Abstract
Background:
Reoperation after breast conserving surgery (BCS) is common and had been partially associated with the lack of consensus on margin definition. We sought to investigate factors associated with reoperations and variation in reoperations rates across breast surgeons at our cancer center.
Methods:
Retrospective analysis of patients with clinical stage I-II breast cancer who underwent BCS between January-December-2014 were conducted prior to the recommendation of no ink on tumor margin. Patient demographics, tumor and surgical data were extracted from medical records. A multivariate regression model was used to identify factors associated with reoperation.
Results:
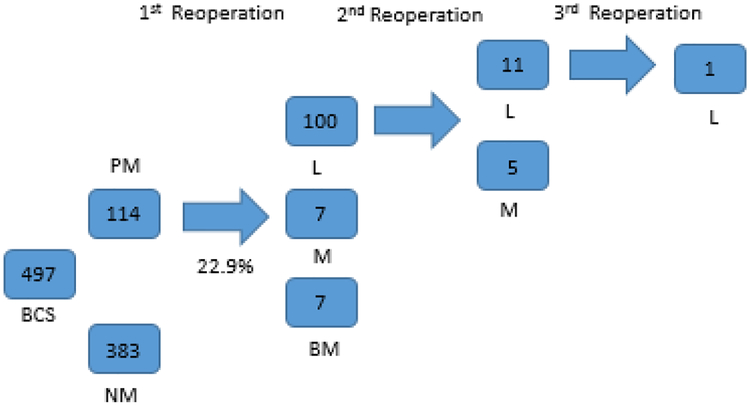
490 patients with stage I (n=408) and stage II (n=89) breast cancer underwent BCS; 7 patients had bilateral breast cancer and underwent bilateral BCS procedures. Median invasive tumor size was 1.1 cm. Reoperation rate was 22.9% (n=114) and varied among surgeons (range 15–40%). In 100 (88%) patients the second procedure was re-excision, followed by unilateral mastectomy (n=7, 6%) and bilateral mastectomy (n=7, 6%). Intraoperative margin techniques (global-cavity or targeted-shaves) were utilized in 50.1% of cases; no specific margin technique was utilized in 49.9% of cases. Median total specimen size was 65.8 cm3 (range 24.5–156.0 cm3). In the adjusted model, patients with multifocal disease were more likely to undergo reoperation (OR=5.78; 95% CI=2.17–15.42). Also, two surgeons were found to have significantly higher reoperation rates (OR=6.41;95% CI=1.94–21.22; OR=3.41;95% CI=1.07–10.85).
Conclusions:
Examination of BCS demonstrated variability in reoperation rates and margin practices among our breast surgeons. Future trials should look at surgeon-specific factors that may predict for reoperations.
Introduction
Breast conserving surgery (BCS) followed by radiation therapy (RT) is a standard treatment option for women with early stage breast cancer. It is estimated that approximately two-thirds of women in the United States diagnosed with operable breast cancer in 2015 received treatment with BCS.[1] Although patients successfully treated with BCS and RT can expect equivalent rates of long-term survival compared to mastectomy, local recurrence (LR) rates with BCS are slightly higher.[2–4] Factors associated with LR vary, but positive surgical margins after lumpectomy remain a major factor. BCS requires tumor excision with margins clear of disease to reduce the risk of LR; however, there is no reliable, or cost effective intraoperative method of obtaining clear margins and re-excision rates range from 15–40% nationwide.[5–11] Reoperations negatively impact patient care by delaying the initiation and completion of adjuvant therapies, increasing health care costs, diminishing aesthetic outcomes, contributing to increased infection rates, and exacerbating patient-specific psychological stressors.[12–14] We sought to investigate factors associated with reoperations and variation in reoperations rates across surgeons at our comprehensive cancer center.
Methods
Setting
We examined the care of women undergoing BCS at two primary surgical sites (Brigham and Women’s Hospital [BWH] and Brigham and Women’s Faulkner Hospital [BWFH]) of Dana-Farber/Brigham and Women’s Cancer Center (DF/BWCC), a Harvard-affiliated NCI-designated comprehensive cancer center located in Boston, Massachusetts. The breast program is organized as a multidisciplinary disease center, with more than 2500 new breast cancer patients per year. At the time of our study, the program included eight breast surgical oncologists who practiced across primary surgical sites.
Cohort Selection
We utilized an internal dataset developed through the integration of administrative and surgeon billing data to identify consecutive women with breast cancer who underwent breast surgery at two primary surgical sites (BWH and BWFH) between January and December 2014. We identified 490 patients with initial clinical stages I and II breast cancer who underwent BCS as their first definitive procedure at our center during the study timeframe; seven patients had bilateral breast cancer and underwent bilateral lumpectomy procedures as their first definitive surgery. Male patients and those who received preoperative therapy were excluded. We also excluded cases of one breast surgeon from the cohort who had less than 20 surgical procedures during the study period. Patient demographics, tumor and surgical data were extracted from medical records. This initiative was undertaken as a quality improvement project and was exempt from review by the Dana-Farber/Harvard Cancer Center Institutional Review Board.
Outcome Measure
Our primary outcome of interest was the rate of reoperation among the patient cohort. Reoperation was defined as re-excision lumpectomy or mastectomy that occurred in patients with positive margins after the first definitive surgery. As this study was developed prior to the new margin definition consensus, a negative margin was considered >2mm during the study period. We also calculated the proportion of patients undergoing two or three reoperations, and assessed reoperation rates and median specimen size among the individual breast surgeons. Specimen size was calculated by multiplying each dimension size in centimeters and included the size of the global cavity shaves or individual specimens when applicable.
Statistical Analysis
Descriptive statistics were generated to compare patient, tumor, and surgery characteristics between patients undergoing a single surgery and those undergoing reoperation. A multivariate regression model was performed to identify factors associated with reoperation. Variables included in the model were age (<40, 40–49, 50–59, 60–69, >70), insurance (private, public, other), race (defined as white vs. non-white), grade (1,2,3), breast cancer subtypes (hormone receptor [HR]+/Her2-; HR-/Her2-; HR+ or HR-/Her2+); invasive tumor size, total lumpectomy size, node status (positive vs. negative), lymph node procedure (axillary node dissection, sentinel node biopsy, none), multifocality (yes vs. no), shave margin technique (global, targeted, none) and individual surgical provider. Of note, one of the surgical providers served as the reference group and was excluded from the multivariate analysis. Adjusted odds ratios (OR), 95% confidence intervals (CI), and p-values were calculated to determine the strength of the association between each variables and reoperation. Individual reoperation rates and median total specimen size among breast surgeons were calculated.
Results
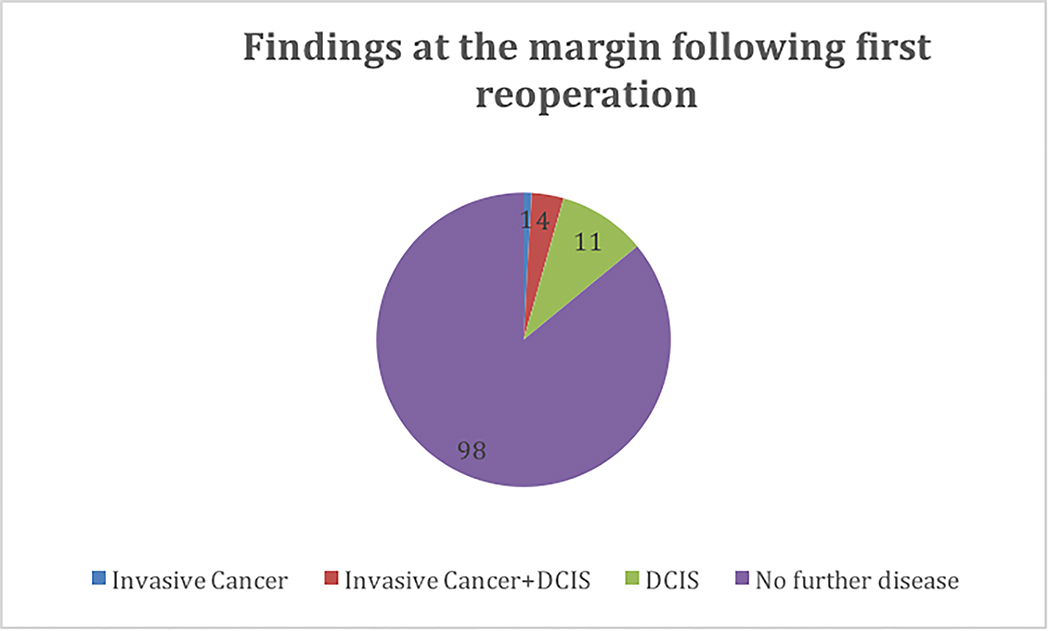
We identified a total of 490 patients with initial clinical stage I (n=408) and stage II (n=89) breast cancer who underwent BCS at our cancer center. Table 1 highlights demographic characteristics. All patients with positive margins underwent reoperation. Overall, the rate of re-operation for positive margins was 22.9 %, ranging from 15.5% to 40.0% by surgeon (Table 2). Re-excision was the most frequent reoperation procedure type performed (n=100, 88%), followed by unilateral mastectomy (n=7, 6%) and bilateral mastectomy (n=7, 6%) (Figure 1). Intraoperative margin techniques, including global cavity or targeted shaves, were utilized in 249 cases (50.1%). Among the total 114 patients who underwent re-excision, 11.7% underwent a second reoperation and 0.7% underwent a third operation (Figure 1). On final pathology after first reoperation, 1 patient (0.9%) had invasive cancer at the margin, 11 (9.6%) had ductal carcinoma in situ at the margin (DCIS) and 4 (3.5%) had both. The remaining 98 patients (86%) had no further disease at the margin. (Figure 2). The median total specimen size in the patient cohort was 65.8 cm3 (range among surgeon was 24.5–156.0 cm3), and the median invasive tumor size ranged between 0.9 to 1.3 cm in the patient cohort operated on by each surgeon (Table 2).
Table 1.
Patient characteristics and multivariate analysis of factors associated with reoperation
| Single Lumpectomy (n=383) N (%) | Repeat Surgery (n=114) N (%) | P-value Univariate |
Adjusted OR (95% CI) | |
|---|---|---|---|---|
| Age | ||||
| <40 | 2 (50.0%) | 2 (50.0%) | 0.007 | 1.43 (0.16–13.19) |
| 40–49 | 38 (62.3%) | 23 (37.7%) | 1.00 | |
| 50–59 | 85 (73.3%) | 31 (26.7%) | 0.83 (0.36–1.90) | |
| 60–69 | 134 (82.7%) | 28 (17.3%) | 0.48 (0.21–1.10) | |
| 70+ | 124 (80.5%) | 30 (19.5%) | 0.85 (0.31–2.32) | |
| Insurance | ||||
| Private | 230 (75.4%) | 75 (24.6%) | 0.24 | 1.00 |
| Public | 148 (80.0%) | 37 (20.0%) | 1.16 (0.57–2.36) | |
| Other | 5 (71.4%) | 2 (28.6%) | 0.72 (0.07–7.77) | |
| Race | ||||
| White | 326 (79.3%) | 85 (20.7%) | 0.03 | 0.83 (0.37–1.86) |
| Non-White | 38 (66.7%) | 19 (33.3%) | 1.00 | |
| Missing | 19 (65.5%) | 10 (34.5%) | 1.32 (0.36–4.79) | |
| Grade | ||||
| I | 128 (82.6%) | 27 (17.4%) | 0.12 | 1.00 |
| II | 167 (75.6%) | 54 (24.4%) | 1.09 (0.57–2.11) | |
| III | 88 (72.7%) | 33 (27.3%) | 1.68 (0.71–4.02) | |
| Tumor Subtype (n=465) | ||||
| HR+/HER2- | 309 (77.4%) | 90 (22.6%) | 0.54 | 1.00 |
| HER2+ | 34 (70.8%) | 14 (29.2%) | 0.63 (0.23–1.70) | |
| HR-/HER2- | 13 (72.2%) | 5 (27.8%) | 1.05 (0.28–3.98) | |
| Invasive Tumor Size (n=479) | 1.25 (0.78) | 1.35 (0.89) | 0.25 | 1.18 (0.84–1.64) |
| Total Lump Size (n=496) | 86.06 (80.98) | 75.33 (85.15) | 0.22 | 1.00 (0.99–1.00) |
| Node Status (n=417) | ||||
| Positive | 41 (68.3%) | 19 (31.7%) | 0.09 | 1.55 (0.77–3.15) |
| Negative | 279 (78.2%) | 78 (21.8%) | 1.00 | |
| Lymph Node Procedure (n=486) | ||||
| ALND | 7 (87.5%) | 1 (12.5%) | 0.76 | 0.31 (0.009–11.04) |
| SLNB | 311 (76.6%) | 95 (23.4%) | 1.23 (0.08–19.89) | |
| None | 56 (77.8%) | 16 (22.2%) | 1.00 | |
| Multifocal | ||||
| Yes | 18 (51.4%) | 17 (48.6%) | 0.0002 | 5.78 (2.17–15.42) |
| No | 365 (79.0%) | 97 (21.0%) | 1.00 | |
| Intraoperative Technique | ||||
| None | 185 (74.6%) | 63 (25.4%) | 0.19 | 1.00 |
| Shaved Global | 125 (82.2%) | 27 (17.8%) | 0.31 (0.07–1.34) | |
| Shaved Targeted | 73 (75.3%) | 24 (24.7%) | 0.67 (0.29–1.52) | |
| FDS Provider | ||||
| Surgeon 1 | 49 (84.5%) | 9 (15.5%) | <0.0001 | 0.92 (0.27–3.09) |
| Surgeon 2 | 62 (80.5%) | 15 (19.5%) | 4.38 (0.80–23.83) | |
| Surgeon 3 | 31 (63.3%) | 18 (36.7%) | 2.57 (0.81–8.22) | |
| Surgeon 4 | 71 (78.9%) | 19 (21.1%) | 3.17 (0.59–16.93) | |
| Surgeon 5 | 21 (60.0%) | 14 (40.0%) | 6.41 (1.94–21.22) | |
| Surgeon 6 | 50 (70.4%) | 21 (29.6%) | 3.41 (1.07–10.85) | |
| Surgeon 7 | 38 (80.9%) | 9 (19.1%) | 3.30 (0.99–11.01) | |
| Surgeon 8 | 54 (85.7%) | 9 (14.3%) | -- |
Abbreviations: ALND, axillary lymph node dissection; BWFH, Brigham and Women’s Faulkner Hospital; BWH, Brigham and Women’s Hospital; HR, hormone receptor; OR, odds ratio; SLNB, sentinel lymph node biopsy.
Table 2.
Detail by provider
| Provider | Total Re-excision | Total Patients | Re-excision Rate | Median Invasive Tumor Size*(IQR)- | Total Specimen Size* (IQR) |
|---|---|---|---|---|---|
| Surgeon 1 | 9 | 58 | 15.5% | 1.3 (1.0) | 91.4 (115.4) |
| Surgeon 2 | 15 | 77 | 19.5% | 1.2 (0.9) | 40.9 (59.3) |
| Surgeon 3 | 18 | 49 | 36.7% | 1.1 (0.9) | 44.0 (54.8) |
| Surgeon 4 | 19 | 90 | 21.1% | 1.2 (1.1) | 24.5 (25.4) |
| Surgeon 5 | 14 | 35 | 40.0% | 0.9 (0.9) | 80.8 (74.9) |
| Surgeon 6 | 21 | 71 | 29.6% | 1.1 (0.9) | 61.6 (69.8) |
| Surgeon 7 | 9 | 47 | 19.1% | 1.1 (0.7) | 156.0 (186.0) |
| Surgeon 8 | 9 | 63 | 14.3% | 1.1 (1.0) | 97.4 (58.9) |
IQR: Interquartile range
Figure 1. Re-operations following initial surgery.
Abbreviations: BCS, Breast Conserving Surgery; PM, Positive Margins; NM, Negative Margins; L, Lumpectomy; M, Mastectomy; BL, Bilateral Mastectomy.
Figure 2. Findings at the margin following first reoperation.
Abbreviations: DCIS, ductal carcinoma in situ
Univariate analysis, demonstrates that age, race, multifocal disease and individual provider where significantly associated with likelihood of reoperation (p-values were 0.007; 0.03, 0.09, 0.0002 and 0.0001 respectively) [Table 1]. However, in the multivariate analysis, multifocal disease (OR=5.78; 95% CI=2.17–15.42) and individual surgical provider (surgeon five OR= 6.41; CI=1.94–21.22; surgeon six OR=3.41; CI=1.07–10.85) were associated with higher reoperation rates. [Table 1].
Discussion
During the study period, there was not a nationwide consensus on the definition of a negative margin; however, our institutional guideline was greater than 2 mm for invasive cancer.[15] We found that the surgical reoperation rate at our comprehensive cancer center during the year prior to adoption of the no ink on tumor margin consensus was 22.9% and found variability among breast surgeons in our practice.
Consistent with previous studies, in our univariate analysis patients <40 years old were more likely undergo reoperation compared to patients between 60–69 years old (50% vs. 17.3% p = 0.007), however, the number of patients was very low in the cohort of patients <40 years old. [16–18] Additionally, multifocality, race and individual surgical provider were also factors significantly associated with reoperation. Patients with multifocal tumors underwent a second operation in 48.6% of cases compared with only 21% of those without multifocal lesions (p = 0.0002)
The association between reoperation and age have been reported in previous studies [19–22], and could be attributed to the reduced sensitivity of standard breast imaging in younger patients, which potentially limits the determination of extent of disease preoperatively. Additionally, surgical decision-making by patient age may influence reoperation rate. Even though current trends suggest younger patients are more likely to undergo mastectomy,[23–24] previous surveys in the literature demonstrated a preference for BCS in younger women and mastectomy in older women.[19–20] Therefore, attempts to conserve more breast tissue in younger women could be another factor contributing to these findings.
In the multivariate analysis, multifocalility and individual surgical provider, were the only factors that continued to be associated with the need for reoperation. Patients with multifocal disease were found to be 5.78 times more likely to undergo reoperation. Multifocality, which is often only identified on post-operative pathologic examination and many times not evident radiographically, has previously been associated with difficulty achieving clear margins. [18–20]. Additionally, surgeons five and six, had patients who were significantly more likely to undergo reoperation, however we are not able to elucidate the reason for this difference.
Other factors, such as the presence of DCIS has also been recognized as playing an important role in achieving negative margins, with the presence of both pure DCIS tumors and mixed tumors impacting rates of reoperations.[23, 25] Even though our study only included patients with initial clinical stage I-II breast cancer, 73.4% of our patients had a DCIS component, and further investigation into the impact that the DCIS component has on reoperation rates at our institution is being investigated.
Our study sought to examine the variability in reoperation rates among a group of surgeons performing only breast surgery (with ≥35 operations annually). We found that the surgical technique used during initial surgery did not affect reoperation rates; however, this finding differs from a recent prospective randomized study by Chapgar et al[26] where shaved margins during BCS reduced the rate of reoperation by 50%. One plausible explanation for this difference may be that during the time period of our study, there was not a standard or consistent intraoperative technique utilized by our providers. A critical analysis of the cause of the differences described above is beyond the scope of our study, however, these results add to the existing literature documenting variability among techniques of margin assessment, and highlights the importance of including the above mentioned variables in future studies.
Since the time this study was performed, surgical practice has changed in our institution. The updated SSO and the ASTRO guidelines for lumpectomy margin width recommending “no ink on tumor” as the new margin standard, has been implemented at our institution along with routine use of cavity shave margins in women undergoing BCS.[27] Following institutional adoption of the new margin definition and cavity shave technique, a preliminary review of our internal database demonstrates that among 961 patients with stage I-III breast cancer undergoing BCS from January 2016 to May 2017, overall rates of reoperation have decreased to 13.2%. These findings suggest the need to continue to evaluate factors associated with reoperation rates and provide further opportunity to examine how standardization of the process of treatment (including use of supplementary preoperative imaging, adoption of the current guidelines for lumpectomy margin width, and surgical technique) can led to less variability among providers.
Our study is subject to several limitations. This is a retrospective study limited to a single year at a single institution. While we limited the study cohort to patients with stage I-II breast cancer, DCIS and invasive disease exist concurrently and multiple studies have suggested that DCIS is associated with higher rates of reoperation.[23, 28] We did not control for the presence of DCIS in the reoperation rates. Reoperations were performed for margins >2mm in three cases due to surgeon discretion (i.e., because the patient decided to avoid radiation). Lastly, we did not examine hospital-specific factors, such as use of MRI, second opinion breast imaging, or presence of multidisciplinary treatment teams that might contribute to differences in reoperation by the likelihood to impact pathologically positive margins after BCS.
Despite these limitations, our study examined a large number of patients during a single year and was derived entirely from one institution. We included breast surgical oncologist focused on a single disease process; with a standard definition for a negative margin. Additionally, in our study, all the patients with positive margins underwent reoperation. All these above-mentioned factors eliminate confounding and institutional differences, such as reporting format and standardized pathologic assessment. Our results emphasize that reoperation following BCS remains a challenge for breast surgeons; the presence of significant provider-level variability in reoperation outcomes highlights an area for further study that could potentially enable improved patient outcomes through more standardized techniques. Further analysis of variation at the hospital- and surgeon -level for BCS operation is warranted.
Conclusions
Our examination of BCS demonstrates variability in reoperation rates and margin practices among breast surgeons within our institution. We found multifocality and individual surgeon provider were significantly associated with rates of reoperation. Since this study, we have implemented cavity shaved margins and incorporated the new pathologic margin guidelines of no ink on tumor. Further analysis of variation at the hospital- and surgeon -for BCS operation is warranted.
SYNOPSIS.
Breast conserving surgery is the most commonly performed procedure for invasive breast cancer and may require reoperation to achieve clear margins. We sought to explore variability in the number of reoperations in women undergoing breast conservation at our institution.
REFERENCES
- 1.Cody HS 3rd, Van Zee KJ. Reexcision--The Other Breast Cancer Epidemic. N Engl J Med 2015; 373: 568–569. [DOI] [PubMed] [Google Scholar]
- 2.Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med 2002; 347: 1227–1232. [DOI] [PubMed] [Google Scholar]
- 3.Early Breast Cancer Trialists’ Collaborative Group, Clarke M, Collins R, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005; 366: 2087–2106. [DOI] [PubMed] [Google Scholar]
- 4.Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 2002; 347: 1233–1241. [DOI] [PubMed] [Google Scholar]
- 5.Fleming FJ, Hill AD, Mc Dermott EW, O’Doherty A, O’Higgins NJ, Quinn CM. Intraoperative margin assessment and re-excision rate in breast conserving surgery. Eur J Surg Oncol 2004; 30: 233–237. [DOI] [PubMed] [Google Scholar]
- 6.Bani MR, Lux MP, Heusinger K, et al. Factors correlating with reexcision after breast-conserving therapy. Eur J Surg Oncol 2009; 35: 32–37. [DOI] [PubMed] [Google Scholar]
- 7.McCahill LE, Single RM, Aiello Bowles EJ, et al. Variability in reexcision following breast conservation surgery. JAMA 2012; 307: 467–475. [DOI] [PubMed] [Google Scholar]
- 8.Russo AL, Arvold ND, Niemierko A, et al. Margin status and the risk of local recurrence in patients with early-stage breast cancer treated with breast-conserving therapy. Breast Cancer Res Treat 2013; 140: 353–361. [DOI] [PubMed] [Google Scholar]
- 9.Kobbermann A, Unzeitig A, Xie XJ, et al. Impact of routine cavity shave margins on breast cancer re-excision rates. Ann Surg Oncol 2011; 18: 1349–1355. [DOI] [PubMed] [Google Scholar]
- 10.Sabel MS, Rogers K, Griffith K, et al. Residual disease after re-excision lumpectomy for close margins. J Surg Oncol 2009; 99: 99–103. [DOI] [PubMed] [Google Scholar]
- 11.Wilke LG, Czechura T, Wang C, Lapin B, Liederbach E, Winchester DP, Yao K. Repeat surgery after breast conservation for the treatment of stage 0 to II breast carcinoma: a report from the National Cancer Data Base, 2004–2010. JAMA Surg 2014; 149: 1296–1305. [DOI] [PubMed] [Google Scholar]
- 12.Deutsch M, Flickinger JC. Patient characteristics and treatment factors affecting cosmesis following lumpectomy and breast irradiation. Am J Clin Oncol 2003; 26: 350–353. [DOI] [PubMed] [Google Scholar]
- 13.Cochrane RA, Valasiadou P, Wilson AR, Al-Ghazal SK, Macmillan RD. Cosmesis and satisfaction after breast-conserving surgery correlates with the percentage of breast volume excised. Br J Surg 2003; 90: 1505–1509. [DOI] [PubMed] [Google Scholar]
- 14.Heil J, Breitkreuz K, Golatta M, et al. Do reexcisions impair aesthetic outcome in breast conservation surgery? Exploratory analysis of a prospective cohort study. Ann Surg Oncol 2012; 19: 541–547. [DOI] [PubMed] [Google Scholar]
- 15.Houssami N, Macaskill P, Marinovich ML, Morrow M. The association of surgical margins and local recurrence in women with early-stage invasive breast cancer treated with breast-conserving therapy: a meta-analysis. Ann Surg Oncol 2014; 21: 717–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wazer DE, Schmidt-Ullrich RK, Ruthazer R, DiPetrillo T, Boyle T, Kanski J, Safaii H. The influence of age and extensive intraductal component histology upon breast lumpectomy margin assessment as a predictor of residual tumor. Int J Radiat Oncol Biol Phys 1999; 45: 885–891. [DOI] [PubMed] [Google Scholar]
- 17.Singletary SE. Surgical margins in patients with early-stage breast cancer treated with breast conservation therapy. Am J Surg 2002; 184: 383–393. [DOI] [PubMed] [Google Scholar]
- 18.Aziz D, Rawlinson E, Narod SA, Sun P, Lickley HL, McCready DR, Holloway CM. The role of reexcision for positive margins in optimizing local disease control after breast-conserving surgery for cancer. Breast J 2006; 12: 331–337. [DOI] [PubMed] [Google Scholar]
- 19.Rutter CE, Park HS, Killelea BK, Evans SB. Growing Use of Mastectomy for Ductal Carcinoma-In Situ of the Breast Among Young Women in the United States. Ann Surg Oncol 2015; 22: 2378–2386. [DOI] [PubMed] [Google Scholar]
- 20.Pesce CE, Liederbach E, Czechura T, Winchester DJ, Yao K. Changing surgical trends in young patients with early stage breast cancer, 2003 to 2010: a report from the National Cancer Data Base. J Am Coll Surg 2014; 219: 19–28. [DOI] [PubMed] [Google Scholar]
- 21.Staradub VL, Hsieh YC, Clauson J, Langerman A, Rademaker AW, Morrow M. Factors that influence surgical choices in women with breast carcinoma. Cancer 2002; 95: 1185–1190. [DOI] [PubMed] [Google Scholar]
- 22.Schifano P, Scarinci M, Borgia P, Perucci CA. Analysis of the recourse to conservative surgery in the treatment of breast tumors. Tumori 2002; 88: 131–136. [DOI] [PubMed] [Google Scholar]
- 23.Jung W, Kang E, Kim SM, et al. Factors Associated with Re-excision after Breast-Conserving Surgery for Early-Stage Breast Cancer. J Breast Cancer 2012; 15: 412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kurniawan ED, Wong MH, Windle I, et al. Predictors of surgical margin status in breast-conserving surgery within a breast screening program. Ann Surg Oncol 2008; 15: 2542–2549. [DOI] [PubMed] [Google Scholar]
- 25.Schiller DE, Le LW, Cho BC, Youngson BJ, McCready DR. Factors associated with negative margins of lumpectomy specimen: potential use in selecting patients for intraoperative radiotherapy. Ann Surg Oncol 2008; 15: 833–842. [DOI] [PubMed] [Google Scholar]
- 26.Chagpar AB, Killelea BK, Tsangaris TN, et al. A Randomized, Controlled Trial of Cavity Shave Margins in Breast Cancer. N Engl J Med 2015; 373: 503–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moran MS, Schnitt SJ, Giuliano AE, et al. Society of Surgical Oncology-American Society for Radiation Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in stages I and II invasive breast cancer. Int J Radiat Oncol Biol Phys 2014; 88: 553–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morrow M, Jagsi R, Alderman AK, et al. Surgeon recommendations and receipt of mastectomy for treatment of breast cancer. JAMA 2009; 302: 1551–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]