Summary
A 49-year-old woman was admitted to the hospital because of cardiac tamponade. The hemorrhagic pericardial effusion was cytologically negative for malignant cells. Cardiac magnetic resonance imaging showed two masses in the anterior and lateral right atrium; however, positron emission tomography (PET) image using 18F-fluorodeoxyglucose revealed strong uptake in the anterior right atrium, without other tumors or metastasis. Intraoperatively, the lateral mass was confirmed as a thrombus, whereas the anterior mass was removed surgically and was diagnosed as an angiosarcoma with histopathological examination. However, she was re-admitted to the hospital 1 month after the operation because of cerebral hemorrhage, suspicious of distant metastasis. PET is useful for the detection of cardiac angiosarcoma.
Keywords: Angiosarcoma, Cardiac tamponade, Radionuclide imaging, 18F-fluorodeoxyglucose
Introduction
Primary cardiac tumor is uncommon with an incidence at autopsy from 0.0017% to 0.033% [1]. Approximately one-fourth of all cardiac primary tumors are considered malignant, and one-third of these malignant tumors are angiosarcomas [2]. Since the signs and symptoms are non-specific, the diagnosis is often delayed, which leads to poor outcomes. Diagnostic imaging such as computed tomography (CT), echocardiography, and magnetic resonance imaging (MRI) have been reported to allow early diagnosis and treatment [3]. We report a case of cardiac angiosarcoma detected by positron emission tomography (PET) imaging, compared with other diagnostic modalities.
Case report
A 49-year-old woman presented at the emergency room with epigastric discomfort and nausea. Her physical examination showed pulsus paradoxus and tachycardia. Electrocardiography demonstrated sinus tachycardia of 120 beats per minute with low-voltage complexes. Laboratory data were within normal limits except for elevated fibrin/fibrinogen degradation products (FDP) (20.7 μg/ml) and carbohydrate antigen (CA)-125 (190.5 U/ml). Transthoracic echocardiography showed massive pericardial effusion and diastolic right ventricular collapse, suggesting the presence of cardiac tamponade. Thoracic CT also showed massive pericardial effusion and bilateral pleural effusion without aortic dissection or lung mass; therefore, pericardiocentesis was performed immediately, and revealed hemorrhagic effusion with no evidence of malignancy, tuberculosis, or infection. Her symptoms, pulsus paradoxus and pericardial effusion on echocardiography subsequently disappeared. A 99mTc-scintigram revealed reduced uptake in the inferior left ventricle; however, coronary angiography and left ventriculography were normal.
The pericardial effusion accumulated again soon after drainage; therefore, various imaging methods were used to examine etiology. Cardiac CT and MRI showed two masses in the anterior right atrium (RA) and pericardial space; however, they were unable to eliminate the possibility of pericardial tumor (Fig. 1A and B). PET was performed using 18F-fluorodeocyglucose (FDG), which revealed strong uptake in the anterior RA, with a standardized uptake value (SUV) of 9.9, suggesting the presence of malignancy (Fig. 1C and D). Neither increased uptake in the pericardium nor other metastasis was visualized. The malignant tumor was suspected to be localized in the RA; therefore, surgery was performed. There was hemorrhagic fluid with coagulation thrombus attached to the RA in the pericardial space (Fig. 2A). The mass, which was removed and rebuilt with an equine pericardial patch, was localized in the RA free wall, consistent with PET imaging (Fig. 2B). Although intraoperative cytology of pericardial effusion was negative for malignancy, histological findings of the tumor showed atypical cells with hyperchoromatic nuclei (Fig. 3A). Immunohistochemical stains of these cells were positive for CD31 (Fig. 3B), CD34, and Ki67, indicating the vascular origin of the tumor, corresponding to angiosarcoma.
Figure 1.
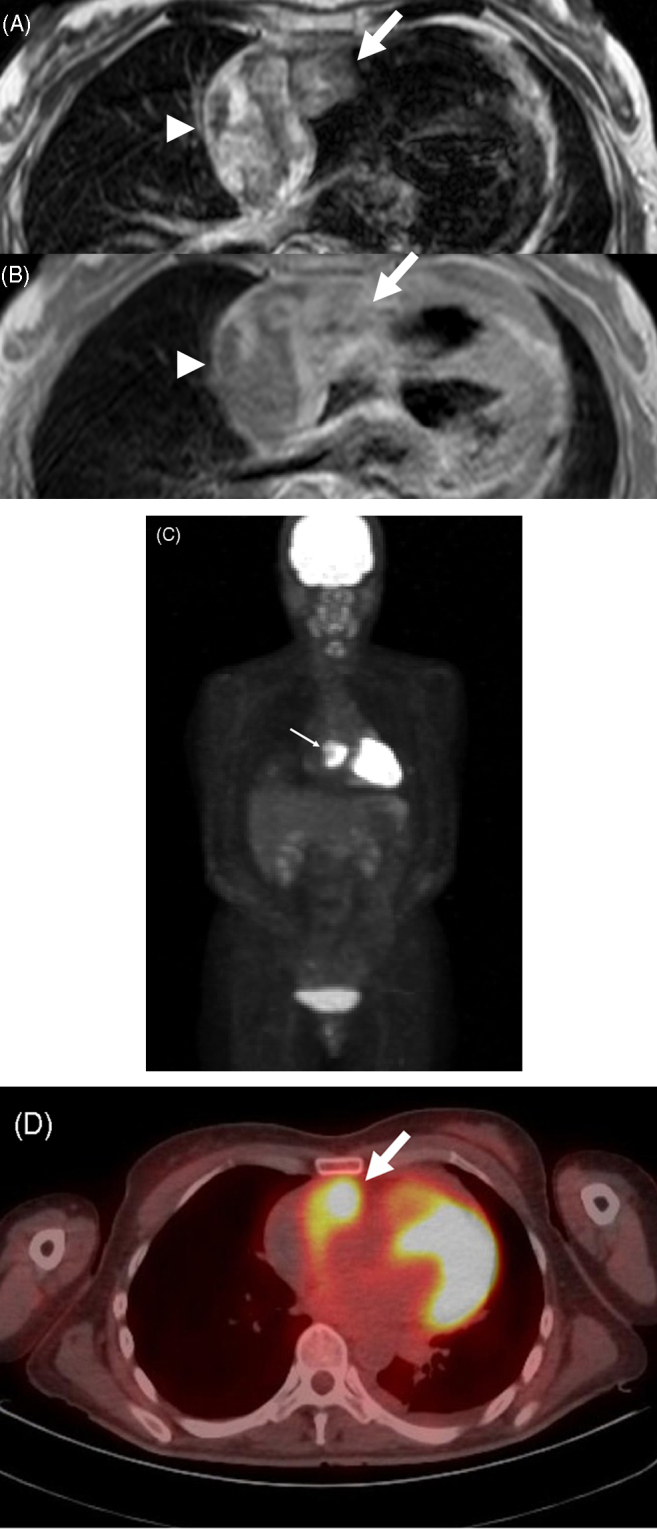
Axial electrocardiography-gated magnetic resonance imaging demonstrates two masses in the anterior right atrium (RA) (arrow) and the right lateral pericardium (arrowhead) ((A) T2-weighted spin echo image; (B) gadolinium-enhanced T1-weighted spin echo image). Positron emission tomography (PET) shows strong uptake of 18F-fluorodeoxyglucose (FDG) in the RA (arrow) (C). PET/computed tomography fusion imaging demonstrates increased uptake of FDG in the anterior RA (arrow) but no uptake in the pericardium (D).
Figure 2.
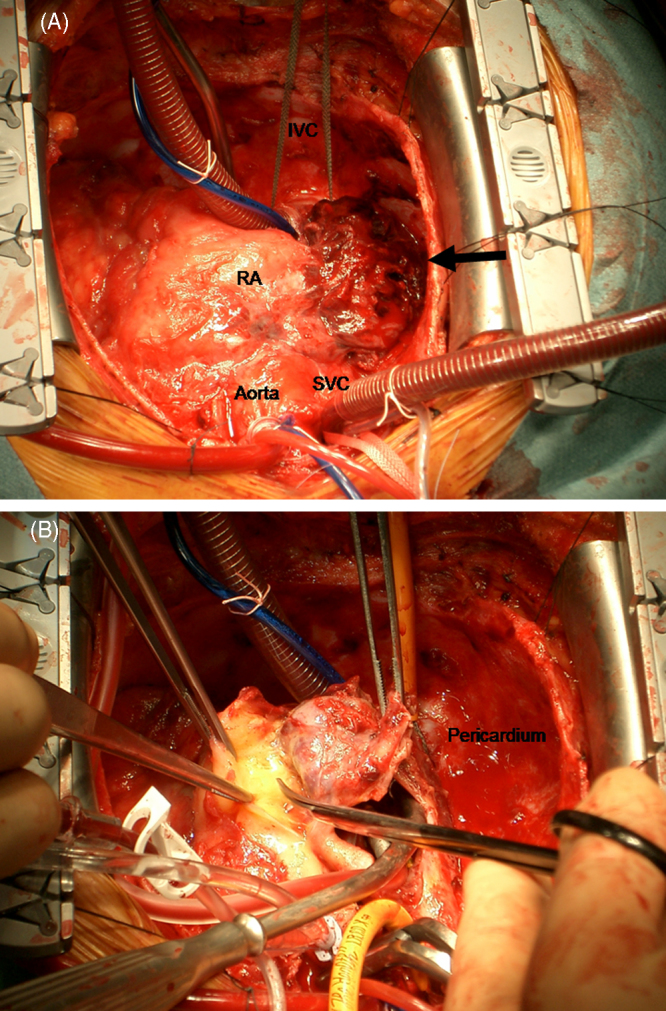
Intraoperative demonstrations show coagulation thrombus (arrow) attached to the right atrium (RA) (A) and a resecting mass from RA (B). SVC, superior vena cava; IVC, inferior vena cava.
Figure 3.
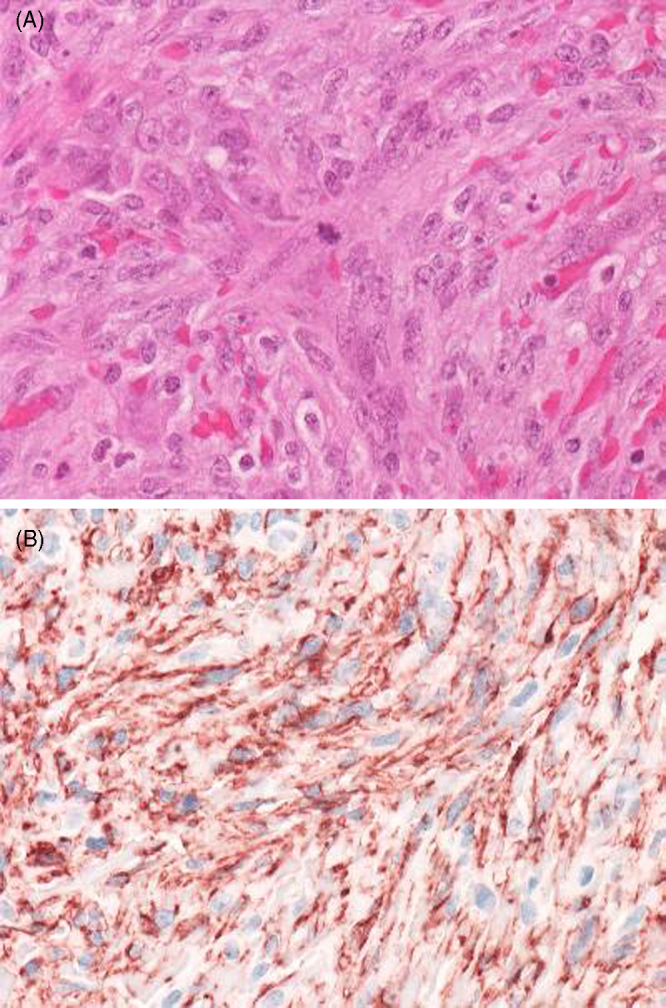
Histopathological appearances of the tumor. The atypical spindle cells are proliferating diffusely. An atypical mitosis can be observed in the center field ((A) Hematoxylin–Eosin, ×40). The tumor cells are strongly positive for CD31 ((B) immunohistochemistry, ×40).
After discharge, she was re-admitted to the hospital on postoperative day (POD) 34, due to cerebral hemorrhage, suspected of a distant metastasis of the tumor. She was discharged for palliative care and died on POD 79.
Discussion
Initial presentations of cardiac angiosarcoma are dyspnea, chest pain, heart murmur, constitutional symptoms, arrhythmias, and congestive heart failure. Because of the propensity of this tumor to involve pericardium, pericardial effusion and cardiac tamponade may also be the finding [4]. Although the pericardial effusion was cytologically negative in this case, the resected tumor was malignant and exposed to pericardium and RA. Without left-side heart lesion, there was a case report of rapid brain metastases after surgery [5]. Owing to the aggressive nature of this tumor, only a small amount of malignant cells may be needed to metastasize.
We utilized various imaging methods to diagnose the cause of recurrent hemorrhagic pericardial effusion. Cardiac MRI was reported to have the advantage for a detection of cardiac tumor over cardiac CT; however, MRI could not differentiate malignant tissue [6]. As shown in some reports [7], [8], [9], this case demonstrated the advantage of PET image for the early detection of cardiac angiosarcoma. SUV is usually defined as the tracer uptake in the tumor divided by the injected dose normalized for the patient weight. Mostly, an SUV > 2.5 is used as the threshold for differentiating benign from malignant [10]. In this case, the SUV of 9.9 was measured in the center of the anterior RA (Fig. 1D), that was consistent with the pathological findings. The metastasis was not detected by PET and MRI pre-operatively; however, the tumor might metastasize at that time, which was not enough for the detection, or the tumor metastasized after the operation rapidly. Another report showed the usefulness of PET and MRI for the detection of local recurrence and metastasis of this tumor [11]; however, the recurrence was 2 years later. To think of the rapid and aggressive behavior of this tumor, it is suggested that more frequent follow-up studies of PET be needed to detect the early distant metastasis.
In this case we demonstrated the usefulness of PET and CT fusion imaging in the detection of a malignant tumor in the RA.
References
- 1.Silverman N.A. Primary cardiac tumors. Ann Surg. 1980;191:127–138. doi: 10.1097/00000658-198002000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herrmann M.A., Shankerman R.A., Edwards W.D., Shub C., Schaff H.V. Primary cardiac angiosarcoma: a clinicopathologic study of six cases. J Thorac Cardiovasc Surg. 1992;103:655–664. [PubMed] [Google Scholar]
- 3.Kurian K.C., Weisshaard D., Parekh H., Berry G.J., Reitz B. Primary cardiac angiosarcoma: case report and review of the literature. Cardiovasc Pathol. 2006;15:110–112. doi: 10.1016/j.carpath.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Nurkalem Z., Gorgulu S., Gumrukcu G., Eren M. Right atrial mass presenting as cardiac tamponade. Int J Cardiol. 2006;112:e20–e22. doi: 10.1016/j.ijcard.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 5.Ikeya E., Taguchi J., Yamaguchi M., Shibuya M., Kanabuchi K. Primary cardiac angiosarcoma: presenting with cardiac tamponade followed by cerebral hemorrhage with brain metastases. Jpn J Thorac Cardiovasc Surg. 2006;54:528–531. doi: 10.1007/s11748-006-0063-9. [DOI] [PubMed] [Google Scholar]
- 6.Inoko M., Iga K., Kyo K., Kondo H., Tamura T., Izumi C., Kitagichi S., Hirozane T., Himura Y., Gen H., Konishi T. Primary cardiac angiosarcoma detected by magnetic resonance imaging but not by computed tomography. Intern Med. 2001;40:391–395. doi: 10.2169/internalmedicine.40.391. [DOI] [PubMed] [Google Scholar]
- 7.Freudenberg L.S., Rosenbaum S.J., Schulte-Herbrüggen J., Eising E.G., Lauenstein T., Wolff A., Bockisch A. Diagnosis of a cardiac angiosarcoma by fluorine-18 fluordeoxyglucose positron emission tomography. Eur Radiol. 2002;12:S158–S161. doi: 10.1007/s00330-002-1478-z. [DOI] [PubMed] [Google Scholar]
- 8.Hori Y., Funabashi N., Miyauchi H., Nakagawa K., Shimura H., Miyazaki M., Kozono H., Nagai Y., Ishikura H., Nagai T., Kobayashi Y., Komuro I. Angiosarcoma in the right atria demonstrated by fusion images of multislice computed tomography and positron emission tomography using F-18 fluoro-deoxyglucose. Int J Cardiol. 2007;123:e15–e17. doi: 10.1016/j.ijcard.2006.11.093. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura-Horigome M., Koyama J., Eizawa T., Kasai H., Kumazaki S., Tsutsui H., Koiwai K., Oguchi K., Kinoshita O., Ikeda U. Successful treatment of primary cardiac angiosarcoma with docetaxel and radiotherapy. Angiology. 2008;59:368–371. doi: 10.1177/0003319707308212. [DOI] [PubMed] [Google Scholar]
- 10.Hellwig D., Graeter T.P., Ukena D., Groeschel A., Sybrecht G.W., Schaefers H.J., Kirsch C.M. 18F-FDG PET for mediastinal staging of lung cancer: which SUV threshold makes sense? J Nucl Med. 2007;48:1761–1766. doi: 10.2967/jnumed.107.044362. [DOI] [PubMed] [Google Scholar]
- 11.Juergens K.U., Hoffmeier A., Riemann B., Maintz D. Early detection of local tumour recurrence and pulmonary metastasis in cardiac angiosarcoma with PET-CT and MRI. Eur Heart J. 2007;28:663. doi: 10.1093/eurheartj/ehl227. [DOI] [PubMed] [Google Scholar]


