent ITT (RPM2). The parameter E is set to a constant and the parameter ITT is a function of MBF. In this work, E=0.6 and where β is a proportionality constant. This functional relationship between ITT and MBF is based on observations in the literature that MBF is a quadratic function of myocardial blood volume, MBF∝MBV2 (Wu et al 1992), and on the Central Volume Principle, ITT=MBV/MBF (Sourbron and Buckley 2012). Presumably, the quadratic relationship between MBF and MBV is due to the dilation of small blood vessels which decreases microvascular resistance and increases blood flow rates. A value of was selected to give the same IRF as RPM1 at MBF=100 mL/min-100g. The RPM2 model has three free parameters: td, MBF, and k.
-
7)Single-compartment model (SCM). The observation region is described by a single lumped compartment in which the contrast agent enters and resides until leaving according to an exponential decay function (Luypaert et al 2011) which is described by three free parameters: td, MBF, and k.
(7) -
8)Fermi model (Fermi). The IRF is defined by a Fermi function (Jerosch-Herold et al 1998), and is described by four free parameters: td; MBF; width parameter (t0, s); and k.
(8)
Model-independent MBF estimation
The two model-independent MBF estimation algorithms, LSVD and MUP, are described.
-
9)
SVD with Tikhonov regularization determined by the L-curve criterion (LSVD). In this implementation, the convolution is discretized in order to formulate the matrix equation for SVD computation. The ti time points are equally spaced with average Δt time increment. The values of Ct(t) and Ca(t) are measured and the convolution is transformed into an algebraic form which is then solved by SVD with Tikhonov regularization to obtain RMBF(t). The regularization factor is determined by the L-curve criterion which balances the effects of noise in RMBF(t) with fit to the original data. The regularization factor is independently determined for each individual TAC for the analysis in this work. As RMBF(t) is flow-scaled and represents the retention of a unit bolus of contrast material, MBF is obtained by taking the maximum value of RMBF(t) (Fieselmann et al 2011).
-
10)Maximum Upslope (MUP). Using an established method (Bindschadler et al 2014), the maximum upslope, dCt/dt, was estimated by fitting a line segment to TAC data from 4 s before the AIF peak to either the minimum of the time-of-peak HU of the myocardial TAC or 7 s after the AIF peak. MBF is computed by the equation below where max(Ca) is the measured peak of the AIF.
(9)
Semi-analytic model implementation
The convolution integral in equation (1) is computed using analytic forms of both RMBF(t) and Ca(t). We used first-order polynomials to construct a linear spline representation of Ca(t) (De Boor 1978) equivalent to linear interpolation between measured data points:
| (10) |
where ai is the constant coefficient in the linear equation, bi is the first-order coefficient or slope, and ti is the time of a sampled data point for time index i=1,2,…,N for N scans. The linear spline is selected over higher-order splines in order to avoid oscillations, i.e. under- or overshoot, between sampled data points and as a tradeoff between computational efficiency and accuracy given the typical noise levels in the data. (However, the method generalizes to higher-order polynomial splines.) Using this approach, the linear spline from equation (10) can be substituted for Ca(t) in the convolution, equation (1), giving the model output corresponding to the impulse response RMBF(t),
| (11) |
where the index j denotes the lower bounded time index for each piece of the linear spline, index j+1 represents the upper bounded time index, and t is on the interval t1≤t≤tN. Each integral has a closed-form analytic solution that depends on the impulse response model used (see Appendix 5 of (Eck 2018)). The model TAC (Cm) is compared to the measured tissue curve (Ct) at sample times ti using the unweighted sum-of-squared difference objective function,
| (12) |
which is minimized when the model curve has reached an optimal fit to the data set across all N scans.
We used a gradient-based optimization algorithm to minimize Φ with respect to model parameters. For robust and efficient performance, analytically determined gradients are supplied (Bard 1974). First, the objective function is differentiated with respect to each model parameter, θs,
| (13) |
in which Cm(ti) is the model output and ∂Cm(ti)/∂θs is the dynamic sensitivity function of the model-predicted TAC with respect to parameter θs. The sensitivity function is computed in a similar fashion as Cm(t),
| (14) |
in which the derivative is derived analytically from the model impulse response function (see Appendix 5 of (Eck 2018)).
References
- Ardle BAM, Dowsley TF, deKemp RA, Wells GA and Beanlands RS 2012. Does Rubidium-82 PET Have Superior Accuracy to SPECT Perfusion Imaging for the Diagnosis of Obstructive Coronary Disease?: A Systematic Review and Meta-Analysis J. Am. Coll. Cardiol 60 1828–37 [DOI] [PubMed] [Google Scholar]
- Bard Y 1974. Nonlinear parameter estimation (Academic Press; ) [Google Scholar]
- Barrett JF and Keat N 2004. Artifacts in CT: Recognition and Avoidance RadioGraphics 24 1679–91 [DOI] [PubMed] [Google Scholar]
- Beister M, Kolditz D and Kalender WA 2012. Iterative reconstruction methods in X-ray CT Phys. Med 28 94–108 [DOI] [PubMed] [Google Scholar]
- Bindschadler M, Modgil D, Branch KR, La Riviere PJ and Alessio AM 2014. Comparison of blood flow models and acquisitions for quantitative myocardial perfusion estimation from dynamic CT. Phys. Med. Biol 59 1533–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calamante F, Gadian DG and Connelly A 2000. Delay and dispersion effects in dynamic susceptibility contrast MRI: simulations using singular value decomposition Magn. Reson. Med 44 466–473 [DOI] [PubMed] [Google Scholar]
- Calamante F, Yim PJ and Cebral JR 2003. Estimation of bolus dispersion effects in perfusion MRI using image-based computational fluid dynamics NeuroImage 19 341–53 [DOI] [PubMed] [Google Scholar]
- Cheong LH, Koh TS and Hou Z 2003. An automatic approach for estimating bolus arrival time in dynamic contrast MRI using piecewise continuous regression models Phys. Med. Biol 48 N83. [DOI] [PubMed] [Google Scholar]
- Chung S-Y, Lee K-Y, Chun EJ, Lee W-W, Park EK, Chang H-J and Choi SI 2010. Comparison of Stress Perfusion MRI and SPECT for Detection of Myocardial Ischemia in Patients With Angiographically Proven Three-Vessel Coronary Artery Disease Am. J. Roentgenol 195 356–62 [DOI] [PubMed] [Google Scholar]
- Coxson PG, Huesman RH and Borland L 1997. Consequences of Using a Simplified Kinetic Model for Dynamic PET Data J. Nucl. Med 38 660–7 [PubMed] [Google Scholar]
- De Boor C 1978. A practical guide to splines (Springer-Verlag; New York: ) [Google Scholar]
- Desiderio MC, Lundbye J, Baker WL, Farrell MB and Heller G 2018. CONTEMPORARY PATIENT RADIATION EXPOSURE FROM PET VERSUS SPECT MPI J. Am. Coll. Cardiol 71 A1489 [Google Scholar]
- Duvall WL, Rai M, Ahlberg AW, O’Sullivan DM and Henzlova MJ 2015. A multi-center assessment of the temporal trends in myocardial perfusion imaging J. Nucl. Cardiol 22 539–51 [DOI] [PubMed] [Google Scholar]
- Eck BL 2018. Myocardial Perfusion Imaging with X-Ray Computed Tomography (Cleveland, Ohio, USA: Case Western Reserve University; ) [Google Scholar]
- Eck BL, Fahmi R, Fuqua C, Vembar M, Dhanantwari A, Bezerra HG and Wilson DL 2015. Low dose dynamic myocardial CT perfusion using advanced iterative reconstruction SPIE Medical Imaging 2015: Biomedical Applications in Molecular, Structural, and Functional Imaging vol 9417 (Orlando, Florida) pp 94170Z-94170Z–11 Online: http://dx.doi.org/10.1117/12.2081418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eck BL, Fahmi R, Levi J, Fares A, Wu H, Li Y, Vembar M, Dhanantwari A, Bezerra HG and Wilson DL 2016. Comparison of quantitative myocardial perfusion imaging CT to fluorescent microsphere-based flow from high-resolution cryo-images SPIE Medical Imaging 2016: Biomedical Applications in Molecular, Structural, and Functional Imaging vol 9788 (San Diego, California) pp 97882F-97882F–9 Online: http://dx.doi.org/10.1117/12.2217027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eurostat. Availability of medical technology — imaging equipment, 2015. Eurostat. 2017 Online: http://ec.europa.eu/eurostat/statistics-explained/index.php?title=File:Availability_of_medical_technology_%E2%80%94_imaging_equipment,_2015_HLTH17.png.
- Fahmi R, Eck BL, Levi J, Fares A, Dhanantwari A, Vembar M, Bezerra HG and Wilson DL 2016. Quantitative myocardial perfusion imaging in a porcine ischemia model using a prototype spectral detector CT system Phys. Med. Biol 61 2407–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahmi R, Eck BL, Vembar M, Bezerra HG and Wilson DL 2014. Dynamic CT myocardial perfusion imaging: detection of ischemia in a porcine model with FFR verification SPIE Medical Imaging 2014: Biomedical Applications in Molecular, Structural, and Functional Imaging vol 9038 (San Diego, California) pp 90380O-90380O–10 Online: http://dx.doi.org/10.1117/12.2043800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fieselmann A, Kowarschik M, Ganguly A, Hornegger J and Fahrig R 2011. Deconvolution-Based CT and MR Brain Perfusion Measurement: Theoretical Model Revisited and Practical Implementation Details Int. J. Biomed. Imaging 2011 1–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraker TD and Fihn SD 2007. 2007 Chronic Angina Focused Update of the ACC/AHA 2002 Guidelines for the Management of Patients With Chronic Stable Angina J. Am. Coll. Cardiol 50 2264–74 [DOI] [PubMed] [Google Scholar]
- Goh V, Halligan S and Bartram CI 2007. Quantitative Tumor Perfusion Assessment with Multidetector CT: Are Measurements from Two Commercial Software Packages Interchangeable? Radiology 242 777–82 [DOI] [PubMed] [Google Scholar]
- Goh V, Shastry M, Engledow A, Reston J, Wellsted DM, Peck J, Endozo R, Rodriguez-Justo M, Taylor SA, Halligan S and Groves AM 2011. Commercial software upgrades may significantly alter Perfusion CT parameter values in colorectal cancer Eur. Radiol 21 744–9 [DOI] [PubMed] [Google Scholar]
- Gramer BM, Muenzel D, Leber V, von Thaden A-K, Feussner H, Schneider A, Vembar M, Soni N, Rummeny EJ and Huber AM 2012. Impact of iterative reconstruction on CNR and SNR in dynamic myocardial perfusion imaging in an animal model Eur. Radiol 22 2654–61 [DOI] [PubMed] [Google Scholar]
- Hainfeld JF, Slatkin DN, Focella TM and Smilowitz HM 2006. Gold nanoparticles: a new X-ray contrast agent Br. J. Radiol 79 248–53 [DOI] [PubMed] [Google Scholar]
- Hansis E and Shao L 2011. Simulation of motion artifacts in offset flat-panel cone-beam CT 2011 IEEE Nuclear Science Symposium Conference Record 2011 IEEE Nuclear Science Symposium Conference Record pp 2471–5 [Google Scholar]
- Hubbard L, Ziemer B, Lipinski J, Sadeghi B, Javan H, Groves EM, Malkasian S and Molloi S 2016. Functional Assessment of Coronary Artery Disease Using Whole-Heart Dynamic Computed Tomographic Perfusion Circ. Cardiovasc. Imaging 9 Online: http://circimaging.ahajournals.org/lookup/doi/10.1161/CIRCIMAGING.116.005325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbell JH and Seltzer SM 1995. Tables of x-ray mass attenuation coefficients and mass energy-absorption coefficients 1 keV to 20 meV for elements z = 1 to 92 and 48 additional substances of dosimetric interest (United States: ) Online: http://www.osti.gov/scitech/servlets/purl/76335 [Google Scholar]
- Huber AM, Leber V, Gramer BM, Muenzel D, Leber A, Rieber J, Schmidt M, Vembar M, Hoffmann E and Rummeny E 2013. Myocardium: Dynamic versus Single-Shot CT Perfusion Imaging Radiology 269 378–86 [DOI] [PubMed] [Google Scholar]
- Idée J-M, Nachman I, Port M, Petta M, Le Lem G, Le Greneur S, Dencausse A, Meyer D and Corot C 2002. Iodinated contrast media: from non-specific to blood-pool agents Contrast Agents II (Springer) pp 151–171 Online: http://link.springer.com/chapter/10.1007/3-540-46009-8_5 [Google Scholar]
- Isola AA, Grass M and Niessen WJ 2010. Fully automatic nonrigid registration-based local motion estimation for motion-corrected iterative cardiac CT reconstruction Med. Phys 37 1093–109 [DOI] [PubMed] [Google Scholar]
- Jerosch-Herold M, Wilke N, Stillman AE and Wilson RF 1998. Magnetic resonance quantification of the myocardial perfusion reserve with a Fermi function model for constrained deconvolution Med. Phys 25 73–84 [DOI] [PubMed] [Google Scholar]
- Jerosch-Herold M, Wilke N, Wang Y, Gong G-R, Mansoor AM, Huang H, Gurchumelidze S and Stillman AE 1999. Direct comparison of an intravascular and an extracellular contrast agent for quantification of myocardial perfusion Int. J. Card. Imaging 15 453–64 [DOI] [PubMed] [Google Scholar]
- Kershaw LE and Cheng H-LM 2010. Temporal resolution and SNR requirements for accurate DCE-MRI data analysis using the AATH model Magn. Reson. Med 64 1772–80 [DOI] [PubMed] [Google Scholar]
- Kiyooka T, Hiramatsu O, Shigeto F, Nakamoto H, Tachibana H, Yada T, Ogasawara Y, Kajiya M, Morimoto T and Morizane Y 2005. Direct observation of epicardial coronary capillary hemodynamics during reactive hyperemia and during adenosine administration by intravital video microscopy Am. J. Physiol.-Heart Circ. Physiol 288 H1437–43 [DOI] [PubMed] [Google Scholar]
- Kroft LJM and de Roos A 1999. Blood pool contrast agents for cardiovascular MR imaging J. Magn. Reson. Imaging 10 395–403 [DOI] [PubMed] [Google Scholar]
- Kudo K, Christensen S, Sasaki M, Østergaard L, Shirato H, Ogasawara K, Wintermark M and Warach S 2013. Accuracy and Reliability Assessment of CT and MR Perfusion Analysis Software Using a Digital Phantom Radiology 267 201–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lardo AC 2006. Contrast-Enhanced Multidetector Computed Tomography Viability Imaging After Myocardial Infarction: Characterization of Myocyte Death, Microvascular Obstruction, and Chronic Scar Circulation 113 394–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T-Y 2002. Functional CT: physiological models Trends Biotechnol. 20 S3–1012570152 [Google Scholar]
- Lortie M, Beanlands RSB, Yoshinaga K, Klein R, DaSilva JN and deKemp RA 2007. Quantification of myocardial blood flow with 82Rb dynamic PET imaging Eur. J. Nucl. Med. Mol. Imaging 34 1765–74 [DOI] [PubMed] [Google Scholar]
- Luypaert R, Sourbron S and de Mey J 2011. Validity of perfusion parameters obtained using the modified Tofts model: A simulation study: Validity of the Modified Tofts Model Magn. Reson. Med 65 1491–7 [DOI] [PubMed] [Google Scholar]
- MATLAB and Optimization Toolbox Release 2016b. (Natick, Massachusetts, United States: The Mathworks, Inc.) [Google Scholar]
- Min JK, Taylor CA, Achenbach S, Koo BK, Leipsic J, Nørgaard BL, Pijls NJ and De Bruyne B 2015. Noninvasive Fractional Flow Reserve Derived From Coronary CT Angiography: Clinical Data and Scientific Principles JACC Cardiovasc. Imaging 8 1209–22 [DOI] [PubMed] [Google Scholar]
- Modat M, Ridgway GR, Taylor ZA, Lehmann M, Barnes J, Hawkes DJ, Fox NC and Ourselin S 2010. Fast free-form deformation using graphics processing units Comput. Methods Programs Biomed 98 278–84 [DOI] [PubMed] [Google Scholar]
- Montalescot G et al. 2013. 2013 ESC guidelines on the management of stable coronary artery disease The Task Force on the management of stable coronary artery disease of the European Society of Cardiology Eur. Heart J 34 2949–3003 [DOI] [PubMed] [Google Scholar]
- Muenzel D. et al. Dynamic CT Perfusion Imaging of the Myocardium: A Technical Note on Improvement of Image Quality. PLOS ONE. 2013;8:e75263. doi: 10.1371/journal.pone.0075263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzic RF and Christian BT 2006. Evaluation of objective functions for estimation of kinetic parameters Med. Phys 33 342–53 [DOI] [PubMed] [Google Scholar]
- Pack JD, Yin Z, Xiong G, Mittal P, Dunham S, Elmore K, Edic PM and Min JK 2016. Motion correction for improving the accuracy of dual-energy myocardial perfusion CT imaging ed B Gimi and A Krol p 97880Z Online: http://proceedings.spiedigitallibrary.org/proceeding.aspx?doi=10.1117/12.2216986
- Patel MR, Dai D, Hernandez AF, Douglas PS, Messenger J, Garratt KN, Maddox TM, Peterson ED and Roe MT 2014. Prevalence and predictors of nonobstructive coronary artery disease identified with coronary angiography in contemporary clinical practice Am. Heart J. 167 846–852.e2 [DOI] [PubMed] [Google Scholar]
- Peters A, Gunasekera R, Henderson B, Brown J, Lavender J, De Souza M, Ash J and Gilday D 1987. Noninvasive measurement of blood flow and extraction fraction. Nucl. Med. Commun 8 823–37 [DOI] [PubMed] [Google Scholar]
- Piccinelli M and Garcia EV 2016. Advances in Single-Photon Emission Computed Tomography Hardware and Software Cardiol. Clin 34 1–11 [DOI] [PubMed] [Google Scholar]
- Prior JO, Allenbach G, Valenta I, Kosinski M, Burger C, Verdun FR, Delaloye AB and Kaufmann PA 2012. Quantification of myocardial blood flow with 82 Rb positron emission tomography: clinical validation with 15 O-water Eur. J. Nucl. Med. Mol. Imaging 39 1037–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie CJ, Godwin JD, Crawford CR, Stanford W, Anno H and Kim Y 1992. Minimum scan speeds for suppression of motion artifacts in CT. Radiology 185 37–42 [DOI] [PubMed] [Google Scholar]
- Riviere PJL and Vargas P 2008. Correction for Resolution Nonuniformities Caused by Anode Angulation in Computed Tomography IEEE Trans. Med. Imaging 27 1333–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo G, Turkheimer FE and Bertoldo A 2013. Multi-scale hierarchical approach for parametric mapping: Assessment on multi-compartmental models NeuroImage 67 344–53 [DOI] [PubMed] [Google Scholar]
- Rochitte CE et al. 2014. Computed tomography angiography and perfusion to assess coronary artery stenosis causing perfusion defects by single photon emission computed tomography: the CORE320 study Eur. Heart J 35 1120–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab F, Ingrisch M, Marcus R, Bamberg F, Hildebrandt K, Adrion C, Gliemi C, Nikolaou K, Reiser M and Theisen D 2015. Tracer kinetic modeling in myocardial perfusion quantification using MRI: Tracer Kinetic Models in Cardiac Perfusion Quantification Magn. Reson. Med 73 1206–15 [DOI] [PubMed] [Google Scholar]
- So A, Hsieh J, Li J-Y, Hadway J, Kong H-F and Lee T-Y 2012. Quantitative myocardial perfusion measurement using CT Perfusion: a validation study in a porcine model of reperfused acute myocardial infarction Int. J. Cardiovasc. Imaging 28 1237–48 [DOI] [PubMed] [Google Scholar]
- So A, Lee T-Y, Imai Y, Narayanan S, Hsieh J, Kramer J, Procknow K, Leipsic J, LaBounty T and Min J 2011. Quantitative myocardial perfusion imaging using rapid kVp switch dual-energy CT: Preliminary experience J. Cardiovasc. Comput. Tomogr 5 430–42 [DOI] [PubMed] [Google Scholar]
- Sourbron SP and Buckley DL 2012. Tracer kinetic modelling in MRI: estimating perfusion and capillary permeability Phys. Med. Biol 57 R1–33 [DOI] [PubMed] [Google Scholar]
- Varga-Szemes A, Meinel FG, De Cecco CN, Fuller SR, Bayer RR and Schoepf UJ 2015. CT Myocardial Perfusion Imaging Am. J. Roentgenol 204 487–97 [DOI] [PubMed] [Google Scholar]
- Vinnakota KC and Bassingthwaighte JB 2004. Myocardial density and composition: a basis for calculating intracellular metabolite concentrations Am. J. Physiol. - Heart Circ. Physiol 286 H1742–9 [DOI] [PubMed] [Google Scholar]
- Wu XS, Ewert DL, Liu YH and Ritman EL 1992. In vivo relation of intramyocardial blood volume to myocardial perfusion. Evidence supporting microvascular site for autoregulation. Circulation 85 730–7 [DOI] [PubMed] [Google Scholar]
- Zhu X-Y, Daghini E, Chade AR, Versari D, Krier JD, Textor KB, Lerman A and Lerman LO 2009. Myocardial microvascular function during acute coronary artery stenosis: effect of hypertension and hypercholesterolaemia Cardiovasc. Res 83 371–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemer BP, Hubbard L, Lipinski J and Molloi S 2015. Dynamic CT perfusion measurement in a cardiac phantom Int. J. Cardiovasc. Imaging 31 1451–9 [DOI] [PMC free article] [PubMed] [Google Scholar]