Abstract
PURPOSE OF REVIEW
Chronic kidney disease (CKD) is associated with bone loss and fractures. The purpose of this review is to provide clinicians with an overview of the underlying pathogenesis of CKD-associated osteoporosis, and a summary of the current diagnostic and therapeutic approaches to this disease.
RECENT FINDINGS
In 2017, the Kidney Disease Improving Global Outcomes Committee on Bone Quality updated their guidelines to include screening for osteoporosis and fracture risk by dual energy X-ray absorptiometry in patients with CKD. Once a diagnosis of osteoporosis and/or fracture risk is established, it is not clear how nephrologists should manage their patients.
SUMMARY
Patients with CKD should be screened for CKD-associated osteoporosis and considered for strategies that prevent bone loss and fractures. Assessment of bone turnover via imaging, biochemical testing or bone biopsy can help guide the choice of therapy. Ran-domized controlled trials are needed to assess safety and efficacy of treatments to prevent bone loss and fractures.
Keywords: CKD-MBD, Renal Osteodystrophy, Osteoporosis
INTRODUCTION
Chronic kidney disease (CKD) affects over 20 million individuals in the United States, and 752 million individuals worldwide (Table 1) 1. Individuals with CKD have a high prevalence of CKD mineral and bone disease (CKD-MBD). CKD-MBD encompasses a range of abnormalities including derangements in mineral metabolism, vascular and soft tissue calcifications and skeletal abnormalities. In patients with CKD, osteoporosis and fracture risk are higher than in the general population2, have negative effects on quality of life and increase the risk of mortality3. While the pathophysiology of bone disease in patients with CKD is complex and not completely clear, clinicians are faced with the need to manage the disorder and initiate patients on treatments to improve their bone health. While the need to develop treatments that decrease risk of fracture in CKD patients is of paramount importance, there have not been advances in the discovery of new treatments and pharmacologic agents that are directed at the CKD population. This review will discuss the effects of CKD on bone, the definition of CKD-associated osteoporosis, invasive and non-invasive diagnostic methods of assessing bone health in CKD, and the currently available therapies for the management of CKD-associated osteoporosis.
Table 1.
Stages of Chronic Kidney Disease
| CKD Stages | Estimated Glomerular Filtration Rate (mL/min/1.73m2) |
|---|---|
| 1 | ≥90 |
| 2 | 60–89 |
| 3A | 45–59 |
| 3B | 30–44 |
| 4 | 15–29 |
| 5 | <15 |
| 5D | On dialysis |
BONE STRENGTH IN CHRONIC KIDNEY DISEASE
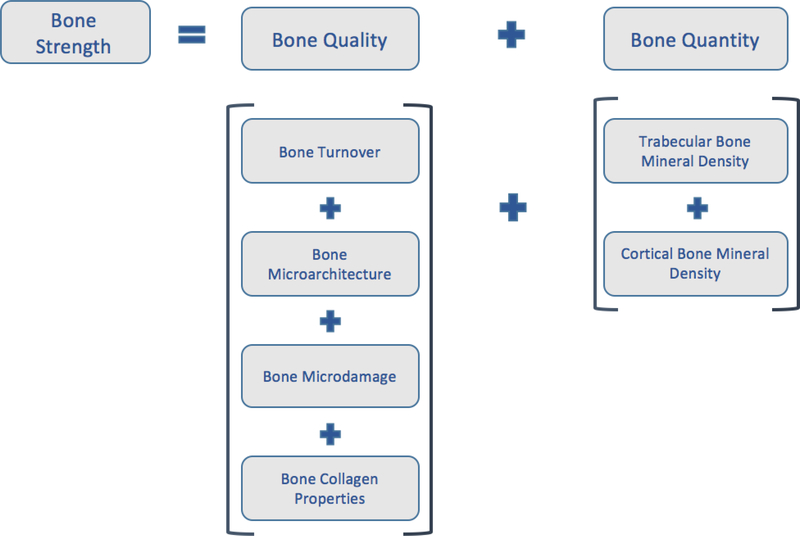
Osteoporosis is defined by the National Institutes of Health (NIH) Consensus Development Panel on Osteoporosis as a skeletal disorder characterized by compromised bone strength predisposing a person to an increased risk of fracture4. Bone strength is determined by bone quantity and bone quality (Figure 1). Bone quantity can be assessed by 2-dimensional areal bone mineral density (BMD) by dual energy X-ray absorptiometry or by 3-dimensional volumetric BMD of cortical and trabecular bone by quantitative computed tomography. Bone quality pertains to bone material properties and includes bone turnover, mineralization, microdamage, collagen properties and cortical and trabecular microarchitecture (Figure 1). CKD is associated with global impairments in bone strength; therefore, bone disease in patients with CKD may be classified as CKD-associated osteoporosis.
Figure 1. The Elements of Bone Strength.
Bone strength is defined by bone quality and bone quantity. Bone quality pertains to bone material properties and includes bone turnover, microarchitecture, microdamange and collagen properties. Bone quantity pertains to the bone mineral density of trabecular and cortical bone.
Several studies using the National Health and Examination Survey (NHANES) reported that prevalence rates of osteoporosis in patients with GFR < 60mL/minute were double those in patients with GFR > 60 mL/minute5, and that more than 80% of women and 50% of men over age 65 years with osteoporosis had a GFR < 35 mL/minute6. Cortical bone comprises around 80% of the skeleton and contributes largely to bone strength7. Hyperparathyroidism of kidney disease leads to increased cortical porosity and to de-creased cortical thickness7–9. Cortical bone loss subsequently results in loss of areal BMD7,10. Trabecular bone also contributes to bone strength. In patients with CKD, older age, hypogonadism and medications used to treat kidney diseases (i.e., glucocorticoids and calcineurin inhibitors) may result in loss of trabeculae. Mineralization defects (i.e., osteomalacia) are not uncommon in CKD; though widespread use of vitamin D analogues seems to have decreased the prevalence of mineralization defects in recent years8. Bone quality in CKD is further impaired as advanced glycation products accumulate and weaken the collagen network11, as damaged bone and microcracks are not adequately repaired, and as oxidative stress levels are heightened12.
In health, bone remodeling is balanced between bone formation by osteoblasts and bone resorption by osteoclasts; mineralization of bone is also necessary to form mature bone. The metabolic derangements present in CKD alter both remodeling and mineralization and are increasingly recognized as occurring in early kidney disease when function is considered normal, even before the development of biochemical evidence of CKD-MBD13–15. Klotho deficiency occurs in CKD and is one of the first derangements of CKD-MBD16. Low expression of Klotho induces fibroblast growth factor (FGF23) resistance, resulting in increased FGF23 levels17. Both FGF23 and Klotho are expressed in osteocytes with a complex inter-relationship, their effects on bone being both dependent and independent of each other18. Klotho deficiency decreases the number and surface area of both osteoblasts and osteoclasts while also enhancing osteoblast activity therefore affecting bone turnover18,19. The exact role of Klotho on bone at different stages of CKD is not well understood yet19. The increased expression of FGF23 expression in CKD negatively impacts bone health by suppressing osteoblast differentiation and matrix mineralization20.
The decline in Klotho and the rise in FGF23 is followed by a rise in parathyroid hormone (PTH) levels, a decline in vitamin D levels and abnormalities in calcium and phosphorus homeostasis. Moreover, metabolic acidosis alters the balance between resorption and formation. CKD is additionally accompanied by chronic inflammation that can be deleterious to bone health via pathways that effect the WNT/β-catenin signaling pathway21,22. The WNT/β-catenin signaling pathway regulates osteogenesis and decreases bone resorption22. The regulation of this pathway is known to occur by two proteins, sclerostin and Dickkopf-related protein 1 (DKK1)22. Sclerostin inhibits WNT/β-catenin signaling via binding to low-density lipoprotein receptor-related protein 5/6 (LRP5/6), a component of the Wnt receptor complex that activates the β-catenin signaling pathway23, thereby inhibiting osteoblast differentiation and causing low bone turnover. Sclerostin also plays a role in stimulating osteoclast activity24. DKK1 is an LRP5/6 antagonist whose role is not completely well characterized in osteoporosis, but which is upregulated in inflammatory diseases such as rheumatoid arthritis and may be a mediator of bone erosions in rheumatoid arthritis25. TNF-α levels and other inflammatory cytokines’ levels are higher at lower levels of kidney function26. Moreover, the expression of TNF-α is related to overexpression of sclerostin and DKK127. This may explain why both sclerostin and DKK1 levels are elevated early in the course of CKD28,29, and can at least partially account for the effect of chronic inflammation of CKD on bone health.
Together, these skeletal abnormalities result in an increased risk of fractures. Fracture risk increases as estimated glomerular filtration rate (eGFR) decreases. The 3-year cumulative incidence of fractures is as high as 5% in men and as high as 9.6% in women aged greater than 65 years with eGFR <15mL/min per 1.73 m2 as compared to 1.6% in men and 4.3% in women with eGFR ≥60mL/min per 1.73 m2 30. In NHANES III, participants with CKD had a >2-fold greater prevalence of hip fracture as compared to participants without CKD31. Fractures in CKD patients are associated with increased economic burden related to hospitalizations, morbidity and mortality. The annual costs for management of fractures in patients with CKD stages 3–5D are estimated to be as high as 556 million dollars3. In the dialysis population, those who sustained a hip fracture had a median survival time of 289 days as compared to a survival time of 714 days in controls matched by age, cardiovascular disease and dialysis duration who had not sustained hip fractures32.
DIAGNOSIS OF RENAL OSTEODYSTROPHY (AND ASSESSMENT OF FRACTURE RISK)
It is important for clinicians to make an accurate assessment of skeletal health in CKD patients. This assessment will guide the therapeutic approach that may mitigate the burden of fractures and other morbidity and mortality associated with CKD-associated osteoporosis.
INVASIVE DIAGNOSIS OF Renal Osteodystrophy
BONE BIOPSY
Renal Osteodystrophy (ROD) refers to abnormal bone pathology in kidney disease33. ROD was historically classified into hyperparathyroid bone disease (osteitis fibrosa cystica), mild hyperparathyroid bone disease, mixed osteodystrophy, low turnover/adynamic bone disease and osteomalacia. High turnover disease results from excessive activity of both osteoclasts and osteoblasts. In contrast, low bone turnover disease is characterized by the absence of osteoclast and osteoblast activity. Tetracycline double-labeled iliac crest bone biopsy is the gold standard for evaluating ROD type and mineralization.
Bone biopsy data from cohorts of patients both with CKD stages 3–5 and CKD stage 5D have elucidated the prevalence of histomorphometric abnormalities in both groups of patients9,34–36. In patients with CKD stages 3–5, normal bone followed by adynamic bone disease are the most common histomorphometric findings34,35. At least in one cohort, osteitis fibrosa cystica was the most common histomorphometric abnormality occurring in 47% of patients in CKD stages 3–436. In CKD stage 5D, the most common type of ROD is low turnover/adynamic bone disease (~60%) and the prevalence of osteomalacia is low (~3%)8,37. In 2009, the Kidney Disease Improving Global Outcomes (KDIGO) committee proposed a new classification system to better describe the bone abnormalities present in CKD and to assist with clinical decision-making. This system was based on the three key histologic features of renal osteodystrophy, bone turnover, mineralization and volume (TMV system; Table 2); histomorphometric abnormalities are reported using standard nomenclature as recommended by the American Society of Bone and Mineral Research38. This shift in classification recognized that the historical definition could not accommodate advances in our understanding of the diverse and complex pathobiology of bone disease in CKD patients. For example, the historic renal osteodystrophy classification system relied on assessments of bone turnover and mineralization but was unable to appreciate the increasingly recognized and important role of bone volume as indicators of bone disease and fracture risk in CKD39. The TMV classification system provides a clinically relevant description of the underlying bone pathology as assessed by histomorphometry, which in turn helps define the pathophysiology and thereby guide therapy. KDIGO recommends performing a bone biopsy in CKD stages 3a-5D if knowledge of the type of bone disease will affect treatment decisions. However, bone biopsy is not routinely used in the clinic because it is costly, invasive, painful, requires time consuming measurements, is only available at few centers worldwide and has not been shown to predict fracture risk. Therefore, non-invasive methods are more commonly used to identify fracture risk and underlying ROD type and inform treatment.
Table 2.
TMV classification system for renal osteodystrophy
| Turnover | Mineralization | Volume |
|---|---|---|
| Low | Normal | Low |
| Normal | Abnormal | Normal |
| High | High |
From: Moe S, Drueke T, Cunningham J, et al. Definition, evaluation, and classification of renal osteodystrophy: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 2006; 69(11):1945–53.
NON-INVASIVE DIAGNOSIS OF ROD
SKELETAL IMAGING
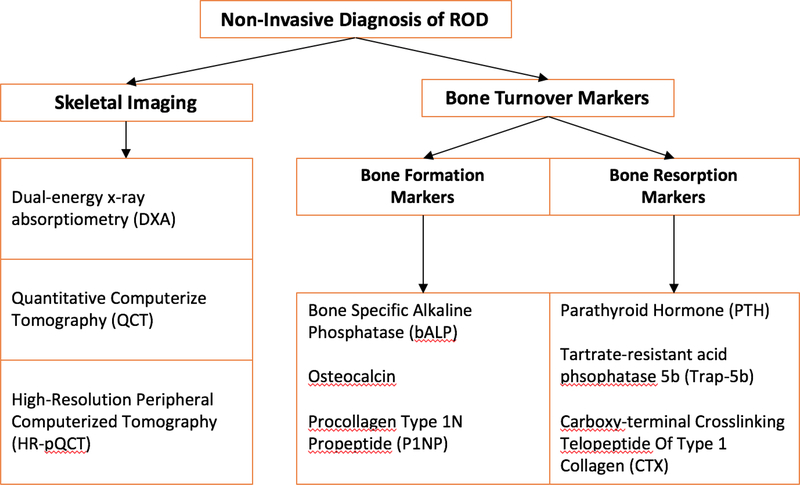
Non-invasive skeletal imaging can be used to assess the presence of skeletal abnormalities and to classify fracture risk. Measurement of areal bone mineral density (BMD) by dual energy X-ray absorptiometry (DXA) risk stratifies patients with CKD for fracture 40–42. In clinical practice, osteoporosis is detected by a DXA T-Score ≤ −2.5 or the presence of a low trauma fracture at any T-Score (Figure 2). In patients with CKD stages 3a5D with evidence of CKD-MBD and/or risk factors for osteoporosis, KDIGO recommends “BMD testing to assess fracture risk if results will impact treatment decisions”43. The optimal frequency of measurement of BMD in CKD patients is unknown. In clinical practice in the general population, BMD is tested every 1–2 years.
Figure 2. Non-Invasive Diagnosis of ROD.
Non-invasive skeletal imaging can be used to assess the presence of skeletal abnor-malities and to classify fracture risk. Measurement of bone mineral density can be done by dual energy X-ray absorptiometry (DXA). Microarchitecture and mineral density of trabecular and cortical bone can be assessed by quantitative computerized tomography (QCT) and by high-resolution peripheral computerized tomography (HR-pQCT).
Alternatively, assessment of turnover type can also be based on bone turnover markers. Bone formation markers, which are markers of osteoblast function, include bone specific alkaline phosphatase (BALP), osteocalcin, and procollagen type-1 N-terminal propeptide (P1NP). Bone resorption markers, which are markers of osteoclast number and function, include tartrate-resistant acid phosphatase 5b (Trap-5b) and C-terminal telopeptides of type I collagen (CTX).
DXA is helpful in assessing areal BMD or bone quantity. However, it is unable to assess bone quality. Peripheral quantitative computed tomography (pQCT) and high resolution pQCT (HRpQCT) are imaging techniques that can assess the skeleton in three dimensions, providing information on microarchitecture and mineral density of both trabecular and cortical bone44. pQCT is widely available while HRpQCT is limited to research settings. The more widespread use of these techniques is limited by the absence of studies showing their superiority over DXA in predicting fractures or in guiding therapy44.
BIOCHEMICAL AND BIOMARKER ASSESSMENT OF BONE TURNOVER
Although bone biopsy is the gold standard for the diagnosis and classification of bone disease, its use in the clinic is limited due to lack of performance by nephrologists and endocrinologists and few centers internationally that process and read bone histomorphometry. Alternatively, assessment of turnover type can also be based on bone turnover markers with a moderate degree of accuracy (Table 3) (Figure 2). The most commonly used marker of turnover by nephrologists is PTH. Bone formation markers, which are markers of osteoblast function, include bone specific alkaline phosphatase (BSAP), osteocalcin, and procollagen type-1 N-terminal propeptide (P1NP). Bone resorption markers, which are markers of osteoclast number and function, include tartrate-resistant acid phosphatase 5b (Trap-5b) and C-terminal telopeptides of type I collagen (CTX).
Table 3.
AUROCs of Circulating Bone Biomarkers to Distinguish High and Low Bone Turnover from Non-high and Non-low Bone Turnover as Assessed by BFR/BS
| Blood Sample Marker | N | AUROC (95% CI) | Best Cutoff |
|---|---|---|---|
| Low vs Non-low | |||
| iPTH, pg/mL | 280 vs 196 | 0.701 (0.653–0.750) | 103.8 |
| wPTH, pg/mL | 260 vs 180 | 0.712 (0.662–0.761) | 48.0 |
| bALP, U/L | 273 vs 190 | 0.757 (0.713–0.801) | 33.1 |
| P1NP, ng/mL | 280 vs 1,197 | 0.650 (0.599–0.701) | 498.9 |
| Combined iPTH + bALP | 272 vs 188 | 0.718 (0.670–0.767) | NA |
| Combined wPTH + bALP | 257 vs 174 | 0.743 (0.695–0.790) | NA |
| High vs Non-hiah | |||
| iPTH, pg/mL | 81 vs 395 | 0.724 (0.663–0.786) | 323.0 |
| wPTH, pg/mL | 75 vs 365 | 0.678 (0.611–0.746) | 61.4 |
| bALP, U/L | 77 vs 386 | 0.711 (0.655–0.767) | 42.1 |
| P1NP, ng/mL | 81 vs 396 | 0.743 (0.689–0.797) | 621.1 |
| Combined iPTH + bALP | 76 vs 384 | 0.718 (0.658–0.779) | NA |
| Combined wPTH + bALP | 72 vs 359 | 0.691 (0.628–0.725) | NA |
Abbreviations: AUROC, area under the receiver operating characteristic curve; bALP, bone-specific alkaline phosphatase; BFR/BS, bone formation rate/bone surface; CI, confidence interval; iPTH, intact parathyroid hormone; NA, not available; P1NP, amino-terminal propeptide of type 1 procollagen; wPTH, whole parathyroid hormone. From Sprague et al, 2015. Diagnostic Accuracy of Bone Turnover Markers and Bone Histology in Patients With CKD Treated by Dialysis. Am J Kidney Dis.
Bone turnover markers can be useful when they are at the extremes of their ranges45. At those extremes, they can help distinguish between low and non-low turnover and high and non-high turnover bone disease with an area under the curve >0.67 (Table 2). Their correlation with bone histomorphometric parameters from bone biopsies is moderate (Table 4).
Table 4.
Diagnostic performance of bone biomarkers compared to bone biopsy histomorphometry
| Biomarker | Assay | Population (N) | Diagnosis | Cutoff | Prevalence (%) | Spec (%) | Sens (%) | NPV (%) | PPV (%) | AUC | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Markers of bone turnover | |||||||||||
| iPTH (pg/ml) | Roche Diagnostics | HD, 476 | Low BFR | <103.8 | 59 | 0.70 | Sprague et al. [7••] | ||||
| iPTH (pg/ml) | Roche Diagnostics | HD, 476 | High BFR | >323.0 | 17 | 0.72 | Sprague et al. [7••] | ||||
| iPTH (pg/ml) | Immulite, DPC | PD, 41 | HTO | >386 | 49 | 62 | 70 | 80 | 77 | 0.77 | de Oliveira et al. [17] |
| iPTH (pg/ml) | Roche Diagnostics | HD, 40 | Non-LTO | NA | 50 | 0.85 | Haarhaus et al. [18] | ||||
| iPTH (pg/ml) | Scantibodies | CKD-5, 60 | Low BFR | <335 | NA | 80 | 40 | 53 | 72 | Cejka et al. [19] | |
| iPTH (pg/ml) | Scantibodies | CKD-5, 60 | High BFR | >391 | NA | 80 | 43 | 84 | 38 | Cejka et al. [19] | |
| iPTH (pg/ml) | Immulite, DPC | hd, n | LTO | <150 | 60 | 50 | 85 | 83 | Barreto et al. [20] | ||
| iPTH (pg/ml) | Immulite, DPC | HD, 97 | HTO | >300 | 37 | 69 | 75 | 62 | Barreto et al. [20] | ||
| iPTH (pg/ml) | Nichols Diagnostics | CKD 3–4, 26 | HTO | >31 | 54 | 100 | 40 | 0.91 | Lehmann et al. [21] | ||
| iPTH (pg/ml) | Nichols Diagnostics | HD - PD, 78 | HTO | >27 | 69 | 100 | 18 | 0.85 | Lehmann et al. [21] | ||
| iPTH (pg/ml) | Biosource | CKD 4–5, 84 | ABD | <237 | 23 | 53 | 78 | 0.75 | Bervoets et al. [22] | ||
| iPTH (pg/ml) | Nichols Diagnostics | HD, 103 | ABD | <150 | 37 | 76 | 81 | 88 | 65 | Couttenye et al. [23] | |
| biPTH (pg/ml) | Scantibodies | HD, 440 | Low BFR | <48 | 59 | 0.71 | Sprague et al. [7••] | ||||
| biPTH (pg/ml) | Scantibodies | HD, 440 | High BFR | >61.4 | 17 | 0.68 | Sprague et al. [7••] | ||||
| biPTH (pg/ml) | Nichols Diagnostics | CKD 3–4, 26 | HTO | >21 | 54 | 100 | 40 | 0.94 | Lehmann et al. [21] | ||
| biPTH (pg/ml) | Nichols Diagnostics | HD + PD, 78 | HTO | >15 | 69 | 100 | 9 | 0.86 | Lehmann et al. [21] | ||
| PTH (1–841/C-PTH | Scantibodies/Nichols | D, 51 | LTO | <1 | 55 | 83 | 100 | 100 | 88 | Monier-Faugere et al. [24] | |
| PTH (1–84)/C-PTH | Scantibodies/N ichols | CKD 3–4, 26 | HTO | >0.76 | 54 | 100 | 0 | 0.54 | Lehmann et al. [21] | ||
| PTH (1—84)/C-PTH | Scantibodies/Nichols | HD - PD, 78 | HTO | >0.8 | 69 | 100 | 22 | 0.55 | Lehmann et al. [21] | ||
| Sclerostin (pg/ml) | R&D Systems | CKD-5, 60 | Low BFR | <2800 | NA | 80 | 35 | 66 | 57 | Cejka et al. [19] | |
| Sclerostin (pg/ml) | R&D Systems | CKD-5, 60 | High BFR | >1925 | NA | 80 | 59 | 40 | 93 | Cejka et al. [19] | |
| Sclerostin (ng/dl) | Quidel/TECOmedical | PD, 41 | HTO | >1.82 | 49 | 62 | 85 | 61 | 68 | 0.70 | de Oliveira et al. [17] |
| Markers of bone formation | |||||||||||
| BsAP (U/l) | Quidel | HD, 463 | Low BFR | <33.1 | 59 | 0.76 | Sprague et al. [7••] | ||||
| BsAP (U/l) | Quidel | HD, 463 | High BFR | >42.1 | 17 | 0.71 | Sprague et al. [7••] | ||||
| BsAP (U/l) | Metra Biosystems | PD, 41 | HTO | >57 | 49 | 96 | 65 | 95 | 92 | 0.79 | de Oliveira et al. [17] |
| BsAP | Quidel | HD, 40 | Non-LTO | NA | 50 | 0.89 | Haarhaus et al. [18] | ||||
| BsAP (U/l) | Electrophoretica | CKD 4–5, 84 | ABD | <23 | 23 | 66 | 83 | 0.79 | Bervoets et al. [22] | ||
| BsAP (U/l) | Electrophoretica | HD, 103 | ABD | <27 | 37 | 86 | 78 | 88 | 75 | Couttenye et al. [23] | |
| Blx | HPLCa | HD, 40 | Non-LTO | NA | 50 | 0.83 | Haarhaus et al. [18] | ||||
| TAP (U/l) | a | PD, 41 | HTO | >107 | 49 | 81 | 65 | 80 | 76 | de Oliveira et al. [17] | |
| TAP (U/l) | Kinetic methoda | CKD 4–5, 84 | ABD | <66 | 23 | 54 | 74 | 0.65 | Bervoets et al. [22] | ||
| TAP (U/l) | Kinetic methoda | HD, 103 | ABD | <123 | 37 | 83 | 75 | Couttenye et al. [23] | |||
| OC (ng/ml) | Biosource | CKD 4–5, 84 | ABD | <41 | 23 | 67 | 83 | 94 | 47 | 0.80 | Bervoets et al. [22] |
| OC (ng/ml) | Diasorin | HD, 103 | ABD | <14 | 37 | 54 | 96 | 97 | 55 | Couttenye et al. [23] | |
| P1NP (ng/ml) | Unknown | HD, 477 | Low BFR | <499 | 59 | 0.65 | Sprague et al. [7••] | ||||
| P1NP (ng/ml) | Unknown | HD, 477 | High BFR | >621 | 17 | 0.74 | Sprague et al. [7••] | ||||
| Markers of bone resorption | |||||||||||
| DPYD (nmol/1) | Quidel | PD, 41 | HTO | >14 | 49 | 62 | 75 | 61 | 65 | 0.72 | de Oliveira et al. [17] |
| DPYD/PYD (nmol/1) | HPLCa | CKD 4–5, 84 | ABD | Bervoets et al. [22] |
From: Curr Osteoporos Rep (2017) 15:178 –186
MANAGEMENT OF KIDNEY RELATED BONE DISEASE AND THERAPEUTIC CONSIDERATIONS
VITAMIN D, PHOSPHORUS AND PTH MANAGEMENT AND LIFE-STYLE MODIFICATION
The first-line therapy for the management of CKD-associated osteoporosis is treatment of the mineral and metabolic abnormalities associated with CKD-MBD. Management of hyperparathyroidism, hyperphosphatemia and vitamin D deficiency need to occur before initiation of medications that are used to treat osteoporosis (see Treatment Section) 43. Secondary hyperparathyroidism has long been considered a major feature of CKD-MBD. It begins early in the course of CKD. Secondary hyperparathyroidism prevalence and severity increase as kidney function declines. In early stages of CKD, management strategies aim at preventing the rise of PTH levels. The KDIGO 2017 guidelines recommend managing hyperphosphatemia, hypocalcemia, high phosphate intake and nutritional vitamin D (25-hydroxy vitamin D) deficiency in CKD stage 3–5 patients with rising PTH levels or PTH levels consistently above the upper limit of normal46. In patients with CKD stage 5D, KDIGO recommends targeting PTH levels of 2 to 9 times the upper normal limit of the assay. Lowering plasma phosphate levels with phosphate binders can partially reverse hyperparathyroidism. When PTH levels fail to decrease or continue to rise, vitamin D receptor activators (VDRA) should be used46,47–49. Using nutritional vitamin D, such as ergocalciferol or cholecalciferol, is possible but not recommended by the guidelines because of unproven benefit46. The optimal serum concentration of nutritional vitamin D is unknown but data in patients with CKD stage 5D suggest that levels of 25hydroxy D > 30 ng/mL may be sufficient50. When PTH levels remain elevated despite sufficient 25-hydroxy D levels, calcimimetics are added. Calcimimetics, such as cinacalcet, can increase BMD51, normalize bone histology52 and may reduce the risk of fractures in CKD patients53. Whether this translates into improved mortality in CKD stage 5D patients is less clear54. Etecalcetide is an intravenous calcimimetic that was recently approved for the treatment of secondary hyperparathyroidism. When compared to cinacalcet in CKD stage 5D patients with PTH levels> 500pg/mL, etecalcetide was superior to cinalacet in reducing PTH levels (p for superiority 0.04)55. Long term effects and safety of eticalcetide have yet to be studied. Finally, non-pharmacologic interventions that include modifying dietary calcium and nutritional vitamin D, smoking cessation, weight bearing exercise, and moderating alcohol intake 56 should be discussed with all patients.
ANTI-RESORPTIVE AGENTS
Antiresorptive agents include bisphosphonates and denosumab. These agents inhibit osteoclast mediated bone resorption making them advantageous for the treatment of patients with high turnover bone disease; they are contraindicated in patients with low turnover or adynamic bone disease. Trials in patients with CKD-MBD are needed to determine their skeletal and extra-skeletal safety57.
BISPHOSPHONATES
Bisphosphonates inhibit farnesyl pyrophosphate synthase, an important enzyme for osteoclast function. By inhibiting farnesyl pyrophosphate, they lead to osteoclast cell death. Long-term use of bisphosphonates is known to decrease the rate of bone turnover and remodeling58,59. As such, the duration of their use is controversial and they are not indicated in patients with low turnover or adynamic bone disease. Bisphosphonates are mainly cleared via the kidneys. Therefore, these agents have not been recommended in patients with CrCl < 30 mL/minute due to concern of excessive accumulation of bisphos-phonate in the skeleton, thus resulting in over suppression of bone remodeling. Finally, intravenous bisphosphonates have been associated with acute kidney injury.
Despite the safety concerns of using bisphosphonates in patients with CKD, a growing body of data suggest that these agents are safe in patients with an eGFR 15 to 59 ml/min/1.73 m2 due to age-related declines in kidney function without CKD-MBD 60,61. Miller et al. conducted a retrospective analysis evaluating the effect of risendronate on post-menopausal women with creatinine clearance of <80 ml/min62. Women with lower creatinine clearance treated with risedronate had a significant increase in BMD and reduction in vertebral fractures compared to women treated with placebo. The rate of kidney and non-kidney adverse events was not higher in women with kidney failure. In Japanese subjects with osteoporosis and eGFR 30 to ≥ 90mL/min/1.73 m2, risendronate administration improved lumbar spine BMD (p<0.001) and suppressed bone turnover markers urine N-terminal telopeptide of type 1 collagen, urine C-terminal telopeptide of type 1 collagen and BSAP (p <0.001). Importantly, kidney function was preserved throughout the treatment duration63. In a secondary analysis of the Fracture Intervention Trial, treatment with alendronate was safe and resulted in an increase in total hip and spine BMD and a reduction in spinal fractures in women with an eGFR <45 mL/minute61. Alendronate administration to patients with CKD stages 3–4 resulted in a significant increase in lumbar spine T-Score (p=0.03) as compared to patients who received placebo64. In CKD stage 5D patients, ibandronate significantly improved lumbar spine T score65. Despite these skeletal benefits, the major concern with the use of bisphosphonates is the development of adynamic bone disease. Ota et al.66 investigated bone-tissue level safety of alendronate in 5/6 nephrectomized rats with CKD stage 4. Alendronate improved femoral trabecular bone volume fraction, the mineral-to-matrix-ratio of the endosteal and periosteal regions of cortical bone, and the carbonate-to-phosphate ratio of both trabecular and cortical bone66. These results are promising but cannot be generalized to our population since alendronate was only administered for 4 weeks, while our patients usually require longer treatment durations.
DENOSUMAB
Denosumab is a monoclonal antibody against the receptor activator of NF-κB ligand (RANKL). Denosumab does not act directly on bone but rather through mimicking the action of osteoprotegerin, a decoy receptor produced by osteoblasts that binds RANK and inhibits its binding to RANKL. Via this mechanism, denosumab inhibits osteoclast proliferation and development, making it a potent antiresportive agent. Denosumab is cleared by the reticuloendothelial system and not by the kidneys. In contrast to bisphos-phonates, it does not accumulate in kidney failure, and therefore does not carry the risk of long-term oversuppression of bone turnover.
The role of denosumab in managing osteoporosis in patients with age-related kidney disease was explored in a post-hoc analysis of The Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months (FREEDOM) Trial 67. The registration trial included 7868 postmenopausal women and demonstrated that treatment with denosumab for 36 months reduced vertebral, hip and non-vertebral fracture risk by 68%, 40%, and 20% respectively 68. In another study, administration of denosumab to CKD stage 5D patients with PTH >1000 pg/mL resulted in an increase in femoral neck and lumbar spine BMD at 6 months69. The effects of denosumab on bone histology in patients with kidney failure have not been studied yet. In the FREEDOM trial, the rate of adverse events did not differ between patients with creatinine clearance less than as compared to greater than 30 ml/min/1.73 m2 67. However, later studies and case reports demonstrated that the administration of denosumab is associated with a significant risk of hypocalcemia particularly at lower creatinine clearance70–72. These risks may be mitigated by adequate supplementation with calcium and vitamin D prior to therapy initiation and by the use of high calcium dialysate baths in CKD stage 5D patients73.
OSTEOANABOLIC AGENTS
Osteoanabolic agents refer to drugs that stimulate bone formation. Teriparatide and Abaloparatide are the two osteoanabolic agents currently used for the management of osteoporosis. They are forms of recombinant PTH that mimic PTH action on osteoblasts. Osteoblast formation is increased and osteoclast death is inhibited following PTH administration74, making the administration of osteoanabolic agents ideal for increasing bone mass in low turnover or adynamic bone disease. However, their use is contraindicated in CKD patients with high turnover bone disease caused by elevated PTH levels, as high PTH levels can lead to CKD-associated osteoporosis via increases in cortical porosity and thinning due to endocortical trabecularization10. The long-term side effects of osteoanabolic agents in patients with CKD have not been studied. Since hyperparathyroidism is associated with cardiovascular calcification and mortality, then it is plausible that osteoanabolic agents would lead to the same consequences. Due to animal skeletal safety data, their use is currently limited to 2 years per lifetime duration. After holding these therapies, data suggest that women with postmenopausal osteoporosis will require another agent to prevent further bone loss75.
TERIPARATIDE
Teriparatide is a recombinant peptide of the first 34 amino terminal residues of PTH. It was the first FDA approved osteoanabolic agent to treat osteoporosis and to prevent fractures in both age-related and glucocorticoid-induced osteoporosis 76,77. Iliac crest bone biopsies in postmenopausal women treated with teriparatide for 19 months showed a significant increase in cancellous bone volume and connectivity density, cortical bone thickness78 and trabecular morphology as compared to biopsies in postmenopausal women receiving placebo79. Our initial knowledge on the safety of use of teriparatide in kidney failure came from studies in post-menopausal women with age-related decline in kidney function. In a post-hoc analysis of the Fracture Prevention Trial80, females with post-menopausal osteoporosis and eGFR 30 to 80 mL/min per 1.73 m2 had significant increases in lumbar spine and femoral neck BMD. Compared to women with eGFR ≥ 80 mL/min per 1.73 m2, they had similar reductions in the risk of vertebral and non-vertebral fractures. Importantly, the subjects with kidney dysfunction did not have a higher incidence of adverse events as compared to the group with normal kidney function. The pharmacokinetic safety profile of teriparatide in kidney failure has since been tested proving that there is no risk of accumulation of once weekly injections of teriparatide in kidney failure81. Data on the use of teriparatide in patients with moderate to severe CKD with MBD is available from small observational studies67,70,82–86. Daily administration of teriparatide for six months to CKD stage 5D patients with biopsy proven adynamic bone disease led to an increase in lumbar spine BMD with significant monthly increases in both lumbar spine and femoral neck BMD84. Administration of once-weekly teriparatide to CKD stage 5D patients with hypoparathyroidism and osteoporosis resulted in an increase in lumbar spine BMD. Resorption markers’ levels increased significantly as well87. The most commonly seen adverse events included hypercalcemia and hyperuricemia, which were more common in patients with the lowest levels of creatinine clearance80. Some patients with kidney failure also experienced transient hypotension87.
ABALOPARATIDE
Abaloparatide is a recombinant PTH-related peptide analogue that shares the first 20 amino acids of the N terminus of PTH-rp. Abaloparatide was designed to have relatively greater affinity for the transient state of PTH/PTH1 receptor; thus, being more purely anabolic 88. In ovariectomized rats, abaloparatide increased bone formation and mass without increasing bone resorption 89,90. Similarly, in ovariectomized monkeys, abaloparatide administration led to increased bone formation and bone mass in vertebral and non-vertebral sites without influencing bone resorption markers91. Postmenopausal women receiving abaloparatide also had significant improvements in BMD at the total hip, femoral hip and lumbar spine92,93. In The Abaloparatide Comparator Trial In Vertebral Endpoints (ACTIVE)93, postmenopausal women receiving subcutaneous abaloparatide for 18 months had a relative risk of vertebral fractures of 0.14 (p<0.001) as compared to women receiving placebo. Bone histomorphometry in post-menopausal women treated with 12–18 months of abaloparatide demonstrated no evidence of excessive osteoid, marrow fibrosis, or abnormalities in mineralization 94. Furthermore, patients treated with abaloparatide had lower eroded surface on histomorphometry versus the placebo group 93, but had equivalent increases in cortical porosity compared to teriparatide 94. These observations are consistent with the clinical trial bone turnover marker data showing that the rise in CTX, a resorption marker, was significantly less pronounced with abaloparatide than with teriparatide 94. The risk of hypercalcemia with abaloparatide can be as much as 50% lower than the risk of hypercalcemia with teriparatide93. There are no published studies of abaloparatide in patients with kidney failure. However, the lower risk of hypercalcemia and hyperuricemia associated with its use in patients with healthy kidney function make it more attractive for use in CKD patients.
DEVELOPMENT OF NEW AGENTS
Inflammation’s role in renal osteodystrophy and skeletal health is increasingly being studied (Refer to section: CKD effects on bone strength). Inhibition of TNF-α decreases levels of DKK1 in rheumatoid arthritis patients95. Similarly, rheumatoid arthritis patients receiving IL-6 inhibitors experience decreases in DKK1 levels and increases in the bone formation marker PINP96.
Clinical trials involving sclerostin inhibitors in post-menopausal osteoporosis increase BMD and decrease the risk of vertebral and non-vertebral fractures 97–99. Despite the large increases in bone mass seen with these agents, we do not recommend their use since the function of sclerostin in the vasculature is not well understood and in one clinical trial there were increased cardiovascular events among patients randomized to the monoclonal antibody against sclerostin99,100.
Cathepsin K antagonists were also developed to decrease bone resorption by inhibiting Cathepsin K, a protease found in osteoclasts. These drugs never reached phase IV trials due to their association with increased risk of cerebrovascular events 101.
While none of these agents were developed with the CKD population in mind, their development sheds light on an exciting future for CKD-associated osteoporosis therapy. They highlight a departure from defining skeletal health in terms of systemic and hormonal derangements to a more bone-centric approach focused on osteocyte cell signaling and interactions. How these new approaches will affect the skeletal and extraskeletal manifestations of CKD needs to be investigated.
CONCLUSION
CKD-associated osteoporosis is a complex disease that confers high morbidity and mortality to patients with kidney disease. Timely diagnosis of the underlying skeletal abnormalities identifies patients for treatments that may prevent future bone loss and decrease fracture risk. All patients with CKD should be managed with strategies that mitigate the derangements that are associated with declining kidney function and CKD-MBD, such as the use of phosphate binders, vitamin D analogs and/or calcimimetics. Based on the 2017 KDIGO Guidelines, fracture risk classification by DXA should be performed for patients with CKD-MBD and/or clinical risk factors for osteoporosis or fractures. The frequency of fracture risk assessment for CKD patients is not known, but based on the general population guidelines, every 2 years may be acceptable. Once fracture risk and/or a diagnosis of osteoporosis has been established, patients should be counseled on lifestyle modifications that are beneficial to the skeleton, including proper nutrition, weight bearing exercise, smoking cessation and limiting alcohol intake. Once the need for treatments that may mitigate bone loss and lower fracture risk are established, determination of ROD type is necessary to inform treatment. Bone turnover markers, when at the extremes of their ranges, can be helpful in distinguishing low from non-low turnover; thus, distinguishing which patients might benefit from an anabolic versus an anti-resorptive agent. When bone turnover markers are unable to assist with identification of underlying ROD type, bone biopsy is necessary. Ultimately, the development and study of treatments that are directed against fractures in patients with CKDassociated osteoporosis are needed to provide evidence-based recommendations that will both change nephrology practice patterns and lead to improved quality of life and decreased mortality in patients with CKD.
Footnotes
Compliance with Ethical Standards
Conflict of Interest
Thomas Nickolas reports grant support and person fees from Amgen and is on the Scientific Advisory Board.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
••Of major importance
- 1.Bikbov B, Perico N, Remuzzi G, on behalf of the GBDGDEG. Disparities in Chronic Kidney Disease Prevalence among Males and Females in 195 Countries: Analysis of the Global Burden of Disease 2016 Study. Nephron 2018:1–6. [DOI] [PubMed] [Google Scholar]
- 2.Alem AM, Sherrard DJ, Gillen DL, et al. Increased risk of hip fracture among patients with end-stage renal disease. Kidney Int 2000;58:396–9. [DOI] [PubMed] [Google Scholar]
- 3.Kim SM, Long J, Montez-Rath M, Leonard M, Chertow GM. Hip Fracture in Patients With Non-Dialysis-Requiring Chronic Kidney Disease. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 2016;31:1803–9. [DOI] [PubMed] [Google Scholar]
- 4.NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy, March 7–29, 2000: highlights of the conference. Southern medical journal 2001;94:569–73. [PubMed] [Google Scholar]
- 5.Nickolas TL, Stein E, Cohen A, et al. Bone mass and microarchitecture in CKD patients with fracture. J Am Soc Nephrol 2010;21:1371–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klawansky S, Komaroff E, Cavanaugh PF, Jr., et al. Relationship between age, renal function and bone mineral density in the US population. Osteoporos Int 2003;14:570–6. [DOI] [PubMed] [Google Scholar]
- 7.Sharma AK, Toussaint ND, Masterson R, et al. Deterioration of Cortical Bone Microarchitecture: Critical Component of Renal Osteodystrophy Evaluation. American journal of nephrology 2018;47:376–84. [DOI] [PubMed] [Google Scholar]
- • 8.Malluche HH, Mawad HW, Monier-Faugere MC. Renal osteodystrophy in the first decade of the new millennium: analysis of 630 bone biopsies in black and white patients. J Bone Miner Res 2011;26:1368–76. This paper defined the characteristics of renal osteodystrophy in the contemporary era, in light of the marked differences that have occurred over the past several decades in the management of CKD-MBD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carvalho C, Magalhaes J, Neto R, et al. Cortical bone analysis in a predialysis population: a comparison with a dialysis population. Journal of bone and mineral metabolism 2016. [DOI] [PubMed] [Google Scholar]
- 10.Nickolas TL, Stein EM, Dworakowski E, et al. Rapid cortical bone loss in patients with chronic kidney disease. J Bone Miner Res 2013;28:1811–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vashishth D, Gibson GJ, Khoury JI, Schaffler MB, Kimura J, Fyhrie DP. Influence of nonenzymatic glycation on biomechanical properties of cortical bone. Bone 2001;28:195–201. [DOI] [PubMed] [Google Scholar]
- 12.McNerny EMB, Nickolas TL. Bone Quality in Chronic Kidney Disease: Definitions and Diagnostics. Current osteoporosis reports 2017;15:207–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wesseling-Perry K, Pereira RC, Tseng CH, et al. Early skeletal and biochemical alterations in pediatric chronic kidney disease. Clinical journal of the American Society of Nephrology : CJASN 2012;7:146–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drueke TB, Massy ZA. Changing bone patterns with progression of chronic kidney disease. Kidney international 2016;89:289–302. [DOI] [PubMed] [Google Scholar]
- 15.Graciolli FG, Neves KR, Barreto F, et al. The complexity of chronic kidney disease-mineral and bone disorder across stages of chronic kidney disease. Kidney Int 2017;91:1436–46. [DOI] [PubMed] [Google Scholar]
- 16.Barker SL, Pastor J, Carranza D, et al. The demonstration of alphaKlotho deficiency in human chronic kidney disease with a novel synthetic antibody. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association 2015;30:223–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakatani T, Sarraj B, Ohnishi M, et al. In vivo genetic evidence for klotho-dependent, fibroblast growth factor 23 (Fgf23) -mediated regulation of systemic phosphate homeostasis. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 2009;23:433–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaludjerovic J, Komaba H, Lanske B. Effects of klotho deletion from bone during chronic kidney disease. Bone 2017;100:50–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Komaba H, Kaludjerovic J, Hu DZ, et al. Klotho expression in osteocytes regulates bone metabolism and controls bone formation. Kidney Int 2017;92:599–611. [DOI] [PubMed] [Google Scholar]
- 20.Wang H, Yoshiko Y, Yamamoto R, et al. Overexpression of fibroblast growth factor 23 suppresses osteoblast differentiation and matrix mineralization in vitro. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 2008;23:939–48. [DOI] [PubMed] [Google Scholar]
- 21.Akchurin OM, Kaskel F. Update on inflammation in chronic kidney disease. Blood purification 2015;39:84–92. [DOI] [PubMed] [Google Scholar]
- 22.Rossini M, Gatti D, Adami S. Involvement of WNT/beta-catenin signaling in the treatment of osteoporosis. Calcified tissue international 2013;93:121–32. [DOI] [PubMed] [Google Scholar]
- 23.Li X, Zhang Y, Kang H, et al. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. The Journal of biological chemistry 2005;280:19883–7. [DOI] [PubMed] [Google Scholar]
- 24.Wijenayaka AR, Kogawa M, Lim HP, Bonewald LF, Findlay DM, Atkins GJ. Sclerostin stimulates osteocyte support of osteoclast activity by a RANKL-dependent pathway. PloS one 2011;6:e25900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang SY, Liu YY, Ye H, et al. Circulating Dickkopf-1 is correlated with bone erosion and inflammation in rheumatoid arthritis. The Journal of rheumatology 2011;38:821–7. [DOI] [PubMed] [Google Scholar]
- 26.Gupta J, Mitra N, Kanetsky PA, et al. Association between albuminuria, kidney function, and inflammatory biomarker profile in CKD in CRIC. Clinical journal of the American Society of Nephrology : CJASN 2012;7:1938–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heiland GR, Zwerina K, Baum W, et al. Neutralisation of Dkk-1 protects from systemic bone loss during inflammation and reduces sclerostin expression. Annals of the rheumatic diseases 2010;69:2152–9. [DOI] [PubMed] [Google Scholar]
- 28.Cejka D, Herberth J, Branscum AJ, et al. Sclerostin and Dickkopf-1 in renal osteodystrophy. Clin J Am Soc Nephrol 2011;6:877–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sabbagh Y, Graciolli FG, O’Brien S, et al. Repression of osteocyte Wnt/beta-catenin signaling is an early event in the progression of renal osteodystrophy. J Bone Miner Res 2012;27:1757–72. [DOI] [PubMed] [Google Scholar]
- 30.Naylor KL, McArthur E, Leslie WD, et al. The three-year incidence of fracture in chronic kidney disease. Kidney international 2014;86:810–8. [DOI] [PubMed] [Google Scholar]
- 31.Nickolas TL, McMahon DJ, Shane E. Relationship between moderate to severe kidney disease and hip fracture in the United States. Journal of the American Society of Nephrology : JASN 2006;17:3223–32. [DOI] [PubMed] [Google Scholar]
- 32.Mittalhenkle A, Gillen DL, Stehman-Breen CO. Increased risk of mortality associated with hip fracture in the dialysis population. American journal of kidney diseases : the official journal of the National Kidney Foundation 2004;44:672–9. [PubMed] [Google Scholar]
- 33.Moe S, Drueke T, Cunningham J, et al. Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 2006;69:1945–53. [DOI] [PubMed] [Google Scholar]
- 34.Spasovski GB, Bervoets AR, Behets GJ, et al. Spectrum of renal bone disease in end-stage renal failure patients not yet on dialysis. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association 2003;18:1159–66. [DOI] [PubMed] [Google Scholar]
- 35.Tomiyama C, Carvalho AB, Higa A, Jorgetti V, Draibe SA, Canziani ME. Coronary calcification is associated with lower bone formation rate in CKD patients not yet in dialysis treatment. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 2010;25:499–504. [DOI] [PubMed] [Google Scholar]
- 36.Lehmann G, Ott U, Kaemmerer D, Schuetze J, Wolf G. Bone histomorphometry and biochemical markers of bone turnover in patients with chronic kidney disease Stages 3 – 5. Clin Nephrol 2008;70:296–305. [DOI] [PubMed] [Google Scholar]
- 37.Sprague SM, Bellorin-Font E, Jorgetti V, et al. Diagnostic Accuracy of Bone Turnover Markers and Bone Histology in Patients With CKD Treated by Dialysis. Am J Kidney Dis 2015. [DOI] [PubMed] [Google Scholar]
- 38.Parfitt AM, Drezner MK, Glorieux FH, et al. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 1987;2:595–610. [DOI] [PubMed] [Google Scholar]
- 39.Barreto FC, Barreto DV, Moyses RM, et al. Osteoporosis in hemodialysis patients revisited by bone histomorphometry: a new insight into an old problem. Kidney Int 2006;69:1852–7. [DOI] [PubMed] [Google Scholar]
- 40.Iimori S, Mori Y, Akita W, et al. Diagnostic usefulness of bone mineral density and biochemical markers of bone turnover in predicting fracture in CKD stage 5D patients--a single-center cohort study. Nephrol Dial Transplant 2012;27:345–51. [DOI] [PubMed] [Google Scholar]
- •• 41.Yenchek RH, Ix JH, Shlipak MG, et al. Bone mineral density and fracture risk in older individuals with CKD. Clin J Am Soc Nephrol 2012;7:1130–6. This was the first study to demonstrate that measurement of BMD by DXA predicted fracture in CKD patients with similar accuracy to that of patients with healthy kidney function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.West SL, Lok CE, Langsetmo L, et al. Bone mineral density predicts fractures in chronic kidney disease. J Bone Miner Res 2015;30:913–9. [DOI] [PubMed] [Google Scholar]
- 43.Ketteler M, Block GA, Evenepoel P, et al. Executive summary of the 2017 KDIGO Chronic Kidney Disease–Mineral and Bone Disorder (CKD-MBD) Guideline Update: what’s changed and why it matters. Kidney International 2017;92:26–36. [DOI] [PubMed] [Google Scholar]
- 44.Jamal SA, Nickolas TL. Bone imaging and fracture risk assessment in kidney disease. Current osteoporosis reports 2015;13:166–72. [DOI] [PubMed] [Google Scholar]
- 45.Nickolas TL, Cremers S, Zhang A, et al. Discriminants of prevalent fractures in chronic kidney disease. J Am Soc Nephrol 2011;22:1560–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ketteler M, Block GA, Evenepoel P, et al. Executive summary of the 2017 KDIGO Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Guideline Update: what’s changed and why it matters. Kidney Int 2017;92:26–36. [DOI] [PubMed] [Google Scholar]
- 47.Coyne D, Acharya M, Qiu P, et al. Paricalcitol capsule for the treatment of secondary hyperparathyroidism in stages 3 and 4 CKD. Am J Kidney Dis 2006;47:263–76. [DOI] [PubMed] [Google Scholar]
- 48.Coyne DW, Goldberg S, Faber M, Ghossein C, Sprague SM. A randomized multicenter trial of paricalcitol versus calcitriol for secondary hyperparathyroidism in stages 3–4 CKD. Clin J Am Soc Nephrol 2014;9:1620–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sprague SM, Llach F, Amdahl M, Taccetta C, Batlle D. Paricalcitol versus calcitriol in the treatment of secondary hyperparathyroidism. Kidney international 2003;63:1483–90. [DOI] [PubMed] [Google Scholar]
- 50.Coen G, Mantella D, Manni M, et al. 25-hydroxyvitamin D levels and bone histomorphometry in hemodialysis renal osteodystrophy. Kidney Int 2005;68:1840–8. [DOI] [PubMed] [Google Scholar]
- 51.Tsuruta Y, Okano K, Kikuchi K, Tsuruta Y, Akiba T, Nitta K. Effects of cinacalcet on bone mineral density and bone markers in hemodialysis patients with secondary hyperparathyroidism. Clinical and experimental nephrology 2013;17:120–6. [DOI] [PubMed] [Google Scholar]
- 52.Behets GJ, Spasovski G, Sterling LR, et al. Bone histomorphometry before and after long-term treatment with cinacalcet in dialysis patients with secondary hyperparathyroidism. Kidney Int 2015;87:846–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moe SM, Abdalla S, Chertow GM, et al. Effects of Cinacalcet on Fracture Events in Patients Receiving Hemodialysis: The EVOLVE Trial. J Am Soc Nephrol 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ballinger AE, Palmer SC, Nistor I, Craig JC, Strippoli GF. Calcimimetics for secondary hyperparathyroidism in chronic kidney disease patients. The Cochrane database of systematic reviews 2014:Cd006254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Block GA, Bushinsky DA, Cheng S, et al. Effect of Etelcalcetide vs Cinacalcet on Serum Parathyroid Hormone in Patients Receiving Hemodialysis With Secondary Hyperparathyroidism: A Randomized Clinical Trial. JAMA 2017;317:156–64. [DOI] [PubMed] [Google Scholar]
- 56.Brauer CA, Coca-Perraillon M, Cutler DM, Rosen AB. Incidence and mortality of hip fractures in the United States. JAMA 2009;302:1573–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wilson LM, Rebholz CM, Jirru E, et al. Benefits and Harms of Osteoporosis Medications in Patients With Chronic Kidney Disease: A Systematic Review and Meta-analysis. Ann Intern Med 2017;166:649–58. [DOI] [PubMed] [Google Scholar]
- 58.Chavassieux PM, Arlot ME, Reda C, Wei L, Yates AJ, Meunier PJ. Histomorphometric assessment of the long-term effects of alendronate on bone quality and remodeling in patients with osteoporosis. The Journal of clinical investigation 1997;100:1475–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Recker RR, Delmas PD, Halse J, et al. Effects of intravenous zoledronic acid once yearly on bone remodeling and bone structure. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 2008;23:6–16. [DOI] [PubMed] [Google Scholar]
- 60.Miller PD, Roux C, Boonen S, Barton IP, Dunlap LE, Burgio DE. Safety and efficacy of risedronate in patients with age-related reduced renal function as estimated by the cockcroft and gault method: a pooled analysis of nine clinical trials. J Bone MinerRes 2005;20:2105–15. [DOI] [PubMed] [Google Scholar]
- 61.Jamal SA, Bauer DC, Ensrud KE, et al. Alendronate Treatment in Women with Normal to Severely Impaired Renal Function: An Analysis of the Fracture Intervention Trial*. J Bone MinerRes 2007. [DOI] [PubMed] [Google Scholar]
- 62.Miller PD, Roux C, Boonen S, Barton IP, Dunlap LE, Burgio DE. Safety and efficacy of risedronate in patients with age-related reduced renal function as estimated by the Cockcroft and Gault method: a pooled analysis of nine clinical trials. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 2005;20:2105–15. [DOI] [PubMed] [Google Scholar]
- 63.Shigematsu T, Muraoka R, Sugimoto T, Nishizawa Y. Risedronate therapy in patients with mild-to-moderate chronic kidney disease with osteoporosis: post-hoc analysis of data from the risedronate phase III clinical trials. BMC nephrology 2017;18:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Toussaint ND, Lau KK, Strauss BJ, Polkinghorne KR, Kerr PG. Effect of alendronate on vascular calcification in CKD stages 3 and 4: a pilot randomized controlled trial. Am J Kidney Dis 2010;56:57–68. [DOI] [PubMed] [Google Scholar]
- 65.Bergner R, Henrich D, Hoffmann M, Schmidt-Gayk H, Lenz T, Upperkamp M. Treatment of reduced bone density with ibandronate in dialysis patients. Journal of nephrology 2008;21:510–6. [PubMed] [Google Scholar]
- 66.Ota M, Takahata M, Shimizu T, et al. Efficacy and safety of osteoporosis medications in a rat model of late-stage chronic kidney disease accompanied by secondary hyperparathyroidism and hyperphosphatemia. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 2017;28:1481–90. [DOI] [PubMed] [Google Scholar]
- 67.Jamal SA, Ljunggren O, Stehman-Breen C, et al. Effects of denosumab on fracture and bone mineral density by level of kidney function. J Bone Miner Res 2011;26:1829–35. [DOI] [PubMed] [Google Scholar]
- 68.Cummings SR, San Martin J, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 2009;361:756–65. [DOI] [PubMed] [Google Scholar]
- 69.Chen CL, Chen NC, Hsu CY, et al. An Open-Label, Prospective Pilot Clinical Study of Denosumab for Severe Hyperparathyroidism in Patients with Low Bone Mass Undergoing Dialysis. The Journal of clinical endocrinology and metabolism 2014:jc20141154. [DOI] [PubMed] [Google Scholar]
- 70.Block GA, Bone HG, Fang L, Lee E, Padhi D. A single-dose study of denosumab in patients with various degrees of renal impairment. J Bone Miner Res 2012;27:1471–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McCormick BB, Davis J, Burns KD. Severe hypocalcemia following denosumab injection in a hemodialysis patient. American journal of kidney diseases : the official journal of the National Kidney Foundation 2012;60:626–8. [DOI] [PubMed] [Google Scholar]
- 72.Salim SA, Nair LR, Thomas L, et al. Denosumab-Associated Severe Hypocalcemia in a Patient With Chronic Kidney Disease. The American journal of the medical sciences 2018;355:506–9. [DOI] [PubMed] [Google Scholar]
- 73.Festuccia F, Jafari MT, Moioli A, et al. Safety and efficacy of denosumab in osteoporotic hemodialysed patients. Journal of nephrology 2017;30:271–9. [DOI] [PubMed] [Google Scholar]
- 74.Lindsay R, Nieves J, Formica C, et al. Randomised controlled study of effect of parathyroid hormone on vertebral-bone mass and fracture incidence among postmenopausal women on oestrogen with osteoporosis. Lancet 1997;350:550–5. [DOI] [PubMed] [Google Scholar]
- 75.Cohen A, Kamanda-Kosseh M, Recker RR, et al. Bone Density After Teriparatide Discontinuation in Premenopausal Idiopathic Osteoporosis. The Journal of clinical endocrinology and metabolism 2015;100:4208–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. The New England Journal of Medicine 2001;344:1434–41. [DOI] [PubMed] [Google Scholar]
- 77.Saag KG, Shane E, Boonen S, et al. Teriparatide or alendronate in glucocorticoid-induced osteoporosis. N Engl J Med 2007;357:2028–39. [DOI] [PubMed] [Google Scholar]
- 78.Jiang Y, Zhao JJ, Mitlak BH, Wang O, Genant HK, Eriksen EF. Recombinant human parathyroid hormone (1–34) [teriparatide] improves both cortical and cancellous bone structure. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 2003;18:1932–41. [DOI] [PubMed] [Google Scholar]
- 79.Chen P, Miller PD, Recker R, et al. Increases in BMD correlate with improvements in bone microarchitecture with teriparatide treatment in postmenopausal women with osteoporosis. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 2007;22:1173–80. [DOI] [PubMed] [Google Scholar]
- 80.Miller PD, Schwartz EN, Chen P, Misurski DA, Krege JH. Teriparatide in postmenopausal women with osteoporosis and mild or moderate renal impairment. Osteoporos Int 2007;18:59–68. [DOI] [PubMed] [Google Scholar]
- 81.Imai H, Watanabe M, Fujita T, Watanabe H, Harada K, Moritoyo T. Pharmacokinetics of teriparatide after subcutaneous administration to volunteers with renal failure: a pilot study. International journal of clinical pharmacology and therapeutics 2014;52:166–74. [DOI] [PubMed] [Google Scholar]
- 82.Ishani A, Blackwell T, Jamal SA, Cummings SR, Ensrud KE, Investigators M. The effect of raloxifene treatment in postmenopausal women with CKD. J Am Soc Nephrol 2008;19:1430–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cejka D, Benesch T, Krestan C, et al. Effect of teriparatide on early bone loss after kidney transplantation. Am J Transplant 2008;8:1864–70. [DOI] [PubMed] [Google Scholar]
- 84.Cejka D, Kodras K, Bader T, Haas M. Treatment of Hemodialysis-Associated Adynamic Bone Disease with Teriparatide (PTH1–34): A Pilot Study. Kidney & blood pressure research 2010;33:221–6. [DOI] [PubMed] [Google Scholar]
- 85.Palcu P, Dion N, Ste-Marie LG, et al. Teriparatide and bone turnover and formation in a hemodialysis patient with low-turnover bone disease: a case report. Am J Kidney Dis 2015;65:933–6. [DOI] [PubMed] [Google Scholar]
- 86.Sumida K, Ubara Y, Hoshino J, et al. Once-weekly teriparatide in hemodialysis patients with hypoparathyroidism and low bone mass: a prospective study. Osteoporosis International 2016;27:1441–50. [DOI] [PubMed] [Google Scholar]
- 87.Sumida K, Ubara Y, Hoshino J, et al. Once-weekly teriparatide in hemodialysis patients with hypoparathyroidism and low bone mass: a prospective study. Osteoporos Int 2016;27:1441–50. [DOI] [PubMed] [Google Scholar]
- 88.Hattersley G, Dean T, Corbin BA, Bahar H, Gardella TJ. Binding Selectivity of Abaloparatide for PTH-Type-1-Receptor Conformations and Effects on Downstream Signaling. Endocrinology 2016;157:141–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bahar H, Gallacher K, Downall J, Nelson CA, Shomali M, Hattersley G. Six Weeks of Daily Abaloparatide Treatment Increased Vertebral and Femoral Bone Mineral Density, Microarchitecture and Strength in Ovariectomized Osteopenic Rats. Calcified tissue international 2016;99:489–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Varela A, Chouinard L, Lesage E, Smith SY, Hattersley G. One Year of Abaloparatide, a Selective Activator of the PTH1 Receptor, Increased Bone Formation and Bone Mass in Osteopenic Ovariectomized Rats Without Increasing Bone Resorption. J Bone Miner Res 2017;32:24–33. [DOI] [PubMed] [Google Scholar]
- 91.Doyle N, Varela A, Haile S, et al. Abaloparatide, a novel PTH receptor agonist, increased bone mass and strength in ovariectomized cynomolgus monkeys by increasing bone formation without increasing bone resorption. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 2018;29:685–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Leder BZ, O’Dea LS, Zanchetta JR, et al. Effects of abaloparatide, a human parathyroid hormone-related peptide analog, on bone mineral density in postmenopausal women with osteoporosis. The Journal of clinical endocrinology and metabolism 2015;100:697–706. [DOI] [PubMed] [Google Scholar]
- 93.Miller PD, Hattersley G, Riis BJ, et al. Effect of Abaloparatide vs Placebo on New Vertebral Fractures in Postmenopausal Women With Osteoporosis: A Randomized Clinical Trial. JAMA 2016;316:722–33. [DOI] [PubMed] [Google Scholar]
- 94.Moreira C, Fitzpatrick LA, Wang Y, Recker RR. Effects of Abaloparatide-SC (BA058) on bone histology and histomorphometry: The ACTIVE phase 3 trial. Bone 2016. [DOI] [PubMed] [Google Scholar]
- 95.Adami G, Orsolini G, Adami S, et al. Effects of TNF Inhibitors on Parathyroid Hormone and Wnt Signaling Antagonists in Rheumatoid Arthritis. Calcified tissue international 2016;99:360–4. [DOI] [PubMed] [Google Scholar]
- 96.Briot K, Rouanet S, Schaeverbeke T, et al. The effect of tocilizumab on bone mineral density, serum levels of Dickkopf-1 and bone remodeling markers in patients with rheumatoid arthritis. Joint, bone, spine : revue du rhumatisme 2015;82:109–15. [DOI] [PubMed] [Google Scholar]
- 97.McClung MR, Grauer A, Boonen S, et al. Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med 2014;370:412–20. [DOI] [PubMed] [Google Scholar]
- 98.Saag KG, Petersen J, Brandi ML, et al. Romosozumab or Alendronate for Fracture Prevention in Women with Osteoporosis. N Engl J Med 2017. [DOI] [PubMed] [Google Scholar]
- 99.Cosman F, Crittenden DB, Adachi JD, et al. Romosozumab Treatment in Postmenopausal Women with Osteoporosis. N Engl J Med 2016;375:1532–43. [DOI] [PubMed] [Google Scholar]
- 100.Langdahl BL, Libanati C, Crittenden DB, et al. Romosozumab (sclerostin monoclonal antibody) versus teriparatide in postmenopausal women with osteoporosis transitioning from oral bisphosphonate therapy: a randomised, open-label, phase 3 trial. Lancet 2017. [DOI] [PubMed] [Google Scholar]
- 101.Eisman JA, Bone HG, Hosking DJ, et al. Odanacatib in the treatment of postmenopausal women with low bone mineral density: three-year continued therapy and resolution of effect. J Bone Miner Res 2011;26:242–51. [DOI] [PubMed] [Google Scholar]