Summary
We describe a case of pulmonary tumor thrombotic microangiopathy (PTTM) associated with lung cancer. A 63-year-old woman, who had been treated for lung cancer, was admitted to our hospital because of progressive dyspnea. Chest CT films showed reticular shadows in the middle and left upper lobes, and echocardiography revealed severe pulmonary hypertension. Because drug induced pneumonitis and either pulmonary thromboembolism or pulmonary tumor embolism were suspected, corticosteroid and anti-coagulant therapy were administered. Despite these treatments, she died 50 days after admission. Postmortem examination revealed PTTM associated with lung cancer. PTTM should be considered in cancer patients who show progressive respiratory failure and pulmonary hypertension.
Keywords: Lung cancer, Pulmonary thromboembolism, Pulmonary tumor embolism, Pulmonary tumor thrombotic microangiopathy
Introduction
Pulmonary tumor thrombotic microangiopathy (PTTM), which is a specific type of pulmonary tumor embolism, is characterized by widespread fibrocellular intimal proliferation of the small pulmonary arteries and arterioles, tumor embolism and the organization and recanalization of thrombosis in patients with metastatic carcinoma [1]. It leads to progressive dyspnea, severe pulmonary hypertension, right-side heart failure and sudden death. It is very difficult to differentiate PTTM from pulmonary thromboembolism due to their similar manifestations [2].
We describe a patient with severe pulmonary hypertension the etiology of which remained elusive during her lifetime and was found to be due to PTTM arising from pulmonary adenocarcinoma on autopsy.
Case report
A 63-year-old woman was diagnosed with lung cancer in 1995, which was histopathologically defined as papillary adenocarcinoma. She underwent a left lower lobe lobectomy and received adjuvant chemotherapy. In June 1998, chest computed tomography (CT) showed mediastinal lymphadenopathy that was suspicious of recurrence. Radiotherapy was performed, and tegafururacil was administered from January 1999 to January 2000. In April 2002, tegafururacil was restarted because new mediastinal lymph node metastases appeared. On October 11, 2002, she was admitted to our hospital because of a 1-month history of progressive cough and dyspnea upon exertion.
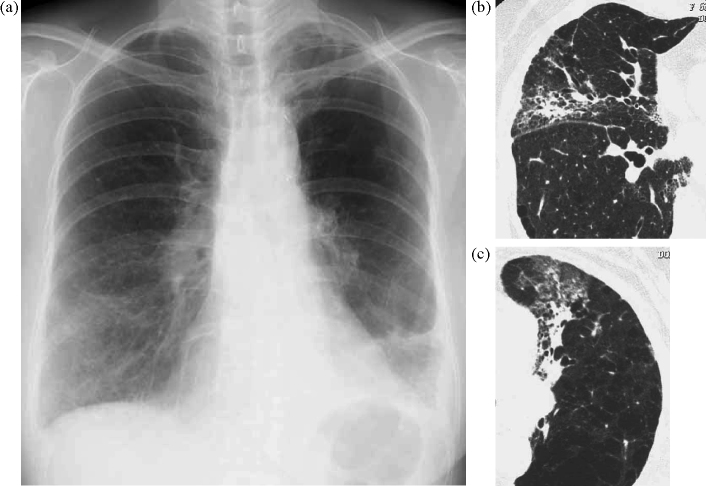
On admission, her pulse was 112/min and her respirations were 28/min. Fine crackles were heard in bilateral lung fields. Laboratory results showed that serum lactate dehydrogenase (432 IU/l) and C-reactive protein (1.65 mg/dl) were elevated. Tumor marker levels were elevated, including CA19-9 (12,470 U/ml) and carcinoembryonic antigen (62.9 mg/dl). Serum KL-6 was also elevated at 670 U/ml. Arterial blood gas values obtained upon breathing room air were: pH 7.46, PaO2 = 56.7 mmHg and PaCO2 = 26.2 mmHg. An electrocardiogram revealed sinus tachycardia (112/min). Chest radiography revealed ground glass shadows in the right lower and left middle lung fields (Fig. 1a). Chest CT films showed reticular shadows in the middle and left upper lobes (Fig. 1b and c).
Figure 1.
(a) Chest radiography on admission showed infiltration shadows in right lower lung field and left middle lung field. (b and c) The chest CT revealed reticular shadows in middle lobe and left upper lobes.
On suspicion of tegafururacil induced pneumonitis, this drug was stopped, and administration of 25 mg of prednisolone was started. However, her symptoms did not improve. Six weeks after admission, her dyspnea was very much worse. Although chest CT showed no evidence of deterioration of the pneumonitis, an electrocardiogram showed sinus tachycardia at a rate of 138/min with an S1Q3T3 pattern. Laboratory findings showed that the percent prothrombin time was slightly reduced (64.9%) and the activated partial thromboplastin time was within normal limits (23.5 s). d-Dimer and thrombin–antithrombin III complex were elevated at 2.7 μg/ml and 7.8 μg/l, respectively. Echocardiography revealed severe pulmonary hypertension (estimated pulmonary artery pressure: 54 mmHg) with a grossly dilated right ventricle. A 99mTc macroaggregate perfusion scan showed multiple peripheral perfusion defects (Fig. 2). Although pulmonary thromboembolism or a pulmonary tumor embolism was suspected, aggravation of her respiratory status prevented performing further examinations for diagnosis. Anti-coagulant therapy was started. However, her respiratory condition deteriorated, and she died 50 days after admission.
Figure 2.
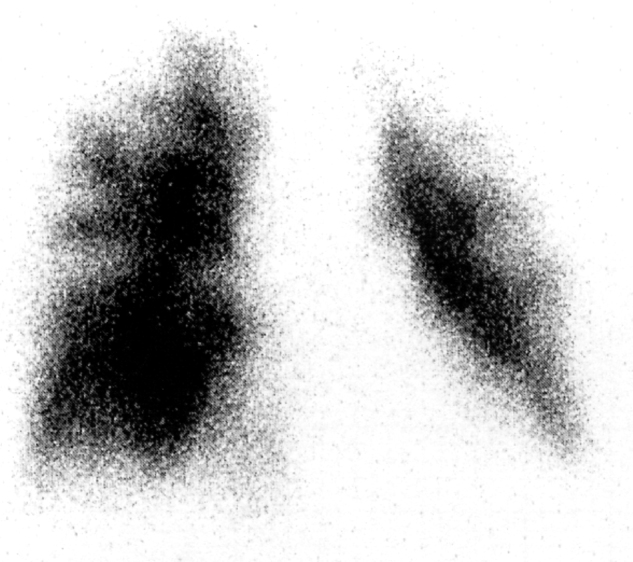
Lung perfusion study demonstrated multiple peripheral perfusion defects.
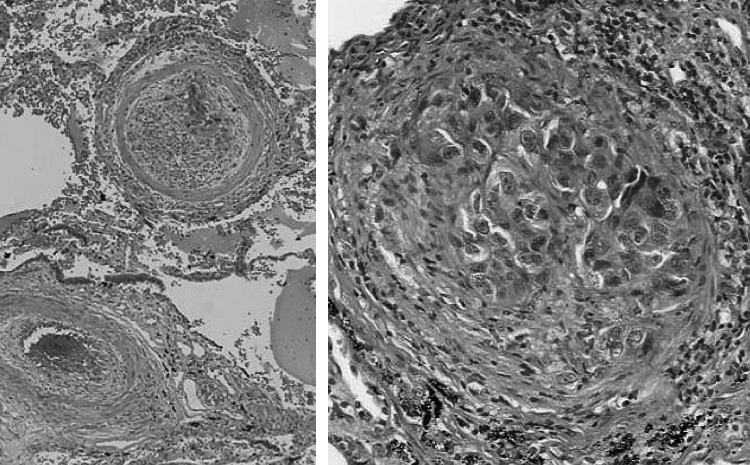
Postmortem macroscopic examinations showed no gross pulmonary emboli or cancer metastases in the pulmonary arteries and main bronchus. However, microscopic examination revealed PTTM that showed fibrocellular intimal embolization with a solid nest of papillary adenocarcinoma in the small arteries of the lungs (Fig. 3). Metastases were observed in the right pulmo-hilar, left cervical, right subclavian and para-lumbar aortic lymph nodes. Interstitial pneumonitis was seen in the middle and left upper lobes as shown on chest CT. We concluded that this was a case of PTTM with lung cancer and drug induced pneumonitis.
Figure 3.
Lung tissue at autopsy revealed pulmonary tumor thrombotic microangiopathy that showed fibrocellular intimal embolization with a solid nest of papillary adenocarcinoma (H.E. stain).
Discussion
We have described a case of PTTM associated with lung cancer. Autopsy revealed diffuse fibrocellular intimal proliferation and tumor emboli in small pulmonary arteries.
PTTM is a rare finding in patients with pulmonary metastatic carcinomas [1]. PTTM has been observed in 3.3% of autopsy cases with malignancy and is frequently diagnosed postmortem [1]. The most common type of neoplasm associated with PTTM is adenocarcinoma, as a large number of gastric cancers are included [1]. Furthermore, histological findings revealed adenocarcinoma cells in all reported cases of PTTM associated with lung cancer [1], [3]. Our case was also diagnosed as PTTM associated with pulmonary adenocarcinoma by autopsy.
It is very difficult to diagnose pulmonary tumor embolism, including PTTM, in patients with known cancers who develop acute or subacute cor pulmonale, because pulmonary tumor embolism (including PTTM) and pulmonary thromboembolism have similar manifestations [2], [4]. The major symptoms of both diseases are a progressive cough and shortness of breath. Although it has been reported that there is a higher incidence of cough in patients with pulmonary tumor embolism than in patients with pulmonary thromboembolism (47% versus 14%; p = 0.02) [4], the diagnostic utility of this symptom is low. Furthermore, laboratory findings, electrocardiography and echocardiography also show similar results for both pulmonary tumor embolism and pulmonary thromboembolism. Our case also could not be distinguished from pulmonary thromboembolism, because the symptoms and results of several examinations closely resembled those of thromboembolism. Lung biopsy might be useful for diagnosing pulmonary tumor embolism and PTTM [3], [5], [6]. However, deterioration of respiratory status often prevents performing a biopsy. In fact, a biopsy could not be performed for our case because her respiratory condition was rapidly deteriorating. Therefore, less invasive, more convenient examinations should be used.
A ventilation-perfusion scan might be a more useful tool for the diagnosis of PTTM than other examinations. It was reported that a perfusion scan typically shows numerous symmetric and peripheral defects (termed the “segmental contour pattern”) in pulmonary tumor embolism, including PTTM, whereas pulmonary thromboembolism usually shows one or more, larger and more centrally located, segmental perfusion defects [3], [6], [7], [8]. A ventilation-perfusion scan of our case showed the segmental contour pattern, which was consistent with PTTM.
Pulmonary microvascular cytological examination using a Swan-Ganz catheter might also be useful to diagnose PTTM. Masson et al. reported that pulmonary microvascular cytology was a less invasive and more convenient tool compared to transbronchial biopsy [9]. The sensitivity and specificity of this technique were reported to be 80–88% and 82–94%, respectively, in patients with lymphangitic carcinomatosis and pulmonary microvascular tumor embolization [9], [10]. In addition, this examination was useful for disease diagnosis in a patient with PTTM who suffered from progressive dyspnea and pulmonary hypertension [8].
Chemotherapy might be the only useful treatment for PTTM, although it has not been established. To our knowledge, there have been only two cases that were successfully treated [3], [6]. One patient was treated with chemotherapy only [3]. The other was treated with corticosteroids, anti-coagulants and an anti-cancer drug [6]. However, our case showed rapid deterioration in spite of corticosteroid and anti-coagulant administration. Anti-coagulants and corticosteroids might be ineffective. Further study is needed to assess the therapeutic strategy for PTTM.
In conclusion, we report a case of PTTM associated with lung cancer. PTTM should also be considered in cancer patients who show progressive respiratory failure and pulmonary hypertension.
Contributor Information
Seigo Miyoshi, Email: seigom@m.ehime-u.ac.jp.
Hironobu Hamada, Email: hhamada@m.ehime-u.ac.jp.
Hitoshi Katayama, Email: hkatayam@uwajima-mh.go.jp.
Naohiko Hamaguchi, Email: n-h-guri@m.ehime-u.ac.jp.
Toru Kadowaki, Email: toruyan@matsue.hosp.go.jp.
Ryoji Ito, Email: ryito@m.ehime-u.ac.jp.
Kazunori Irifune, Email: kirifune@m.ehime-u.ac.jp.
Tatsuhiko Miyazaki, Email: miyazaki@m.ehime-u.ac.jp.
Jitsuo Higaki, Email: jhigaki@m.ehime-u.ac.jp.
References
- 1.von Herbay A., Illes A., Waldherr R., Otto H.F. Pulmonary tumor thrombotic microangiopathy with pulmonary hypertension. Cancer. 1990;66:587–592. doi: 10.1002/1097-0142(19900801)66:3<587::aid-cncr2820660330>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 2.Seppala N., Cala A., Klebe S. Unusual presentation of pulmonary tumor thrombotic microangiopathy with no detectable primary tumor. J Postgrad Med. 2009;55:38–40. doi: 10.4103/0022-3859.48439. [DOI] [PubMed] [Google Scholar]
- 3.Goldhaber S.Z., Dricker E., Buring J.E., Eberlein K., Godleski J.J., Mayer R.J., Hennekens C.H. Clinical suspicion of autopsy-proven thrombotic and tumor pulmonary embolism in cancer patients. Am Heart J. 1987;114:1432–1435. doi: 10.1016/0002-8703(87)90548-5. [DOI] [PubMed] [Google Scholar]
- 4.Kang C.H., Choi J.A., Kim H.R., Oh Y.H., Kim H.K., Kang E.Y. Lung metastases manifesting as pulmonary infarction by mucin and tumor embolization: radiographic, high-resolution CT, and pathologic findings. J Comput Assist Tomogr. 1999;23:644–646. doi: 10.1097/00004728-199907000-00029. [DOI] [PubMed] [Google Scholar]
- 5.Uruga H., Morokawa N., Enomoto T., Takaya H., Miyamoto A., Kishi K., Kurosaki A., Fujii T., Yoshimura K. A case of pulmonary tumor thrombotic microangiopathy associated with lung adenocarcinoma diagnosed by CT-guided lung biopsy. Nihon Kokyuki Gakkai Zasshi. 2008;46:928–933. [PubMed] [Google Scholar]
- 6.Miyano S., Izumi S., Takeda Y., Tokuhara M., Mochizuki M., Matsubara O., Kuwata H., Kobayashi N., Kudo K. Pulmonary tumor thrombotic microangiopathy. J Clin Oncol. 2007;25:597–599. doi: 10.1200/JCO.2006.09.0670. [DOI] [PubMed] [Google Scholar]
- 7.Chen W.L., Cherng S.C., Hwang W.S., Wang D.J., Wei J. Perfusion scan in pulmonary tumor microembolism: report of a case. J Formos Med Assoc. 1991;90:863–866. [PubMed] [Google Scholar]
- 8.Ota K., Matsuyama M., Kokuho N., Masuko H., Hayashi H., Iizuka T., Hayashibara K., Saito T., Kawabata Y. An autopsy case of pulmonary tumor thrombotic microangiopathy complicated with interstitial pneumonia and lipoid pneumonia. Nihon Kokyuki Gakkai Zasshi. 2009;47:518–523. [PubMed] [Google Scholar]
- 9.Masson R.G., Krikorian J., Lukl P., Evans G.L., McGrath J.M. Pulmonary microvascular cytology in the diagnosis of lymphangitic carcinomatosis. NEJM. 1989;321:71–76. doi: 10.1056/NEJM198907133210202. [DOI] [PubMed] [Google Scholar]
- 10.Abati A., Landucci D., Danner R.L., Solomon D. Diagnosis of pulmonary microvascular metastasis by cytologic evaluation of pulmonary artery catheter-derived blood specimens. Hum Pathol. 1994;25:257–262. doi: 10.1016/0046-8177(94)90197-x. [DOI] [PubMed] [Google Scholar]