Summary
An 84-year-old female patient with a past medical history significant for hypertension and diabetes mellitus, was admitted to the Emergency Department with acute coronary syndrome and complete atrioventricular block. She underwent a successful primary percutaneous coronary intervention. Ten minutes following tirofiban administration, the patient complained of hemoptysis and severe dyspnea. After chest X-ray and diagnostic bronchoscopy, she was diagnosed with diffuse alveolar hemorrhage. She died because of respiratory insufficiency on the third day of hospitalization. We present the first tirofiban-related diffuse alveolar hemorrhage case caused with half of the recommended dose of tirofiban used in the setting of non-ST-elevation myocardial infarction.
Keywords: Glycoprotein IIb/IIIa antagonist, Tirofiban, Acute coronary syndrome, Diffuse alveolar hemorrhage
Introduction
Platelet activation and aggregation play a key role in acute coronary syndromes (ACS). Glycoprotein IIb/IIIa (GP IIb/IIIa) receptor complex, which is found in large amounts on the activated platelets, forms fibrinogen bridges between platelets. This process constitutes the final common pathway in platelet aggregation regardless of stimulus type and is targeted by many antiplatelet regimens in cardiology. Now, GP IIb/IIIa antagonists are used frequently and effectively in ACS to inhibit platelet aggregation and thrombus formation. Although they increase minor hemorrhagic complications, we present an uncommon and lethal hemorrhagic complication of tirofiban in a patient who underwent primary percutaneous coronary intervention.
Case report
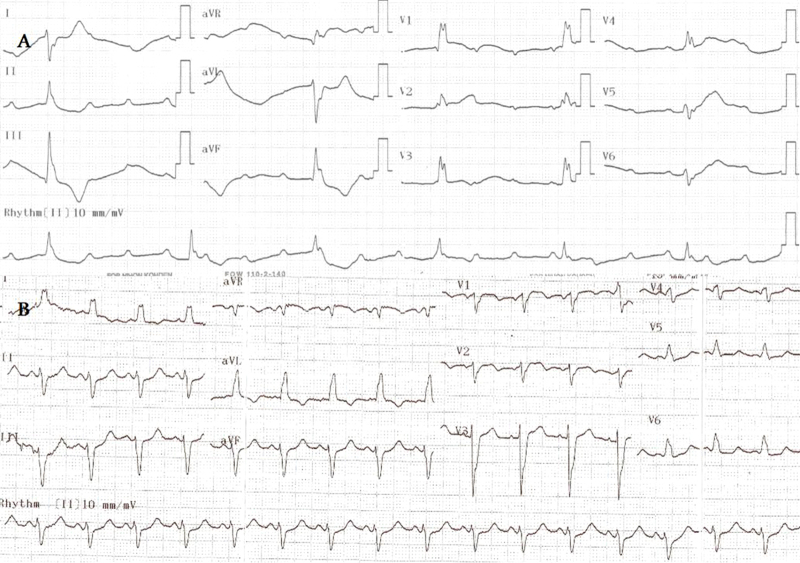
An 84-year-old female patient with a past medical history significant for hypertension and diabetes mellitus was admitted to the emergency service because of squeezing chest pain lasting 2 h. Electrocardiogram (ECG) showed complete atrioventricular block, escape rhythm with a rate of 38 bpm (Fig. 1A). The patient was diagnosed with ACS and then sent to the catheterization laboratory following 300 mg acetylsalicylic acid and 600 mg clopidogrel loading. After transvenous pacemaker insertion and 10,000 IU intravenous heparin, successful primary percutaneous coronary intervention (PCI) was performed to the total occlusion, proximal to the left anterior descending coronary artery with a door–balloon time of 20 min. Angiography of the left coronary system also showed critical proximal obtuse marginal branch 2 lesion. Left ventricular end–diastolic pressure was 18 mm Hg at that time.
Figure 1.
Twelve-lead electrocardiography showing complete atrioventricular block and escape rhythm (A) and sinus rhythm with left bundle branch block after primary percutanous coronary intervention (B).
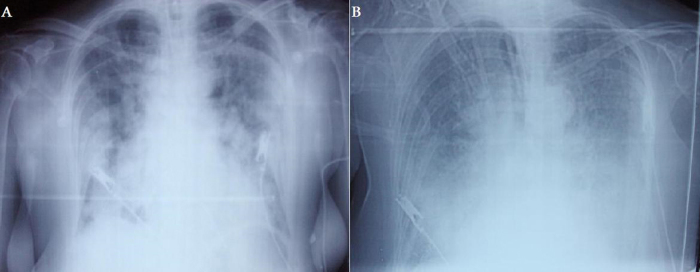
Following primary PCI, ECG in the coronary care unit revealed sinus rhythm with left bundle branch block (Fig. 1B). Heart rate and blood pressure were 110 bpm and 85/50 mm Hg, respectively. O2 saturation was 95% with pulse oxymetry probe and she was not complaining of breathlessness. Tirofiban was administered along with other antiischemic drugs to prevent thrombotic complications. Because of increased risk of hemorrhage due to gender and age, half of the recommended bolus and infusion doses were administered, but 10 min following tirofiban administration, the patient complained of hemoptysis and dyspnea. Tirofiban infusion was immediately stopped. However, she had respiratory arrest after rapid decrease of pulse oxymetry values. After endotracheal intubation, aspiration material was noted to be bloody and bedside chest X-ray showed diffuse infiltration of the lungs (Fig. 2A). Activated clotting time (ACT) with Actalyke® ACT system (Helena Laboratories, Beaumont, TX, USA) and platelet number were 328 s and 193,000/μl respectively at that time. Creatinine was 0.87 mg/dL (reference 0.5–0.9 mg/dL). Transthoracic echocardiography revealed hypokinesia of interventricular septum, anterior and anterolateral walls with a left ventricular ejection fraction of 25%. Hematocrit level progressively decreased from 32.5% to 23.8% (reference 35–47%) on the same day and two units of erythrocyte suspension were transfused. The working diagnosis was acute left heart failure with pulmonary edema on that day.
Figure 2.
Bedside chest X-ray showing diffuse bilateral pulmonary infiltrations on the first day (A) and confluence of infiltrations in the third day. Cardiac silhouette cannot be seen (B).
On the second day, need for high concentrations of inspired oxygen fraction (FiO2) disappeared and arterial blood gas measurements showed progressive improvement under continuous positive airway pressure mode of mechanical ventilation; 75 mg clopidogrel and 2000 IU enoxaparine bid were prescribed, but bronchoscopy reported diffuse blood contamination of the bronchial tree without any active bleeding point on the same day. At that time, the working diagnosis was diffuse alveolar hemorrhage. Acetylsalicylic acid and enoxaparine were stopped.
On the third day, only 75 mg clopidogrel was prescribed as an antithrombotic agent to prevent stent thrombosis according to almost normal arterial blood gas tests and good medical condition. Then unexpectedly, oxygen saturation became worse even with 100% FiO2 in the following hours. Bedside chest X-ray showed increase of infiltrations in both lungs (Fig. 2B). Her respiratory condition did not show any progress and she died on the same day.
Discussion
In our patient, tirofiban was felt to be the responsible agent for diffuse alveolar hemorrhage. She had no history of smoking, hemoptysis, or lung disease. Previous chest X-rays were normal. However, hemoptysis, widespread pulmonary consolidations, and severe dyspnea developed in only 10 min following tirofiban administration. On the other hand, although seven cases of diffuse alveolar hemorrhage following tirofiban administration have been reported in the literature, no cases with diffuse alveolar hemorrhage as a complication of isolated acetylsalicylic acid, clopidogrel, or heparin administration have been presented to date. A large-scale retrospective analysis of 5458 acute myocardial infarction (AMI) patients treated with abciximab or eptifibatide and 4136 AMI patients treated without GP IIb/IIIa inhibitors found that GP IIb/IIIa inhibitors statistically increase the risk of diffuse alveolar hemorrhage [1]. A total of 11 patients showed typical signs (dyspnea, hemoptysis, arterial hypoxemia, pulmonary radiographic changes, and, if possible, bronchoscopic signs) of diffuse alveolar hemorrhage in the GP IIb/IIIa inhibitor group. Only one patient in the control group presented with a similar clinical picture but that patient had received thrombolytic therapy with reteplase and underwent emergent coronary artery bypass graft.
GP IIb/IIIa that is expressed on the surface of activated platelets has a central role in platelet aggregation through binding fibrinogen and von willebrand factor to platelets and crosslinking them. Since the presence of platelet rich thrombus and benefit of acetylsalicylic acid in ACS are well known, GP IIb/IIIa antagonists have been used to inhibit this final common pathway. So, the adjunctive use of GP IIb/IIIa antagonists is recommended for patients with ST-elevation MI at the time of primary PCI and for high-risk angina pectoris/non-ST-elevation MI patients for whom invasive strategy is planned [2], [3].
Bleeding is the most frequent side effect due to GP IIb/IIIa antagonists. The ADMIRAL (Abciximab before Direct Angioplasty and Stenting in Myocardial Infarction Regarding Acute and Long-term Follow-up) study reported some complications in acute ST-elevation MI patients in whom abciximab had been used during primary PCI. A total of 300 patients with AMI were randomly assigned, in a double-blind fashion, either to abciximab plus stenting (149 patients) or placebo plus stenting (151 patients) before they underwent coronary angiography. In the abciximab group, there was 0.7% major bleeding, 12.1% minor bleeding, 6% groin hematoma with minor bleeding, 4.7% thrombocytopenia, and 1.3% severe thrombocytopenia. A presumably more lethal complication of GP IIb/IIIa antagonists is diffuse alveolar hemorrhage, which is, fortunately, encountered rarely in daily clinical practice.
The first diffuse alveolar hemorrhage cases due to abciximab and tirofiban were reported in 1997 by Sitges and Villa [4] and in 2000 by Ali et al. [5], respectively. Although the exact incidence is unknown, in a study examining 1020 patients who received GP IIb/IIIa antagonists and underwent PCI, seven patients were diagnosed with diffuse alveolar hemorrhage and two of them expired. The incidence of pulmonary hemorrhage for abciximab, eptifibatide, and tirofiban was 0.7%, 0.5%, and 0.9%, respectively [6]. Another study examining 2553 patients who underwent coronary angiography and received abciximab, reported the incidence of severe pulmonary hemorrhage as 0.27% [7]. In the same study, there was no severe pulmonary hemorrhage in the control group, which consisted of 5412 patients that underwent coronary interventions without abciximab. On the other hand, Iskandar et al. [1] reported diffuse alveolar hemorrhage incidence as 0.33% (6 of 1810) for eptifibatide and 0.14% (5 of 3648) for abciximab in the setting of AMI.
Tirofiban is one of the most commonly used GP IIb/IIIa antagonists. It is a non-peptide competitive antagonist of the GP IIb/IIIa receptor and most of its inhibitory effects disappear after 4 h.
Even though the exact pathophysiology is still not fully understood, increased pulmonary capillary wedge pressure (PCWP) is a common risk factor in almost all patients. Left ventricular systolic dysfunction and congestive heart failure [6], [8], sudden myocardial stunning due to ACS, as in our case [8], [9], [10], chronic obstructive pulmonary disease and related pulmonary hypertension are leading causes of increased PCWP and main predisposing factors for diffuse alveolar hemorrhage during GP IIb/IIIa inhibitor administration [7], [8]. Most of the patients with this catastrophic complication had a prolonged ACT time during GP IIb/IIIa inhibitor administration [6], [7], [8], [9] that can provoke or maintain the hemorrhage. In our case, ACT was prolonged and we propose that it contributed to rapid initiation of the hemorrhage within 10 min following tirofiban administration.
GP IIb/IIIa antagonist-related diffuse alveolar hemorrhage has a high mortality rate that was calculated as 33–44% from two patient series [7], [8]. Only seven tirofiban-related severe alveolar hemorrhage patients have been reported to date and four of them expired. So with our patient, tirofiban-related severe alveolar hemorrhage mortality can be calculated as 62%.
New antithrombotic drugs and new doses of these drugs are increasingly used in cardiology practise. So predetermined GP IIb/IIIa inhibitor doses in primary PCI may need some changes. Different loading doses of clopidogrel may affect the hemorrhagic complications of GP IIb/IIIa antagonists. We could not find any study comparing diffuse alveolar hemorrhage rates after 300 mg versus 600 mg clopidogrel loading in patients undergoing primary PCI with GP IIb/IIIa antagonists. In our case half of the recommended bolus and infusion doses of tirofiban caused diffuse alveolar hemorrhage.
In conclusion, diffuse alveolar hemorrhage is a rare but potentially lethal complication of GP IIb/IIIa antagonists. They should be used very carefully especially in patients having increased PCWP and when other antithrombotic drugs are used. It is clear that weight-adjusted heparin to obtain ACT of 200–250 s will also decrease the risk [11]. Early diagnosis may prevent mortality in some patients. As the signs and the symptoms of this condition are rather non-specific, high clinical suspicion is needed to establish the correct diagnosis. Opacifications in chest X-rays and dyspnea are also common in acute MI patients, but all invasive cardiologists should also consider diffuse alveolar hemorrhage especially in the presence of hemoptysis and/or a sudden fall in hemoglobin levels. Thorax computed tomography and bronchoscopy give valuable information for differential diagnosis. Treatment is mainly supportive and involves discontinuation of all possible antithrombotic agents, respiratory support, blood transfusion, and platelet infusion especially in abciximab-related hemorrhages. But more importantly, possible benefits and risks of GP IIb/IIIa antagonists should be evaluated carefully in patients having multiple predisposing factors, such as elderly patients using multiple antithrombotic drugs or patients with high PCWP. In our case, half of the recommended bolus and infusion doses of tirofiban caused diffuse alveolar hemorrhage. So, even lower doses of GP IIb/IIIa antagonists may not be safe in this population.
References
- 1.Iskandar S.B., Kasasbeh E.S., Mechleb B.K., Garcia I., Jackson A., Fahrig S., Albalbissi K., Henry P.D. Alveolar hemorrhage: an underdiagnosed complication of treatment with glycoprotein IIb/IIIa inhibitors. J Interv Cardiol. 2006;19:356–363. doi: 10.1111/j.1540-8183.2006.00161.x. [DOI] [PubMed] [Google Scholar]
- 2.Kushner F.G., Hand M., Smith S.C., Jr., King S.B., 3rd., Anderson J.L., Antman E.M., Bailey S.R., Bates E.R., Blankenship J.C., Casey D.E., Jr., Green L.A., Hochman J.S., Jacobs A.K., Krumholz H.M., Morrison D.A. A report of the American college of cardiology foundation/American heart association task force on practice guidelines. Circulation Electronic Publication; 2009. Focused updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (Updating the 2005 guideline and 2007 focused update) November 18. [DOI] [PubMed] [Google Scholar]
- 3.Anderson J.L., Adams C.D., Antman E.M., Bridges C.R., Califf R.M., Casey D.E., Jr., Chavey W.E., 2nd, Fesmire F.M., Hochman J.S., Levin T.N., Lincoff A.M., Peterson E.D., Theroux P., Wenger N.K., Wright R.S. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: a report of the American college of cardiology/American heart association task force on practice guidelines (writing committee to Revise the 2002 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction): developed in collaboration with the American college of emergency physicians, the society for cardiovascular angiography and interventions, and the society of thoracic surgeons: endorsed by the American association of cardiovascular and pulmonary rehabilitation and the society for academic emergency medicine. Circulation. 2007;116:e148–e304. doi: 10.1161/CIRCULATIONAHA.107.181940. [DOI] [PubMed] [Google Scholar]
- 4.Sitges M., Villa F.P. Massive pulmonary hemorrhage in a patient treated with a platelet glycoprotein IIb/IIIa inhibitor. Int J Cardiol. 1997;62:269–271. doi: 10.1016/s0167-5273(97)00228-3. [DOI] [PubMed] [Google Scholar]
- 5.Ali A., Patil S., Grady K.J., Schreiber T.L. Diffuse alveolar hemorrhage following administration of tirofiban or abciximab: a nemesis of platelet glycoprotein IIb/IIIa inhibitors. Catheter Cardiovasc Interv. 2000;49:181–184. doi: 10.1002/(sici)1522-726x(200002)49:2<181::aid-ccd14>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 6.Ali A., Hashem M., Rosman H.S., Kazmouz G., Gardin J.M., Schrieber T.L. Use of platelet glycoprotein IIb/IIIa inhibitors and spontaneous pulmonary hemorrhage. J Invasive Cardiol. 2003;15:186–188. [PubMed] [Google Scholar]
- 7.Kalra S., Bell M.R., Rihal C.S. Alveolar hemorrhage as a complication of treatment with abciximab. Chest. 2001;120:126–131. doi: 10.1378/chest.120.1.126. [DOI] [PubMed] [Google Scholar]
- 8.Ener R.A., Bruno N., Dadourian D., Wolf N., Van Decker W., Burke J., Styler M., Topolsky D. Alveolar hemorrhage associated with platelet glycoprotein IIb/IIIa receptor inhibitors. J Invasive Cardiol. 2006;18:254–261. [PubMed] [Google Scholar]
- 9.Yilmaz M.B., Akin Y., Biyikoglu S.F., Guray U., Korkmaz S. Diffuse alveolar hemorrhage following administration of tirofiban in a patient with acute coronary syndrome: a fatal complication. Int J Cardiol. 2004;93:81–82. doi: 10.1016/s0167-5273(03)00130-x. [DOI] [PubMed] [Google Scholar]
- 10.Gill D.S., Ng K., Ng K.S. Massive pulmonary haemorrhage complicating the treatment of acute coronary syndrome. Heart. 2004;90:e15. doi: 10.1136/hrt.2003.028589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferguson J.J., Kereiakes D.J., Adgey A.A., Fox K.A., Hillegass W.B., Jr., Pfisterer M., Vassanelli C. Safe use of platelet GP IIb/IIIa inhibitors. Eur Heart J. 1998;19(Suppl.):D40–51. [PubMed] [Google Scholar]