Summary
A 45-year-old woman complaining of consciousness disturbance demonstrated multiple brain infarctions. Echocardiogram showed vegetation on the posterior mitral leaflet. Infectious endocarditis was initially suspected and we started empirical antibiotics. However, mitral vegetation grew rapidly and caused severe mitral regurgitation. Acute heart failure was so poorly controlled by conservative treatment that we concluded cardiac surgery was indicated. Mitral valve replacement was safely performed, and there was no sign of heart failure or recurrent thromboembolism during the postoperative course. Thereafter, multiple hepatic masses and a solid lesion in the pancreatic head were detected by computed tomography. The patient finally died of multiple organ failure that presumably resulted from malignancy in the terminal stage. The clinical course of this case can be explained by the pathology of nonbacterial thrombotic endocarditis (NBTE). The standard treatment for NBTE consists of systemic anticoagulation as well as controlling the underlying malignancy. However, we could not diagnose this case as NBTE before surgery. Although mitral valve replacement was finally effective to control acute heart failure in this case, NBTE should be exactly diagnosed as quickly as possible and the treatment policy should be deliberated.
Keywords: Vegetation, Mitral valve, Heart failure, Surgery, Nonbacterial thrombotic endocarditis
Introduction
Nonbacterial thrombotic endocarditis (NBTE) was first described by Ziegler in 1888 and further described by Libman who termed it a “terminal type” valve disease or marantic endocarditis. The pathologic event was thought to be the deposition of fibrin on healthy or superficially degenerating heart valves. Neoplastic complications, mostly seen in adenocarcinoma of the lung [1] and pancreas [2], but also in other types of cancer [3], and associated hypercoagulability are thought to cause NBTE, therefore fulfilling the criteria for Trousseau syndrome. Diagnosis of NBTE requires a high degree of clinical suspicion as well as a careful examination of echocardiography to detect the presence of valvular thrombi [4]. When NBTE is diagnosed, treatment consists of systemic anticoagulation as well as controlling the underlying malignancy. The consensus about the indication for surgical treatment of NBTE has not been established yet. However, patients with large mobile vegetations, valvular dysfunction, or recurrent embolic events despite anticoagulation are in general considered eligible for surgery.
Case report
A 45-year-old woman was admitted to our hospital complaining of aphasia, apathy, and decreased attentiveness. She had history of breast cancer and depressive psychosis. Her blood pressure was 130/72 mmHg, the pulse rate was 79/min with regular rhythm, and the body temperature was 36.1 °C. Chest radiogram and electrocardiogram were within normal limits. There were no abnormal findings in brain computed tomography (CT) at admission. Laboratory studies showed moderate inflammatory signs, increased value of liver enzymes, and abnormality in the clotting system (Table 1). Progressive consciousness disturbance in a middle-aged woman with few risk factors obliged us to start antibiotics and antiviral drug infusion on the suspicion of encephalitis or myelitis. Blood cultures performed consecutively three times each showed negative findings. Cerebrospinal fluid did not show any sign of infection either. On day 3 of hospitalization, ultrasound cardiography (UCG) showed tiny vegetation on the posterior mitral leaflet with mild mitral valve regurgitation. Based on these findings, we diagnosed infective endocarditis (IE) and changed antibiotics to empirical therapy for IE as described in the guidelines. At the same time, brain CT showed the emergence of multiple low-density areas of various sizes (Fig. 1). During the next 2 days, holosystolic cardiac murmur at the apex became audible and the patient developed acutely progressive respiratory failure (Fig. 2B). UCG demonstrated that the size of frail vegetation on the mitral valve had increased and caused severely exacerbated mitral regurgitation (Fig. 3). Indices suggestive of volume overload in the left atrium and right-sided heart were confirmed. Staff in both neurology and cardiology concurred on the urgent need for surgery and the patient underwent open heart surgery for mitral valve replacement. During surgery, the mitral valve leaflets were found to be entirely normal, though attached thrombi were shown on the atrial surface. Histopathologic examination of the valve specimen demonstrated slight signs of inflammatory reaction and there were no findings indicating bacteriologic colony formation. Bacterial culture of the surgical specimen also showed negative findings. The postoperative course was uneventful with no sign of heart failure or recurrent thromboembolism. Abdominal CT demonstrated multiple hepatic low-density areas and a 3.2 cm × 2.1 cm solid lesion in the pancreatic head (Fig. 4). Findings of carcinomatous peritonitis were also demonstrated. Multiple organ failure probably caused by malignancy in the terminal stage progressed gradually and she died 30 days after cardiac surgery.
Table 1.
Laboratory data on admission.
| Na | 134 mmol/dl | WBC | 17,160/μl |
| K | 4.7 mmol/dl | RBC | 441 × 104/μl |
| Cl | 97 mmol/dl | Hgb | 12.2 g/dl |
| BUN | 17.8 mg/dl | Hct | 36.4% |
| Cre | 0.63 mg/dl | PLT | 19.2 × 104/μl |
| Amy | 25 IU/l | BNP | 172 pg/ml |
| CPK | 137 IU/l | ||
| AST | 9 IU/l | PT | 65% |
| ALT | 59 IU/l | APTT | 25.6 s |
| LDH | 829 IU/l | Fibrinogen | 161 mg/dl |
| ALP | 660 IU/l | FDP | 392.7 μg/ml |
| T-Bil | 0.7 mg/dl | D-dimer | 22.33 μg/ml |
| CRP | 13.4 mg/dl | TAT | 48.2 ng/ml |
| BS | 129 mg/dl | AT-3 | 92% |
Amy, amylase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; ALT, alanine aminotransferase; APTT, activated partial thromboplastin time; AT-3, antithrombin-3; BNP, brain natriuretic peptide; BS, blood sugar; BUN, blood urea nitrogen; CPK, creatine phosphokinase; Cre, serum creatinine; CRP, C-reactive protein; FDP, fibrin/fibrinogen degradation products; Hct, hematocrit; Hgb, hemoglobin; LDH, lactate dehydrogenase; PLT, platelet count; PT, prothrombin time; RBC, red blood cell count; TAT, thrombin-antithrombin III complex; T-Bil, total bilirubin; WBC, white blood cell count.
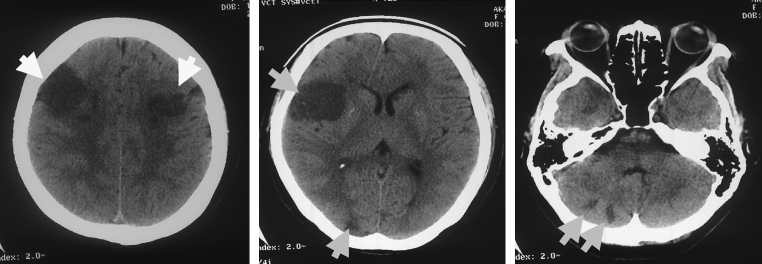
Figure 1.
Brain computed tomography demonstrated multiple disseminated low-density areas of various sizes on day 5 of hospitalization.
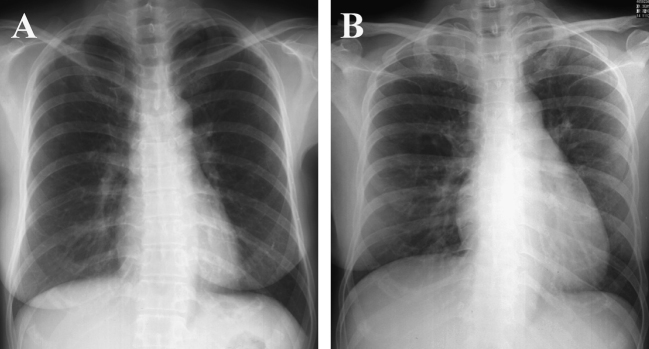
Figure 2.
Transition of images on chest radiogram. Normal image on admission (A) turned out to show cardiomegaly on day 5 of hospitalization (B). Pulmonary congestion and pleural effusion were not apparent.
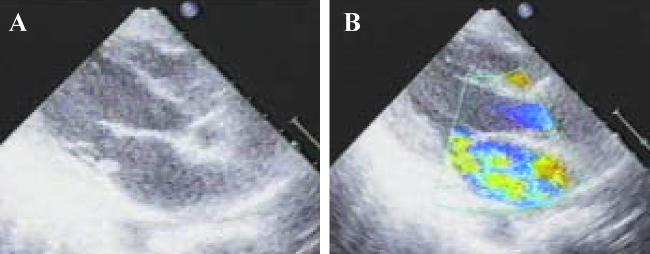
Figure 3.
Echocardiogram on day 5 of hospitalization demonstrated high echoic frail vegetation on posterior mitral leaflet (A). Severe mitral regurgitation was also observed (B), and resulted in volume overload in the left atrium and right-sided heart.
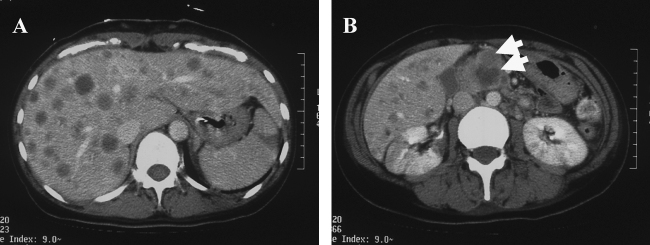
Figure 4.
Abdominal computed tomography showed multiple low-density areas in the liver (A), and a slightly enhanced mass lesion in the head of pancreas (B).
Discussion
This report describes a case of NBTE in which emergent mitral valve replacement was effective for controlling severe heart failure and preventing recurrent embolic events. The standard treatment for NBTE consists of systemic anticoagulation to improve symptoms and prevent recurrent thromboembolism as well as control of the underlying malignancy. However, we experienced difficulties in distinguishing NBTE from IE because findings typical of IE such as fever and positive blood cultures are not always present in IE like our case. Moreover, we had not detected the underlying malignancy until surgical treatment became indispensable to overcome the situation. Accordingly, we could not attempt systemic anticoagulation before surgery.
The indication and appropriate timing for cardiac surgery in NBTE have not been formally defined. Rabinstein et al. reported that the presence of large vegetations and the progression to severe valvular dysfunction as well as the occurrence of recurrent embolism despite optimal anticoagulation require surgical excision of vegetation with or without valve replacement [5]. Although systemic anticoagulation had not been performed, the timing of cardiac surgery was considered optimal in our case because of the severity of heart failure. Surgical treatment was effective in relieving the critical conditions. However, our patient with a poor overall prognosis had to undergo open heart surgery. Was this good or bad for the patient and her family?
In order to improve the situation, an accurate differential diagnosis between NBTE and IE as soon as possible is very important. Some reports have recommended transesophageal echocardiography (TEE) if NBTE is suspected [6]. Valvular vegetations along coaptation lines without destruction of valvular tissue are suggestive of NBTE. Presence of both left- and right-sided vegetations is also more consistent with NBTE than with IE [7]. It is reported that valve dysfunction due to NBTE is rare unless the affected valve has previous damage such as old rheumatic changes or degenerative alterations. In our case, however, we speculate that mobile vegetations along the closure line of leaflet increased in size and extensiveness by continuous fibrin accumulation and created valve dysfunction. Empirical antibiotics therapy and mitral valve surgery were not effective for improving the inflammatory markers, which is also suggestive that the pathology could not have resulted from IE. Increased inflammatory markers in our case are thought to be derived from neoplasm. Cerebrovascular disease as the first manifestation of cancer as in our case is a rare clinical entity. Outcome is very poor and correlates with both the severity of neurologic disability and the stage of tumor [8]. Characteristic stroke patterns in NBTE and IE were demonstrated via magnetic resonance imaging (MRI). IE-associated strokes show a variety of MRI patterns, including single cortical stroke, punctate lesions, and disseminated patterns, whereas NBTE strokes frequently have multiple disseminated lesions of various sizes [9].
Edoute at el. reported that up to 19% of non-selected patients with solid tumors had cardiac valvular vegetations [10]. Many factors are known to contribute to a thrombophilic state in cancer patients [11]. The occurrence of venous thrombosis in malignant tumors has been well documented. However, acute arterial thromboembolism in cancer patients is less often recognized. Schattner et al. reported that only 2 of 311 autopsy cases of pancreatic adenocarcinoma demonstrated evidence of arterial thromboembolism [12]. This pathological condition seems more often to be a terminal or pre-terminal stage as demonstrated in our case. Although surgical treatment in our case was effective in terms of controlling severe heart failure, cardiac surgery for NBTE should be fundamentally considered in patients with potentially curable cancer. This case demonstrates that NBTE should be accurately diagnosed as rapidly as possible and the treatment policy should be considered carefully.
References
- 1.Martin-Martorell P., Insa-Molla A., Chirivella-Gonzalez M.I., Cervera-Miguel J.I. Nonbacterial thrombotic endocarditis associated with lung adenocarcinoma. Clin Transl Oncol. 2007;9:744–746. doi: 10.1007/s12094-007-0133-1. [DOI] [PubMed] [Google Scholar]
- 2.Chen L., Li Y., Gebre W., Lin J.H. Myocardial and cerebral infarction due to nonbacterial thrombotic endocarditis as an initial presentation of pancreatic adenocarcinoma. Arch Pathol Lab Med. 2004;128:1307–1308. doi: 10.5858/2004-128-1307-MACIDT. [DOI] [PubMed] [Google Scholar]
- 3.Singh V., Bhat I., Havlin K. Marantic endocarditis (NBTE) with systemic emboli and paraneoplastic cerebellar degeneration: uncommon presentation of ovarian cancer. J Neurooncol. 2007;83:81–83. doi: 10.1007/s11060-006-9306-y. [DOI] [PubMed] [Google Scholar]
- 4.el-Shami K., Griffiths E., Streiff M. Nonbacterial thrombotic endocarditis in cancer patients: pathogenesis, diagnosis, and treatment. Oncologist. 2007;12:518–523. doi: 10.1634/theoncologist.12-5-518. [DOI] [PubMed] [Google Scholar]
- 5.Rabinstein A.A., Giovanelli C., Romano J.G., Koch S., Forteza A.M., Ricci M. Surgical treatment of nonbacterial thrombotic endocarditis presenting with stroke. J Neurol. 2005;252:352–355. doi: 10.1007/s00415-005-0660-z. [DOI] [PubMed] [Google Scholar]
- 6.Dutta T., Karas M.G., Segal A.Z., Kizer J.R. Yield of transesophageal echocardiography for nonbacterial thrombotic endocarditis and other cardiac sources of embolism in cancer patients with cerebral ischemia. Am J Cardiol. 2006;97:894–898. doi: 10.1016/j.amjcard.2005.09.140. [DOI] [PubMed] [Google Scholar]
- 7.Aryana A., Esterbrooks D.J., Morris P.C. Nonbacterial thrombotic endocarditis with recurrent embolic events as manifestation of ovarian neoplasm. J Gen Intern Med. 2006;21:C12–C15. doi: 10.1111/j.1525-1497.2006.00614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taccone F.S., Jeangette S.M., Blecic S.A. First-ever stroke as initial presentation of systemic cancer. J Stroke Cerebrovasc Dis. 2008;17:169–174. doi: 10.1016/j.jstrokecerebrovasdis.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 9.Singhal A.B., Topcuoglu M.A., Buonanno F.S. Acute ischemic stroke patterns in infective and nonbacterial thrombotic endocarditis: a diffusion-weighted magnetic resonance imaging study. Stroke. 2002;33:1267–1273. doi: 10.1161/01.str.0000015029.91577.36. [DOI] [PubMed] [Google Scholar]
- 10.Edoute Y., Haim N., Rinkevich D., Brenner B., Reisner S.A. Cardiac valvular vegetations in cancer patients: a prospective echocardiographic study of 200 patients. Am J Med. 1997;102:252–258. doi: 10.1016/S0002-9343(96)00457-3. [DOI] [PubMed] [Google Scholar]
- 11.Bick R.L. Cancer-associated thrombosis. N Engl J Med. 2003;349:109–111. doi: 10.1056/NEJMp030086. [DOI] [PubMed] [Google Scholar]
- 12.Schattner A., Klepfish A., Huszar M., Shani A. Two patients with arterial thromboembolism among 311 patients with adenocarcinoma of the pancreas. Am J Med Sci. 2002;324:335–338. doi: 10.1097/00000441-200212000-00009. [DOI] [PubMed] [Google Scholar]