Summary
Echocardiographic examination of patients with granulomatous endocarditis in patients with Wegener's granulomatosis (WG) reveals vegetation-like lesions that may be misdiagnosed as infective endocarditis resulting in inappropriate therapy. Three-dimensional transesophageal echocardiography aids differential diagnosis. Here, we report the case of a WG patient with associated mitral and aortic granulomatous endocarditis. Although the patient was treated with prednisolone and cyclophosphamide, serial echocardiography did not reveal any significant changes in disease course.
Keywords: Mitral valve, Endocarditis, Wegener's granulomatosis
Introduction
We present a patient with Wegener's granulomatosis exhibiting a vegetation-like mass on the mitral valve. The misdiagnosis of valvular lesions would have resulted in inappropriate surgical intervention.
Case report
A 67-year-old man presented with a 2-month history of fever, malaise, and appetite loss. He also had long histories of hypertension, hyperuricemia, and sinus problems, with the latter including several bleeding episodes from the right nostril. On admission, his blood pressure, pulse, and body temperature were 169/97 mmHg, 96 beats/min, and 37.7 °C, respectively. There were many raised purpura on the anterior aspects of both legs. Auscultation revealed a grade II/VI systolic murmur at the apex. Neurologic and abdominal findings from his examinations were unremarkable. Initial laboratory data were as follows: leukocyte count, 3.6 × 103/mm3; hemoglobin, 9.7 g/dl; hematocrit, 28.2%; platelets, 7.4 × 103/mm3; blood urea nitrogen (BUN), 60.5 mg/dl; creatinine, 2.7 mg/dl; total protein, 7.3 mg/dl; albumin, 2.8 mg/dl; C-reactive protein, 2.0 mg/dl; C3, 32.3 mg/dl (normal: 80–160); and C4, 6.4 mg/dl (normal: 10–40). The patient was positive for rheumatoid factor and negative for anti-nuclear antibody. His serum tested strongly positive for anti-neutrophil cytoplasmic antibodies (ANCA) directed against PR3 (PR3-ANCA) with a titer of 104 U/ml (normal < 10 EU). Urinalysis revealed 1–4 white blood cells/high-power field, hematuria (>100 red blood cells/high power field) and proteinuria. Erythrocyte sedimentation rate was 90 mm/h and serial blood cultures were negative.
Chest X-ray revealed blunting of the costophrenic angle bilaterally. Chest examination by computed tomography (CT) demonstrated consolidation and ground-glass opacity in the lower lobes bilaterally and bronchial wall thickening in the right lower lobe (Fig. 1a). Body CT revealed pneumatosis cystoides intestinalis although he had no abdominal symptoms (Fig. 1b). A transthoracic echocardiogram revealed a vegetation-like lesion, 5 mm × 8 mm, on the posterior mitral leaflet with severe mitral regurgitation with preserved left ventricular function (ejection fraction 74%, fraction shortening 44%) and structure (end-diastolic/end-systolic dimension 55 mm/33 mm). A three-dimensional transesophageal echocardiogram revealed thickened P2 scallop of the mitral valve with torn chordae (Fig. 2) and a non-homogenous rigid mass, 2 mm × 2 mm in size, on the right coronary cusp of the aortic valve. Renal biopsy showed membranous glomerulonephritis with lymphocytic infiltration.
Figure 1.
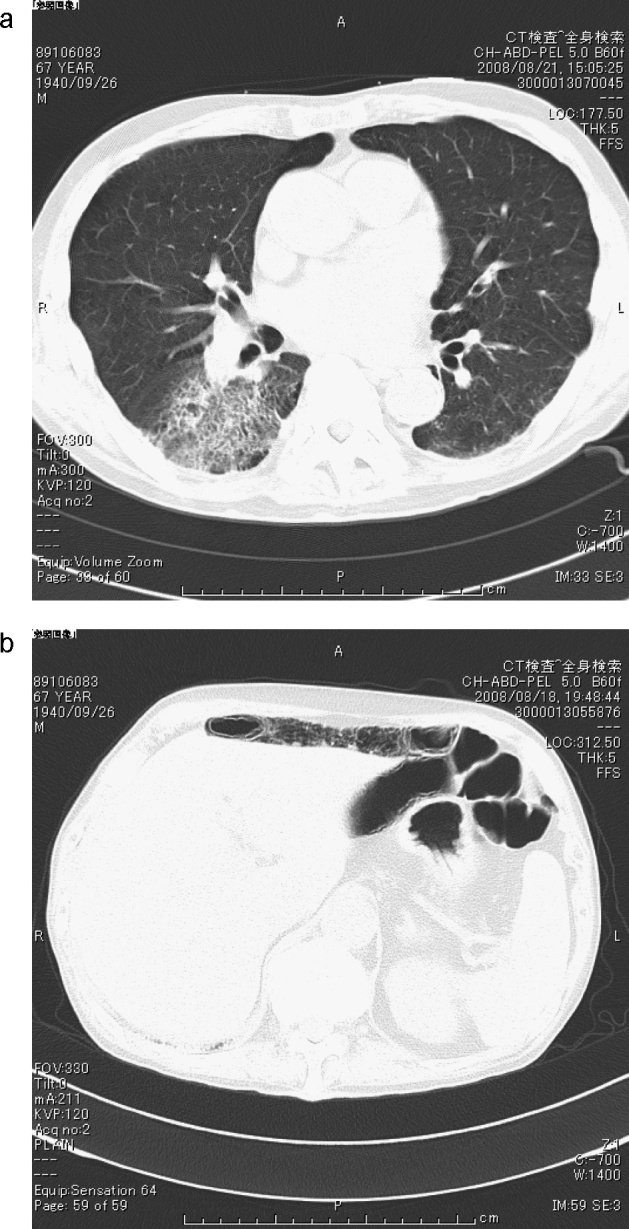
(a) Computed tomography (CT) images showing consolidation and ground-glass opacity on the right lower lobe. (b) Body-CT demonstrated pneumatosis cystoides intestinalis.
Figure 2.
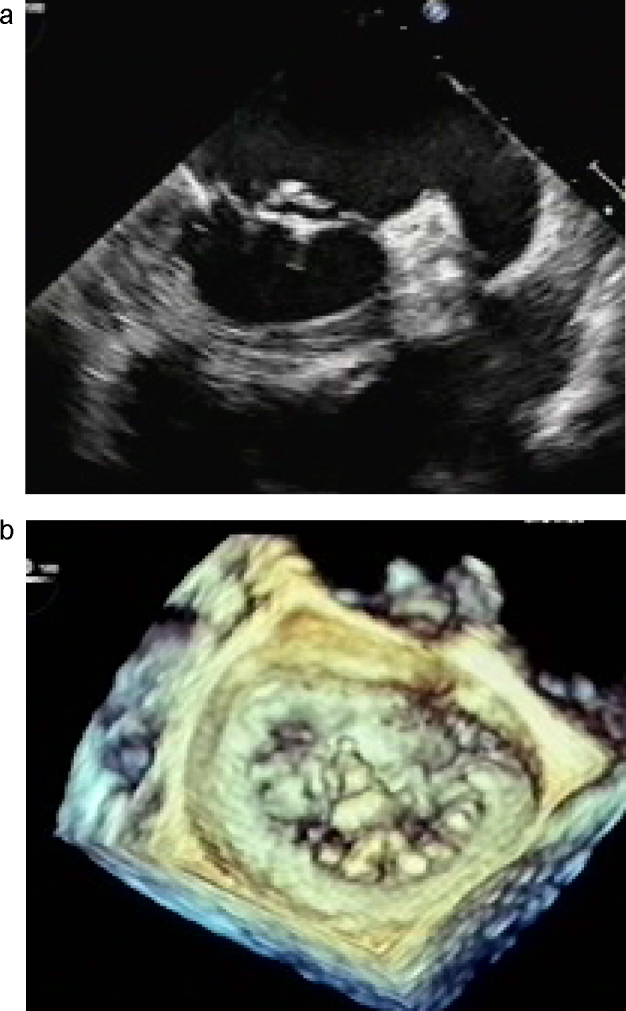
2-Dimensional (a) and 3-dimensional (b) transesophageal echocardiogram of the mitral valve. 3-Dimensional echocardiographic findings clearly show localized thickening of the P2 scallop with torn chordae.
Following our diagnosis of WG, he received an intravenous pulse dose of 400 mg cyclophosphamide and was started on a regimen of prednisolone, 40 mg/day. Seventeen days after cyclophosphamide pulse therapy, his BUN and creatinine levels increased, respectively, up to 144.0 mg/dl and 6.3 mg/dl. As a result, hemodialysis was initiated three times weekly. The patient's condition gradually improved and his BUN and creatinine levels decreased again to 65.6 mg/dl and 2.9 mg/dl, respectively, and he was weaned from hemodialysis. His chest CT improved with regard to the consolidation and ground-glass opacity in the lower lobes bilaterally. Anti-PR3-ANCA titer fell to 47 EU, and C3 and C4 levels increased to 87.1 mg/dl and 24.8 mg/dl, respectively. The dose of prednisolone was reduced gradually, and he was given a regimen of prednisolone (20 mg/day) at discharge, 78 days after the original cyclophosphamide treatment.
Serial transthoracic echocardiography performed at 2-month intervals at an outpatient care facility revealed no evidence of any progression of the mitral valve lesion or changes in left ventricular function for 15 months. He suddenly developed gastrointestinal bleeding 17 months after discharge and died before endoscopic examination could be performed. His family did not allow an autopsy.
Discussion
There have been several reports of cardiac involvement in patients with WG [1], but only a few have described mitral valve disease [2], [3], [4]. Granulomatous valvular lesions have been frequently reported in histopathological studies of WG. Herbst et al. performed surgery to replace the mitral valve of a patient suffering from a large mass (10 mm) on the anterior mitral leaflet, masquerading as left atrial myxoma with moderate mitral regurgitation [2]. The patient's condition deteriorated post-operatively, and histopathological examination revealed an intense inflammatory infiltrate with aggregates of neutrophils and eosinophils, leading to the diagnosis of WG. Koyalakonda et al. reported a case in which the patient underwent mitral and aortic valve replacement (types of prostheses were not mentioned) due to progressive granuloma of the anterior mitral leaflet and a non-stenotic aortic valve with moderate aortic regurgitation [3]. The anterior mitral leaflet showed acute and chronic inflammatory changes with myxoid and fibrinous degeneration and perivascular eosinophilic infiltration consistent with WG. Attaran et al. described a WG patient with an extensive mitral mass resulting in mild stenosis as well as moderate regurgitation; despite immunosuppressant and steroid treatment, the lesion increased in size and the patient underwent mitral valve replacement with a mechanical prosthesis [4].
Ruling out infective endocarditis is an important diagnostic differentiation in WG patients. Chirinos et al. reported a patient with ANCA-positive aortic valve endocarditis caused by Enterococcus faecalis in which the ANCA test became negative after aortic valve replacement (the histology of the mitral valve was not mentioned although previous histological reports were discussed) [5].
Even if serial blood cultures are negative, the possibility of infective endocarditis should always be considered in patients with persistent fever accompanied by inflammation and valvular heart disease. In the patient reported here, three-dimensional echocardiography demonstrated a thickened, curled, deformed P2 scallop with torn chordae rather than a thread-like, mobile, fragile, embolic mass; the form and size of the granulomatous lesion on P2 remained unchanged on serial transthoracic echocardiograms over a 15-month period. Because left ventricular function and structure did not deteriorate, mitral valve surgery was not recommended due to pulmonary and renal risks.
The mechanism of valvular changes in patients with WG is still unclear, but the histological findings suggest that granulomatous lesions are associated with autoimmune inflammation, responsible for myxoid degeneration on the leaflet and subvalvular apparatus. The ruptured chordae tendineae in the present case may have been caused by a decrease in the stretching threshold of the chordae due to deformation of the P2 scallop or inflammation of the chordae. Serial echocardiograms did not show any progression of the valvular lesion, and we speculate that medical treatment to control the disease prevented further deterioration.
Although the cause of death in this patient was not clear, one possibility is an association with pneumatosis cystoides intestinalis. This condition causes few abdominal complaints but can nevertheless lead to gastrointestinal bleeding, mostly caused by occult upper gastrointestinal lesions such as peptic erosions or ulcer [6].
In summary, vegetation-like lesions revealed by echocardiography characterize WG granulomatous endocarditis and its misdiagnosis as infective endocarditis can result in inappropriate therapy. In the present case, three-dimensional transesophageal echochardiography revealed inflammatory deformation of the mitral leaflet and helped inform the differential diagnosis. Medical treatment to control disease activity may be very helpful in preventing the progression of granulomatous endocarditis in WG patients.
References
- 1.Goodfield M.E.R., Bhanadari S., Plant W.D., Morley-Davies A., Sutherland G.R. Cardiac involvement in Wegener's granulomatosis. Br Heart J. 1995;73:110–115. doi: 10.1136/hrt.73.2.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herbst A., Padilla M.T., Prasad A.R., Morales M.C., Copeland J.G. Cardiac Wegener's granulomatosis masquerading as left atrial myxoma. Ann Thorac Surg. 2003;75:1321–1323. doi: 10.1016/s0003-4975(02)04662-3. [DOI] [PubMed] [Google Scholar]
- 3.Koyalakonda S.P., Krishnan U., Hobbs W.J. A rare instance of multiple valvular lesions in a patient with Wegener's granulomatosis. Cardiology. 2010;117:28–30. doi: 10.1159/000319603. [DOI] [PubMed] [Google Scholar]
- 4.Attaran S., Desmond M., Ratnasingham J., Scawn N., Pullan D.M. Mitral valve involvement in Wegener's granulomatosis. Ann Thorac Surg. 2010;90:996–997. doi: 10.1016/j.athoracsur.2010.02.103. [DOI] [PubMed] [Google Scholar]
- 5.Chirinos J.A., Corrales-Medina V.F., Garcia S., Lichtstein D.M., Bisno A.L., Chakko S. Endocarditis associated with antineutrophil cytoplasmic antibodies: a case report and review of the literature. Clin Rheumatol. 2007;26:590–595. doi: 10.1007/s10067-005-0176-z. [DOI] [PubMed] [Google Scholar]
- 6.Gabel A., Mueller S., Haentsche K., Daeuwel N. Pneumatosis cystoides intestinalis: an unexpected finding in intestinal bleeding under therapy with phenprocroumon. Digestion. 2000;61:215–218. doi: 10.1159/000007760. [DOI] [PubMed] [Google Scholar]


