Abstract
Objective:
Evaluate the association between exposure to oral corticosteroids (CSs) and future healthcare resource utilization (HCRU) and costs for patients with systemic lupus erythematosus (SLE).
Methods:
Adults diagnosed with SLE (index date) between January 1, 2008 and June 30, 2013 and naive to oral CSs with continuous health plan enrollment for ≥6 months pre- and ≥5 years post-index were identified from a large health plan claims database. Per-patient monthly average daily dose (ADD) of oral CSs (prednisone or its equivalent) was calculated for the first 2 years post-index to categorize patients into 4 steroid exposure cohorts: low (≤5 mg/day), medium (6–20 mg/day), high (>20 mg/day) and no-steroids. Differences in HCRU and total healthcare costs during the third year post-index across CS exposure cohorts were modeled with adjustment for baseline characteristics.
Results:
Study included 18,618 SLE patients (163 high-dose, 1,127 medium-dose, 6,717 low-dose, and 10,611 no-steroids). Compared to low-dose CS users, high-dose CS users were more likely to have emergency room visits (39.3% vs. 29.7%; p=0.0085) and to be hospitalized (21.5% vs. 12.3%; p=0.0005). After adjustment for baseline characteristics, they also had significantly greater average annual total healthcare costs ($60,366 vs. $18,777; p<0.0001). A one milligram increase in CS average daily dose was associated with 1.07 times the average annual costs after adjusting for baseline characteristics (p<0.0001).
Conclusion:
Long-term high-dose oral CS use was associated with significantly greater future HCRU and costs. Judicious reduction in daily steroid dose may decrease the imminent economic burden associated with high-dose steroid use in SLE.
Keywords: Lupus, SLE, Corticosteroids, Costs, Resource Utilization, Burden
INTRODUCTION
Systemic lupus erythematosus (SLE) is a serious chronic autoimmune disease characterized by widespread inflammation in multiple organs.1,2 It occurs primarily in young women of child-bearing age.1,3 According to estimates of the Lupus Foundation of America, 1.5 million Americans have a form of lupus and more than 16,000 new cases are reported every year.4 SLE is associated with compromised health-related quality of life (HRQoL) and significant economic burden, with mean annual direct costs reported to range from $13,735 to $20,926 per person in the United States (US) (adjusted to 2009 US dollars).5,6,7,8,9,10,11,12,13,14 Mean annual medical costs for patients with SLE are reported to be 3 to 4 times greater compared with those for people without SLE.15
Current treatment options manage symptoms of disease and reduce the number and severity of flares, with corticosteroids (CSs) often being the cornerstones of treatment.16,17,18,19 Despite this, few studies have evaluated the impact of CS dose on healthcare resource utilization (HCRU) and costs among SLE patients in the US. They have reported greater doses of CSs to be associated with increased risk/incidence of various CS-associated adverse events and greater annual HCRU and costs.20,21,22,23,24 Although the clinical burden and toxicity of CSs are well-established in the literature, none of these studies have evaluated the HCRU and costs among SLE patients newly initiating oral CS therapy and receiving long-term high-dose/medium-dose/no CSs vs. low-dose oral CSs. Published large administrative database studies that evaluated CS-associated HCRU and costs in SLE also used relatively older data21,23, while US payers are interested in recent data. As CSs are commonly prescribed in SLE patients and many patients take them chronically, it is important to generate evidence on the current clinical and economic burden associated with long-term CS use. Thus, the goal of this study was to evaluate the association between exposure to oral CSs measured over 24 months and future HCRU and costs in commercially insured SLE patients newly initiating oral CS treatment in the US.
PATIENTS AND METHODS
DATA SOURCE
This observational, retrospective cohort study was conducted using the IQVIA PharMetrics Plus (PMTX+) Health Plan Claims Database from July 1, 2007, to June 30, 2016. This database comprises adjudicated medical and pharmacy claims for more than 150 million unique health plan members across the US, providing a diverse representation of geography, employers, payers and providers. Data elements include inpatient and outpatient diagnoses and procedures, retail and mail order prescription records, pharmacy and medical benefit (co-pay, deductible) information, inpatient stay and provider details, demographic variables, product type, payer type, and start and stop dates of health-plan enrollment. Amounts charged by the providers and allowed and paid by the health plans are available for all services rendered. The database is nationally representative of the US commercially insured population in terms by age and sex. Patients in each three-digit zip code and every metropolitan statistical area of the US are included, with data from 90% of US hospitals, 80% of all US doctors, and representation from 85% of the Fortune 100 companies. All data are compliant with the Health Insurance Portability and Accountability Act (HIPAA) to protect patients’ privacy.
SAMPLE SELECTION
Patients aged ≥18 years with ≥2 non–same-day medical claims with a diagnosis of SLE (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] code: 710.0x) between January 1, 2008, and June 30, 2013 (selection period) were identified in the database. The date of the first observed SLE diagnosis code (≥2 non-same-day medical claims with a diagnosis of SLE [ICD-9 710.0x] were required) was defined as the index date (prevalent SLE population). For inclusion, patients needed to have continuous health plan enrollment for ≥180 days immediately preceding the index date (pre-index period) and ≥1,080 days immediately following the index date (post-index period). Patients were excluded from the study if they had ≥1 pharmacy claim for oral steroids during the 180-day pre-index period; were aged ≥65 years and not covered by Medicare Risk, had Medicare Cost coverage or State Children’s Health Insurance Program (SCHIP); or had data quality issues (missing drug quantity information or invalid year of birth, sex, or health plan enrollment dates).
DEFINING CORTICOSTEROID EXPOSURE GROUPS
The first 2 years of the post-index period were used to create oral CS exposure groups for comparison (exposure period), while the third year of the post-index period was used to evaluate outcome measures. Three of the four exposure groups of interest were based on evidence of ≥1 pharmacy claim for an oral CS (betamethasone, budesonide, cortisone acetate, deflazacort, dexamethasone, hydrocortisone, methylprednisolone, prednisolone, prednisolone acetate, prednisolone sodium phosphate, prednisone or triamcinolone) during the exposure period and following their first SLE diagnosis. The first oral CS prescription/administration was defined as the patient’s treatment index date. All patients with no evidence of oral CSs in the exposure period formed the fourth, “no-steroid user” cohort and retained their SLE diagnosis-based index dates.
To account for both length of exposure as well as variable dosing, patients’ average daily dose (ADD) of prednisone (the most commonly used oral CS in SLE patients) for each of the first 24 months post-index was calculated using the following formula derived from pharmacy claims: (Strength x Quantity) / Days Supply.
The per-patient average monthly ADD was then calculated for the 24-month period, and the resultant values were used to categorize patients into three mutually exclusive exposure groups (based on literature review and inputs from clinical experts):
-
1)
“Low-dose”, defined as ≤5mg/day
-
2)
“Medium-dose”, defined as 6–20mg/day
-
3)
“High-dose”, defined as >20mg/day
ADDs of other oral CSs (e.g., methylprednisolone) were converted into the prednisone equivalent.25
STUDY MEASURES
All-cause HCRU and all-cause healthcare costs were evaluated in the post-index period during the third year. HCRU included the mutually exclusive categories of pharmacy services (Rx), emergency room (ER) visits, outpatient (OP) visits and inpatient (IP) visits. Costs were assessed for each of these HCRU services. Only direct health care costs for services covered by the patient’s insurance benefit were reported, using allowed amounts on the claims, which represented the contracted reimbursable amount for covered medical services or supplies that the health plan agrees to pay to service providers. Costs were converted to 2016 US dollar using the medical component of the Consumer Price Index.
Patient demographics and baseline clinical characteristics were identified during the pre-index period. These included age at index date, sex, health plan type, payer type, region, index year, physician specialty (that recorded the SLE diagnosis), rheumatology visit, primary care physician (PCP) visit, hematology visit, nephrology visit, Charlson Comorbidity Index (CCI) score, comorbidities, concomitant medications (non-steroidal anti-inflammatory drugs [NSAIDs], anti-malarials, and immunosuppressants [e.g., cyclophosphamide, mycophenolate mofetil, azathioprine, cyclosporine, tacrolimus), total medical costs and total pharmacy costs.
STATISTICAL ANALYSES
For categorical measures, data were reported as the frequency (number of cases [N]) and percentage (%) of total patients observed in each category. For continuous variables, data were reported as the mean, standard deviation (SD), and median. Differences in the distribution of these variables were tested for statistical significance using the chi-squared test for categorical variables and the non-parametric Wilcoxon rank-sum test for continuous variables. A p-value of ≤0.05 was considered statistically significant. Generalized linear modelling (with a log link function and gamma error term distribution) was used to evaluate differences in total healthcare costs during the third year post-index among CS exposure cohorts. Models were adjusted for age, gender, CCI score, cardiovascular disease (e.g., acute myocardial infarction, coronary atherosclerosis, aneurysm, pulmonary embolism, pericarditis, valve disorders, cardiomyopathy, tachycardia, atrial fibrillation, congestive heart failure, embolism) renal disease (e.g., glomerulonephritis, nephrotic syndrome, nephritis, nephropathy, acute kidney failure, chronic kidney disease, end state renal disease, renal failure, renal sclerosis), pre-index total medical costs and pre-index use of prescription NSAIDs and anti-malarial agents. These specific variables were selected for adjustment as they confound the association between steroid dose and HCRU/costs; furthermore, they have been previously demonstrated to be associated with HCRU and costs in SLE.21,23
RESULTS
STUDY ATTRITION
In total, 109,817 patients were initially identified as having evidence of SLE, of whom 91,199 (83%) were excluded for the reasons listed in Figure 1. The remaining 18,618 patients included 163 high-dose oral CS patients, 1,127 medium-dose oral CS patients, 6,717 low-dose oral CS patients, and 10,611 no-steroid use patients (Table 1).
Figure 1:
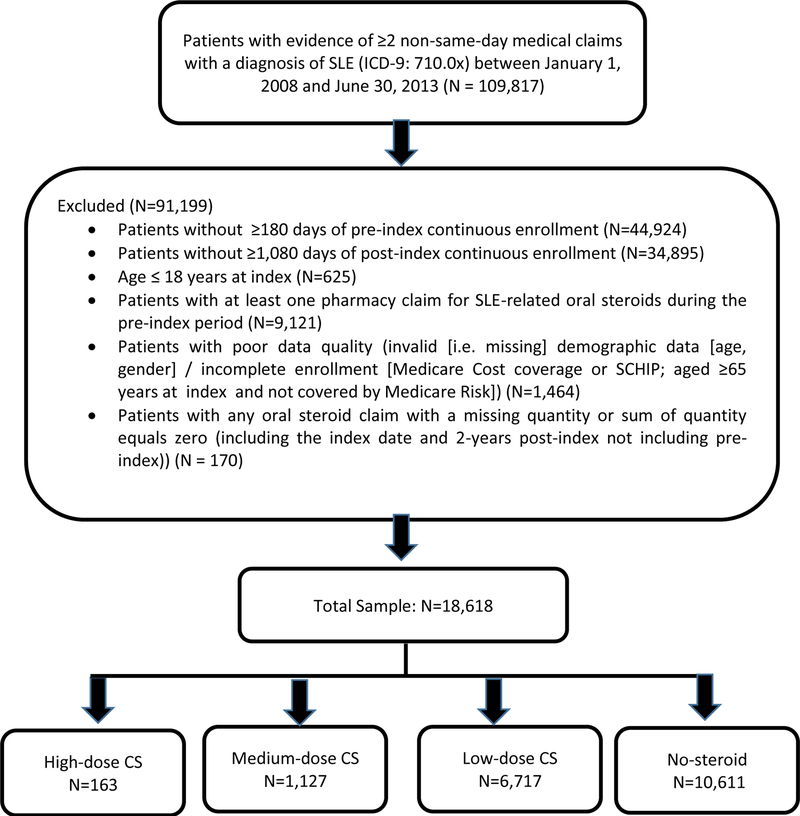
Sample Selection
Table 1.
Baseline characteristics of the study sample
| Patient cohort | High Dose CS (HD) |
Medium Dose CS (MD) |
Low Dose CS (LD) |
No-steroid Dose (NS) |
p- value (HD vs. LD) |
p- value (MD vs. LD) |
p- value (NS vs. LD) |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (N = 163) | (N = 1,127) | (N = 6,717) | (N = 10,611) | ||||||||
| Age Group (n, %) | |||||||||||
| 18–34 years | 46 | 28.2% | 249 | 22.1% | 978 | 14.6% | 1,505 | 14.2% | † | * | † |
| 35–44 years | 35 | 21.5% | 283 | 25.1% | 1,648 | 24.5% | 2,408 | 22.7% | |||
| 45–54 years | 47 | 28.8% | 369 | 32.7% | 2,426 | 36.1% | 3,764 | 35.5% | |||
| 55–64 years | 34 | 20.9% | 221 | 19.6% | 1,642 | 24.4% | 2,890 | 27.2% | |||
| 65+ years | 1 | 0.6% | 5 | 0.4% | 23 | 0.3% | 44 | 0.4% | |||
| Age (years) | |||||||||||
| Mean ± SD | 43.3 ± 12.4 | 44.1 ± 11.4 | 46.3 ± 10.5 | 46.9 ± 10.7 | † | * | † | ||||
|
Gender, (n, %) Female |
143 | 87.7% | 987 | 87.6% | 6,190 | 92.2% | 9,523 | 89.7% | † | * | * |
|
Health Plan Type (n, %) |
|||||||||||
| Consumer-directed | 0 | 0.0% | 9 | 0.8% | 23 | 0.3% | 50 | 0.5% | † | * | |
| HMO | 24 | 14.7% | 193 | 17.1% | 925 | 13.8% | 1,797 | 16.9% | |||
| Indemnity | 5 | 3.1% | 31 | 2.8% | 165 | 2.5% | 356 | 3.4% | |||
| POS | 10 | 6.1% | 53 | 4.7% | 239 | 3.6% | 455 | 4.3% | |||
| PPO | 121 | 74.2% | 833 | 73.9% | 5,300 | 78.9% | 7,837 | 73.9% | |||
| Other/Unknown | 3 | 1.8% | 8 | 0.7% | 65 | 1.0% | 116 | 1.1% | |||
| Payer Type (n, %) | |||||||||||
| Commercial | 89 | 54.6% | 647 | 57.4% | 3,886 | 57.9% | 6,064 | 57.1% | † | ||
| Medicaid | 9 | 5.5% | 57 | 5.1% | 264 | 3.9% | 565 | 5.3% | |||
| Medicare | 1 | 0.6% | 8 | 0.7% | 36 | 0.5% | 60 | 0.6% | |||
| Self-insured | 61 | 37.4% | 410 | 36.4% | 2,496 | 37.2% | 3,845 | 36.2% | |||
| Other/Unknown | 3 | 1.8% | 5 | 0.4% | 35 | 0.5% | 77 | 0.7% | |||
| Region (n, %) | |||||||||||
| Northeast | 39 | 23.9% | 284 | 25.2% | 1,576 | 23.5% | 3,018 | 28.4% | † | * | |
| Midwest | 48 | 29.4% | 279 | 24.8% | 1,603 | 23.9% | 2,856 | 26.9% | |||
| South | 63 | 38.7% | 425 | 37.7% | 2,917 | 43.4% | 3,536 | 33.3% | |||
| West | 13 | 8.0% | 139 | 12.3% | 621 | 9.2% | 1,201 | 11.3% | |||
| Index Year (n, %) | |||||||||||
| 2008 | 60 | 36.8% | 450 | 39.9% | 2,787 | 41.5% | 4,861 | 45.8% | * | ||
| 2009 | 26 | 16.0% | 179 | 15.9% | 1,045 | 15.6% | 1,754 | 16.5% | |||
| 2010 | 32 | 19.6% | 165 | 14.6% | 939 | 14.0% | 1,392 | 13.1% | |||
| 2011 | 20 | 12.3% | 156 | 13.8% | 874 | 13.0% | 1,227 | 11.6% | |||
| 2012 | 16 | 9.8% | 117 | 10.4% | 711 | 10.6% | 913 | 8.6% | |||
| 2013 | 9 | 5.5% | 60 | 5.3% | 361 | 5.4% | 464 | 4.4% | |||
|
Physician Specialty (n, %) |
|||||||||||
| Rheumatologist | 31 | 19.0% | 231 | 20.5% | 1,450 | 21.6% | 2,075 | 19.6% | † | ||
| Cardiologist | 1 | 0.6% | 2 | 0.2% | 28 | 0.4% | 45 | 0.4% | |||
| Pulmonologist | 0 | 0.0% | 7 | 0.6% | 14 | 0.2% | 21 | 0.2% | † | ||
| Nephrologist | 4 | 2.5% | 24 | 2.1% | 49 | 0.7% | 90 | 0.8% | † | * | |
| Hematologist | 1 | 0.6% | 0 | 0.0% | 4 | 0.1% | 5 | 0.0% | |||
| Neurologist | 0 | 0.0% | 6 | 0.5% | 21 | 0.3% | 25 | 0.2% | |||
| Primary Care** | 36 | 22.1% | 205 | 18.2% | 1,459 | 21.7% | 2,048 | 19.3% | † | † | |
| Other | 90 | 55.2% | 652 | 57.9% | 3,692 | 55.0% | 6,302 | 59.4% | * | ||
|
Rheumatology Visit (n, %) |
29 | 17.8% | 220 | 19.5% | 1,414 | 21.1% | 1,756 | 16.5% | * | ||
| PCP Visit (n, %) | 78 | 47.9% | 537 | 47.6% | 3,542 | 52.7% | 4,634 | 43.7% | † | * | |
|
Hematology Visit (n, %) |
0 | 0.0% | 8 | 0.7% | 31 | 0.5% | 37 | 0.3% | |||
|
Nephrology Visit (n, %) |
7 | 4.3% | 36 | 3.2% | 126 | 1.9% | 199 | 1.9% | † | † | |
|
Charlson Comorbidity Index (CCI) Score (n, %) |
|||||||||||
| 0 | 72 | 44.2% | 461 | 40.9% | 2,966 | 44.2% | 5,006 | 47.2% | † | † | |
| 1 | 54 | 33.1% | 406 | 36.0% | 2,526 | 37.6% | 3,793 | 35.7% | |||
| 2 | 16 | 9.8% | 136 | 12.1% | 725 | 10.8% | 1,006 | 9.5% | |||
| 3+ | 21 | 12.9% | 124 | 11.0% | 500 | 7.4% | 806 | 7.6% | |||
| Mean + SD | 1.0 + 1.2 | 1.0 + 1.2 | 0.9 + 1.1 | 0.8 + 1.1 | * | † | |||||
|
Comorbidities of Interest: (n, %) |
|||||||||||
| Cardiovascular disease |
30 | 18.4% | 178 | 15.8% | 910 | 13.5% | 1,147 | 10.8% | † | * | |
| Stroke | 6 | 3.7% | 40 | 3.5% | 193 | 2.9% | 283 | 2.7% | |||
| Myocardial infarction | 4 | 2.5% | 16 | 1.4% | 73 | 1.1% | 101 | 1.0% | |||
| Peripheral vascular disease |
12 | 7.4% | 85 | 7.5% | 465 | 6.9% | 663 | 6.2% | |||
| Cerebrovascular disease |
6 | 3.7% | 37 | 3.3% | 190 | 2.8% | 290 | 2.7% | |||
| Osteoporosis | 5 | 3.1% | 30 | 2.7% | 233 | 3.5% | 422 | 4.0% | |||
| Infection | 71 | 43.6% | 467 | 41.4% | 2,951 | 43.9% | 3,829 | 36.1% | * | ||
| Diabetes | 13 | 8.0% | 90 | 8.0% | 461 | 6.9% | 809 | 7.6% | |||
| Hypertension | 39 | 23.9% | 285 | 25.3% | 1,659 | 24.7% | 2,525 | 23.8% | |||
| Renal disease | 21 | 12.9% | 115 | 10.2% | 269 | 4.0% | 470 | 4.4% | * | * | |
|
Pre-index (6-month) Total Medical Costs |
|||||||||||
| Mean ± SD | $11,977 ± $47,389 |
$9,467 + $27,462 |
$5,997 + $22,541 |
$5,195 + $21,958 |
† | * | † | ||||
| Median | $2,248 | $2,308 | $1,944 | $1,441 | † | * | |||||
|
Pre-index (6-month) Total Pharmacy Costs |
|||||||||||
| Mean + SD | $1,504 + $3,499 |
$1,612 + $3,376 |
$1,620 + $2,984 |
$1,207 + $2,865 |
* | ||||||
| Median | $373 | $411 | $630 | $345 | † | * | * | ||||
|
Concomitant Medications: (n, %) |
|||||||||||
| Corticosteroids | 0 | 0.0% | 0 | 0.0% | 7 | 0.1% | 4 | 0.0% | |||
| NSAIDs | 26 | 16.0% | 286 | 25.4% | 1853 | 27.6% | 1965 | 18.5% | † | * | |
| Anti-malarials | 36 | 22.1% | 277 | 24.6% | 2245 | 33.4% | 3338 | 31.5% | † | * | † |
| Immunosuppressants | 9 | 5.5% | 70 | 6.2% | 251 | 3.7% | 285 | 2.7% | † | * | |
| Cyclophosphamide | 0 | 0.0% | 2 | 0.2% | 1 | 0.0% | 1 | 0.0% | |||
| Mycophenolate mofetil |
7 | 4.3% | 36 | 3.2% | 129 | 1.9% | 159 | 1.5% | † | † | † |
Abbreviations. HMO=Health Maintenance Organization, PPO=Preferred Provider Organization, POS=Point of Service, NSAIDs=Nonsteroidal Anti-Inflammatory Drugs
=p<0.0001
=p<0.05
Primary care: primary care physician/family physician/general practitioner, internal medicine physician
LOW-DOSE ORAL CSS VS. HIGH- AND MEDIUM-DOSE ORAL CSS
Baseline characteristics
Compared with patients receiving low-dose CSs, patients receiving high-dose CSs were younger (46 vs. 43 years; p=0.0002), had a higher risk of renal disease (4.0% vs. 12.9%; p<0.0001) and had greater mean pre-index total medical costs ($5,997 vs. $11,977; p=0.0013). Compared with patients receiving low-dose CSs, patients receiving medium-dose CSs were also younger (46 vs. 44 years; p<0.0001), had a higher risk of renal disease (4.0% vs. 10.2%; p<0.0001) and had higher mean pre-index total medical costs ($5,997 vs. $9,467; p<0.0001). Table 1 provides additional details on the baseline characteristics of patients eligible for inclusion into our study.
Unadjusted HCRU and costs in the third year post-index
A significantly greater percentage of patients receiving high-dose oral CS used ER services (39.3% vs. 29.7%; p=0.0085) and had ≥1 IP hospitalization (21.5% vs. 12.3%; p=0.0005) in the third year post-index compared to those receiving low-dose oral CS. Mean healthcare utilization (Rx, ER, OP and IP services) and mean all-cause total costs ($60,366 vs. $18,777; p<0.0001) in the third year post-index were significantly greater for patients receiving high-dose oral CS. Table 2 and Figure 2 provide additional details.
Table 2.
Healthcare resource utilization for the third year post-index
| Patient cohort | High Dose CS (HD) | Medium Dose CS (MD) |
Low Dose CS (LD) | No-steroid Dose (NS) | p- value (HD vs. LD) |
p- value (MD vs. LD) |
p- valu e (NS vs. LD) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (N = 163) | (N = 1,127) | (N = 6,717) | (N = 10,611) | ||||||||||||
|
PHARMACY SERVICES (NDC codes) |
|||||||||||||||
| Corticosteroids (n %) | 105 | 64.4% | 779 | 69.1% | 2,764 | 41.1% | 1,676 | 15.8% | * | * | * | ||||
| Mean + SD, Median |
3.3 + 4.1 |
2 | 3.8 + 4.3 |
2 | 1.0 + 2.0 |
0 | 0.3 + 0.9 |
0 | * | * | * | ||||
| Immunosuppressants (n, %) |
50 | 30.7% | 374 | 33.2% | 522 | 7.8% | 434 | 4.1% | * | * | * | ||||
| Mean + SD, Median |
1.6 + 3.4 |
0 | 2.2 + 4.1 |
0 | 0.4 + 1.9 |
0 | 0.2 + 1.5 |
0 | * | * | * | ||||
| Cyclophosphamide (n, %) |
2 | 1.2% | 5 | 0.4% | 1 | 0.0% | 3 | 0.0% | † | † | |||||
| Mean + SD, Median |
0.1 + 1.6 |
0 | 0.0 + 0.3 |
0 | 0.0 + 0.2 |
0 | 0.0 + 0.0 |
0 | * | † | |||||
| Mycophenolate mofetil (n, %) |
32 | 19.6% | 220 | 19.5% | 268 | 4.0% | 234 | 2.2% | * | * | * | ||||
| Mean + SD, Median |
0.9 + 2.2 |
0 | 1.1 + 2.8 |
0 | 0.2 + 1.1 |
0 | 0.1 + 0.9 |
0 | * | * | * | ||||
| Anti-malarials (n, %) | 78 | 47.9% | 642 | 57.0% | 3,175 | 47.3% | 3,950 | 37.2% | * | * | |||||
| Mean + SD, Median |
3.0 + 3.9 |
0 | 3.4 + 4.1 |
2 | 2.7 + 3.7 |
0 | 2.1 + 3.5 |
0 | * | * | |||||
| Biologics (n, %) | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | |||||||
| Mean + SD, Median |
0.0 + 0.0 |
0 | 0 | 0.0 + 0.0 |
0 | 0.0 + 0.0 |
0 | 0.0 + 0.0 |
0 | ||||||
| NSAIDs (n, %) | 48 | 29.4% | 322 | 28.6% | 2,499 | 37.2% | 2,695 | 25.4% | † | * | * | ||||
| Mean + SD, Median |
0.8 + 1.8 |
0 | 1.2 + 2.7 |
0 | 1.4 + 2.7 |
0 | 0.9 + 2.2 |
0 | † | † | * | ||||
| All other pharmacy (n, %) |
158 | 96.9% | 1,090 | 96.7% | 6,400 | 95.3% | 8,886 | 83.7% | † | * | |||||
| Mean + SD, Median |
42.1 + 39.9 |
28 | 40.7 + 36.2 |
30 | 34.4 + 32.8 |
25 | 22.5 + 26.5 |
14 | † | * | * | ||||
|
EMERGENCY ROOM SERVICES (n, %) |
64 | 39.3% | 378 | 33.5% | 1,996 | 29.7% | 2,381 | 22.4% | † | † | * | ||||
| Mean + SD, Median |
1.0 + 2.1 |
0 | 0.8 + 2.4 |
0 | 0.6 + 1.9 |
0 | 0.4 + 1.7 |
0 | † | † | * | ||||
|
OUTPATIENT SERVICES (n, %) |
|||||||||||||||
| Physician office visits (n, %) |
160 | 98.2% | 1,108 | 98.3% | 6,557 | 97.6% | 10,140 | 95.6% | * | ||||||
| Mean + SD, Median |
22.8 + 30.8 |
13 | 18.6 + 20.9 |
13 | 15.7 + 16.4 |
11 | 12.8 + 17.2 |
8 | * | * | * | ||||
| Rheumatology (n, %) |
52 | 31.9% | 445 | 39.5% | 2,240 | 33.3% | 2,651 | 25.0% | * | * | |||||
| Mean + SD, Median |
1.0 + 2.1 |
0 | 1.4 + 2.4 |
0 | 1.0 + 1.8 |
0 | 0.6 + 1.4 |
0 | * | * | |||||
| All others (n, %) | 159 | 97.5% | 1,096 | 97.2% | 6,517 | 97.0% | 10,089 | 95.1% | * | ||||||
| Mean + SD, Median |
21.8 + 30.5 |
12 | 17.2 + 20.8 |
11 | 14.7 + 16.3 |
10 | 12.2 + 17.1 |
8 | * | * | * | ||||
| Laboratory and pathology (n, %) |
157 | 96.3% | 1,079 | 95.7% | 6,259 | 93.2% | 9,491 | 89.4% | † | * | |||||
| Mean + SD, Median |
45.2 + 48.4 |
34 | 39.1 + 40.6 |
28 | 25.4 + 27.4 |
19 | 20.3 + 26.2 |
14 | * | * | * | ||||
| Radiology examinations (n, %) |
135 | 82.8% | 872 | 77.4% | 5,243 | 78.1% | 7,678 | 72.4% | * | ||||||
| Mean + SD, Median |
5.8 + 10.1 |
3 | 4.3 + 7.0 |
2 | 4.0 + 5.5 |
2 | 3.2 + 5.4 |
2 | * | * | |||||
| Surgical services (n, %) |
98 | 60.1% | 622 | 55.2% | 3,722 | 55.4% | 4,851 | 45.7% | * | ||||||
| Mean + SD, Median |
2.6 + 4.4 |
1 | 2.0 + 3.6 |
1 | 1.9 + 3.3 |
1 | 1.4 + 2.8 |
0 | † | * | |||||
| Ancillary services (n, %) |
157 | 96.3% | 1,075 | 95.4% | 6,251 | 93.1% | 9,584 | 90.3% | † | * | |||||
| Mean + SD, Median |
33.0 + 62.8 |
14 | 20.7 + 31.9 |
11 | 14.6 + 20.9 |
8 | 11.8 + 23.9 |
6 | * | * | * | ||||
| Dialysis (n, %) | 8 | 4.9% | 13 | 1.2% | 43 | 0.6% | 57 | 0.5% | * | ||||||
| Mean + SD, Median |
1.5 + 11.8 |
0 | 1.4 + 15.9 |
0 | 0.3 + 6.4 |
0 | 0.4 + 8.8 |
0 | † | * | |||||
| HCPCS SERVICES (J codes) |
|||||||||||||||
| Corticosteroids (n, %) | 42 | 25.8% | 347 | 30.8% | 2,217 | 33.0% | 2,110 | 19.9% | * | ||||||
| Mean + SD, Median |
0.7 + 2.0 |
0 | 0.8 + 2.0 |
0 | 0.8 + 1.7 |
0 | 0.4 + 1.4 |
0 | * | ||||||
| Immunosuppressants (n, %) |
6 | 3.7% | 32 | 2.8% | 32 | 0.5% | 22 | 0.2% | † | * | † | ||||
| Mean + SD, Median |
0.1 + 0.7 |
0 | 0.1 + 1.1 |
0 | 0.0 + 0.2 |
0 | 0.0 + 0.4 |
0 | * | * | |||||
| Cyclophosphamide (n, %) |
4 | 2.5% | 20 | 1.8% | 19 | 0.3% | 13 | 0.1% | † | * | † | ||||
| Mean + SD, Median |
0.1 + 0.4 |
0 | 0.1 + 0.6 |
0 | 0.0 + 0.2 |
0 | 0.0 + 0.2 |
0 | † | * | |||||
| Mycophenolate mofetil (n, %) |
1 | 0.6% | 6 | 0.5% | 10 | 0.1% | 5 | 0.0% | † | † | |||||
| Mean + SD, Median |
0.0 + 0.1 |
0 | 0.0 + 0.4 |
0 | 0.0 + 0.1 |
0 | 0.0 + 0.1 |
0 | † | ||||||
| Anti-malarials (n, %) | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 1 | 0.0% | |||||||
| Mean + SD, Median |
0.0 + 0.0 |
0 | 0.0 + 0.0 |
0 | 0.0 + 0.0 |
0 | 0.0 + 0.0 |
0 | |||||||
| Biologics (n, %) | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | |||||||
| Mean + SD, Median |
0.0 + 0.0 |
0 | 0.0 + 0.0 |
0 | 0.0 + 0.0 |
0 | 0.0 + 0.0 |
0 | |||||||
| All other HCPCS (n, %) |
77 | 47.2% | 502 | 44.5% | 2,637 | 39.3% | 2,989 | 28.2% | † | † | * | ||||
| Mean + SD, Median |
4.9 + 11.6 |
0 | 5.5 + 21.4 |
0 | 2.9 + 12.6 |
0 | 2.2 + 15.1 |
0 | * | † | |||||
|
NUMBER OF INPATIENT HOSPITALIZATIONS |
|||||||||||||||
| One or more hospitalizations (n, %) |
35 | 21.5% | 198 | 17.6% | 828 | 12.3% | 1,007 | 9.5% | † | * | * | ||||
| Mean + SD, Median |
0.7 + 2.4 |
0 | 0.4 + 1.2 |
0 | 0.2 + 0.7 |
0 | 0.1 + 0.7 |
0.7 | 0 | * | * | * | |||
| Total Days (Mean + SD, Median) |
6.1 + 21.6 |
0 | 2.2 + 10.0 |
0 | 1.2 + 9.1 |
0 | 0.9 + 6.5 |
0 | * | † | † | ||||
Abbreviations. HCPCS=Healthcare Common Procedure Coding System
=p<0.0001
=p<0.05
Figure 2.
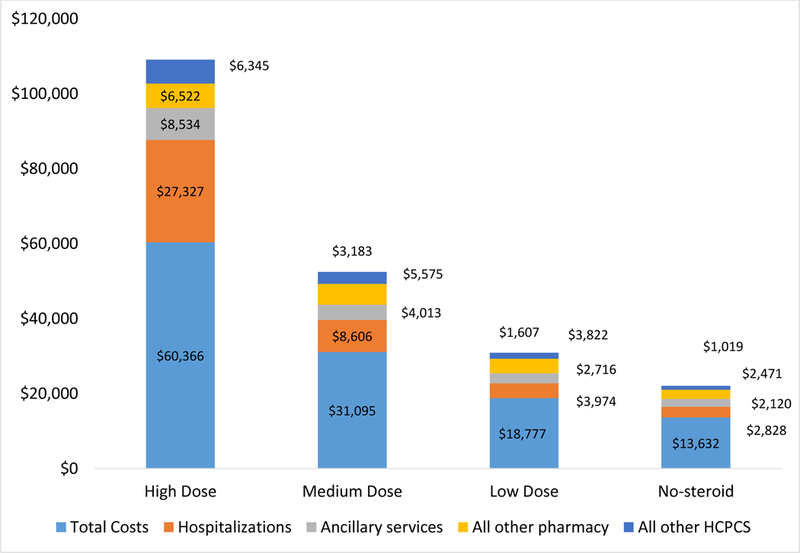
Healthcare component costs among patients with SLE, by CS dose exposure category (third year of follow up)
A significantly greater percentage of patients receiving medium-dose oral CS used ER services (33.5% vs. 29.7%; p=0.0097) and had ≥1 IP hospitalization (17.6% vs. 12.3%; p<0.0001) in the third year post-index compared to those receiving low-dose oral CS. Mean healthcare utilization (Rx, ER, OP and IP services) and mean all-cause total costs ($31,095 vs. $18,777; p<0.0001) in the third year post-index were significantly greater for patients receiving medium-dose oral CS. Table 2 and Figure 2 provide additional details.
Multivariate models
In multivariate adjusted analysis, patients in the high-dose and medium-dose groups had 2.8 times (cost ratio [CR]: 2.80, 95% confidence interval [CI]: 2.290–3.420) and 1.7 times (CR: 1.71, 95% CI: 1.571–1.850) the average annual all-cause healthcare costs, respectively, compared with those in the low-dose oral CSs group (Table 3). In a separate multivariate adjusted analysis, a 1-mg/day increase in steroid ADD increased the total all-cause healthcare costs by 1.07 times in the third year post-index (CR: 1.07, 95% CI: 1.062–1.074; p<0.0001) (Figure 3).
Table 3:
Generalized linear model of total all-cause healthcare costs during the third year post-index
| 95% Confidence Limits | ||||
|---|---|---|---|---|
| Variable | Cost Ratio (95% CI) |
Lower Limit | Upper Limit | p-value |
| Steroid Exposure Group (Reference group: low) | ||||
| Non-Steroid Use | 0.72 (0.70– 0.75) |
0.697 | 0.754 | * |
| Medium | 1.71 | 1.571 | 1.850 | * |
| High | 2.80 | 2.290 | 3.420 | * |
| Age (continuous) | 1.01 | 1.004 | 1.008 | * |
| Gender (Reference group: Male) | 1.01 | 0.951 | 1.080 | |
| Charlson comorbidity index | 1.19 | 1.165 | 1.214 | * |
| Cardiovascular disease | 1.28 | 1.209 | 1.363 | * |
| Renal disease | 1.66 | 1.507 | 1.824 | * |
| Log (Total medical costs) | 1.06 | 1.054 | 1.065 | * |
| NSAIDs | 1.11 | 1.060 | 1.160 | * |
| Anti-malarials | 0.84 | 0.802 | 0.870 | * |
=p<0.0001
=p<0.05
Figure 3:
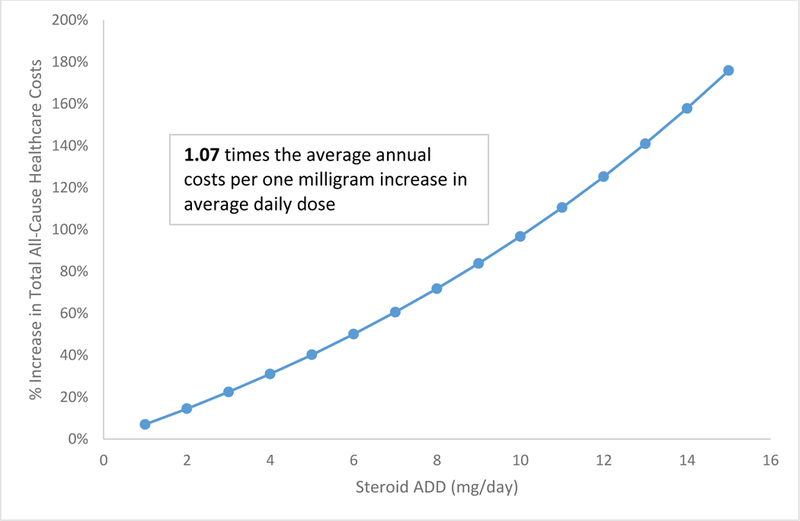
Total all-cause healthcare costs with one mg/day increase in steroid average daily dose
LOW-DOSE ORAL CSS VS. NO-STEROIDS
Baseline characteristics
Compared with patients receiving low-dose CSs, patients in the no-steroids group were older (46 vs. 47 years; p=0.0003) with a lower proportion of patients having CV disease (13.5% vs. 10.8%; p<0.0001) and infection (43.9% vs. 36.1%; p<0.0001) in the 6-month pre-index period. Mean pre-index total medical and pharmacy costs were significantly lower for patients in the no-steroids group compared to those receiving low-dose CS ($5,195 vs. $5,997; p=0.0206 and $1,207 vs. $1,620; p<0.0001, respectively). Table 1 provides additional details.
Unadjusted HCRU and costs in the third year post-index
A significantly lower percentage of patients in the no-steroids groups used ER services (22.4% vs. 29.7%; p<0.0001) and had ≥1 IP hospitalization (9.5% vs. 12.3%; p<0.0001) in the third year post-index compared to those receiving low-dose oral CS. Mean healthcare utilization (Rx, ER, OP and IP services) and mean all-cause total costs ($13,632 vs. $18,777; p<0.0001) in the third year post-index were significantly lower for patients in the no-steroids group. Table 2 and Figure 2 provide additional details.
Multivariate model
In multivariate adjusted analysis, patients in the no-steroids group had 0.7 times (CR: 0.72, 95% CI: 0.697–0.754) the average annual all-cause healthcare costs compared with those in the low-dose oral CSs group (Table 3).
DISCUSSION
In this study, patients in the high-dose and medium-dose oral CS groups had significantly greater (2.8 times and 1.7 times, respectively) average annual total healthcare costs compared with patients in the low-dose group. Patients in the no-steroids group had significantly lower (0.7 times) average annual total healthcare costs compared with patients in the low-dose group. Although previous studies have evaluated the economic burden of SLE associated with the use of oral CSs7,8,10,11,21, none of these studies have evaluated the impact of long-term exposure to CSs in SLE patients newly initiating oral CS treatment. Chen et al.23 conducted a cross-sectional study among adult SLE patients using a large US insurance claims database and associated oral GC use with greater annual HCRU and costs. Mean total healthcare costs during a 1-year follow-up period were reported to double from patients receiving low-dose GC to patients receiving greater-dose GC.23 Compared to this study, our study included SLE patients new to oral CSs and evaluated the impact of long-term exposure to CSs on HCRU and costs (i.e. the first 2 years after SLE diagnosis were used to create CS exposure groups of interest and the third year post-index was used to evaluate the outcomes of interest). We also used different definitions that were agreed upon by rheumatology experts to identify high, medium and low-dose steroid use.
In our study sample, significantly greater percentage of patients receiving high-dose and medium-dose oral CSs consulted a nephrologist as their prescribing physician specialty, had renal disease, had greater number of nephrology visits and had significantly greater total medical costs in the pre-index period compared with those receiving low-dose oral CSs. Patients in the non-steroid group had significantly lower rheumatology visits, PCP visits and total medical and pharmacy costs in the pre-index period compared with those receiving low-dose oral CSs. Therefore, it was required to adjust for baseline characteristics, including pre-index medical costs and the CCI, in order to account for the covariate imbalance that exists when evaluating the association between steroid dose and HCRU/costs.
Compared to patients receiving low-dose oral CSs, a significantly greater percentage of patients receiving high-dose and medium-dose oral CSs used ER services and had ≥1 IP hospitalization with a longer mean length of stay in the third year post-index. On the other hand, a significantly lower percentage of patients in the non-steroid group used ER services, had ≥1 IP hospitalization and had a shorter mean length of hospital stay compared with those receiving low-dose oral CSs. Patients receiving CSs may need more regular monitoring, which may result in increased HCRU.20,26 More frequent ER visits and hospitalizations for patients who received greater CS doses may be attributed to greater disease severity, flares, worst general health status or treatment of adverse events.19,27,28
The International Task Force for SLE recommends prescription of the lowest GC dose needed for disease control and to withdraw GC completely, if possible.29 In addition, duration of exposure to CSs should be minimized.30 Pre-existing comorbidities that may increase the risk of experiencing CS-related adverse events in the future should be evaluated prior to initiating CS therapy.30 These actions may help reduce complications and economic burden associated with CS use for SLE patients and may improve HRQoL.31 Steroid-sparing agents can accelerate the process of decreasing steroid requirements in SLE patients, yet tolerable and effective treatment options are limited. Treatment options that will help decrease CS use in SLE patients and improve their clinical, economic, and HRQoL outcomes are needed.
Antimalarial use is lower in our cohort (22.1%−33.4%) in comparison to other cohorts. For example, between 61–83% of SLE patients in the Lupus in Minorities, Nature versus Nurture (LUMINA) cohort reported hydroxychloroquine use.32 In a Medicaid administrative database study of patients with SLE receiving immunosuppressive therapy (i.e., MMF, azathioprine or cyclophosphamide), 30.1%−54.4% were on hydroxychloroquine.33 The differences in antimalarial treatment utilization among the different cohorts are likely due to differences in patient samples included in the studies. The LUMINA cohort was primarily recruited from rheumatology clinics and had to meet the American College of Rheumatology definition of SLE for inclusion. In contrast, we identified SLE patients based on a previously validated method using provider-reported ICD-9 diagnosis codes. The SLE Medicaid population also has a very different sociodemographic (including age, income, race/ethnicity) profile than our cohort. For instance, the mean age of our study cohort is 45 while the mean age of the Medicaid cohort is 35.
This study had several limitations. First, administrative claims data sets are not designed for the primary purpose of conducting research. The internal validity is typically not sufficient to make positive inferences of cause and effect. Second, the analytic focus was on patients who met the continuous observation criteria (6 months pre-index and 36 months post-index), with a potential to eliminate patients who may have different treatment patterns coincident with observation patterns. Results may not be generalizable to those without consistent access to care, for instance. Third, patients selected for one particular treatment rather than another may have very different characteristics. Some of these differences were measured in our study (such as age and sex) but some are not measurable or not available in the dataset (e.g., patient preferences). Fourth, treatments may not be captured within the data-set and there may be incomplete encounter histories for patients selected for this study. Fifth, medical billing codes used to indicate diagnoses and procedures are subject to non-clinical influences. Finally, although adjustment for relevant baseline characteristics in the multivariate modelling was done, the opportunity for residual confounding remains.
In conclusion, in a contemporary cohort of SLE patients new to oral CSs, high- and medium-dose CS use was associated with significantly greater future healthcare utilization and costs relative to low-dose steroid use, after adjustment for baseline demographic and clinical characteristics. No-steroid use was associated with significantly lower future healthcare utilization and costs relative to low-dose steroid use. Patients with SLE receiving CSs require greater resource use for disease and medication management. Minimizing the daily steroid use of SLE patients while concomitantly controlling their disease activity may reduce the looming economic burden associated with CS use.
Summary.
This is the largest US claims database study evaluating the healthcare resource utilization (HCRU) and costs associated with long-term exposure to different doses of oral corticosteroids (CSs) for adult patients with systemic lupus erythematosus (SLE) newly initiating oral CSs.
High-dose (>20mg/day) and medium-dose (6–20 mg/day) oral CS use was associated with significantly greater future healthcare costs relative to low-dose (≤5 mg/day) oral CS use.
No-steroid use was associated with significantly lower future healthcare costs relative to low-dose oral CS use.
A one milligram increase in CS average daily dose was associated with 1.07 times the average annual costs after adjusting for baseline characteristics (p<0.0001).
Acknowledgments
Grant(s) or other financial supporter(s) of the study: IQVIA received research funding from AstraZeneca. EV received fees for consulting services related to the design and conduct of the study.
References
- 1.Centers for Disease Control and Prevention. Systemic lupus erythematosus (SLE) Available online at: http://www.cdc.gov/arthritis/basics/lupus.htm [Accessed September 6, 2017].
- 2.Centers for Disease Control and Prevention. Lupus Basic Fact Sheet Available online at: http://www.cdc.gov/lupus/basics/index.html [Accessed September 6, 2017].
- 3.Dall’Era M Systemic lupus erythematosus. In: Imboden JB, Hellman DB, Stone JH, editors. Current Rheumatology Diagnosis and Treatment third ed. New York (NY): McGraw-Hill; 2013. Chapter 21. [Google Scholar]
- 4.Lupus Foundation of America. Lupus facts and statistics Available online at: http://www.resources.lupus.org/entry/facts-and-statistics [Accessed September 6, 2017].
- 5.Slawsky KA, Fernandes AW, Fusfeld L, Manzi S, Goss TF. A structured literature review of the direct costs of adult systemic lupus erythematosus in the US. Arthritis Care Res (Hoboken) 2011;63:1224–32. [DOI] [PubMed] [Google Scholar]
- 6.Turchetti G, Yazdany J, Palla I, Yelin E, Mosca M. Systemic lupus erythematosus and the economic perspective: a systematic literature review and points to consider. Clin Exp Rheumatol 2012;30:S116–22. [PMC free article] [PubMed] [Google Scholar]
- 7.Garris C, Jhingran P, Bass D, Engel-Nitz NM, Riedel A, Dennis G. Healthcare utilization and cost of systemic lupus erythematosus in a US managed care health plan. Journal of Medical Economics;16(5):667–677, DOI:10.3111/13696998.2013.778270 [DOI] [PubMed] [Google Scholar]
- 8.Narayanan S, Wilson K, Ogelsby A, Juneau P, Durden E. Economic burden of systemic lupus erythematosus flares and comorbidities in a commercially insured population in the United States. J Occup Environ Med 2013;55:1262–70. [DOI] [PubMed] [Google Scholar]
- 9.Zhu TY, Tam LS, Li EK. Cost of illness studies in systemic lupus erythematosus: A systematic review. Arthritis Care Res (Hoboken) 2011;63:751–760. [DOI] [PubMed] [Google Scholar]
- 10.Carls G, Li T, Panopalis P, Wang S, Mell AG, Gibson TB, et al. Direct and indirect costs to employers of patients with systemic lupus erythematosus with and without nephritis. J Occup Environ Med 2009;51:66–79. [DOI] [PubMed] [Google Scholar]
- 11.Li T, Carls GS, Panopalis P, Wang S, Gibson TB, Goetzel RZ. Long-term medical costs and resource utilization in systemic lupus erythematosus and lupus nephritis: A five-year analysis of a large medicaid population. Arthritis Rheum 2009;61:755–763. [DOI] [PubMed] [Google Scholar]
- 12.Pelletier EM, Ogale S, Yu E, Brunetta P, Garg J. Economic outcomes in patients diagnosed with systemic lupus erythematosus with versus without nephritis: Results from an analysis of data from a US claims database. Clin Ther 2009;31:2653–2664. [DOI] [PubMed] [Google Scholar]
- 13.Thumboo J, Strand V. Health-related quality of life in patients with systemic lupus erythematosus: An update. Ann Acad Med Singapore 2007;36:115–122. [PubMed] [Google Scholar]
- 14.McElhone K, Abbott J, Teh LS. A review of health related quality of life in systemic lupus erythematosus. Lupus 2006;15:633–643. [DOI] [PubMed] [Google Scholar]
- 15.Furst DE, Clarke A, Fernandes AW, Bancroft T, Gajria K, Greth W, et al. Resource utilization and direct medical costs in adult systemic lupus erythematosus patients from a commercially insured population. Lupus 2013;22:268–78. [DOI] [PubMed] [Google Scholar]
- 16.Lam NC, Ghetu MV, Bieniek ML. Systemic lupus erythematosus: primary care approach to diagnosis and management. Am Fam Physician 2016;94:284–94. [PubMed] [Google Scholar]
- 17.Kalunian K, Joan TM. New directions in the treatment of systemic lupus erythematosus. Curr Med Res Opin 2009;25:1501–1514. [DOI] [PubMed] [Google Scholar]
- 18.D’Cruz DP, Khamashta MA, Hughes GR. Systemic lupus erythematosus. Lancet 2007;369:587–96. [DOI] [PubMed] [Google Scholar]
- 19.Panopalis P, Gillis JZ, Yazdany J, Trupin L, Hersh A, Julian L, et al. Frequent use of the emergency department among persons with systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2010;62:401–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petri M, Bechtel B, Dennis G, Shah M, McLaughlin T, Kan H, et al. Burden of corticosteroid use in patients with systemic lupus erythematosus: results from a Delphi panel. Lupus 2014;23:1006–13. [DOI] [PubMed] [Google Scholar]
- 21.Shah M, Chaudhari S, McLaughlin TP, Kan HJ, Bechtel B, Dennis GJ, et al. Cumulative burden of oral corticosteroid adverse effects and the economic implications of corticosteroid use in patients with systemic lupus erythematosus. Clin Ther 2013;35:486–97. [DOI] [PubMed] [Google Scholar]
- 22.Sarnes E, Crofford L, Watson M, Dennis G, Kan H, Bass D. Incidence and US costs of corticosteroid-associated adverse events: a systematic literature review. Clin Ther 2011;33:1413–32. [DOI] [PubMed] [Google Scholar]
- 23.Chen SY, Choi CB, Li Q, Yeh WS, Lee YC, Kao AH, et al. Glucocorticoid use in patients with systemic lupus erythematosus: association between dose and health care utilization and costs. Arthritis Care Res (Hoboken) 2015;67:1086–94. [DOI] [PubMed] [Google Scholar]
- 24.Manson SC, Brown RE, Cerulli A, Vidaurre CF. The cumulative burden of oral corticosteroid side effects and the economic implications of steroid use. Respir Med 2009;103:975–94. [DOI] [PubMed] [Google Scholar]
- 25.Schimmer BP, Parker KL. Adrenocorticotropic hormone; adrenocortical steroids and their synthetic analogs; inhibitors of the synthesis and actions of adrenocortical hormones. The pharmacological basis of therapeutics 2006;11:1587. [Google Scholar]
- 26.Mosca M, Tani C, Aringer M, Bombardieri S, Boumpas D, Brey R, et al. European league against rheumatism recommendations for monitoring patients with systemic lupus erythematosus in clinical practice and in observational studies. Ann Rheum Dis 2010;69:1269–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kan HJ, Song X, Johnson BH, Bechtel B, O’Sullivan D, Molta CT. Healthcare utilization and costs of systemic lupus erythematosus in Medicaid. Biomed Res Int 2013:808391. [DOI] [PMC free article] [PubMed]
- 28.Ow MY, Ho PC, Thumboo J, Wee HL. Factors associated with health services utilization in patients with systemic lupus erythematosus: a systematic review. Clin Exp Rheumatol 2010;28:892–904. [PubMed] [Google Scholar]
- 29.van Vollenhoven RF, Mosca M, Bertsias G, Isenberg D, Kuhn A, Lerstrøm K, et al. Treat-to-target in systemic lupus erythematosus: recommendations from an international task force. Ann Rheum Dis 2014;73:958–67. [DOI] [PubMed] [Google Scholar]
- 30.Kasturi S, Sammaritano LR. Corticosteroids in lupus. Rheum Dis Clin North Am 2016;42:47–62. [DOI] [PubMed] [Google Scholar]
- 31.Ruiz-Irastorza G, Danza A, Khamashta M. Glucocorticoid use and abuse in SLE. Rheumatology (Oxford) 2012;51:1145–53. [DOI] [PubMed] [Google Scholar]
- 32.Fernandez et al. A multiethnic, multicenter cohort of patients with systemic lupus erythematosus as a model for the study of ethnic disparities in SLE. Arthritis & Rheumatism 2007;57(4):576–584. [DOI] [PubMed] [Google Scholar]
- 33.Feldman et al. Comparative rates of serious infections among patients with systemic lupus erythematosus receiving immunosuppressive medications. Arthritis & Rheumatology 2017;69(2):387–397. [DOI] [PMC free article] [PubMed] [Google Scholar]


