Abstract
A 53-year-old Japanese man presented with severe chest pain. He had suffered from persistent fever, muscle pain, arthralgia, and dyspnea on exertion (New York Heart Association class I) for two and half months prior to admission. He had been treated with several antibiotics for two months and prednisolone for almost one month prior to admission. On the day of admission, he had suffered from chest pain at rest, and had come to our hospital. Electrocardiography showed a normal sinus rhythm with significant ST segment elevation in leads V3–6 and abnormal Q waves in leads V4–6. Transthoracic echocardiography demonstrated left ventricular ejection fraction of 52% with severe mitral regurgitation and an 18-mm vegetation on the anterior mitral valve leaflet. Multiple blood cultures identified Streptococcus sanguis. The diagnosis was acute myocardial infarction and mitral regurgitation associated with infective endocarditis (IE). The incidence of acute coronary syndrome caused by IE is quite low in patients with native valves. After a 6-week course of antibiotics, mitral valve replacement and partial cardiomyotomy were performed. Two years after the surgery, follow-up echocardiography showed almost normal left ventricle function and no mitral regurgitation, and the patient has been living an active life without any complications.
Keywords: Infective endocarditis, Myocardial infarction, Septic emboli
1. Introduction
Infective endocarditis (IE) is still associated with high in-hospital mortality, ranging from 16% to 25%, and a high incidence of embolic events, ranging from 13% to 49%, despite recent improvements in diagnostic and therapeutic strategies [1]. Such wide ranges of incidence of mortality events emphasize the heterogeneity of the disease and the critical need for baseline risk stratification in order to focus aggressive management toward the high risk subsets of patients. IE is well known to cause many types of complication [2]. Systemic embolism is a common complication of IE, most frequently involving the central nervous system, spleen, kidney, liver, and iliac or mesenteric arteries, whereas acute coronary syndrome is infrequently encountered [3]. Acute myocardial infarction (AMI) complicated by septic coronary embolism from IE is a rare and fatal condition. The incidence of coronary septic embolism is difficult to estimate. Three series found that 11 (10.6%) of 104 Russian patients had AMI as a result of septic embolism [4], only 14 (2.9%) of 586 Spanish patients had acute coronary syndrome, and half were associated with prosthetic valves, 1.5% of cases of acute coronary syndrome occurred with native valves, and embolism was the cause in only 3 (0.51%) of 586 patients [5]. And only 2 (0.52%) of 384 patients had coronary embolisms in a multicenter prospective European study [1]. Therefore, the incidence of acute coronary syndrome is quite low in patients with native valves.
Here, we report a case of AMI complicated by septic coronary embolism caused by IE of the mitral valve.
2. Case report
A 53-year-old Japanese man presented with severe chest pain. He had suffered from persistent fever, muscle pain, arthralgia, and dyspnea on exertion (New York Heart Association class II) for two and a half months prior to admission. He had been treated with several antibiotic agents including cefditoren pivoxil, azithromycin, or cefpodoxime proxetil for two months at another clinic, but his fever had persisted. A primary physician had suspected vasculitis, and prescribed prednisolone (20 mg/day) for almost one month. He had suffered from chest pain at rest on the day of admission, and had come to our hospital.
On admission, body temperature was 36.7 °C, heart rate was 80 beats per minute, and blood pressure was 130/90 mmHg. Respiratory rate was 26 per minute with oxygen saturation of 95% breathing ambient air. Physical examination found coarse breath sounds and bilateral crackles, and a grade III systolic murmur at the left sternal border. The abdomen was normal. No peripheral edema was present in the legs. Skin examination revealed numerous petechiae, and multiple small, peripheral necrotic lesions on the feet (Fig. 1a). Conjunctival petechiae were identified without typical Roth's spots (Fig. 1b). No other physical or neurological abnormalities were found, although he had dysphasia 1 week prior to admission which had improved. His coronary risk factors were hypertension, dyslipidemia, and current smoking. He had been treated with valsartan (80 mg/day) and fluvastatin (10 mg/day).
Fig. 1.
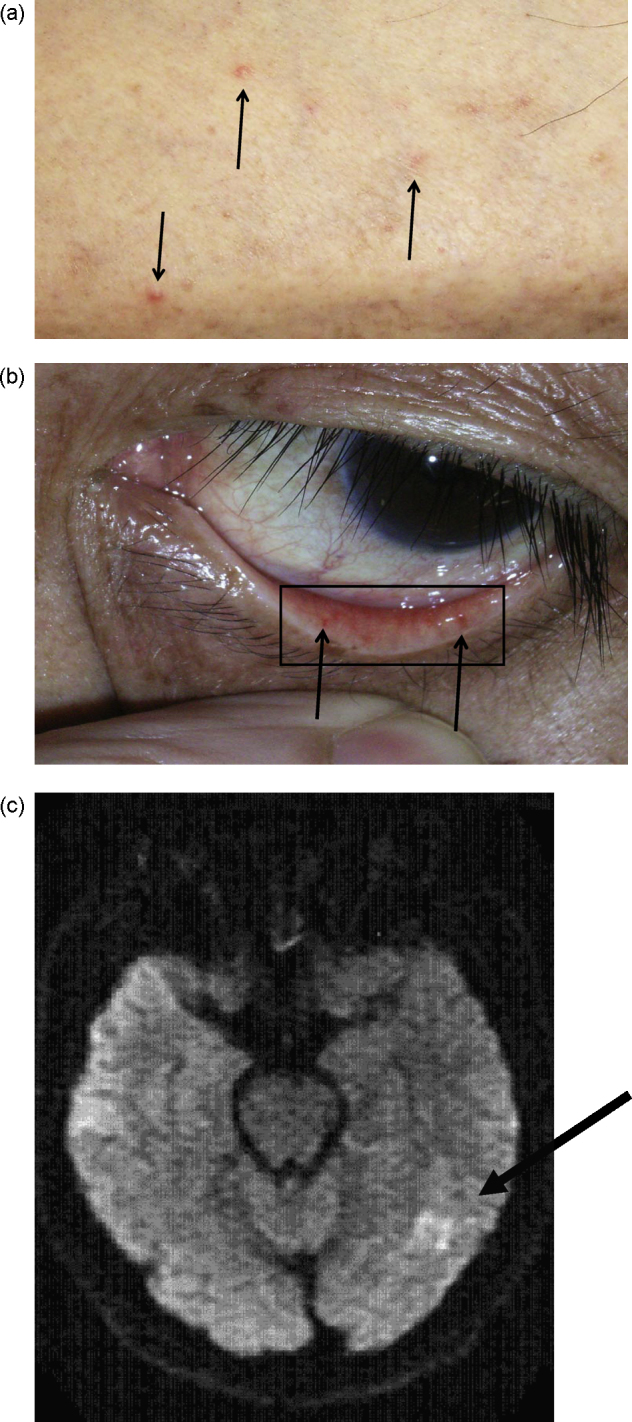
(a) Painful papules were present at both extremities (arrows). (b) Petechiae were present on the palpebral conjunctiva (arrows). (c) Diffusion-weighted magnetic resonance images showed scattered regions of hyperintensity in the left middle cerebral artery territory (arrow). Intraluminal filling defects were not seen.
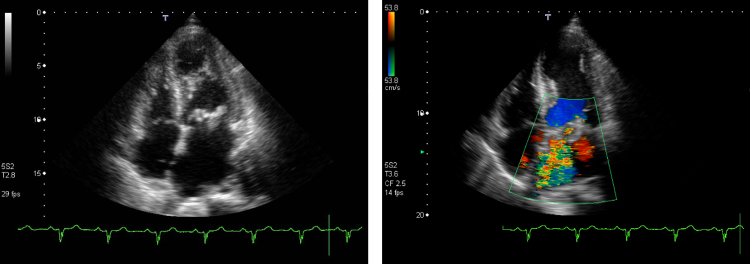
The results of laboratory tests are shown as follows. The white blood cell count was 17,000 mm−3 with 89% neutrophils. The level of creatine kinase had increased to 458 IU and troponin T test showed positive finding. Electrocardiography showed a normal sinus rhythm with significant ST segment elevation in leads V3–6 and abnormal Q waves in leads V4–6 (Fig. 2a). Chest radiography showed cardiomegaly and marked pulmonary edema. Bedside transthoracic echocardiography demonstrated left ventricular ejection fraction of 52% with severe mitral regurgitation and an 18-mm vegetation on the anterior mitral valve leaflet (Fig. 3). The periannular valve was intact. Hypokinesis was observed in the apical anterior of the left ventricle. Brain magnetic resonance imaging revealed cerebral infarctions in the distal portion of the left middle cerebral artery (Fig. 1c). Brain magnetic resonance angiography did not reveal any mycotic aneurysm.
Fig. 2.
(a) Electrocardiogram on admission showed significant ST segmental elevation in leads V3–6, and abnormal Q waves in leads V4–6. (b) Coronary angiogram done 6 weeks after the onset of acute myocardial infarction (cranial view) showed total occlusion of the distal left anterior descending artery (arrow).
Fig. 3.
Transthoracic echocardiographic images, four chamber view, showed highly mobile vegetations and hypokinesia of the left ventricular apex (left). The vegetation size was 18 mm. Severe mitral regurgitation was seen in the Doppler flowmetry image (right).
Six hours after the onset of chest pain, the electrocardiographic findings and serum enzyme measurements strongly suggested anterolateral AMI. However, we did not perform coronary angiography because of the presence of heart failure, and could not rule out the possibility of the vegetation in the aortic valve. We initiated conservative treatment with diuretics and planned an elective mitral valve operation because he had cerebral infarctions due to septic emboli. After obtaining two sets of blood samples, intravenous administration of ampicillin and gentamicin, and continuous infusion of carperitide at a dose of 0.1 μg/kg/min were begun. Multiple blood cultures identified Streptococcus sanguis.
The course of intravenous antibiotics was continued for 6 weeks, with a good clinical response. He was then transferred to Juntendo University Hospital for mitral valve replacement. Before the operation, coronary angiography demonstrated that the distal left anterior descending artery was totally occluded (Fig. 2b). The diagnosis was embolic AMI and mitral regurgitation associated with IE.
The surgical procedure is briefly described here. Standard cardiopulmonary bypass with mild hypothermia was used. After establishing complete cardiac arrest with blood cardioplegia, both atrial incisions were made, and the mitral and tricuspid valves were evaluated. The mitral valve had fragile, easily mobilized vegetations (Fig. 4). The mitral valve was completely excised and replaced with an ATS 27 mm mechanical valve. The multiple vegetations on the left atrial endocardium were also widely debrided and left ventricular aneurysmectomy was performed at the area of myocardial infarction.
Fig. 4.
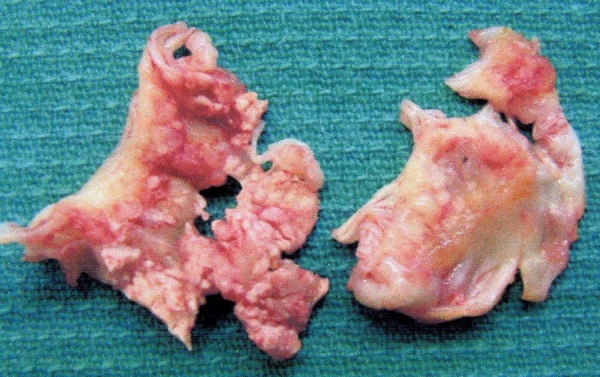
Macroscopic view of the mitral valve with vegetations. The vegetation is the large irregular, red swelling on the valve leaflet. Leaflet perforation is seen.
The postoperative course was uneventful. After the surgery, the patient was treated with ampicillin and gentamycin intravenously for 2 weeks. His symptoms significantly improved and repetitive blood cultures were negative. Postoperative echocardiography revealed normal function of the prosthetic mitral valve and hypokinesis of the apex of the left ventricle. One and a half years after the surgery, follow-up echocardiography showed no abnormalities in the regional wall motion of the left ventricle, and he has been living an active life without complications.
3. Discussion
Systemic embolism occurs in 22–50% of patients with IE, the majority (up to 65%) in the central nervous system, but other major arterial beds may be involved, including the coronary arteries. Most coronary embolisms occur in the left ascending coronary artery (13 of 14 cases) because of the downward course of the left ascending coronary artery compared with the right coronary artery or left circumflex artery, which originate at 90° to the aortic cusp [5]. Septic emboli are more frequent with mitral valve infection (25%) than with aortic valve infection (10%) [6].
We believe that the present case of AMI was caused by embolism from IE of the mitral valve. The excised myocardium did not involve the left coronary artery. Therefore we could not confirm the vegetation origin of the coronary embolism by pathological examination. However, we identified systemic embolization in the patient, and coronary angiography clearly showed the typical appearance of embolization in the left coronary artery (Fig. 2b).
Coronary angiography can establish the diagnosis of septic emboli in the coronary artery. However, contact between the catheter and the valve surface with vegetation may release systemic emboli. Therefore coronary angiography in patients with IE was reported to be safe if no vegetation is observed on the aortic valve [7]. But percutaneous intervention is not the definitive therapeutic strategy. The indication mainly depends on the infarct size and the grade of congestive heart failure due to myocardial infarction itself because another embolic complication might be induced by the catheterization. Balloon or stent procedures may allow mycotic aneurysm to develop at the site, resulting in complications including coronary rupture or sudden death [8].
Furthermore, evaluation of the cerebral embolism is essential. Preoperative cerebral embolism requires modification of the timing of surgery. The rate of exacerbation of cerebral complications decreased to 10% in patients who underwent surgical treatment more than 15 days after cerebral infarction, and to 2.3% in those who underwent surgical treatment more than 4 weeks later [9].
In this case, we decided to continue medical treatment because the infarct area was relatively small [distal left anterior descending artery occlusion; ejection fraction 52% (Simpson method); and maximum creatine phosphokinase level, 890 IU].
Surgical embolectomy using direct coronary incision is possible if the patient is in unstable condition, and was successful in one case [10]. And a possible demerit of the conservative strategy might be an increased risk of bacterial myocarditis followed by cardiac rupture [11]. However, surgical treatment carries high operative risk in this case.
We reported the rare case of a patient with IE had developed AMI. If the patient with fever of unknown origin has chest pain, the possibility of IE in patients with AMI should be considered, and the dissemination of septic emboli should be evaluated including before coronary angiography. Especially, the evaluation of the cerebral embolism is essential. The therapeutic strategy depends on the clinical manifestations or signs of systemic embolism.
References
- 1.Thuny F., Di Salvo G., Belliard O., Avierinos J.F., Pergola V., Rosenberg V., Casalta J.P., Gouvernet J., Derumeaux G., Iarussi D., Ambrosi P., Calabró R., Riberi A., Collart F., Metras D. Risk of embolism and death in infective endocarditis: prognostic value of echocardiography: a prospective multicenter study. Circulation. 2005;112:69–75. doi: 10.1161/CIRCULATIONAHA.104.493155. [DOI] [PubMed] [Google Scholar]
- 2.Takaya T., Takeuchi Y., Okamoto M., Hata K., Kojima Y., Nakajima K., Kita T., Ito M., Nakajima H., Takaoka R., Nomura T., Iwahashi K., Kawashima S., Seo T. Mitral regurgitation resulting from the consecutive multiple perforations by infective endocarditis mimicking the isolated anterior mitral cleft. J Cardiol. 2008;52:159–162. doi: 10.1016/j.jjcc.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 3.Herzog C.A., Henry T.D., Zimmer S.D. Bacterial endocarditis presenting as acute myocardial infarction: a cautionary note for the era of reperfusion. Am J Med. 1991;90:392–397. [PubMed] [Google Scholar]
- 4.Tiurin V.P., Korneev N.V. The mechanisms of the development and diagnosis of myocardial infarct in septic endocarditis. Ter Arkh. 1992;64:55–58. (in Russian) [PubMed] [Google Scholar]
- 5.Manzano M.C., Vilacosta I., San Román J.A., Aragoncillo P., Sarriá C., López D., López J., Revilla A., Manchado R., Hernández R., Rodríguez E. Acute coronary syndrome in infective endocarditis. Rev Esp Cardiol. 2007;60:24–31. (in Spanish) [PubMed] [Google Scholar]
- 6.Bayer A.S., Bolger A.F., Taubert K.A., Wilson W., Steckelberg J., Karchmer A.W., Levison M., Chambers H.F., Dajani A.S., Gewitz M.H., Newburger J.W., Gerber M.A., Shulman S.T., Pallasch T.J., Gage T.W. Diagnosis and management of infective endocarditis and its complications. Circulation. 1998;98:2936–2948. doi: 10.1161/01.cir.98.25.2936. [DOI] [PubMed] [Google Scholar]
- 7.Welton D.E., Young J.B., Raizner A.E., Ishimori T., Adyanthaya A., Mattox K.L., Chahine R.A., Miller R.R. Value and safety of cardiac catheterization during active infective endocarditis. Am J Cardiol. 1979;44:1306–1310. doi: 10.1016/0002-9149(79)90445-4. [DOI] [PubMed] [Google Scholar]
- 8.McGee M.B., Khan M.Y. Ruptured mycotic aneurysm of a coronary artery. A fatal complication of Salmonella infection. Arch Intern Med. 1980;140:1097–1098. [PubMed] [Google Scholar]
- 9.Eishi K., Kawazoe K., Kuriyama Y., Kitoh Y., Kawashima Y., Omae T. Surgical management of infective endocarditis associated with cerebral complications. Multi-center retrospective study in Japan. J Thorac Cardiovasc Surg. 1995;110:1745–1755. doi: 10.1016/S0022-5223(95)70038-2. [DOI] [PubMed] [Google Scholar]
- 10.Beak M.J., Kim H.K., Yu C.W., Na C.Y. Mitral valve surgery with surgical embolectomy for mitral valve endocarditis complicated by septic coronary embolism. Eur J Cardiothorac Surg. 2008;33:116–118. doi: 10.1016/j.ejcts.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 11.LeLeiko R.M., Bower D.J., Larsen C.P. MRSA-associated bacterial myocarditis causing ruptured ventricle and tamponade. Cardiology. 2008;111:188–190. doi: 10.1159/000121602. [DOI] [PubMed] [Google Scholar]