Summary
A 65-year-old male, who had been diagnosed to have myasthenia gravis (MG) 25 years previously, was admitted to our hospital with faintness. Cardiac ultrasonography showed decreased left ventricular function. Magnetic resonance imaging depicted delayed contrast enhancement in localized regions. No significant coronary artery stenosis was found, and due to the reproducible susceptibility for sustained ventricular tachycardia, he underwent cardioverter-defibrillator implantation. Although relatively uncommon, cardiac manifestations should not be overlooked in MG patients, as they may be associated with ventricular arrhythmias and cardiac dysfunction.
Keywords: Myasthenia gravis, Cardiac dysfunction, Delayed gadolinium-based contrast enhancement, Cardioverter-defibrillator implantation
Introduction
Myasthenia gravis (MG) is an autoimmune disease with a prevalence of 400 per million, and it is characterized by abnormal muscular fatigability caused by autoantibodies to the nicotinic acetylcholine receptor (AChR) in the motor end-plates of skeletal muscles [1]. Although it is known that MG primarily affects skeletal muscle, but not cardiac muscle, MG may be associated with various cardiac manifestations; these include myocarditis, pericarditis, and cardiac arrhythmias. In the current paper, we report a case with MG who presented with left ventricular dysfunction. We also discuss the possible underlying pathophysiological abnormality that potentially causes cardiac manifestations of this autoimmune disorder.
Case report
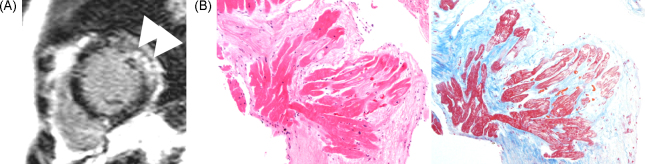
A Japanese male was diagnosed to have MG and underwent thymectomy at 40 years of age. Subsequently, he started corticosteroid therapy. At 65 years of age, he felt ill, and he was admitted to a nearby hospital. Non-sustained ventricular tachycardia was documented (data not shown). His left ventricular function was mildly reduced on cardiac ultrasonography. Magnetic resonance imaging (MRI) showed delayed gadolinium enhancement and local thinning of the posterior region of the left ventricle (Figure 1A). On the other hand, no abnormal finding was observed by gallium-67 scintigraphy (data not shown).
Figure 1.
Gadolinium-DTPA (diethylene triamine pentaacetic acid)-enhanced magnetic resonance imaging (MRI) and endomyocardial biopsy specimen. (A) Apical short-axis delayed enhanced MRI showed thinning of the apical posterior wall (arrowhead) and delayed enhancement in posterior region (arrows). (B) Hematoxylin-eosin staining and azan staining of an endomyocardial biopsy specimen. Subendocardial fibrosis and degeneration of cardiomyocytes are shown.
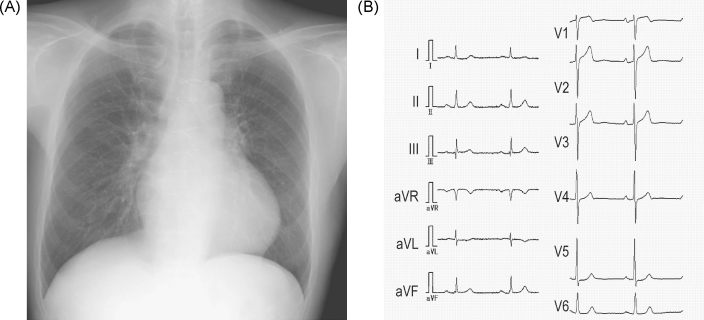
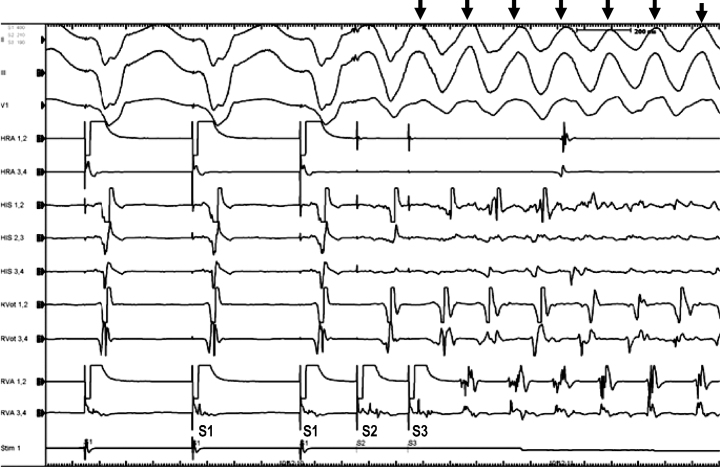
He was referred to our hospital for further evaluation. On admission, his blood pressure was 120/76 mmHg, and systolic heart murmurs were auscultated at the third left sternal border and apex. His chest radiograph showed cardio-thoracic ratio of 53.1%. Electrocardiogram at rest showed ST depression in I, aVL, and V5-6 (Figure 2). Bilateral ptosis and weakness of orbicularis oculi were observed. His alcohol consumption was estimated to be about 360 g per week. The following data were obtained: leukocyte count, 7600/μL; hemoglobin, 14.5 g/dL; and serum brain-type natriuretic peptide level, 107.7 pg/mL. Serum levels of blood urea nitrogen, creatinine, C-reactive protein, serum alpha-galactosidase activity, angiotensin-converting enzyme, and lysozyme were all within normal ranges. Serum tested positive for antibody against AChR, but negative for antibody against ryanodine receptor (RyR), as measured by a sandwich enzyme-linked immunoassay procedure. Coronary artery stenosis was absent on coronary angiography. An endomyocardial biopsy specimen in the posterior wall region of the left ventricle, where delayed contrast-enhancement was demonstrated by MRI, showed increased subendocardial fibrosis and degenerated cardiomyocytes; however epithelioid granulomatous regions were absent (Figure 1B). Left ventriculography showed an ejection fraction of 47%. Electrophysiological study showed reproducible provocation of a sustained ventricular tachycardia (Figure 3); therefore, the patient underwent implantation of a cardioverter-defibrillator.
Figure 2.
Chest radiograph and electrocardiogram on admission to our hospital. (A) Chest radiograph. (B) Electrocardiogram. There is a slight ST segment depression in leads I, aVL, and V5-6.
Figure 3.
Electrophysiology study. After a double extra stimulation from right ventricular apex, ventricular tachycardia was provoked (arrows). The interval between S1–S1, S1–S2, and S2–S3 is 400, 210, and 190 milliseconds. Il, lll, limb lead of body surface electrocardiogram; V1, chest lead of body surface electrocardiogram; HRA, high right atrium; HIS, His bundle electrocardiogram; RVOT, right ventricular outflow tract; RVA, right ventricular apex.
Discussion
We have described a case of an individual with MG, diagnosed 25 years previously, with impaired left ventricular function, delayed gadolinium enhancement MRI, and easily provoked ventricular tachycardia.
MG is an autoimmune disease caused by antibodies that react mainly with the AChR. This autoantibody is thought to have specific affinity for the AChR in skeletal muscle, but not in the heart muscle. Cardiac manifestations are, in general, considered to be relatively uncommon in MG patients; however, it has been reported that approximately 16% of MG patients have various cardiac manifestations, such as arrhythmias and heart failure [2]. There are several other papers reporting that histologic signs of myocarditis may be observed in between 20% and 60% of all MG cases at post-mortem examination [3]. It is also known that cardiac involvement can be found more frequently in MG patients with thymoma than those without thymoma. In fact, it has been reported that 50% of MG patients with thymoma have cardiac involvement without obvious etiology [2]. Autoimmune-mediated myocarditis is known to develop especially in patients with thymoma-associated MG, and it may underlie various cardiac manifestations. Antistriational autoantibodies include those against β1- and β2-adrenergic receptors, RyR, titin, and Kv1.4 [4], [5]. RyR antibodies were found to be negative in our patient, whether other autoantibodies are present is under investigation.
There are autoimmunological mechanisms considered to be participating in the cardiac involvement in MG. For example, RyR is a calcium-channel protein located in the sarcoplasmic reticulum membrane [1]. Although skeletal and cardiac muscle RyRs are antigenically different, the anti-RyR antibodies in MG patients may cross-react with cardiac and skeletal muscle RyR [1]. Similarly, certain IgG antibodies in MG patients may react with both β1- and β2-adrenergic receptors [4]. Anti-titin antibodies are reported to be associated with older-onset MG. Kv1.4 is a muscular voltage-gated potassium channel [5]. Clinical features associated with anti-Kv1.4 antibody-positive MG patients may include myocarditis, QT prolongation, and thymoma [6].
We are unable to specify the pathophysiological background of cardiac manifestations in our case; however, there are several possibilities. In our case, enhanced fibrosis in the cardiac biopsy was demonstrated; however, infiltration of immune cells was not apparent. It has been reported that various degrees of infiltration of inflammatory cells may be observed in MG positive for antistriational autoantibodies [5], indicating that autoimmune-mediated cardiomyositis cannot completely be ruled out in our case. As alcohol consumption of more than 90 g per day may cause cardiac dysfunction [7], alcohol-induced cardiomyopathy may be present in our patient, who had been consuming about 360 g of alcohol per week. Another possibility is cardiac sarcoidosis. The findings of delayed contrast-enhancement in MRI [8], localized thinning of the ventricular wall, impaired left ventricular function, and non-sustained ventricular tachycardia might also be due to cardiac sarcoidosis. Although non-caseating epithelioid granuloma was not demonstrated in our case, this possibility remains, because characteristic histological findings may be observed in fewer than 20% of cases on endomyocardial biopsy [9]. In addition, there is a case report showing the coexistence of sarcoidosis and MG, although whether this co-occurrence was just a coincidence was not clear. Furthermore, an MG case complicated with giant cell myocarditis after thymectomy in MG has been reported [10]. Giant cell myocarditis is a rare and fatal disorder characterized by rapidly progressive congestive heart failure, and it is sometimes associated with autoimmune disorders such as MG. Therefore, giant cell myocarditis should also be considered in the case of MG with progressive heart failure, although our patient was less likely to be this case. We could not find a paper reporting delayed gadolinium enhancement MRI in alcohol-induced or MG-associated cardiomyopathy. In order to investigate the mechanisms underlying observed cardiac manifestations in our case, we may need to examine MG-related antistriational autoantibodies, such as those against titin, β1- and β2-adrenergic receptors, and Kv1.4.
In summary, we have presented a case of a patient with MG who developed faintness and cardiac dysfunction. Cardiac ultrasonography revealed impaired cardiac function, and MRI showed delayed contrast-enhancement. Although relatively uncommon, cardiac manifestations should not be overlooked in MG patients, as they may be associated with ventricular arrhythmias and cardiac dysfunction.
References
- 1.Mygland A., Tysnes O.B., Matre R., Aarli J.A., Gilhus N.E. Anti-cardiac ryanodine receptor antibodies in thymoma-associated myasthenia gravis. Autoimmunity. 1994;17:327–331. doi: 10.3109/08916939409010673. [DOI] [PubMed] [Google Scholar]
- 2.Hofstad H., Ohm O.J., Mork S.J., Aarli J.A. Heart disease in myasthenia gravis. Acta Neurol Scand. 1984;70:176–184. doi: 10.1111/j.1600-0404.1984.tb00817.x. [DOI] [PubMed] [Google Scholar]
- 3.Mygland A., Aarli J.A., Hofstad H., Gilhus N.E. Heart muscle antibodies in myasthenia gravis. Autoimmunity. 1991;10:263–267. doi: 10.3109/08916939109001899. [DOI] [PubMed] [Google Scholar]
- 4.Xu B.Y., Pirskanen R., Lefvert A.K. Antibodies against beta1 and beta2 adrenergic receptors in myasthenia gravis. J Neuroimmunol. 1998;91:82–88. doi: 10.1016/s0165-5728(98)00159-3. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki S., Utsugisawa K., Yoshikawa H., Motomura M., Matsubara S., Yokoyama K., Nagane Y., Maruta T., Satoh T., Sato H., Kuwana M., Suzuki N. Autoimmune targets of heart and skeletal muscles in myasthenia gravis. Arch Neurol. 2009;66:1334–1338. doi: 10.1001/archneurol.2009.229. [DOI] [PubMed] [Google Scholar]
- 6.Suzuki S., Satoh T., Yasuoka H., Hamaguchi Y., Tanaka K., Kawakami Y., Suzuki N., Kuwana M. Novel autoantibodies to a voltage-gated potassium channel Kv1.4 in a severe form of myasthenia gravis. J Neuroimmunol. 2005;170:141–149. doi: 10.1016/j.jneuroim.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 7.Piano M.R. Alcoholic cardiomyopathy: incidence, clinical characteristics, and pathophysiology. Chest. 2002;121:1638–1650. doi: 10.1378/chest.121.5.1638. [DOI] [PubMed] [Google Scholar]
- 8.Niida T., Isoda K., Sasaki M., Horikawa M., Hayashi K., Ohsuzu F. Late gadolinium enhanced high resolution magnetic resonance imaging reveals pathophysiological condition of cardiac sarcoidosis. Int Heart J. 2009;50:263–266. doi: 10.1536/ihj.50.263. [DOI] [PubMed] [Google Scholar]
- 9.Uemura A., Morimoto S., Hiramitsu S., Kato Y., Ito T., Hishida H. Histologic diagnostic rate of cardiac sarcoidosis: evaluation of endomyocardial biopsies. Am Heart J. 1999;138:299–302. doi: 10.1016/s0002-8703(99)70115-8. [DOI] [PubMed] [Google Scholar]
- 10.Joudinaud T.M., Fadel E., Thomas-de-Montpreville V., Mussot S., Flecher E.M., Dartevelle P.G. Fatal giant cell myocarditis after thymoma resection in myasthenia gravis. J Thorac Cardiovasc Surg. 2006;131:494–495. doi: 10.1016/j.jtcvs.2005.09.035. [DOI] [PubMed] [Google Scholar]