Abstract
Identifying HIV-1-associated B cell defects and responses to activation may direct interventions to circumvent their impaired antibody responses to infection and vaccines.
Among 34 viremic HIV-1-infected and 20 seronegative control adults, we measured baseline frequencies and activation of B and T cell subsets, expression of activation-induced cytidine deaminase (AID), potential determinants of B cell activation in vivo and B and T cell responses in vitro.
At baseline, HIV-1 infection was associated with increased IgM memory and decreased anergic cell frequencies, as well as increased activation in all 10 B cell subsets compared with controls. HIV-1 status, TFH activation, and BAFF were significant potential drivers of B cell activation. Despite high baseline activation among HIV-1-infected subjects, stimulation in vitro with combined surrogates for antigen (anti-IgM), cognate (CD40 ligand) and soluble T cell factors (IL-4) elicited comparable B cell activation, transitions from naïve to class-switched memory cells and AID expression in both groups.
In summary, viremic HIV-1 infection perturbs circulating B cell subsets and activation at each stage of B cell maturation. However, that appropriate stimulation of B cells elicits effective activation and maturation provides impetus for advancing vaccine development to prevent secondary infections by circumventing early B cell defects.
Keywords: Cellular immunology, B cells, T cells, HIV, Cellular activation
Introduction
HIV-1 infection is associated with early and consistent B cell dysfunction with morbid consequences, including an increased incidence of secondary infections, autoimmune disease and B cell lymphomas. Circulating B cells exhibit polyclonal hyperactivity, resulting in overproduction of total and HIV-1-specific antibodies, initial expansion of B cell areas in lymphoid tissue, and increased activation, proliferation, and terminal differentiation (1, 2). Furthermore, whereas immature, transitional, activated subsets and plasma cells may be expanded compared with those in healthy adults, memory subsets are often contracted (3). Increased B cell death may be due to both decreased survival (4) and increased apoptosis (5, 6). These defects coalesce to result in poor responsiveness to antigens in vivo (such as pneumococcal vaccines (7)) and B cell stimuli in vitro, such as microbial ligands that often initiate immune responses (8, 9).
Increased levels of B cell activation at all stages of HIV-1 infection may also impede effective humoral responses (1, 10). Such activation may raise the threshold for subsequent stimulation with individual stimuli or mandate the need to elicit further activation and differentiation via several pathways. Moreover, the specific determinants of B cell activation during HIV-1 infection have not been well characterized, although HIV-1 gp120 (11), T cell-derived CD40 ligand on the viral envelope (12), increased exposure to microbial antigens (5, 9, 13) and T cell activation (10) have been proposed. Effects seen in circulating B cells and impaired responses to infection and vaccine likely have their origin in germinal centers, which are compromised from early in the course of HIV-1 infection (14, 15).
Effective B cell activation and differentiation in response to specific antigen in germinal centers (GC) results from the interaction between GC B cells and T follicular helper cells (TFH) in vivo (16), cells that have functionally-related counterparts detectable in the circulation (17). Despite increased numbers of circulating TFH cells (18), a dysfunctional interaction between TFH and B cells in HIV-1-infection may result in a loss of ability to generate appropriate humoral responses to infection and vaccines (19).
To address several outstanding issues of B cell phenotype and function, we characterized the subsets, activation and responsiveness of B cells from viremic HIV-1-infected adults and control subjects. High levels of activation characterized all circulating subsets of B cells. However, in the presence of in vitro stimuli that served as surrogates for antigen engagement (anti-IgM) and both cognate and soluble T cell help (anti-CD40 and IL-4, respectively), these cells could be driven to further activation and maturation, including production of activation-induced cytidine deaminase (AID). This B cell-specific enzyme is required for enhancing the quality and function of antibodies by mediating both affinity maturation through somatic hypermutation (SHM) of the antigen-binding variable regions of immunoglobulin genes and for class switch recombination of IgM to IgG or IgA. These stimulated values were comparable to those in cells from healthy control subjects. Our data suggest that B cells from patients with relatively advanced HIV-1 infection, even with viremia and prior to antiretroviral therapy, can respond to appropriately-configured external stimuli that engage complementary pathways.
Materials and Methods
Study population.
We enrolled 20 HIV-1-seronegative control and 34 HIV-1-infected subjects with detectable plasma HIV-1 RNA (viremic for > 6 months) largely matched for age, gender and ethnicity (Table I). Exclusion criteria included any acute medical illness at the time of enrollment, and for control subjects, any high-risk behaviors for acquisition of HIV-1 infection. Written informed consent was obtained with protocols approved by the Combined Institutional Review Board covering the University of Colorado Denver, Denver Veterans Affairs Medical Center, and Denver Health and Hospital Authority.
Table I.
Demographics of HIV-1-infected and Control subjects
| Control subjects (n = 20) |
HIV-1-infected (n = 34) |
|
|---|---|---|
|
Age (years) Mean (std dev) |
38 (9.4) | 41.4 (10.1) |
| Sex (F:M) | 3:14 | 1:28 |
| Race (Non-white:White) | 7:10 | 13:16 |
|
CD4+ T cells/μL Mean (range) |
ND | 291.9 (3–1007)* |
| Number (%) with <200 CD4+ T cells/μL | NA | 12 (41%)* |
|
CD19+ B cells (percent of lymphocytes) Mean (range) |
2.9% (1.05–8.19%) |
4.3% (0.36–15.8%) |
|
Plasma HIV-1 RNA copies/mL Mean (range) |
NA | 356,967 (553–3,793,362)* |
ND = no data; NA = not applicable
data not available for 1 subject
Peripheral blood mononuclear cells (PBMC).
PBMC were separated from 60mL of fresh whole blood by density centrifugation and washed twice. Immunophenotyping for lineage and activation was performed by flow cytometry on ice as described below. For RNA, 3–5×106 cells were suspended in RNALater (Applied Biosystems Inc., Foster City, CA) and stored at −80oC. RNA was extracted using an RNEasy RNA Extraction Kit (Qiagen, Inc., Valencia, CA; manufacturer’s protocol), quantified using a NanoDrop spectrophotometer (NanoDrop Technologies Inc., Wilmington, DE), and stored at −80oC.
In vitro stimulation.
PBMC (2×106 cells/mL) in RPMI media (Invitrogen) with 10% heat-inactivated FCS (HyClone Laboratories) and 10 μg/mL gentamicin (Invitrogen) were cultured in media alone or stimulated with a combination of anti-IgM (1.3 μg/mL; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA), anti-CD40 (1 μg/mL; BD-Biosciences-Pharmingen, San Diego, CA) and IL-4 (10 ng/mL; Peprotech, Inc., Rocky Hill, NJ) at 37oC for 4 days and divided into tubes for FACS analysis or RNA extraction.
Flow cytometric analysis.
PBMC (1–4×106 cells/tube) were stained with monoclonal antibodies to B cell markers (CD19-AF700, IgM-PerCP-Cy5.5, IgD-PE-Cy7 (Biolegend), CD38-FITC, CD21-PE, CD10-PE-CF594, CD40-APC, CD86-BV421 (BD Pharmingen)) and T cell markers (CD3-AF700, CD4PacBlue (Biolegend), CD8-APC-AF750 (Invitrogen), CD45RA-ECD, CD27-PE-Cy5 (Beckman Coulter), CD27-BV650, CD38-FITC, HLA-DR-PE-Cy7, PD-1-APC, CXCR5-PE (R&D Systems)) for 40 minutes at room temperature. Stained cells were subsequently washed twice and fixed in 1% paraformaldehyde for 10 minutes at 4°C. Data was acquired within 4 hours using a BD LSRII flow cytometer (BD Biosciences – Immunocytometry Systems, San Jose, CA). Data were analyzed using Flow Jo Software (Tree Star Inc., Ashland, OR) with the gating scheme as per Supplemental Figure 1.
Quantitative real-time PCR for AID mRNA.
RNA was extracted from 2–10 × 106 cells using Qiagen All Prep RNA/DNA/Protein extraction kit following the manufacturer recommended protocol using syringe and 20-gauge needle homogenization. The RNA was quantified using Nanodrop (Thermo Fisher Scientific, Waltham, MA). Reverse transcription of RNA was performed using the First Strand cDNA synthesis Kit (Invitrogen) and random hexamers (Invitrogen) per the manufacturer’s protocol. Quantitative PCR analysis was performed using TaqMan Gene Expression Master Mix and TaqMan primer probes targeting human β-actin, β2-microglobulin, and exon 2 of human AID (aicda; all from Thermo Fisher Scientific) in triplicate, using 1μL of template cDNA per reaction on an Applied Biosystems 7500 Fast Real-time PCR System (Thermo Fisher Scientific). AID expression was determined relative to endogenous controls β-actin and β2-microglobulin by subtracting the average cycle threshold (Ct) for β-actin and β2-microglobulin from that for AID using the following equation: Relative AID mRNA expression =AID/avg (β-actin, β2-microglobulin) =2-(Ct(sample)-Avg Ct(reference)) =2(ΔCt). The median slope of all qRT-PCR reactions was −3.33 (range = −3.18 to −3.83). The median efficiency of the reactions was 99.9% (range = 82.5%−106.3%).
Staining for intracellular AID protein by flow cytometry.
CD19+ B cells in PBMCs or purified B cells were stained with a viability dye for 30 minutes at room temperature (Fixable Viability Dye eFluor 520, eBioscience, San Diego, CA), washed, stained with surface B cell markers as above for 20 minutes at room temperature, fixed with fixation medium (Medium A, Invitrogen) at room temperature for 10 minutes, washed and permeabilized with 5% methanol on ice for 10 minutes, which allowed optimal permeabilization and recognition of intracellular AID without affecting surface antigen expression. After washing with FACS buffer, cells were stained with rat anti-AID antibody (1:333; EK2 5G9, Cell Signaling, Danvers, MA) for 30 minutes at room temperature, washed, detected with anti-rat IgG2b-PE (1:100; R2B-7C3, eBioscience) for 20 minutes on ice, washed twice with FACS buffer then data was acquired using flow cytometry. Results with CD3+ T cells and secondary anti-rat IgG alone served as negative controls.
ELISA for cytokines.
Levels of the TNF superfamily member 13B B cell activating factor (BAFF) (R&D Systems, Minneapolis, MN) and IL-21 (eBioscience) were measured in serum by enzyme-linked immunosorbent assays (ELISA) per manufacturer protocols.
B cell purification.
To determine the ability of stimuli to activate B cells and induce AID production in the absence of T and accessory cell help, we purified B cells from approximately 50 million PBMC each from 5 additional viremic HIV-1-infected subjects (age 45–48 years; median 248 CD4+ T cells/μL (range 90–435); plasma HIV-1 RNA 136,000–946,000 copies/mL) and 5 age- and gender-matched HIV-1-seronegative control subjects with a negative selection kit (STEMCELL Technologies, Vancouver, BC). The median yield of B cells was 2.5 × 106 (range 1.85–3.1 × 106) and the median purity was 96.4% (range 92.4–98.5%) as measured by flow cytometry (Supplemental Figure 2). Cells were stimulated and stained as above.
Statistics.
Data were analyzed using R statistical software (GNU General Public License). Assessment of the differences between control and HIV-1-infected subjects were determined using Welch’s unpaired two-sample t-tests and linear regression models to obtain estimates, 95% confidence intervals and p-values.
Results
Subjects.
We enrolled 20 HIV-1-uninfected control and 34 viremic HIV-1-infected subjects with a broad range of plasma HIV-1 RNA and the CD4+ T cell numbers and no active secondary infections or cancer (Table I). Age, ethnicity, and gender were similar in both groups.
B cell subsets in fresh blood at baseline.
In freshly isolated cells, the distribution of circulating B cell subsets, as defined in Table II, were generally similar in HIV-1-infected and control subjects (Figure 1A). Naïve cells represented a comparable majority of the B cells in both groups, followed by class switch memory (IgD−IgM−) (43–53% and 17–20%, respectively). The majority of class switch memory cells lacked CD27 expression in both groups, whereas the majority of IgM memory cells (IgD−IgM+) expressed CD27. The proportion of IgM memory B cells was significantly greater in patients with HIV-1 infection, independent of the presence of CD27. IgM+IgD+CD27+ cells (20, 21), proposed as a prominent source of B cells reactive with pneumococcal polysaccharides (22), were maintained at comparable frequencies in both groups. However, another subset of IgD+ B cells, circulating BND cells (IgD+IgMCD27−), considered to be both autoreactive and typically anergic in healthy adults (23), were significantly less prevalent with HIV-1 infection. However, these latter two subsets make up a minority of the B cell population in both groups, as did circulating germinal center B cells (0.58–0.75% of B cells), and IgD memory cells (0.87–1.02%; not shown).
Table II.
Surface Markers Defining 10 B cell Subsets
| B cell Subset | IgD | IgM | CD27 | CD10 | CD38 |
|---|---|---|---|---|---|
| Transitional | + | + | − | + | |
| Naïve | + | + | − | − | |
| Germinal Center | − | + | + | + | |
| IgM+IgD+CD27+ | + | + | + | − | |
| IgM Memory* | − | + | +/− | − | |
| BND anergic | + | − | − | − | |
| IgD Memory | + | − | + | − | |
| Class Switch Memory* (IgG, A, E) | − | − | +/− | − |
IgM memory and Class switch memory are each divided into two subsets based on CD27 expression.
FIGURE 1. Distribution of B cell subsets in 13 control and 17 HIV-1-infected subjects.
B cell subsets as defined by Table II were measured by multiparameter flow cytometry. Values shown as mean and standard deviation. Groups were compared by unpaired t-tests. (A) Subsets at baseline (day 0). * denotes a significant difference between groups at baseline, p <0.0024. (B) Subsets after stimulation with anti-IgM, anti-CD40, and IL-4 for 4 days. + denotes subsets that showed a significant change between baseline and day 4, p <0.03. * denotes a significant difference between groups at day 4, p <0.031. ° denotes a significant difference in magnitude of change pre- vs post-stimulation between groups, p <0.0023.
B cell subsets in response to stimulation in vitro.
An essential element of B cell function is their ability to be activated by exogenous and endogenous stimuli. We used a system of surrogate antigen (anti-IgM to crosslink the B cell receptor), cognate (anti-CD40) and soluble (IL-4) T cell factors to focus on the ability of individual B cell subsets in PBMC to respond given sufficient stimuli. In both groups, stimulation in vitro was associated with a significant evolution from predominantly naïve and transitional B cell populations (Figure 1A) to a preponderance of CD27+, and to a lesser extent, CD27− class switch memory cells at 4 days (Figure 1B). The magnitude of change in each subset was comparable in both control and HIV-1-infected subjects for all B cell subsets, except the IgM memory, in which the change (decline) was greater with HIV-1 infection. Neither the frequencies nor the differences in BND cells between groups changed significantly with stimulation. These data suggest that B cells from patients with viremic HIV-1 infection can respond if given appropriate complementary stimuli. Of note, soluble antibodies were measured in the supernatant of stimulated PBMCs, but this combination of stimuli did not elicit differentiation to plasma cells and antibody production.
B cell activation.
By flow cytometry, B cells freshly isolated from blood of HIV-1-infected subjects showed consistently increased levels of activation at baseline, with significantly lower densities of both CD21 and CD40 compared with those from controls (Figures 2A and 2B). The decreased expression of CD21 characterized each of the 10 B cell subsets studied in the HIV-1-infected group (30-76% CD21+) compared with those of the controls (62–97% CD21+; p <0.022 for all) (Figure 2C). CD40+ expression decreased significantly only in the IgD memory and class switch memory 27− subsets (p = 0.03 and p = 0.01, respectively; not shown). CD86+ expression was somewhat more selectively increased (though not significantly so) on these fresh B cell subsets (Figure 2B), particularly on transitional, naïve, IgM+IgD+CD27+, germinal center, IgM memory 27+, and class switch memory 27− subsets (p <0.39 for all; not shown). Thus, selective skewing of minority subsets and activation in each subset were relatively consistent and prominent features of circulating B cells among adults with viremic HIV infection.
FIGURE 2. B cell activation markers in 13 control and 20 HIV-1-infected subjects by flow cytometry.
(A) Gating scheme for CD21 and CD86 comparing representative data from a representative control and HIV-1-infected subject pre- vs post-stimulation with anti-IgM, anti-CD40, and IL-4. (B) Percentage of CD19+ B cells expressing CD21, CD40, and CD86 pre- (day 0) and post- (day 4) stimulation with anti-IgM, anti-CD40, and IL-4. Values shown as mean and standard deviation. Groups were compared by unpaired t-tests. * denotes a significant difference between groups, p <0.031. ° denotes a significant difference within groups, pre- vs post-stimulation, p <0.0033. (C) Percentage of each B cell subset (as defined by table II) in freshly isolated cells expressing CD21 at baseline (day 0). Values shown as mean and standard deviation. Groups were compared by unpaired t-tests. * denotes that all subsets were significantly different between groups; (p <0.022).
Despite high levels of activation at baseline, engagement of the B cell receptors with anti-IgM, as a surrogate for antigen and exposure to surrogates for both cognate (anti-CD40) and soluble T cell factors (IL-4) in vitro elicited significant activation of B cells from both HIV-1-infected and control subjects. CD21 and CD40, expressed by the majority of resting B cells, decreased significantly from day 0 to day 4 of stimulation (Figures 2A and 2B). Similarly, expression of CD86, less prominent on resting cells, increased significantly to comparable frequencies in both groups. Indeed, despite differences prior to stimulation, the magnitude of change (percentage expression) of these three activation markers in the total B cell population did not differ significantly between groups. For the minority germinal center subset, CD21 was significantly lower in HIV-1-infected than in control adults pre- and post-stimulation (p =0.0002 and p = 0.036, respectively), as was CD40 expression on the IgD class switch subset poststimulation (46% vs 28% of cells, p = 0.04). CD86 expression increased comparably in both groups after stimulation overall (Figure 2B) and in each B cell subset (not shown).
AID.
The phenotypic increase in B cell activation extended to increased expression of mRNA for activation-induced cytidine deaminase (AID) (Figure 3). AID is the B cell-specific enzyme expressed most prominently in vivo in activated germinal center B cells and is required for isotype class-switch and somatic hypermutation, both of which are essential for effective antibody maturation and antigen binding.
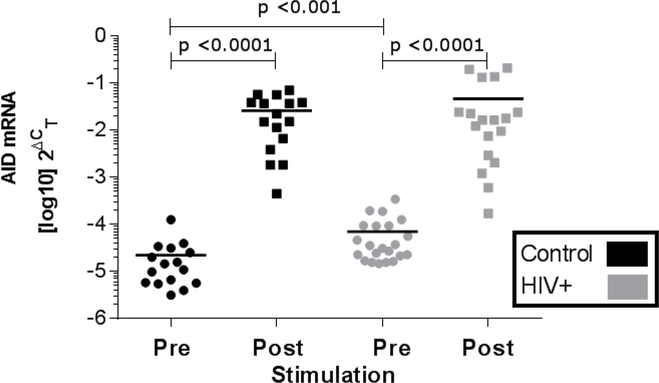
FIGURE 3. AID expression in PBMC by qRT-PCR in 14 control and 21 HIV-1-infected subjects.
Each dot represents values from one subject and horizontal bars represent mean. Values pre- (day 0) vs post-stimulation (day 4) with anti-IgM, anti-CD40, and IL-4 were compared within groups by paired ttest and between groups at each time by unpaired t-tests.
Consistent with these results, and despite increased baseline values, levels of AID mRNA in B cells from HIV-1-infected subjects increased significantly with stimulation compared with baseline values, a relevant functional response to stimulation. These stimulated values of AID were similar in magnitude to those of B cells from control subjects, but the change was lower with HIV infection, consistent with increased activation at baseline (p = 0.039) (Figure 3). Although these stimuli are directed primarily to B cells, these changes in B cell subset distribution, activation and AID expression occurred in the context of PBMC, which also include other cell populations, most prominently T cells.
Response of purified B cells to stimulation.
To determine whether these responses to primarily B cell stimuli (anti-IgM, anti-CD40 and IL-4) directly affected the B cells or were dependent in part on T cells or other accessory cells, we purified B cells by negative selection from 5 additional viremic HIV-1-infected subjects (age 45–48 years; CD4+ T cells 90–435/μL and plasma HIV-1 RNA 136,000–946,000 copies/mL) and 5 age-matched control subjects. Upon stimulation, purified B cells and those in PBMC showed similar degrees of activation, based on downregulation of CD21 and upregulation of CD86. B cells within both cell preparations showed a significant change in these marked with activation compared with baseline values (Figures 4A, B).
FIGURE 4. Impact of Stimulation on Activation of PBMC vs Purified B cells from 5 control vs 5 viremic HIV-1-infected subjects.
(A) Percentage of total B cells expressing CD21 pre- (day 0) and post- (day 5) stimulation with anti-IgM, anti-CD40, and IL-4 was measured by multiparameter flow cytometry. Values shown as mean and standard deviation. Groups were compared by unpaired t-tests. * denotes a significant difference between groups, p <0.021. (B) Percentage of total B cells expressing CD86 pre- (day 0) and post- (day 5) stimulation with anti-IgM, anti-CD40, and IL-4 was measured by multiparameter flow cytometry. Values shown as mean and standard deviation. Groups were compared by unpaired t-tests. * denotes a significant difference within groups, p <0.028.
Consistent with mRNA results, expression of intracellular AID protein increased in B cells upon stimulation (Figures 5A, B). That AID protein did not increase in CD19− cells or CD3+ T cells, nor in the absence of the primary antibody (not shown) highlights the specificity of this staining. The low but relatively increased levels of AID mRNA at baseline noted by qRT-PCR in HIV-1 infection was not in evidence with protein staining, perhaps due in part to the lower sensitivity of the flow cytometry. Nevertheless, the frequency of B cells expressing AID protein increased significantly and to a similar extent in B cells within PBMC from HIV-1-infected and control subjects, consistent with mRNA results (Figure 5B). However, although these frequencies increased in purified B cells from the control subjects, purified B cells from HIV-1-infected adults showed few AID-expressing cells overall. Purified B cells from these subjects are well-recognized to undergo increased rates of apoptosis (3), so although activated, they may have been dying. Staining with viability dye in the same cells revealed an increased proportion of dead cells in the purified B cell population from subjects with HIV-1 infection (Supplemental Figure 3). Nevertheless, B cells from HIV-1-infected patients can show significant and largely comparable response to appropriate stimuli both in the mixed cell context of PBMC and as highly purified B cell populations. We next considered the contribution of T cell activation and other factors to the baseline activation of circulating B cells.
FIGURE 5. AID Expression in Subsets of Purified B cells from 5 control vs 5 viremic HIV-1infected subjects.
(A) Representative flow plots showing shift in AID+ population with stimulation. (B) Percentage of total CD19+ B cells expressing intracellular AID were measured pre- (day 0) and post- (day 5) stimulation with anti-IgM, anti-CD40, and IL-4 by multiparameter flow cytometry. Values shown as mean and standard deviation. Groups were compared by unpaired t-tests. There were no statistically significant differences within groups.
T cell phenotype and activation.
CD4+ T cell subsets in fresh PBMC from adults with and without HIV-1 infection differed in the degree of differentiation. Central memory cells (CD45RACD27+) comprised almost half of these cells in each group (Figure 6B). However, naïve CD4+ T cells (CD45RA+CD27+) were significantly less prominent in the HIV-1-infected subjects, a difference accounted for in part by a significant increase in effector memory cells (CD45RA−CD27−) (Figure 6A, B). As shown above in B cells and long-recognized in T cells (24, 25), CD4+ and CD8+ T cells from adults with HIV-1 viremia showed significantly increased activation based on co-expression of HLA-DR and CD38 (Figures 7B,C). As with B cells, such activation extended to each subset of CD4+ T cells, with increasing activation along the spectrum of maturation (Figure 6C).
FIGURE 6. Phenotype of CD4+ T cells from 13 control and 19 viremic HIV-1-infected adults.
(A) Gating strategy using CD45RA and CD27 expression on circulating CD4+ T cells in blood from a representative control and an HIV-1-infected subject at baseline. (B) Distribution of CD3+CD4+ T cell subsets based on surface expression of CD3, CD4, CD45RA and CD27. Circulating TFH cells were identified as CD4+CXCR5+PD-1+. (C) Activation of CD4+ T cell subsets based on co-expression of HLADR and CD38. Differences in activation between control (white) and HIV-infected (gray) subjects. p <.006 for all subsets, and p ≤.03 for naïve cells. Values between groups were compared by unpaired student t test. CM (central memory), EM (effector memory), TDEM (terminally differentiated effector memory), TFH (T follicular helper cells).
FIGURE 7. Potential determinants of HIV-associated B cell activation in 13 control (black) vs 20 HIV-1-infected (gray) subjects at baseline (day 0).
Values shown as mean and standard deviation. A, B and C are results by flow cytometry of PBMC and F) mRNA from PBMC by qRT-PCR. D) and E) are protein values from serum. Groups were compared by unpaired t-tests. p-values are listed individually.
Most relevant to B cells, CD4+ T follicular helper cells (TFH) are primary drivers of B cell differentiation and maturation, as well as AID expression that drives class switch recombination (CSR) and somatic hypermutation (SHM) in germinal centers (16, 26). Recent data suggest that circulating cells with a comparable phenotype (CD4+CXCR5+PD-1+) may share the ability of their putative germinal center counterparts to provide help to B cells (reviewed in (16)). These circulating TFH-like cells (herein designated TFH cells) comprised a small but similar proportion of CD4+ T cells in subjects with and without HIV-1-infection (mean ± SEM; 1.39 ± .32 vs. 1.13 ± .19%, respectively). The vast majority of these cells in both groups shared the central memory phenotype (94% in controls vs 78% in HIV-1infected, not shown), but TFH cells from HIV-1-infected subjects more often showed effector memory cell markers (not shown), as well as a significant increase in TFH activation (Figure 7A).
Determinants of B cell activation.
We considered whether activation of T cells, particularly TFH cells, as well as B cell-stimulating soluble proteins in serum, the largely TFH-derived IL-21 protein and mRNA and the T-independent BAFF, were associated with B cell activation. All were significantly higher in HIV-1-infected subjects compared with controls, consistent with the increased B cell activation at baseline (Figures 7D, E, F).
Using linear regression models, each potential driver (HIV-1 infection, TFH activation, BAFF, CD4+ activation, CD8+ activation), but not IL-21 mRNA or protein, was significantly and inversely associated with baseline B cell activation, as determined by CD21 expression, by univariable analysis (Table III). Plasma HIV-1 RNA did not show a significant inverse correlation with CD21 expression among infected patients (not shown), but did correlate in part with a decrement in class-switch memory cells (2.3% decrease in CS memory cells per 105 increase in copies/mL of HIV-1 RNA, p = 0.11). Furthermore, expression of the activation marker CD86 significantly correlated with levels of HIV-1 RNA (3.6% increase in CD86+ per 105 increase in copies/mL of HIV-1 RNA, p = 0.0018). Using the three variables that were significantly associated with baseline B cell activation based on univariable analysis and the most biologically plausible in a multivariable comparison, only HIV-1 infection and TFH activation remained significantly associated with B cell activation (Table III).
Table III.
Linear Regression Models for Potential Determinants of CD21 Expression on B cells at Baseline
| Variable | Univariable Analysis | Multivariable Analysis | ||||
|---|---|---|---|---|---|---|
| Coefficient | 95% Confidence Interval | p-value | Coefficient | 95% Confidence Interval | p-value | |
| HIV-1 infection | −305 | (−448, −162) | 0.0025 | −168 | (−334, −2.2) | 0.047 |
| TFH activation | −7.4 | (−11.0, −3.9) | 0.0026 | −4.1 | (−8.2, −0.11) | 0.045 |
| Serum BAFF | −0.22 | (−0.38, −0.058) | 0.046 | −0.088 | (−0.23, 0.052) | 0.21 |
| + CD4 T cell activation | −8.8 | (−13.7, −4.0) | 0.0086 | |||
| + CD8 T cell activation | −4.5 | (−8.1, −0.88) | 0.0089 | |||
| IL-21 mRNA ΔCt | 42 | (−24.7, 108.9) | 0.094 | |||
Coefficient = percent change in CD21 expression on CD19+ B cells between groups per 10% increase in TFH, CD4, and CD8 activation (as measured by HLA-DR+, CD38+), 100pg/mL increase for BAFF, and ΔCt for IL-21 mRNA.
B cell activation is an early step in the maturation of B cells to generate antibodies of high specificity and avidity (strength of binding) and function. The latter are dependent on the activity of AID that mediates SHM of the antigen-binding variable regions of the heavy and light chains of immunoglobulins, as well as for CSR of the effector Fc region from IgM to IgG or IgA. CSR enhances tissue-specific antibody activity and pathogen clearance (27). As anticipated, expression of AID mRNA was very low in fresh blood cells, albeit significantly higher in cells from HIV-1-infected subjects (Figure 3). As with baseline B cell activation, levels of mRNA were significantly associated with the presence of HIV-1 infection (but not directly with copies of HIV-1 RNA in infected patients), with levels of BAFF, CD4+, CD8+ and TFH cell activation, but not plasma IL-21 protein or cellular mRNA (Table IV). None of these potential determinants remained significant in multivariable linear regression, suggesting a complementary contribution of these and likely other factors to this outcome.
Table IV.
Linear Regression Models for Potential Determinants of AID Production at Baseline
| Variable | Univariable Analysis | Multivariable Analysis | ||||
|---|---|---|---|---|---|---|
| Coefficient | 95% Confidence Interval | p-value | Coefficient | 95% Confidence Interval | p-value | |
| HIV-1 infection | 45 | (13.8, 110.8) | 0.0025 | 23.9 | (7.6, 75.2) | 0.13 |
| TFH activation | 10.4 | (10.2, 10.7) | 0.0026 | 10.2 | (9.8, 10.6) | 0.15 |
| Serum BAFF | 10.0 | (10.0, 10.02) | 0.046 | 10.00 | (9.99, 10.01) | 0.62 |
| CD4+ T cell activation | 10.4 | (10.1, 10.7) | 0.0086 | |||
| CD8+ T cell activation | 10.3 | (10.1, 10.5) | 0.0089 | |||
| IL-21 mRNA ΔCt | 7.7 | (5.7, 10.5) | 0.094 | |||
Coefficient = unit change in AID ΔCt RQ between per 10% increase in TFH, CD4, and CD8 activation (as measured by HLA-DR+, CD38+), 100pg/mL increase for BAFF, and ΔCt for IL-21 mRNA.
Overall, these data confirm that circulating B cells from viremic HIV-1-infected adults show substantial activation in vitro, a phenotype that extends to virtually all B cell subsets, including naïve cells. However, B cells from almost all subsets have the potential to generate relatively normal activation responses when given sufficient stimuli, despite increased activation at baseline. That such responses were largely intact, even with highly purified B cells, suggests that B cells from HIV-1-infected adults can be activated and differentiate with appropriate stimuli, perhaps independent of T cell dysfunction under the right conditions.
Discussion
We identify significant perturbations of circulating B cell subsets and abnormal activation in viremic HIV-1-infection compared with those in control subsets, confirming the earliest manifestations described in adults with HIV infection (1) as confirmed by others (2). However, despite increased baseline activation and active HIV-1 viremia, a striking finding of these studies is that in vitro stimulation of B cells from HIV-1-infected subjects resulted in changes in B cell subsets and activation markers comparable to those of control subjects. Indeed, the changes with in vitro stimulation with surrogate antigen in concert with cognate and soluble T cell factors extended to most of the B cell subsets tested. These findings are important because both viremic and aviremic HIV-1 infection is associated with an increased incidence of bacterial pneumonia and impaired responses to vaccination (28), but such responses could potentially be enhanced with appropriate vaccine formulations and/or immune modulation.
We found that the majority of B cell subsets were similar between controls and HIV-1-infected subjects, with the only significant differences in minority subsets. In contrast to several previous studies, we did not confirm the increased frequency of transitional B cells, the earliest stage of mature circulating B cells (29) (30) (31), nor the decrease in naïve cells reported (28), although these findings are inconsistent (32, 33). Consistent with our results, Moir, et. al., reported expansion of the CD27+ IgM memory subset with HIV-1 infection (3) as did D’Orsogna, et. al., in those with higher CD4+ T cell numbers (34). We confirmed similar but not greater frequencies of IgM+IgD+CD27+ cells compared with controls (35). The more mature class switch memory cells may be decreased in HIV-1-infected patients (3, 29–32, 34), irrespective of ART use and correlate with CD4+ T cell counts (34) and vaccine responses, whereas no such deficits were noted in infected Malawian adults (28), ART-treated adults (36), nor among our subjects. Thus, the literature reveals substantial diversity of B cell phenotype, differences that likely reflect the lack of clinical, immunologic and viral uniformity among subjects and between studies, as well as the varied effects of the infection itself in mixed populations.
Among the minority B cell subsets, the frequency of BND cells, a population not heretofore characterized in this context, were fewer among HIV-1-infected adults at baseline. Although considered anergic, these cells can be induced to differentiate into plasma cells secreting autoantibodies (23), which are often identified in these subjects (37). Thus, the multiple factors that stimulate B cells in vivo (10) may contribute to their differentiation and apparent depletion. However, the stimulation conditions used in our study may not have been of sufficient potency or duration to induce these BND cells to differentiate in vitro.
A prominent observation was the increased activation of B cells in adults with HIV-1 viremia than in controls, as consistently reported (2, 28, 38–40), effects that persist with ART (7). We extend these findings to show that such activation extends to each of the 10 subsets examined. Of note, the presence of HIV-1 infection and TFH activation were most closely correlated with this activation, whereas levels of T cell-independent factors, such as plasma HIV-1 RNA and BAFF, were not directly associated, suggesting that a range of immunologic perturbations, likely in inductive lymphoid tissues, drive B cell activation. That robust B cell activation could be elicited in vitro, but e.g., vaccine responses are limited in vivo, also directs attention to the integrity of the lymphoid follicles as the source of humoral immune dysfunction (19), rather than sustained defects in the B cells themselves. In support of this conclusion, purified B cells showed comparable evidence of activation (decreased CD21 and CD40, increased CD86) in response to stimulation in vitro in both groups. One potential weakness of our methods is the potential for masking of CD40 detection on flow cytometry by unlabeled anti-CD40 used in the stimulation cocktail. However, the decrease of CD40 expression with stimulation we found was expected and commensurate with the decrease seen in CD21 and increase seen in CD86 expression.
Indeed, the most notable aspect of these data is the similar rigor with which B cells from both groups responded to stimulation. Almost all B cell subsets, other than BND cells, showed significantly increased changes with stimulation, particularly with loss of naïve cells and gain of class switch memory cells, with few differences between groups. These data support the hypothesis that, given appropriate stimulation, B cells from viremic HIV-1-infected individuals can and do respond similarly to those of uninfected individuals in vitro, despite increased baseline activation. Post-stimulation, expression of CD21 and CD40 decreased and CD86 increased to comparable levels in both groups, consistent with induced B cell activation in most B cell subsets. Such significant responses in B cells in PBMC and their similarity between groups extended to expression of AID mRNA, and, in a subgroup tested, intracellular AID protein. AID is required for SHM which drives antibody affinity and function, as well as CSR of antibody isotypes that also determines function in specific body compartments. Whether the activation derived from the use of anti-IgM, anti-CD40 and IL-4 will enhance the compromised ability of B cells from viremic adults to provide costimulation to CD4+ T cells (39) is under investigation. That AID protein was lower in the purified B cells from HIV-1-infected patients with stimulation but not in PBMC may result from the increased susceptibility of B cells (particularly isolated B cells) from these patients to apoptosis (41), as confirmed by their lower viability.
Activation-associated downregulation of the membrane protein CD21 (complement receptor 2; CR2) may compromise the ability of B cells to respond to microbial and vaccine antigens. Engagement of the trimolecular complex of CD21, CD81, and the CD19 enhances B cell receptor signaling (42). In addition, crosslinking CD21 by C3d and BCR by antigen (such as HIV-1) in immune complexes could also underlie and amplify the B cell activation observed during HIV-1 infection. A deficit of CD21 impairs both T cell-dependent and -independent antibody responses. However, despite low levels of CD21 and CD40, B cells from HIV-1-infected patients can be activated with appropriate stimuli, with further downregulation of CD21, CD40 and increased CD86 expression, suggesting that engaging specific receptors may direct development of more effective vaccines in persons living with HIV-1 infection.
Akin to the B cells at baseline, CD4+ T cells showed a less naïve and more activated memory phenotype in the HIV-1-infected group. Two earlier studies showed a trend toward an increase in CD4+ effector memory (TEm) T cells as observed in our patients with HIV-1 viremia and not the decline in naïve cells (43, 44). However, those studies had fewer subjects and used slightly different maturation markers (CD45RA with CCR7 rather than CD27). Increased activation has been described in central memory, and to a somewhat greater degree in effector memory cells, of HIV-1 viremic than in controls (44). CD8+ T cell activation is associated with disease progression (25) and persists, albeit at a lower level, after effective antiretroviral therapy. The increased activation extended to all CD4+ T cell subsets, including TFH cells.
TFH cells are the key CD4+ memory subset that drives B cell activation and differentiation through both soluble (IL-4 and IL-21) and cognate (CD40 ligand) interactions. As activated memory CD4+ cells, TFH cells may be the preferential targets of HIV-1 infection in germinal centers (45), where they are increased, rather than decreased, in number with HIV-1 infection (19, 46, 47). Increased numbers of circulating TFH are associated with development of broadly neutralizing antibodies against HIV-1(48). However, depletion of selected TFH subpopulations (49) and expression of the inhibitory PD-1 molecule on germinal center TFH cells limits IL-21 production and B cell responses ex vivo, including antibody production and differentiation into plasma cells (19). These reports identify a suppressive effect of TFH cells whereas we identified increased TFH activation and IL-21 in association with B cell activation. Such constitutive activation may be counterproductive by limiting the ability of these cells to focus productive responses to new stimuli, such as infections and vaccines. Investigators are also now beginning to distinguish between the frequency and function of TFH vs T regulatory cells, including T follicular regulatory cells, that share several common markers (50–52), including with HIV-1 infection (47, 53, 54). Moreover, overstimulation of TFH and AID may have the effect of stimulating and “selecting” B cells in the germinal centers that express antibodies of lower affinity and avidity, thereby limiting the quality and function of the antibodies produced (55).
Factors other than HIV-1 virus and TFH activation may be drivers of B cell activation (10). Levels of B cell activating factor (BAFF), a potent T cell-independent B cell activating cytokine (56), were significantly higher in HIV-1-infected sera compared with controls at baseline, as were constitutive IL-21 mRNA and protein, among the most seminal and potent inducers of B cell differentiation and function (57, 58). By univariable linear regression models, each potential driver except IL-21 (mRNA and protein) was significantly associated with baseline B cell activation (as measured by loss of CD21 expression). Using the three significant and biologically-plausible variables (HIV infection, TFH activation, and BAFF) in a multivariable comparison, only HIV-1 infection and TFH activation remained significantly associated with B cell activation.
As with baseline B cell activation, levels of AID mRNA were significantly associated with the presence of HIV-1 infection, consistent with previous studies (36), but not directly with number of copies of HIV-1 RNA in infected patients, and with levels of BAFF, CD4+, CD8+ and TFH cell activation, but not plasma IL-21 mRNA or protein (Table IV). None of these potential determinants remained significant in multivariable linear regression, suggesting a complementary contribution of each to this outcome or, potentially, other drivers. Despite the difference in baseline AID mRNA between groups, AID mRNA and protein increased significantly and comparably post-stimulation levels in both groups, again indicating the capacity for B cells from HIV-1-infected subjects to respond comparably to controls. The ability of B cells to generate antibodies of high specificity and avidity and function is dependent on the activity of AID for somatic hypermutation (SHM) of the antigen-binding variable regions of the heavy and light chains of immunoglobulins, as well as for CSR of the effector Fc region from IgM to IgG or IgA (or IgE). CSR enhances tissue-specific antibody activity and pathogen clearance (27).
Potential limitations of this study include a relatively small sample size and the absence of data on selected variables in some subjects. We chose to evaluate viremic subjects rather than virologically suppressed HIV-1-infected subjects to identify the role of the virus itself and to identify potentially remediable defects in B cell biology. Documenting the duration of infection also would have been a relevant characteristic that may affect the outcomes studied.
Conclusions
In summary, despite baseline perturbations in B cell populations from HIV-1-infected subjects, particularly increased activation compared with control subjects, B cells from these subjects possess the capacity to generate robust changes in activation and phenotype, as well as expression of AID mRNA and protein when stimulated with a combination of surrogate antigen, T cell cognate and soluble factors in vitro. That viremic HIV-1-infected individuals respond suboptimally to vaccinations in vivo highlights the potential to design more effective vaccine formulations that incorporate features of this stimulation panel. Newer vaccines that utilize both protein conjugates and TLR and other innate ligands have shown increased immunogenicity (59) and some efficacy (60) in HIV-1-infected patients. Building on these in vivo and in vitro results could limit the range of opportunistic that continue to compromise and kill patients with HIV-1 infection in both industrialized, and particularly, resource-limited countries that bear the largest burden of HIV-1 disease.
Supplementary Material
Funding/Acknowledgements:
This work was supported by the Veterans Affairs Research Service (I01CX001464), NIH grant R01 AI108479, and the University of Colorado Denver Mucosal and Vaccine Research Program Colorado (MAVRC) (ENJ).
Footnotes
Conflicts of Interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lane HC, Masur H, Edgar LC, Whalen G, Rook AH, Fauci AS. 1983. Abnormalities of B-cell activation and immunoregulation in patients with the acquired immunodeficiency syndrome. N Engl J Med 309: 453–8 [DOI] [PubMed] [Google Scholar]
- 2.Moir S, Malaspina A, Ogwaro KM, Donoghue ET, Hallahan CW, Ehler LA, Liu S, Adelsberger J, Lapointe R, Hwu P, Baseler M, Orenstein JM, Chun TW, Mican JA, Fauci AS. 2001. HIV-1 induces phenotypic and functional perturbations of B cells in chronically infected individuals. Proc Natl Acad Sci U S A 98: 10362–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moir S, Fauci AS. 2008. Pathogenic mechanisms of B-lymphocyte dysfunction in HIV disease. J Allergy Clin Immunol [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moir S, Malaspina A, Pickeral OK, Donoghue ET, Vasquez J, Miller NJ, Krishnan SR, Planta MA, Turney JF, Justement JS, Kottilil S, Dybul M, Mican JM, Kovacs C, Chun TW, Birse CE, Fauci AS. 2004. Decreased survival of B cells of HIV-viremic patients mediated by altered expression of receptors of the TNF superfamily. J Exp Med 200: 587–99 [PubMed] [Google Scholar]
- 5.Ruffin N, Lantto R, Pensieroso S, Sammicheli S, Hejdeman B, Rethi B, Chiodi F. 2012. Immune activation and increased IL-21R expression are associated with the loss of memory B cells during HIV-1 infection. J Intern Med [DOI] [PubMed] [Google Scholar]
- 6.Samuelsson A, Sonnerborg A, Heuts N, Coster J, Chiodi F. 1997. Progressive B cell apoptosis and expression of Fas ligand during human immunodeficiency virus type 1 infection. AIDS Res Hum Retroviruses 13: 1031–8 [DOI] [PubMed] [Google Scholar]
- 7.Johannesson TG, Sogaard OS, Tolstrup M, Petersen MS, Bernth-Jensen JM, Ostergaard L, Erikstrup C. 2012. The impact of B-cell perturbations on pneumococcal conjugate vaccine response in HIV-infected adults. PLoS One 7: e42307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang W, Lederman MM, Mohner RJ, Rodriguez B, Nedrich TM, Harding CV, Sieg SF. 2008. Impaired naive and memory B-cell responsiveness to TLR9 stimulation in human immunodeficiency virus infection. J Virol 82: 7837–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siewe B, Keshavarzian A, French A, Demarais P, Landay A. 2013. A role for TLR signaling during B cell activation in antiretroviral-treated HIV individuals. AIDS Res Hum Retroviruses 29: 1353–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haas A, Zimmermann K, Oxenius A. 2011. Antigen-dependent and -independent mechanisms of T and B cell hyperactivation during chronic HIV-1 infection. J Virol 85: 12102–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He B, Qiao X, Klasse PJ, Chiu A, Chadburn A, Knowles DM, Moore JP, Cerutti A. 2006. HIV-1 envelope triggers polyclonal Ig class switch recombination through a CD40independent mechanism involving BAFF and C-type lectin receptors. J Immunol 176: 3931–41 [DOI] [PubMed] [Google Scholar]
- 12.Epeldegui M, Thapa DR, De la Cruz J, Kitchen S, Zack JA, Martinez-Maza O. 2010. CD40 ligand (CD154) incorporated into HIV virions induces activation-induced cytidine deaminase (AID) expression in human B lymphocytes. PLoS One 5: e11448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haas A, Zimmermann K, Graw F, Slack E, Rusert P, Ledergerber B, Bossart W, Weber R,Thurnheer MC, Battegay M, Hirschel B, Vernazza P, Patuto N, Macpherson AJ, Gunthard HF, Oxenius A. 2011. Systemic antibody responses to gut commensal bacteria during chronic HIV-1 infection. Gut [DOI] [PubMed] [Google Scholar]
- 14.Levesque MC, Moody MA, Hwang KK, Marshall DJ, Whitesides JF, Amos JD, Gurley TC,Allgood S, Haynes BB, Vandergrift NA, Plonk S, Parker DC, Cohen MS, Tomaras GD,Goepfert PA, Shaw GM, Schmitz JE, Eron JJ, Shaheen NJ, Hicks CB, Liao HX, Markowitz M, Kelsoe G, Margolis DM, Haynes BF. 2009. Polyclonal B cell differentiation and loss of gastrointestinal tract germinal centers in the earliest stages of HIV-1 infection. PLoS Med 6: e1000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schacker TW, Nguyen PL, Beilman GJ, Wolinsky S, Larson M, Reilly C, Haase AT. 2002Collagen deposition in HIV-1 infected lymphatic tissues and T cell homeostasis. J Clin Invest 110: 1133–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crotty S 2014. T Follicular Helper Cell Differentiation, Function, and Roles in Disease. Immunity 41: 529–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morita R, Schmitt N, Bentebibel SE, Ranganathan R, Bourdery L, Zurawski G, Foucat E,Dullaers M, Oh S, Sabzghabaei N, Lavecchio EM, Punaro M, Pascual V, Banchereau J, Ueno H. 2011. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity 34: 108–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lindqvist M, van Lunzen J, Soghoian DZ, Kuhl BD, Ranasinghe S, Kranias G, Flanders MD, Cutler S, Yudanin N, Muller MI, Davis I, Farber D, Hartjen P, Haag F, Alter G, Schulze zurWiesch J, Streeck H. 2012. Expansion of HIV-specific T follicular helper cells in chronic HIV infection. J Clin Invest 122: 3271–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cubas RA, Mudd JC, Savoye AL, Perreau M, van Grevenynghe J, Metcalf T, Connick E, Meditz A, Freeman GJ, Abesada-Terk G, Jr., Jacobson JM, Brooks AD, Crotty S, Estes JD, Pantaleo G, Lederman MM, Haddad EK. 2013. Inadequate T follicular cell help impairs B cell immunity during HIV infection. Nat Med 19: 494–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weill JC, Weller S, Reynaud CA. 2009. Human marginal zone B cells. Annu Rev Immunol 27: 267–85 [DOI] [PubMed] [Google Scholar]
- 21.Descatoire M, Weller S, Irtan S, Sarnacki S, Feuillard J, Storck S, Guiochon-Mantel A, Bouligand J, Morali A, Cohen J, Jacquemin E, Iascone M, Bole-Feysot C, Cagnard N, Weill JC, Reynaud CA. 2014. Identification of a human splenic marginal zone B cell precursor with NOTCH2-dependent differentiation properties. J Exp Med 211: 987–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kruetzmann S, Rosado MM, Weber H, Germing U, Tournilhac O, Peter HH, Berner R,Peters A, Boehm T, Plebani A, Quinti I, Carsetti R. 2003. Human Immunoglobulin MMemory B Cells Controlling Streptococcus pneumoniae Infections Are Generated in the Spleen. J Exp Med 197: 939–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duty JA, Szodoray P, Zheng NY, Koelsch KA, Zhang Q, Swiatkowski M, Mathias M, Garman L, Helms C, Nakken B, Smith K, Farris AD, Wilson PC. 2009. Functional anergy in a subpopulation of naive B cells from healthy humans that express autoreactive immunoglobulin receptors. J Exp Med 206: 139–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giorgi JV, Liu Z, Hultin LE, Cumberland WG, Hennessey K, Detels R. 1993. Elevated levels of CD38+ CD8+ T cells in HIV infection add to the prognostic value of low CD4+ T cell levels: results of 6 years of follow-up. The Los Angeles Center, Multicenter AIDS Cohort Study. J Acquir Immune Defic Syndr 6: 904–12 [PubMed] [Google Scholar]
- 25.Liu Z, Cumberland WG, Hultin LE, Kaplan AH, Detels R, Giorgi JV. 1998. CD8+ Tlymphocyte activation in HIV-1 disease reflects an aspect of pathogenesis distinct from viral burden and immunodeficiency. J Acquir Immune Defic Syndr Hum Retrovirol 18: 332–40 [DOI] [PubMed] [Google Scholar]
- 26.Hale JS, Ahmed R. 2015. Memory T follicular helper CD4 T cells. Front Immunol 6: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stavnezer J, Schrader CE. 2014. IgH Chain Class Switch Recombination: Mechanism and Regulation. J Immunol 193: 5370–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iwajomo OH, Finn A, Ogunniyi AD, Williams NA, Heyderman RS. 2013. Impairment of pneumococcal antigen specific isotype-switched Igg memory B-cell immunity in HIV infected Malawian adults. PLoS One 8: e78592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moir S, Buckner CM, Ho J, Wang W, Chen J, Waldner AJ, Posada JG, Kardava L, O’Shea MA, Kottilil S, Chun TW, Proschan MA, Fauci AS. 2010. B cells in early and chronic HIV infection: evidence for preservation of immune function associated with early initiation of antiretroviral therapy. Blood 116: 5571–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hart M, Steel A, Clark SA, Moyle G, Nelson M, Henderson DC, Wilson R, Gotch F, Gazzard B, Kelleher P. 2007. Loss of discrete memory B cell subsets is associated with impaired immunization responses in HIV-1 infection and may be a risk factor for invasive pneumococcal disease. J Immunol 178: 8212–20 [DOI] [PubMed] [Google Scholar]
- 31.Suryani S, Fulcher DA, Santner-Nanan B, Nanan R, Wong M, Shaw PJ, Gibson J, Williams A, Tangye SG. 2010. Differential expression of CD21 identifies developmentally and functionally distinct subsets of human transitional B cells. Blood 115: 519–29 [DOI] [PubMed] [Google Scholar]
- 32.Amu S, Lavy-Shahaf G, Cagigi A, Hejdeman B, Nozza S, Lopalco L, Mehr R, Chiodi F. 2014. Frequency and phenotype of B cell subpopulations in young and aged HIV-1 infected patients receiving ART. Retrovirology 11: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amu S, Ruffin N, Rethi B, Chiodi F. 2013. Impairment of B-cell functions during HIV-1 infection. AIDS 27: 2323–34 [DOI] [PubMed] [Google Scholar]
- 34.D’Orsogna LJ, Krueger RG, McKinnon EJ, French MA. 2007. Circulating memory B-cell subpopulations are affected differently by HIV infection and antiretroviral therapy. AIDS 21: 1747–52 [DOI] [PubMed] [Google Scholar]
- 35.Titanji K, De Milito A, Cagigi A, Thorstensson R, Grutzmeier S, Atlas A, Hejdeman B, Kroon FP, Lopalco L, Nilsson A, Chiodi F. 2006. Loss of memory B cells impairs maintenance of long-term serologic memory during HIV-1 infection. Blood 108: 1580–7 [DOI] [PubMed] [Google Scholar]
- 36.Cagigi A, Du L, Dang LV, Grutzmeier S, Atlas A, Chiodi F, Pan-Hammarstrom Q, Nilsson A. 2009. CD27(−) B-cells produce class switched and somatically hyper-mutated antibodies during chronic HIV-1 infection. PLoS One 4: e5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iordache L, Launay O, Bouchaud O, Jeantils V, Goujard C, Boue F, Cacoub P, Hanslik T, Mahr A, Lambotte O, Fain O, Associated a. 2014. Autoimmune diseases in HIV-infected patients: 52 cases and literature review. Autoimmun Rev 13: 850–7 [DOI] [PubMed] [Google Scholar]
- 38.Kardava L, Moir S, Shah N, Wang W, Wilson R, Buckner CM, Santich BH, Kim LJ, Spurlin EE, Nelson AK, Wheatley AK, Harvey CJ, McDermott AB, Wucherpfennig KW, Chun TW, Tsang JS, Li Y, Fauci AS. 2014. Abnormal B cell memory subsets dominate HIV-specific responses in infected individuals. J Clin Invest 124: 3252–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Malaspina A, Moir S, Kottilil S, Hallahan CW, Ehler LA, Liu S, Planta MA, Chun TW, Fauci AS. 2003. Deleterious effect of HIV-1 plasma viremia on B cell costimulatory function. J Immunol 170: 5965–72 [DOI] [PubMed] [Google Scholar]
- 40.de Bree GJ, Wheatley AK, Lynch RM, Prabhakaran M, Grijsen ML, Prins JM, Schmidt SD, Koup RA, Mascola JR, McDermott AB. 2017. Longitudinal dynamics of the HIV-specific B cell response during intermittent treatment of primary HIV infection. PLoS One 12: e0173577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ho J, Moir S, Malaspina A, Howell ML, Wang W, DiPoto AC, O’Shea MA, Roby GA, Kwan R, Mican JM, Chun TW, Fauci AS. 2006. Two overrepresented B cell populations in HIVinfected individuals undergo apoptosis by different mechanisms. Proc Natl Acad Sci U S A 103: 19436–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fearon DT, Carroll MC. 2000. Regulation of B lymphocyte responses to foreign and selfantigens by the CD19/CD21 complex. Annu Rev Immunol 18: 393–422 [DOI] [PubMed] [Google Scholar]
- 43.Palmer BE, Boritz E, Wilson CC. 2004. Effects of sustained HIV-1 plasma viremia on HIV-1 Gag-specific CD4+ T cell maturation and function. J Immunol 172: 3337–47 [DOI] [PubMed] [Google Scholar]
- 44.Potter SJ, Lacabaratz C, Lambotte O, Perez-Patrigeon S, Vingert B, Sinet M, Colle JH, Urrutia A, Scott-Algara D, Boufassa F, Delfraissy JF, Theze J, Venet A, Chakrabarti LA. 2007. Preserved central memory and activated effector memory CD4+ T-cell subsets in human immunodeficiency virus controllers: an ANRS EP36 study. J Virol 81: 13904–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brenchley JM, Vinton C, Tabb B, Hao XP, Connick E, Paiardini M, Lifson JD, Silvestri G, Estes JD. 2012. Differential infection patterns of CD4+ T cells and lymphoid tissue viral burden distinguish progressive and nonprogressive lentiviral infections. Blood 120: 4172–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ruffin N, Hani L, Seddiki N. 2017. From dendritic cells to B cells dysfunctions during HIV-1 infection: T follicular helper cells at the crossroads. Immunology 151: 137–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miles B, Miller SM, Connick E. 2016. CD4 T Follicular Helper and Regulatory Cell Dynamics and Function in HIV Infection. Front Immunol 7: 659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Locci M, Havenar-Daughton C, Landais E, Wu J, Kroenke MA, Arlehamn CL, Su LF, Cubas R, Davis MM, Sette A, Haddad EK, International AVIPCPI, Poignard P, Crotty S. 2013. Human circulating PD-1+CXCR3-CXCR5+ memory Tfh cells are highly functional and correlate with broadly neutralizing HIV antibody responses. Immunity 39: 758–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boswell KL, Paris R, Boritz E, Ambrozak D, Yamamoto T, Darko S, Wloka K, Wheatley A, Narpala S, McDermott A, Roederer M, Haubrich R, Connors M, Ake J, Douek DC, Kim J, Petrovas C, Koup RA. 2014. Loss of circulating CD4 T cells with B cell helper function during chronic HIV infection. PLoS Pathog 10: e1003853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chung Y, Tanaka S, Chu F, Nurieva RI, Martinez GJ, Rawal S, Wang YH, Lim H, Reynolds JM, Zhou XH, Fan HM, Liu ZM, Neelapu SS, Dong C. 2011. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat Med 17: 983–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Linterman MA, Pierson W, Lee SK, Kallies A, Kawamoto S, Rayner TF, Srivastava M, Divekar DP, Beaton L, Hogan JJ, Fagarasan S, Liston A, Smith KG, Vinuesa CG. 2011. Foxp3+ follicular regulatory T cells control the germinal center response. Nat Med 17: 975–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sage PT, Francisco LM, Carman CV, Sharpe AH. 2012. The receptor PD-1 controls follicular regulatory T cells in the lymph nodes and blood. Nat Immunol 14: 152–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aandahl EM, Michaelsson J, Moretto WJ, Hecht FM, Nixon DF. 2004. Human CD4+ CD25+ regulatory T cells control T-cell responses to human immunodeficiency virus and cytomegalovirus antigens. J Virol 78: 2454–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weiss L, Donkova-Petrini V, Caccavelli L, Balbo M, Carbonneil C, Levy Y. 2004. Human immunodeficiency virus-driven expansion of CD4+CD25+ regulatory T cells, which suppress HIV-specific CD4 T-cell responses in HIV-infected patients. Blood 104: 3249–56 [DOI] [PubMed] [Google Scholar]
- 55.Victora GD, Schwickert TA, Fooksman DR, Kamphorst AO, Meyer-Hermann M, Dustin ML, Nussenzweig MC. 2010. Germinal center dynamics revealed by multiphoton microscopy with a photoactivatable fluorescent reporter. Cell 143: 592–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Buchanan RM, Popowych Y, Arsic N, Townsend HG, Mutwiri GK, Potter AA, Babiuk LA, Griebel PJ, Wilson HL. 2011. B-cell activating factor (BAFF) promotes CpG ODN-induced B cell activation and proliferation. Cell Immunol 271: 16–28 [DOI] [PubMed] [Google Scholar]
- 57.Pallikkuth S, Pilakka Kanthikeel S, Silva SY, Fischl M, Pahwa R, Pahwa S. 2011. Upregulation of IL-21 receptor on B cells and IL-21 secretion distinguishes novel 2009 H1N1 vaccine responders from nonresponders among HIV-infected persons on combination antiretroviral therapy. J Immunol 186: 6173–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Avery DT, Deenick EK, Ma CS, Suryani S, Simpson N, Chew GY, Chan TD, Palendira U,Bustamante J, Boisson-Dupuis S, Choo S, Bleasel KE, Peake J, King C, French MA,Engelhard D, Al-Hajjar S, Al-Muhsen S, Magdorf K, Roesler J, Arkwright PD, Hissaria P, Riminton DS, Wong M, Brink R, Fulcher DA, Casanova JL, Cook MC, Tangye SG. 2010. B cell-intrinsic signaling through IL-21 receptor and STAT3 is required for establishing longlived antibody responses in humans. J Exp Med 207: 155–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Offersen R, Melchjorsen J, Paludan SR, Ostergaard L, Tolstrup M, Sogaard OS. 2012. TLR9-adjuvanted pneumococcal conjugate vaccine induces antibody-independent memory responses in HIV-infected adults. Hum Vaccin Immunother 8: 1042–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.French N, Gordon SB, Mwalukomo T, White SA, Mwafulirwa G, Longwe H, Mwaiponya M, Zijlstra EE, Molyneux ME, Gilks CF. 2010. A trial of a 7-valent pneumococcal conjugate vaccine in HIV-infected adults. N Engl J Med 362: 812–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.