Summary
The Functional Annotation of Animal Genomes (FAANG) project aims to identify genomic regulatory elements in both sexes and across multiple stages of development in domesticated animals. This study represents the first stage of the FAANG project for the horse, Equus caballus. A biobank of 80 tissue samples, two cell lines and six body fluids was created from two adult Thoroughbred mares. Ante-mortem assessments included full physical examinations, lameness, ophthalmologic and neurologic evaluations. Complete blood counts and serum biochemistries were also performed. At necropsy, in addition to tissue samples, aliquots of serum, ethylenediaminetetraacetic acid plasma, heparinized plasma, cerebrospinal fluid, synovial fluid, urine and microbiome samples from all regions of the gastrointestinal and urogenital tracts were collected. Epidermal keratinocytes and dermal fibroblasts were cultured from skin samples. All tissues were grossly and histologically evaluated by a board-certified veterinary pathologist. The results of the clinical and pathological evaluations identified subclinical eosinophilic and lymphocytic infiltration throughout the length of the gastrointestinal tract as well as a mild clinical lameness in both animals. Each sample was cryo-preserved in multiple ways, and nuclei were extracted from selected tissues. These samples represent the first published systemically healthy equine-specific biobank with extensive clinical phenotyping ante- and post-mortem. The tissues in the biobank are intended for community-wide use in the functional annotation of the equine genome. The use of the biobank will improve the quality of the reference annotation and allow all equine researchers to elucidate unknown genomic and epigenomic causes of disease.
Keywords: horse, genome regulation, tissue collection, nuclei isolation, necropsy, biorepository
Introduction
The Encyclopedia of DNA Elements (ENCODE) Consortium was established in 2003 to identify functional elements within the human genome (ENCODE Project Consortium 2004). At the conclusion of the pilot phase, in 2007, it was abundantly clear that the human genome was composed of more than just protein-coding genes (ENCODE Project Consortium et al. 2007). Information gained from the project allowed researchers to assign a biochemical function to 80% of the genome (ENCODE Project Consortium 2012). According to the ENCODE website, as of July 2017 ENCODE data have been utilized in over 700 publications linking functional data to disease in humans (ENCODE Project Consortium 2017). The number of publications will continue to expand as additional genomic regions associated with disease are identified. Realizing the need for better annotation to advance discovery in animal species, the animal genomic research community developed the Functional Annotation of Animal Genomes (FAANG) Consortium
The FAANG Consortium, organized in 2014, is working to use the assays and analysis techniques developed during the ENCODE project to annotate the majority of functional elements in the genomes of domesticated animal species (Andersson et al. 2015). Biobanks have been created for human (Bao et al. 2013) and porcine datasets (Abbott 2015; Albl et al. 2016). These biobanks have aimed to standardize tissue collection and preservation to improve downstream molecular analyses. The FAANG consortium has worked to standardize tissue sets, methods of collection, assays and meta-data analyses (Tuggle et al. 2016). The necessity of these datasets in the horse cannot be overstated.
Putative causal variants in coding regions have been identified for simple, Mendelian diseases in the horse such as hyperkalemic periodic paralysis (Ptacek et al. 1994), hereditary equine regional dermal asthenia (Tryon et al. 2007) and dwarfism in Frisian horses (Leegwater et al. 2016). However, complex inherited diseases, such as fracture risk (Blott et al. 2014), osteochondrosis (van Grevenhof et al. 2009; Lykkjen et al. 2010; Teyssedre et al. 2012; McCoy et al. 2016) and recurrent laryngeal neuropathy (Dupuis et al. 2011) have failed to be localized to coding regions of the genome despite extensive research. Better annotation of genes and functional elements within the genome will help to identify variations causative of complex diseases such as these. The objective of the current study was to create a biobank of tissues from two systemically healthy Thoroughbred mares to be used in the functional annotation of the equine genome.
Materials and Methods:
Animals
Two Jockey Club registered Thoroughbred mares deemed systemically healthy by the University of California Davis (UCD) Veterinary Medical Teaching Hospital were donated to the project. The horses (ECA_UCD_AH1 and ECA_UCD_AH2) were five and four years of age respectively at the time of necropsy. Neither mare had a history of traumatic injuries nor had they been bred. The animals were more than one year out of athletic training programs prior to euthanasia. All protocols were approved by the UCD Institutional Animal Care and Use Committee (Protocol #19037). A pedigree for both animals was obtained prior to their donation to the project.
Clinical phenotyping
Both mares were evaluated at the UC Davis Veterinary Hospital prior to euthanasia. Clinical evaluations were performed by a board-certified internist (CJF), surgeon (SAK) and ophthalmologist (ML) for signs of abnormalities in dermatologic, cardiopulmonary, gastrointestinal, neurologic, urogenital, lymphatic, orthopedic or ophthalmic systems. Videos were taken of the lameness and neurological examinations. A complete blood count and serum biochemistry were also performed on each horse.
Karyotyping
Metaphase chromosome spreads were prepared from Pokeweed-stimulated blood lymphocyte cultures according to standard protocols (Raudsepp & Chowdhary 2008). Chromosomes were stained with Giemsa for initial counting. Sex chromosomes were identified by CBG banding (Arrighi & Hsu 1971). Refined chromosome analysis and karyotyping were carried out by GTG banding (Seabright 1971). Twenty cells were captured and analyzed for each experiment using isis V5.2 (MetaSystems GmbH) software.
PCR analysis of sex chromosomes
Genomic DNA was isolated from blood lymphocyte cultures. PCR analysis was carried out using primers for the equine sex determining region Y (SRY) and androgen receptor (AR) gene, as described earlier (Raudsepp et al. 2004,2010).
Peripheral blood mononuclear cell collection and preservation
Peripheral blood mononuclear cells were isolated from 10 ml of heparinized blood using Histopaque® and density gradient centrifugation (Hida et al. 2002).
Fluid collection
Blood was collected ante-mortem from an intravenouscatheter into ethylenediaminetetraacetic acid, heparin and additive-free vacutainers. The blood was shielded from light, preserved on ice and returned to the lab for centrifugation. Immediately post-mortem, cerebrospinal fluid (CSF), synovial fluid and urine were collected. CSF was collected at the atlanto-occipital site, as previously described (Finno et al. 2015). Synovial fluid was collected via syringe aspiration of the carpal and tarsal joints from ECA_UCD_AH1. For ECA_UCD_AH2 synovial fluid was collected via syringe aspiration of the carpal joint. All blood, CSF and synovial fluid samples were centrifuged at 2000 g for 10 min at 4 °C, and the supernatant was flash frozen in 1-ml aliquots. Plasma, serum and buffy coat were transferred to storage tubes and flash frozen. Urine was freely caught postmortem. All fluid samples are stored at –80 °C.
Tissue collection
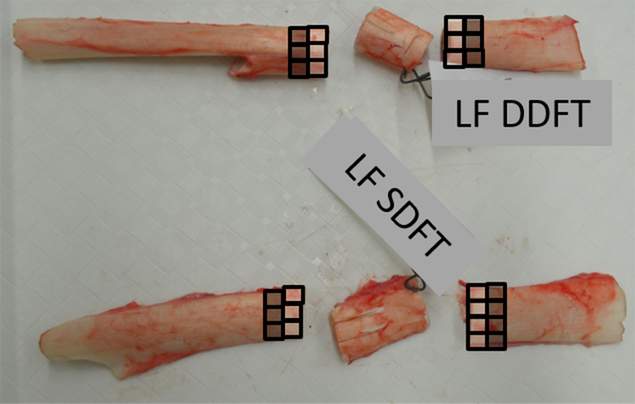
Tissue samples for cell culture were collected concurrently with CSF and synovial fluid. All tissues to be collected were assigned to one of seven collection stations based on organ system (Table 1). Organs or organ samples were labeled and delivered to the stations by veterinarians ensuring proper identification. All tissues were examined by a board-certified pathologist (VKA) for any evidence of gross pathology. Additionally, tissue samples for histopathology were collected. Two sets of samples were then collected for the biobank: the first set was directly proximal and the second set distal to the sampling site for histopathology (Fig. 1). For each biobank sample, 1-cm3 aliquots of tissues were collected. At least two, and a maximum of 12, aliquots of each tissue were preserved.
Table 1.
Tissues from each organ system of the body were sorted into stations based on when they became available during a necropsy. This organization of tissues into stations allowed for an expedited collection process.
| Station | Organ system collected |
|---|---|
| Station 1 | Cell culture biopsies |
| Station 1B | Integumentary |
| Station 2 | Musculoskeletal |
| Station 3 | Neurological |
| Station 4 | Respiratory and cardiovascular |
| Station 5 | Gastrointestinal |
| Station 6 | Urogenital |
Figure 1.
Depiction of collection sites using superficial and deep digital flexor tendon of the left forelimb. The middle sections of tissue were labeled and preserved in formalin for pathological evaluation. The white squares represent proximal samples and the black squares represent the distal samples.
Tissue preservation
After collection, the samples were preserved multiple ways. Histopathologic samples were labeled and preserved in 10% buffered formalin. All but two of the proximal site samples were flash frozen in liquid nitrogen and stored at –80 °C until further use. The two remaining proximal site samples were maintained on ice before cross-linking and preservation for ChIP-seq using a modification of the iDEAL chip-seq kit protocol from Diagenode®. The samples collected distal to the histopathologic sites were sectioned, flash frozen and are stored at –80 °C. Nuclei isolation was carried out, according to the standards developed in the mouse ENCODE project (Yue et al. 2014), on 16 of the tissues from a tertiary sample collected distal to the histopathologic site (Table 2).
Table 2.
A complete list of tissues collected from ECA_UCD_AH1 and ECA_UCD_AH2. Tissues with nuclei isolated and preserved are denoted with an asterisk (*). Tissues in bold have been prioritized based on the FAANG guidelines for RNA-seq and ChIP-seq of four histone modifications (H3K4me3, H3K4me1, H3K27ac and H3K27me3).
| Tissues collected | ||
|---|---|---|
| Integumentary system | Cardiovascular system | Pituitary |
| Neck skin | Left lung* | Cerebellum vermis |
| Dorsum (over back) skin | Heart left atrium | Cerebellum Lateral Hemisphere* |
| Loin adipose* | Heart left ventricle* | Pons |
| Gluteal adipose | Heart right atrium | Thalamus |
| Heart right ventricle | Hypothalamus | |
| Musculoskeletal system | Mitral valve | Dura mater |
| Gluteal muscle | Tricuspid valve | Corpus callosum |
| Sacrocaudalis muscle | Aortic valve | C1 spinal cord |
| Longissimus muscle* | Pulmonic valve | C6 spinal cord* |
| Rib bone marrow | Trachea | T8 spinal cord |
| Long bone marrow | L1 spinal cord | |
| Coronary band | Digestive system | L6 spinal cord |
| Hoof wall | Tongue | Dorsal root ganglia |
| Lamina* | Epiglottis | |
| Metacarpal bone diaphysis | Esophagus | Urogenital System |
| Sesamoid bone | Stomach | Urinary bladder |
| Cartilage | Duodenum | Uterus |
| Superficial digital flexor* | Jejunum* | Ovary* |
| Suspensory ligament | Ileum | Oviduct |
| Deep digital flexor | Cecum | Cervix |
| Right ventral colon | Vagina | |
| Abdominal/thoracic organs | Left ventral colon | Mammary gland |
| Sciatic nerve | Left dorsal colon | |
| Liver* | Right dorsal colon* | Cell Culture |
| Spleen* | Small colon | Keratinocytes |
| Adrenal cortex | Fibroblasts | |
| Adrenal medulla | Nervous system | |
| Kidney cortex | Cornea | Other |
| Kidney medulla* | Retina | Peripheral blood mononuclear cellsł |
| Larynx | Frontal cortex* | |
| Pancreas | Parietal cortex | |
| Thyroid | Occipital cortex | |
| Lymph node* | Temporal cortex | |
Sample only collected from UCDAH1.
Cell isolation and preservation
Immediately post-mortem, skin above the gluteal muscle and the medial aspect of the upper hindlimb were cleaned with phosphate-buffered saline (PBS), shaved and sterilized. Full-thickness strips were excised from each region and stored in media appropriate for isolation of either epidermal keratinocytes or dermal fibroblasts (Caldelari & Muller 2010; Raimondi et al. 2011). Keratinocyte cultures were established from upper hind-limb biopsies after being sectioned into 0.5 cm × 1 cm pieces and treated with dispase (Roche) for 24 hours at 4 °C. The epidermis was then separated from the dermis and incubated in CnT Accutase100 (CELLnTEC Advanced Cell Systems AG) at room temperature. The keratinocytes were detached, collected in a cell suspension, passed through a cell strainer, centrifuged and resuspended to count. Keratinocytes were seeded at 5 × 104 cells/cm2 in CnT-09 complete medium (CELLnTEC Advanced Cell Systems AG), which was changed every two days until confluence. To passage, keratinocytes were treated with Accutase100, centrifuged, counted and re-seeded at 5 × 104 cells/cm2. Keratinocytes were cryopreserved after passages 2 and 3 (ECA_UCD_AH1) or passage 4 (ECA_UCD_AH2) using CnT-CRYO-50 (CELLnTEC Advanced Cell Systems AG), according to manufacturer instructions, and were stored in cryotubes in liquid nitrogen (Caldelari & Müller 2010).
To collect dermal fibroblasts, skin biopsies were washed three times in ice cold PBS containing 3× antibiotics (100× penicillin and streptomycin, Sigma-Aldrich). Dermis was then separated, and 2–3mm2 fragments were placed into a 24-well tissue-culture-treated plate. Complete medium (Dulbecco’s Minimum Essential Medium, 20% fetal bovine serum, 2× non-essential amino acids, 2 mm L-glutamine, 2× penicillin/streptomycin, 2 μg/ml amphotericin B and 1 μg/ml fluconazole) was added to cover each tissue fragment. For the first week, complete medium was added to keep the tissue covered. Afterwards, medium was changed weekly until confluence, at which point the cells were trypsinized, counted and seeded in a 12-well plate. With each passage, cells were seeded in a larger well size: 1.9 cm2 (primary), 3.8 cm2 (passage 1), 9.5 cm2 (passage 2), 25 cm2 (passage 3) and 75 cm2 (passage 4). Dermal fibroblasts were cryopreserved after passages 3 and 4 for both horses using DMSO-based freezing medium (Raimondi et al. 2011) and stored in liquid nitrogen.
Pathological evaluation
The tissues preserved in formalin were embedded in paraffin, sectioned and evaluated by a board certified veterinary pathologist (VKA). The sections were stained using hematoxylin and eosin (HE) then visualized using light microscopy.
Results
Clinical phenotyping
The lameness examinations revealed a grade 2 out of 5 lameness in both horses (Table S1). ECA_UCD_AH1 was lame on the right hind leg. No tissues were collected from this leg. ECA_UCD_AH2 was bilaterally lame on both forelimbs (see supplementary video at https://youtu.be/OrfyVtYr1iQ), but it was noted that the horse’s front shoes were removed the day prior to the exam. Results of the serum biochemistry, complete blood count, neurological exam (see supplementary Video at https://youtu.be/seWYe69ZhUs) and ophthalmic exam were all within normal limits (Table S1). A karyotype and PCR analysis of sex chromosomes was completed for each individual (TR) with no abnormalities detected. Interpretation of all clinical examinations determined both mares were systemically healthy at the time of euthanasia.
Tissue and fluid collection and preservation
All tissues were collected within three hours of euthanasia. Eighty tissues and six body fluids were collected. The samples were preserved via one of four methods (Table 2) and subsequently stored at –80 °C. Serum, plasma, urine, CSF, synovial fluid and buffy coat were aliquoted after centrifugation and stored at –80 °C. Dermal fibroblasts and epidermal keratinocytes established from skin biopsies were cryopreserved in liquid nitrogen (Table S2). Peripheral blood mononuclear cells’s were isolated from UCDAH1 only and stored at–−80 °C (Table 2).
Pathology report
The conclusions from the pathology report for each tissue of each horse are listed in Table S3. Most of the tissues collected showed no significant abnormalities. However, the gastrointestinal tract of both horses contained substantial, subclinical, eosinophilic and lymphocytic inflammatory cell infiltrate in the lamina propria and submucosa (Fig. S1). This inflammation extended from the duodenum through the small colon in both horses (Table S3).
Discussion
Biobanks have been created for samples from healthy humans (Triendl 2003; Ronningen et al. 2006; Jaddoe et al. 2007; Roden et al. 2008; Sudlow et al. 2015), diseased humans (Triendl 2003; Garcia-Merino et al. 2009), a diabetic pig model (Albl et al. 2016), cancer cell lines (Barretina et al. 2012; van de Wetering et al. 2015) and canine mammary tumors (Milley et al. 2015). In humans, the list continues to grow, but the availability of published biobanks for domesticated animals is much more limited (Groeneveld et al. 2016). The 80 tissue samples, two cell lines and six body fluids in the biobank described here are intended to be used by the equine research community in the functional annotation of the equine genome. This report also provides guidelines on the tissue sampling and collection to ensure congruency with future biobanks. Samples from the biobank may be used by any interested researchers in the equine community to further the annotation of the genome.
The phenotyping and histopathologic results are intended to provide context for future sequencing and related molecular analyses. The lameness observed in both horses was evaluated for plausible causes to determine if samples should be excluded from the biobank. No plausible cause of the right hind lameness for UCDAH1 could be elucidated. Therefore, all musculoskeletal limb samples were collected from the left legs for UCDAH1. UCDAH2 had her shoes removed the day prior to her lameness exam, which could have contributed to the lameness. Hoof tester examination was positive bilaterally on UCDAH2’s soles, and the lameness improved with the addition of shoes prior to euthanasia. For this reason, it is suspected the lameness was due to the shoe removal and the samples were included in the biobank.
Underlying pathology will have a significant impact on the annotation in abnormal tissues. For example, the eosinophilic and lymphocytic infiltration within the gastrointestinal tract of these two horses may result in a difference in observed gene expression and epigenetic modifications as compared to healthy animals, as the presence of these inflammatory cells changes the cellular composition of the tissue. The presence of eosinophils and lymphocytes is indicative of underlying inflammation; therefore, transcriptional changes such as an increase in the expression of interleukins, cytokines and chemokines (Davanian et al. 2012; Brady et al. 2015) should be expected in future RNA-sequencing data. Along with an elevation of transcription of these genes, it is possible that histone modifications, methylation, transcription factor binding and open chromatin regions will also be altered because these modifications influence transcription (ENCODE Project Consortium et al. 2007). Observation of inflammatory cells post-mortem underscores the importance of histological evaluation in the establishment of a biobank.
This is the first published report of a systemically healthy equine specific biobank and the first non-human biobank to include extensive ante- and post-mortem phenotypic data. The use of this biobank in the functional annotation of the equine genome will lead to advances in both equine and human medicine. Similar to ENCODE pioneering the field of personalized human medicine, FAANG will advance individualized care of animals for production and companion purposes. Researchers can access sample availability and associated metadata on data.faang.org. It is recommended to then contact the corresponding author to coordinate shipping of samples. Accession information for each horse can be found in Table S1.
Supplementary Material
Table S1 Results from clinical examinations and phenotyping of ECA_UCD_AH1 and ECA_UCD_AH2.
Table S2 Description of cryopreserved cells from cell culture.
Table S3 Detailed description of the histopathologic report for every tissue from each individual horse.
Figure S1 A hematoxylin and eosin stained section of the duodenum of ECA_UCD_AH1 demonstrating the eosinophilic and lymphocytic infiltration observed in both horses.
Acknowledgements
The authors would like to acknowledge members of the Zhou and Ross laboratories at UC Davis for their technical assistance
Funding Sources
Funding for sample collection was provided by the Grayson Jockey Club Foundation, USDA NRSP-8 and the UC Davis Center for Equine Health. Support for M.J.M. was provided by UC Davis Agriculture Experiment Station. Support for C.J.F. was provided by the National Institutes of Health (NIH) (1K01OD015134and L40 TR001136). E.N.B was supported by USDA NIFA National Need Fellowship Award #20143842021796. All listed funding agencies provided support for sample collection or salary support for the investigators listed above. None of the funding agencies had any role in the design of the study, analysis, interpretation of the data or writing of the manuscript.
References
- Abbott A (2015) Inside the first pig biobank. Nature 519, 397–8. [DOI] [PubMed] [Google Scholar]
- Albl B, Haesner S, Braun-Reichhart C, Streckel E, Renner S, Seeliger F, Wolf E, Wanke R & Blutke A (2016) Tissue sampling guides for porcine biomedical models. Toxicologic Pathology 44, 414–20. [DOI] [PubMed] [Google Scholar]
- Andersson L, Archibald AL, Bottema CD et al. (2015) Coordinated international action to accelerate genome-to-phenome with FAANG, the Functional Annotation of Animal Genomes project. Genome Biologu 16, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrighi FE & Hsu TC (1971) Localization of heterochromatin in human chromosomes. Cytogenetics 10, 81–6. [DOI] [PubMed] [Google Scholar]
- Bao WG, Zhang X, Zhang JG, Zhou WJ, Bi TN, Wang JC, Yan WH & Lin A (2013) Biobanking of fresh-frozen human colon tissues: impact of tissue ex-vivo ischemia times and storage periods on RNA quality. Annals of Surgical Oncology 20, 1737–44. [DOI] [PubMed] [Google Scholar]
- Barretina J, Caponigro G, Stransky N et al. (2012) The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature 483, 603–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blott SC, Swinburne JE, Sibbons C, Fox-Clipsham LY, Helwegen M, Hillyer L, Parkin TD, Newton JR & Vaudin M (2014) A genome-wide association study demonstrates significant genetic variation for fracture risk in Thoroughbred racehorses. BMC Genomics 15, 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady RA, Bruno VM & Burns DL (2015) RNA-Seq analysis of the host response to Staphylococcus aureus skin and soft tissue infection in a mouse model. PLoS One 10, e0124877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldelari R & Muller EJ (2010) Short- and long-term cultivation of embryonic and neonatal murine keratinocytes. Methods in Molecular Biology 633, 125–38. [DOI] [PubMed] [Google Scholar]
- Davanian H, Stranneheim H, Bage T, Lagervall M, Jansson L, Lundeberg J & Yucel-Lindberg T (2012) Gene expression profiles in paired gingival biopsies from periodontitis-affected and healthy tissues revealed by massively parallel sequencing. PLoS ONE 7, e46440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuis MC, Zhang Z, Druet T, Denoix JM, Charlier C, Lekeux P & Georges M (2011) Results of a haplotype-based GWAS for recurrent laryngeal neuropathy in the horse. Mammalian Genome 22, 613–20. [DOI] [PubMed] [Google Scholar]
- ENCODE Project Consortium (2004) The ENCODE (ENCyclopedia Of DNA Elements) Project. Science 306, 636–40. [DOI] [PubMed] [Google Scholar]
- ENCODE Project Consortium (2012) An integrated encyclopedia of DNA elements in the human genome. Nature 489, 57–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENCODE Project Consortium (2017) encodeproject.org.
- ENCODE Project Consortium, Birney E, Stamatoyannopoulos JA et al. (2007) Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature 447, 799–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finno CJ, Estell KE, Katzman S, Winfield L, Rendahl A, Textor J, Bannasch DL & Puschner B (2015) Blood and cerebrospinal fluid alpha-tocopherol and selenium concentrations in neonatal foals with neuroaxonal dystrophy. Journal of Veterinarian Internal Medicine 29, 1667–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Merino I, de Las Cuevas N, Jimenez JL, Gallego J, Gomez C, Prieto C, Serramia MJ, Lorente R, Munoz-Fernandez MA & Spanish HIVB (2009) The Spanish HIV BioBank: a model of cooperative HIV research. Retrovirology 6, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groeneveld LF, Gregusson S, Guldbrandtsen B, Hiemstra SJ, Hveem K, Kantanen J, Lohi H, Stroemstedt L & Berg P (2016) Domesticated animal biobanking: land of opportunity. PLoS Biology 14, e1002523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hida N, Maeda Y, Katagiri K, Takasu H, Harada M & Itoh K (2002) A simple culture protocol to detect peptide-specific cytotoxic T lymphocyte precursors in the circulation. Cancer Immunology, Immunotherapy 51, 219–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaddoe VW, Bakker R, van Duijn CM et al. (2007) The Generation R Study Biobank: a resource for epidemiological studies in children and their parents. European Journal of Epidemiology 22, 917–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leegwater PA, Vos-Loohuis M, Ducro BJ et al. (2016) Dwarfism with joint laxity in Friesian horses is associated with a splice site mutation in B4GALT7. BMC Genomics 17, 839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lykkjen S, Dolvik NI, McCue ME, Rendahl AK, Mickelson JR & Roed KH (2010) Genome-wide association analysis of osteochondrosis of the tibiotarsal joint in Norwegian Standardbred trotters. Anim Genet 41 Suppl 2, 111–20. [DOI] [PubMed] [Google Scholar]
- McCoy AM, Beeson SK, Splan RK, Lykkjen S, Ralston SL, Mickelson JR & McCue ME (2016) Identification and validation of risk loci for osteochondrosis in standardbreds. BMC Genomics 17, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milley KM, Nimmo JS, Bacci B, Ryan SD, Richardson SJ & Danks JA (2015) DogMATIC--a remote biospecimen collection kit for biobanking. Biopreservation and Biobanking 13, 247–54. [DOI] [PubMed] [Google Scholar]
- Ptacek LJ, Tawil R, Griggs RC et al. (1994) Dihydropyridine receptor mutations cause hypokalemic periodic paralysis. Cell 77, 863–8. [DOI] [PubMed] [Google Scholar]
- Raimondi E, Piras FM, Nergadze SG, Di Meo GP, Ruiz-Herrera A, Ponsa M, Ianuzzi L & Giulotto E (2011) Polymorphic organization of constitutive heterochromatin in Equus asinus (2n = 62) chromosome 1. Hereditas 148, 110–3. [DOI] [PubMed] [Google Scholar]
- Raudsepp T & Chowdhary BP (2008) FISH for mapping single copy genes. Methods in Molecular Biology 422, 31–49. [DOI] [PubMed] [Google Scholar]
- Raudsepp T, Durkin K, Lear TL, Das PJ, Avila F, Kachroo P & Chowdhary BP (2010) Molecular heterogeneity of XY sex reversal in horses. Animal Genetics 41 Suppl 2, 41–52. [DOI] [PubMed] [Google Scholar]
- Raudsepp T, Lee EJ, Kata SR, Brinkmeyer C, Mickelson JR, Skow LC, Womack JE & Chowdhary BP (2004) Exceptional conservation of horse-human gene order on X chromosome revealed by high-resolution radiation hybrid mapping. Proceedings of the National Academy of Sciences of the United States of America 101, 2386–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roden DM, Pulley JM, Basford MA, Bernard GR, Clayton EW, Balser JR & Masys DR (2008) Development of a large-scale de-identified DNA biobank to enable personalized medicine. Clinical Pharmacology & Therapeutics 84, 362–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronningen KS, Paltiel L, Meltzer HM, Nordhagen R, Lie KK, Hovengen R, Haugen M, Nystad W, Magnus P & Hoppin JA (2006) The biobank of the Norwegian Mother and Child Cohort Study: a resource for the next 100 years. European Journal of Epidemiology 21, 619–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seabright M (1971) A rapid banding technique for human chromosomes. Lancet 2, 971–2. [DOI] [PubMed] [Google Scholar]
- Sudlow C, Gallacher J, Allen N et al. (2015) UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Medicine 12, e1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teyssedre S, Dupuis MC, Guerin G, Schibler L, Denoix JM, Elsen JM & Ricard A (2012) Genome-wide association studies for osteochondrosis in French Trotter horses. Journal of Animal Science 90, 45–53. [DOI] [PubMed] [Google Scholar]
- Triendl R (2003) Japan launches controversial Biobank project. Nature Medicine 9, 982. [DOI] [PubMed] [Google Scholar]
- Tryon RC, White SD & Bannasch DL (2007) Homozygosity mapping approach identifies a missense mutation in equine cyclophilin B (PPIB) associated with HERDA in the American Quarter Horse. Genomics 90, 93–102. [DOI] [PubMed] [Google Scholar]
- Tuggle CK, Giuffra E, White SN et al. (2016) GO-FAANG meeting: a gathering on functional annotation of animal genomes. Animal Genetics 47, 528–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Wetering M, Francies HE, Francis JM et al. (2015) Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell 161, 933–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Grevenhof EM, Schurink A, Ducro BJ, van Weeren PR, van Tartwijk JM, Bijma P & van Arendonk JA (2009) Genetic variables of various manifestations of osteochondrosis and their correlations between and within joints in Dutch warmblood horses. Journal of Animal Science 87, 1906–12. [DOI] [PubMed] [Google Scholar]
- Yue F, Cheng Y, Breschi A et al. (2014) A comparative encyclopedia of DNA elements in the mouse genome. Nature 515, 355–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Results from clinical examinations and phenotyping of ECA_UCD_AH1 and ECA_UCD_AH2.
Table S2 Description of cryopreserved cells from cell culture.
Table S3 Detailed description of the histopathologic report for every tissue from each individual horse.
Figure S1 A hematoxylin and eosin stained section of the duodenum of ECA_UCD_AH1 demonstrating the eosinophilic and lymphocytic infiltration observed in both horses.