Abstract
The acute respiratory distress syndrome (ARDS) remains a serious clinical problem with the current treatment being supportive in the form of mechanical ventilation. However, mechanical ventilation can be a double-edged sword; if set properly, it can significantly reduce ARDS associated mortality but if set improperly it can have unintended consequences causing a secondary ventilator induced lung injury (VILI). The hallmark of ARDS pathology is a heterogeneous lung injury, which predisposes the lung to a secondary VILI. The current standard of care approach is to wait until ARDS is well established and then apply a low tidal volume (LVt) strategy to avoid over-distending the remaining normal lung. However, even with the use of LVt strategy, the mortality of ARDS remains unacceptably high at ~40%. In this review, we analyze the lung pathophysiology associated with ARDS that renders the lung highly vulnerable to a secondary VILI. The current standard of care LVt strategy is critiqued as well as new strategies used in combination with LVt to protect the lung. Using the current understanding of alveolar mechanics (i.e. the dynamic change in alveolar size and shape with tidal ventilation) we provide a rationale for why the current protective ventilation strategies have not further reduced ARDS mortality. New strategies of protective ventilation based on dynamic physiology in the micro-environment (i.e. alveoli and alveolar ducts) are discussed. Current evidence suggests that alveolar inflation and deflation is viscoelastic in nature, with a fast and slow phase in both alveolar recruitment and collapse. Using this knowledge, a ventilation strategy with a prolonged time at inspiration would recruit alveoli and a brief release time at expiration would prevent alveolar collapse, converting heterogeneous to homogeneous lung inflation significantly reducing ARDS incidence and mortality.
Mechanical Ventilation and Lung Injury
Identification of ARDS as a syndrome and the possibility that a mechanical ventilator setting, could reduce ARDS mortality led to a large number of physiologic studies investigating the optimal ventilator setting for the ARDS patient.(1, 2) What these studies found was that mechanical ventilation can significantly reduce ARDS morbidity and mortality when set properly, but can exacerbate lung damage by causing a secondary ventilator induced lung injury (VILI) when set improperly.(1) Dreyfuss and Saumon described the transpulmonary pressure (Ptp) gradient across the alveolus caused alveolar strain (i.e. alveolar volume change) as the major mechanism of VILI rather than just the magnitude of airway pressure (Volutrauma vs Barotrauma).(3) Further work from Gattinoni et al. in ARDS patients demonstrated that dependent lung areas demonstrate preferential loss of lung volume, whereas non-dependent lung areas have relatively normal ventilation.(4, 5) The term ‘baby lung’ was coined for this remaining normal residual lung tissue, and it was postulated that over-distension would occur in the more compliant ‘baby lung’ if a normal size tidal volume (Vt) were delivered to the ARDS patient. The mechanism of atelectrauma is excessive shear-stress as the collapsed alveolar walls pull apart during inflation and stress-concentration, which occurs between patent and totally collapsed or edema filled alveoli.(6)
These physiologic and clinical studies suggested that VILI might be minimized if: a) Ptp was reduced in order to lower alveolar strain; b) Vt and plateau pressure (Pplat) were lowered to prevent lung over-distension; and c) adequate PEEP was used to minimize atelectrauma.(7, 8) Using these data as proof-of-concept, several small clinical trials were conducted with some studies showing no improvement in lung protection with low Vt (LVt) strategy using 6ml/kg,(9–11) while other studies suggested that LVt was lung protective.(12–14) In 2000, the ARDS Network (ARDSnet) published a large randomized controlled trial demonstrating that LVt significantly reduced mortality in ARDS patients (from 39% to 31%). Since this study, LVt has remained the current standard of care protective ventilation strategy for patients with established ARDS.(15)
Stagnation in Reducing ARDS Mortality
Understanding the pathophysiology associated with ARDS and how it can be exacerbated with mechanical ventilation led to the development of protective ventilation strategies.(15) These strategies combined with better hemodynamic and fluid resuscitation management significantly reduced mortality from 1967 when ARDS was first identified until present.(16) However, there has not been a further significant reduction in ARDS-related mortality over the last 17 years.(16–21) The lack of reduced mortality suggests that the pathophysiology caused by mechanical ventilation in alveoli and alveolar ducts [the micro-environment] is not fully understood.(22) Without this knowledge it is impossible deduce the optimal combination of macro-components including Vt, Ptp, Pplat, PEEP, set on the ventilator needed to block mechanical damage to the micro-environment of the pulmonary parenchyma.
There remain significant gaps in our knowledge of the precise characterization of VILI-induced lung tissue damage including: 1) the significance of each mechanical breath profile (MBP) component (i.e. all airway pressures, volumes, flows, rates and the duration that they are applied during both inspiration and expiration) on lung injury or protection,(23) and 2) the potential synergy among MBP components and other factors, such as increased inflammation (Biotrauma) secondary to the mechanical injury (Volutrauma and Atelectrauma), that injure or protect the lung. Since the lung changes volume as a dynamic viscoelastic system,(6) the macro-components of the mechanical breath that are dialed into the ventilator must account for the dynamic nature of lung physiology, with adjustments being continually made directed by changes in this physiology. Without the ability to continually adjust components of the MBP with evolving lung pathophysiology (as the lung gets either better or worse) it will be impossible to protect alveoli and alveolar ducts in the micro-environment, which is necessary to reduce VILI and decrease ARDS mortality.(23–26)
Genetic predisposition is another risk factor for developing ARDS. It has been shown that patients with ARDS can be separated into two phenotypes and mortality for each is different. In addition four biomarkers have been identified that can predict these two phenotypes.(27) A new hypothesis on the inflammatory mechanism of ARDS is purinergic signaling (i.e. extracellular release of ATP following cellular damage), which may be the molecular focal point driving progressive acute lung injury. (28) A recent publication has demonstrated that the TCAV protocol significantly reduced lung Diffuse Alveolar Damage (DAD) score, expression of biomarkers, and extracellular matrix homeostasis in both primary and secondary ARDS rat models.(29)
Pathophysiology of ARDS
Since the physiologic state of the lung is critical to understanding the impact of the mechanical breath on lung pathophysiology, the key pathologic components associated with ALI must be understood. There are four well-accepted components of ARDS pathology that result in significant changes in the anatomical, mechanical and functional aspects the lung. These include (1) increased vascular permeability, (2) alveolar flooding with edema, (3) loss of pulmonary surfactant function that causes alveolar collapse (atelectasis) and (4) alveolar instability altering dynamic alveolar ventilation and ultimately resulting in repetitive alveolar collapse and expansion (RACE) (Fig 1).(30) This tetrad of pathology is interdependent in that increased vascular permeability, leads to alveolar flooding, causing surfactant deactivation, which impacts alveolar mechanics. Surfactant can be deactivated by pulmonary edema, which in turn reduces surfactant production by Type II cells. Since these pathologies result in a heterogeneous lung injury it produces a wide range of both alveolar opening and collapse time constants, making protective mechanical ventilation of the heterogeneously lung without causing VILI very difficult.
Figure 1.
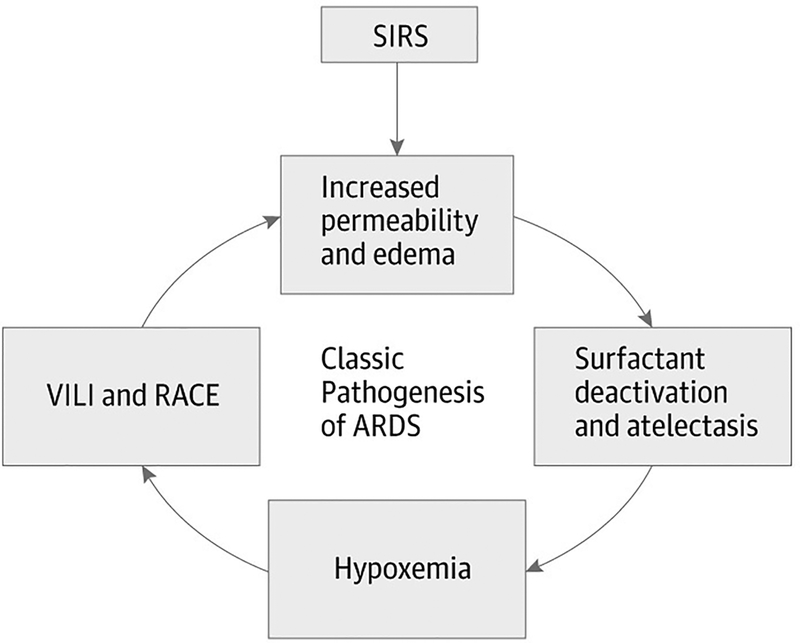
The Tetrad of ARDS Pathophysiology. A major insult (severe sepsis, hemorrhagic shock, trauma, burns, etc.) causes a systemic inflammatory response syndrome (SIRS), which initially increases pulmonary vascular permeability. Increased permeability results in alveolar flooding with edema fluid, which is known to deactivate surfactant function. The combination of edema and atelectasis caused from surfactant dysfunction results in hypoxemia. Functional surfactant is necessary for normal alveolar mechanics so that loss of function results in significant alveolar collapse and instability, with alveoli collapsing and reopening with each breath. Each of these pathologic components contributes to the development of ARDS individually and synergistically.(30)
The micro-environment of the pulmonary parenchyma is complex with alveoli sharing walls in a honeycomb fashion forming an interdependent structure.(31) This interdependence greatly adds to the structural integrity of each individual alveolus, but this structural integrity is lost with heterogeneous alveolar instability or collapse. There is a current misconception that alveoli change volume in a linear elastic fashion much like a balloon whereas alveoli actually change volume non-linearly being a part of the viscoelastic lung system.(32–34)
The importance of this knowledge cannot be overestimated when designing the optimally protective mechanical breath. Viscoelastic behavior dictates that there will be a fast and slow component to both alveolar recruitment and collapse. Thus, a percentage of alveoli will recruit rapidly with the applied force (i.e. Vt), but many others will take a much longer time to open at the same airway pressure. Conversely, when the force is removed, a percentage of alveoli will collapse very rapidly while other will take a much longer time to collapse at the same airway pressure. Therefore, it is not only the airway pressure and flow that are important but also the duration during which they are applied. An extended time at inspiration would gradually recruit alveoli, opening the lung, while minimal time at expiration would prevent alveolar collapse, stabilizing the lung.(6) When combined with the knowledge that both alveolar opening(35) and closing(23, 36) time constants are dramatically modified in ALI, the importance of inspiratory and expiratory time in the protective mechanical breath becomes apparent.
Clinically measured lung dynamics in the heterogeneously injured lung are the summed effect of all lung areas, normal, unstable and atelectatic. This brings into question if we can continually adjust the MBP ‘on the fly’ by measuring changes in the macro-components, which are a summation of the changes in a heterogeneous microenvironment. However, with our preemptive ventilation strategy of ‘never give the lung a chance to collapse’, the microenvironment becomes identical (i.e. all alveoli inflated) and the clinically measured macro-dynamics now represent the entire lung.
LVt as a Protective Ventilation Strategy
Assuming that VILI is a core pathology driving progressive acute lung injury and increasing ARDS mortality, why has the current LVt standard of care ventilation strategy been ineffective at reducing ARDS mortality even further for nearly two decades? (16–21) From a physiologic perspective, the ARDSnet Protocol may not be an effective strategy to block components of the ARDS Tetrad (Fig 1) for a number of reasons:
First, the strategy is not preemptive but rather is typically applied after the patient has significant lung injury with a suggested target for treatment a P/F ratio of <300 or arterial oxygen saturation <88%.(15) Thus, lung disease has already progressed to the point of heterogeneous collapse with stress-risers and unstable alveoli, both key VILI mechanisms. The ARDSnet Protocol was designed to protect the remaining healthy ‘baby lung’, rather than to ‘open the lung and keep it open’, which is the key pathology associated with ARDS that makes the lung so vulnerable to a secondary VILI.
Second, the one-size-fits-all concept that 6ml/kg Vt is optimal for all patients is not likely true. Indeed, Deans and Minneci found there were 2,587 patients that met enrollment criteria in the ARDSnet study but were not enrolled in the study for various technical reasons.(37) These patients were subjected to routine treatment including ventilation with Vt 10ml/kg. Thus, 10ml/kg Vt was the actual standard of care for patients, not 12cc/kg that was used in the ARDSnet study.(15) Thus, the observational cohort receiving 10ml/kg Vt would be a more accurate group to compare against the 6ml/kg LVt treatment group. This standard of care group (Vt 10 ml/kg) and LVt strategy (6 ml/kg) were found to have almost identical mortality rates. They also showed that the patients with the more compliant lungs at the time of randomization did poorly with LVt, whereas if the compliance was worse the patients did better with LVt. Thus, a one-size-fits-all approach to mechanical ventilation, standardizing every patient to 6ml/kg may not be ideal and suggests that Vt should be directed by changes in lung physiology such as compliance. The LVt strategy can even be harmful if used in patients with more compliant lungs, as evident by increased mortality.(38)
Third, changes in PEEP and Vt are set by changes in blood oxygenation on a sliding scale.(15) Oxygenation is not a reliable marker of altered alveolar mechanics (i.e. the dynamic change in alveolar size and shape during tidal ventilation) in the acutely injured lung.(39–42) This fact may help to explain the reason why in the ARDSnet study oxygenation improved in the 12ml/kg group but mortality was increased.(15) It has been shown that a better strategy would be to personalize the size of the Vt to the physiologic parameters of the patient’s lung, using parameters such as compliance and driving pressure.(37, 43) (44)
Fourth, LVt strategy for ARDS causes loss of alveolar surface area resulting in hypercapnia and acidosis, and the impact on patient outcome of this acidosis is unknown. Although studies have suggested that hypercapnia may be lung protective reducing mortality,(45–47) it has also been suggested that hypercapnia is harmful and increases mortality.(9) A recent clinical study showed that hypercapnic acidosis during the first 24 hours in the intensive care unit (ICU) is associated with increased mortality,(48) thus the acidosis associated with LVt may be one explanation for the sustained high mortality of ARDS.
With this understanding, protective ventilation must change from the current standard-of-care strategy of allowing the acutely injured lung to remain totally collapsed and attempting and to not cause VILI in the remaining normal lung tissue (i.e. the ARDSnet protocol).(15) The novel strategy must prevent the lung from every collapsing or rapidly (within hours) reopen the lung as soon as the patient is intubated. This would eliminate all of the pathologic problems associated with ventilating a heterogeneously collapse lung. Preemptive ventilation would be a paradigm shift in protective ventilation from treating the acutely injured lung, to preventing ARDS from ever developing.
A recent review discussed the physiology and methods used to personalize PEEP to lung pathology.(32) The goal is to develop a feedback loop using changes in lung physiology to maintain an open and stable lung (Fig 2). A novel feedback loop approach used to stabilize the lung is known as Time Controlled-PEEP (TC-PEEP).(49) With this method, the clinician does not directly set PEEP on the ventilator but rather sets an expiratory time to be sufficiently brief not to allow the lung to fully empty, thereby maintaining lung volume and an end expiratory pressure (TC-PEEP). An advantage of this method is that the expiratory duration may be targeted to be less than the collapse time constant of the alveoli such that, in addition to the end expiratory pressure, the alveolus does not have time to collapse. Thus, TC-PEEP stabilizes the lung using two mechanisms: the brief expiratory duration does not allow sufficient time for alveoli collapse while maintaining a positive end expiratory pressure.(30) TC-PEEP is one of the components of the Time Controlled Adaptive Ventilation (TCAV) Protocol that will be discussed in detail below (Fig 3).(49, 50)
Figure 2.
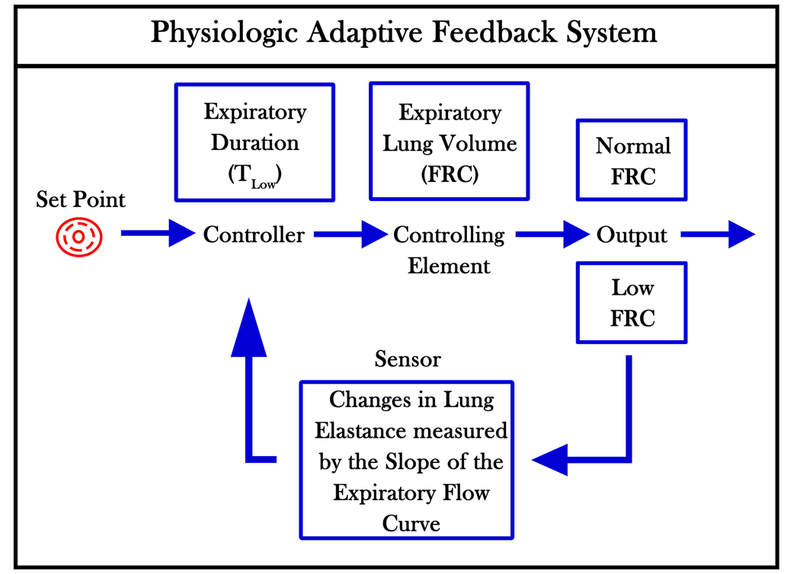
A schematic of a Physiologic Adaptive Feedback System used to maintain lung Functional Residual Capacity (FRC) during expiration. The Set Point is the physiologic FRC in normal humans. The Controller in this feedback system is the expiratory duration and impacts the Controlling Element, which is the volume of FRC. The Output is either a lung with Normal or Low FRC volume. Low FRC will be detected as a change in Lung Elastance, which is measured by the Slope of the Expiratory Flow Curve (Fig 1B). A steeper slope will trigger the Controller to shorten the expiratory duration that will cause an increase in FRC.(32)
Figure 3.
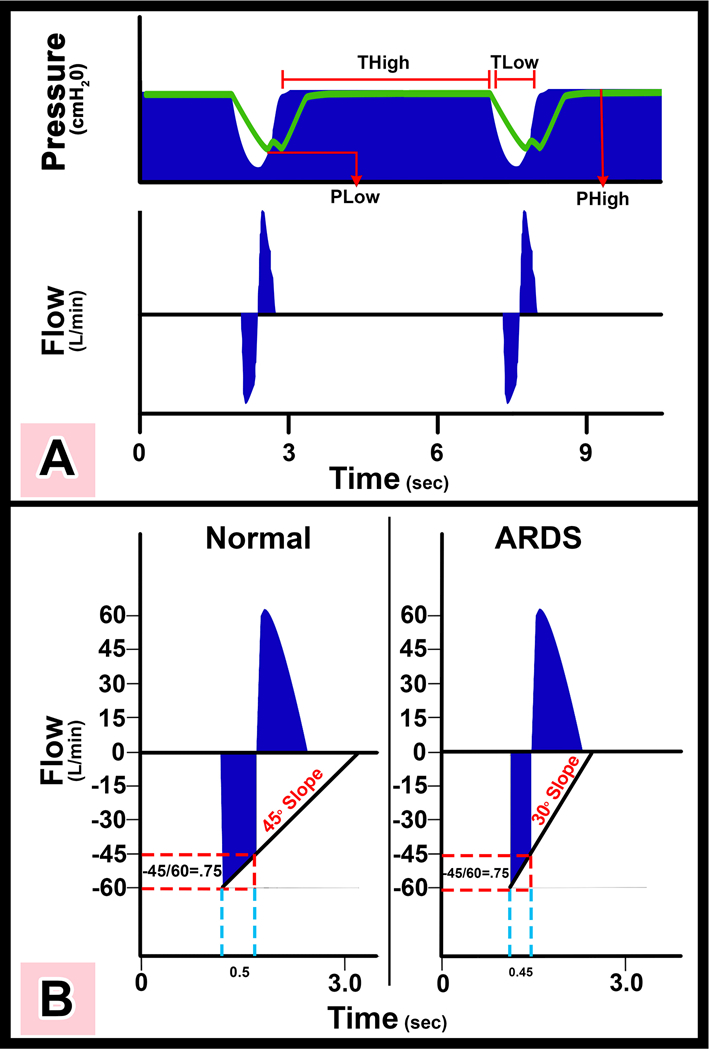
Representative airway pressure and flow curves with APRV set by the TCAV protocol (spontaneous breaths are not shown) a) There is an extended time at inspiration (THigh) and minimal time at expiration (TLow). The high pressure (PHigh) combined with the THigh determines the magnitude and duration of the continuous positive airway pressure (CPAP). The end expiratory airway pressure (TLow) is always set to 0cmH2O, which allows unrestricted expiratory flow for accurate assessment of lung respiratory system elastance determined by the expiratory flow curve. However, PLow never reaches 0cmH2O because TLow is set sufficiently short to maintain both lung volume and pressure at end expiration. The green line is the measured tracheal pressure, which is the actual end expiratory pressure seen by the alveolus. We have found that if expiratory duration is set properly that the end expiratory pressure (the actual PLow) is approximately ½ of the PHigh. b) Using the slope of the expiratory flow curve (SEFC) to set the expiratory duration necessary to stabilize the lung. The SEFC of the Normal lung is approximately 45°, which decreases to 30° in ARDS. Expiratory duration is calculated by terminating expiration at 75% of the peak expiratory flow (−60L/min), which in this example would be at −45L/min. Note that using this same ratio in both Normal and ARDS lungs the expiratory duration is shorter (0.45 vs. 0.5 sec) in the ARDS lung because of the steeper SEFC.(49)
With TC-PEEP resulting in a high degree of alveolar stability how is CO2 effectively cleared? During the extended time at inspiration in the TCAV protocol CO2 diffuses from the alveoli into the large airways and trachea. During the very brief expiratory release phase a large volume of CO2 is removed due the high concentration in the large airway. Thus, the TCAV protocol stabilizes alveoli using TC-PEEP without causing problems in ventilation.
Although several clinical trials have shown that open lung strategies in patients with ARDS did not reduce mortality,(51–53) more recent evidence suggests a survival benefit.(54, 55) Thus, it appears that ventilation strategies that ‘open the lung and keep it open’ may result in a mortality reduction, a hypothesis that is supported by the physiologic understanding of VILI.(56–61) However, a recent clinical trial has shown that a RM combined with titrated PEEP actually increased mortality as compared with the Low PEEP group.(62) The inconsistency in reducing mortality using conventional volume-assist control ventilation with RM and titrated PEEP to, ‘open the lung and keep it open’ suggests that there is a problem with the ventilation strategy. It is possible that the treatment is applied too late in disease progression to be of much benefit. In an editorial on this paper Sahetya and Brower(63) suggest that since there are now four failed clinical trials using RM plus PEEP that novel protective ventilation strategies need to be tested. Data from our laboratory suggest that the TCAV Protocol may be the novel more effective lung protective strategy that Drs. Sahetya and Brower suggest.
Apply Protective Ventilation Preemptively
Although LVt and LVt combined with open lung strategies have reduced mortality from the time ARDS was first identified in 1967,(15) there has not been a continual reduction in mortality over the past two decades.(16–21) The recent failed clinical trial showing that an Open Lung Approach (OLA) actually increased mortality suggests that once ARDS is established even an optimally protective ventilation strategy may not be effective at reducing mortality.(62) Thus, a better strategy would be to ‘Never give the lung a chance to collapse’ in patients ventilated with normal lungs but at high-risk of developing ARDS. This would shift the paradigm from treating established ARDS to preventing ARDS before it developed.
Many recent studies have shown the benefit of applying preemptive protective ventilation strategies on patients in the early stages of ALI, before clinical symptoms develop.(64–68) It is now known that ARDS is not a binary construct (i.e. it is either present or it is not) but rather is progressive, evolving through multiple stages.(69) Thus, early intervention with lung protective ventilation would block progressive lung damage, just as it is better to implement prophylaxis to minimize the chance for a deep vein thrombosis (DVT) rather than to treat the DVT or pulmonary embolism after it has formed. Indeed, preemptive protective mechanical ventilation has been continually applied earlier and has now been shown to be effective when delivered in the operating(70) and emergency rooms.(71)
Determining the Optimal Preemptive Protective Ventilation
To our knowledge, all studies performed with preemptive mechanical ventilation have used the same protective ventilation strategy that is currently used in established-ARDS: LVt, PEEP and RMs. Although recent studies have shown that a combination of preemptive LVt, PEEP and RM in patients without ARDS reduced the development of lung injury(64–68), clinical outcomes have been inconsistent, demonstrating both an increase and decrease in mortality.(65, 72, 73) Neto et al conducted a retrospective study in 2,184 patients that were ventilated without ARDS categorizing the ventilation strategy into low (≤7ml/kg) intermediate (>7 and <10ml/kg) and high (≥10ml/kg) Vt groups.(65) They found a dose-response relationship between lower Vt and a reduction in major pulmonary complications such as ARDS and pneumonia, which were associated with fewer ICU and hospital-free days and reduced mortality. However, another study showed that if lower Vt was combined with lower PEEP, in anesthetized surgery patients the 30-day mortality rates were increased.(72) In contrast, Neto et al conducted a meta-analysis that confirmed the use of LVt protective in surgical patients but found that no added protection was offered by higher PEEP.(65)
The work of Neto et al was supported by the multicenter PROVHILO randomized control trial, showing that neither higher PEEP nor RMs reduce postoperative complications and suggested using LVt with low PEEP and no RMs as a protective preemptive ventilation strategy.(73) Combined these data clearly demonstrate that preemptive protective mechanical ventilation applied in the ICU or the operating room can reduce the incidence and severity of lung injury in patients at risk for ARDS development.
Time-Controlled Adaptive Ventilation (TCAV) Protocol
It is important to understand that, like the ARDSnet Protocol, our TCAV Protocol is an all inclusive mechanical ventilation strategy that consists of the ventilator mode, the setting within that mode, and the adjustments made to these settings based on changes in lung physiology. Often in the literature the mode is analyzed and critiqued in isolation, without regard to the entire protocol being employed. For example, Volume-Assist Control (V-AC) is the mode used within the ARDSnet protocol but the mode itself means nothing without a detailed list of the settings and how these settings will be adjusted in response to changes in the patient’s lung pathology (i.e. the ARDSnet Protocol). The same is true for airway pressure release ventilation (APRV), which is the mode used within the TCAV protocol.
The first APRV publication was in 1987(74) and since its inception, many vastly different setting have been used in both animal and clinical studies that have all been termed ‘APRV’.(49) Jain et al discussed these difference in a paper that reviewed the 30-year history of APRV and demonstrated the ventilator settings that were all called an ‘APRV’ breath had significantly disparate settings.(49) Figure 4 clearly shows the large differences in APRV settings used in 4 published studies.(74–77) These differences include, but not limited to, vastly different peak and end pressure, respiratory rate, and inspiratory and expiratory durations (Fig 4). It is obvious that the settings in any ventilator mode are key to lung injury or protection.
Fig 4.
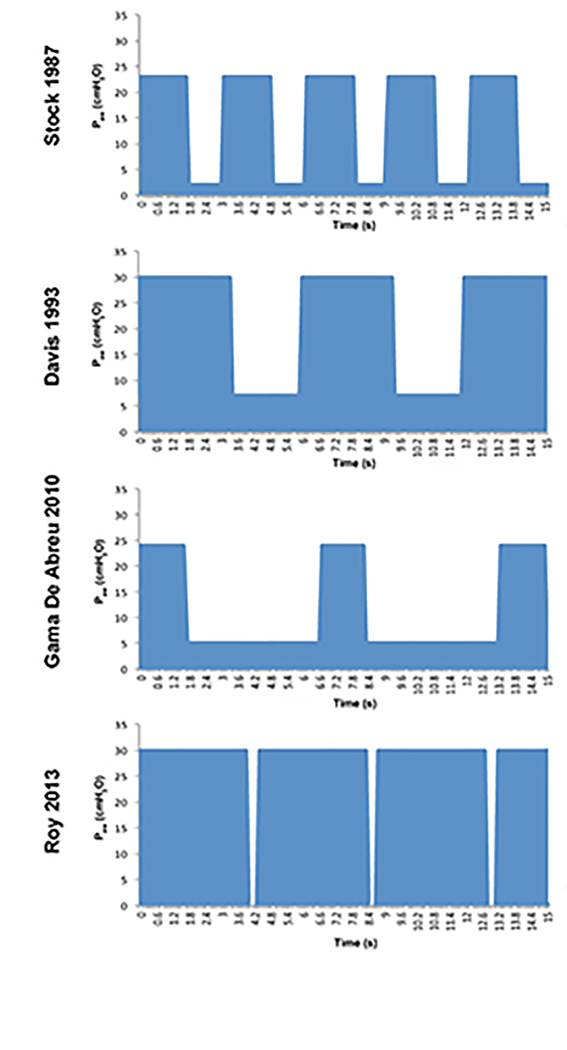
Airway Pressure Release Ventilation (APRV) airway pressure waveforms, illustrating the dramatic variability APRV setting used in various protocols. Stock in 1987 used a TLow of 1.27 seconds, 60% CPAP with and a respiratory rate (RR) of 20. Davis in 1993 used a similar %CPAP, prolonging THigh and TLow, which decreased the RR. Gama de Abreau in 2010 with a prolonged TLow and short THigh, which essentially simulated conventional ventilation. Roy in 2013 used the TCAV protocol comprised of a very brief TLow and 90% CPAP.(49)
Almost all mechanical ventilation review papers discuss the APRV mode in isolation, not as a treatment protocol. In addition these review papers often use a very broad definition APRV, such as any mechanical breath with an inverse I-E ratio, regardless of what this ratio is or if vastly different ventilator settings are being used with the same I-E ratio (Fig 4).(78) For these reasons we named our protective ventilation strategy the TCAV Protocol so that it will be analyzed as a whole, rather than just the ventilator mode within the protocol. The TCAV name is descriptive as to the impact it has on lung physiology: The Time Controlled component (TCAV) of our protocol is the use of an extended inspiratory time to open the lung and a very short expiratory time to keep the lung open. The Adaptive component (TCAV) of our strategy are two fold: 1) since our high pressure is simply continuous positive airway pressure (CPAP) with a quick release we do not set a Vt. Rather the size of the Vt is adaptive to changes in lung volume – if lung volume is low, there is a small release volume (i.e. the volume of gas expired during the brief expiratory release, which is analogous to the set Vt using conventional ventilation), if lung volume is high, release volume will be much larger; thus, Vt is not arbitrarily set on the ventilator but rather adapts to changes in lung volume, and 2) the expiratory duration is set by changes in the slope of the expiratory flow curve, which is a measure of respiratory system compliance. The faster the lung collapse the shorter the expiratory duration so that lung stability is adaptive to changes in lung physiology, regardless if the patient’s lung is getting better or worse.(49, 50) Details of the TCAV Protocol have been published previously (49, 50) and will be discussed below.
TCAV Protocol used as a Preemptive Ventilation Strategy
We now understand that the components of ARDS pathology that render the lung susceptible to a secondary VILI are the loss of lung volume, heterogeneous ventilation and alveolar stability. This knowledge combined with our understanding of dynamic alveolar physiology in the acutely injured lung has directed us away from the standard of care LVt strategy. Instead we have moved toward a mechanical breath that features the component of time at inspiration and expiration to open the lung and keep it open. This is critical since VILI would be dramatically reduced in a homogeneously ventilated lung. Our TCAV Protocol focuses on maintaining adequate lung volume and homogeneous ventilation using an extended time at inspiration and minimal time at expiration, rather than focusing on a specific size of tidal volume. Indeed, a LVt strategy would favor lung collapse with concomitant alveolar instability and heterogeneity. In addition, the TCAV Protocol is personalized to the specific pathology of each patients lung and adaptive as the patient’s lung gets better or worse.(49) We postulate that there are 3-main MBP components necessary for optimal preemptive ventilation and that the TCAV Protocol encompasses all three of these criteria.
The first component of the optimal preemptive ventilation strategy is it must be comfortable for the patient with relatively normal lungs so that the patient can spontaneously breath with minimal resistance. This would eliminate the high frequency oscillatory ventilation (HFOV) mode since the patient cannot breathe spontaneously and must often be heavily sedated or paralyzed using neuromuscular blocking agents. Also a LVt with RM and higher PEEP protocol would not be comfortable or well tolerated in patients with early ALI and relatively normal lungs.
The TCAV Protocol is simply continuous positive airway pressure (CPAP) with a brief release phase. Because of the open breathing system with CPAP, patients can spontaneously breathe (SB) with comfort at any point throughout the entire respiratory cycle eliminating asynchrony as there is no physiologic stimulus (i.e. the lung is not collapsed and the blood gases are in the normal range) to trigger a strong inspiratory effort with an open homogeneously ventilated lung. The CPAP phase maintains lung volume, thereby satiating mechanoreceptors and preventing large inspiratory pressure swings (56). Patients can easily SB on an appropriate amount of CPAP without asynchrony or potential lung over-distension since there are no triggered mechanical breaths with the TCAV Protocol. Indeed, CPAP is used by patients with sleep apnea and is well tolerated in the awake patient with perfectly normal lungs. Since the TCAV Protocol is very comfortable for the patient, it can be applied preemptively and it will keep the lung open, thereby meeting the first criterion of comfort for a preemptive protective breath strategy.
The second component of the optimal preemptive ventilation strategy is that it must be able to recruit and maintain an open lung, since loss of lung surface area results in a strong respiratory drive leading to patient-ventilator asynchrony,(56) In addition a homogeneously ventilated lung would eliminate stress-risers and RACE, two key mechanisms of VILI as well as normalize oxygenation and ventilation. Decreasing Vt using the LVt strategy would collapse, rather than recruit the lung. PEEP is an expiratory phenomenon and prevents collapse but is not associated with lung recruitment. To open the lung recruitment maneuvers (RM) are used and PEEP applied to keep the newly recruited alveoli open.(54) Direct visualization of subpleural alveoli has shown that a RM would open collapsed alveoli but, unless adequate PEEP was added, these newly recruited alveoli would derecruit or be subjected to RACE.(79) Lung recruitment has been shown to be highly variable in ARDS patients(80) raising the question of how often and at what pressure RMs are required to open the lung and keep the it open. A decremental PEEP titration following a RM is believed to be the best method of setting PEEP.(54) However, the optimal PEEP level will change as the patient’s lung progressively improves or worsens, mandating dynamic RM and PEEP titration. This may help explain the results of a recent clinical trial demonstrating that RM and titrated PEEP increases ARDS mortality.(62)
Since alveoli recruit as a viscoelastic system the longer the applied force (i.e. inflation pressure) the more alveoli that will recruit.(6) The TCAV Protocol maintains a CPAP for ~90% of each respiratory cycle to maximize the recruiting force (Fig 3a). We have shown in a rat ARDS model that alveoli continually recruit over a 40sec CPAP phase without an increase of airway pressure (Fig 4).(35) The CPAP phase will gradually recruit alveoli with each breath, slowly ‘nudging’ the lung open without injury. Therefore, the TCAV Protocol fits the second criteria of a preemptive protective breath strategy by maintaining an open, homogeneously ventilated lung.
The third component of the optimal preemptive ventilation strategy is that the lung once recruited, must be kept open. Although an appropriate level of PEEP can be effective at stabilizing the lung once it is open, the ability for PEEP to stabilize alveoli following a RM worsens with progressive ALI and (81) recent a clinical trial combining a RM with titrated PEEP was shown to increase ARDS mortality.(62)
The TCAV Protocol uses the slope of the expiratory flow curve to determine the duration of the release phase, or expiratory duration, necessary to maintain lung stability and prevent alveolar collapse (Fig 3b).(49, 50) The slope of the expiratory curve is a function of lung elastance, where a higher elastance correlates with a faster collapse time constant (thus requiring a shorter expiratory phase).(82, 83) Mechanical compliance and resistance of the lung-thorax can be calculated from the flow recorded during passive expiration.(84). In addition, elastance is a better correlate of residual lung volume (i.e. baby lung) than is predicted body weight.(85) Using the slope of the expiratory curve personalizes the expiratory duration to the pathophysiology of each patient’s lung without the need for any special maneuvers and is adaptive as the lung pathology changes (improves/worsens) (Fig 3b).(49, 50) The brief expiratory duration stabilizes the lung by two mechanisms: time and pressure (i.e. alveoli don’t have time to collapse and TC-PEEP)(32). We have demonstrated that this dual mechanism of lung stabilization is more effective at stabilizing alveoli and preventing their collapse than is high set PEEP with conventional ventilation.(23)
We have found that APRV set using TCAV Protocol generates a TC-PEEP with the dual stabilizing action of pressure and time will prevent alveolar collapse and thus fulfills the third criteria of a preemptive protective breath strategy of not allowing the open lung to collapse. A recent study has shown that the TCAV protocol reduces acute lung injury and inflammation in a primary and secondary rat endotoxin induced ARDS models as compared with volume-controlled ventilation.(86) Do to the sometimes counter intuitive components of APRV, set according to the TCAV protocol, based on the assumptions made in the ARDSnet protocol many misconceptions have been generated on level of comfort, use of TC-PEEP, how to set TC-PEEP, and the size of the tidal volume (Vt). We have discussed these misconceptions in Table 1.
Table 1.
Misconceptions of Airway Pressure Release Ventilation (APRV) as Set Using the TCAV Protocol:
|
Comfort: When set properly for the patient’s lung pathology, patients are typically comfortable on APRV. APRV provides increased comfort by: 1) an open exhalation system allows patients to exhale at any point during the respiratory cycle without pressure limiting and is not confined to the release phase; 2) allows inspiration at any time during the respiratory cycle eliminating inspiratory efforts during a fixed flow ventilation and high pleural pressure efforts; and 3) APRV provides CPAP for 90% of pressure-time profile, which allows for a protective form of spontaneous breathing during mechanical ventilation. (90) APRV set according to the TCAV protocol is essentially CPAP with a brief release phase and no trigger to deliver a mechanical or assisted breath. Because APRV set according to the TCAV protocol is a comfortable ventilation strategy, it can be used preemptively as the primary mode as soon as the patient is intubated, and throughout their entire course of mechanical ventilation, from intubation to weaning. |
| Time Controlled PEEP (TC-PEEP): |
| In the TCAV method of APRV, the release phase is used to dynamically manage the evolving time constants of the respiratory system. This can be determined by the slope of the expiratory flow curve, which reflects [in real-time] the elastance and resistance of the respiratory system at any given time throughout the course of the patient’s illness. Because of the viscoelastic behavior of the lung, set PEEP allows progressive airway closure at a given PEEP level over the prolonged end expiratory period. TC-PEEP eliminates this weakness by controlling time and eliminating airway closure, thereby maintaining a stable static end-expiratory lung volume. Because 90% of the total cycle time in APRV is CPAP, dynamic hyperinflation is decreased. |
| Tidal Volume (Vt): Since APRV, set according to the TCAV protocol, is simply CPAP with a brief release, without any triggers to deliver a mechanical breath, Vt is not set but rather is dependent on the pathophysiology of the lung. If the patient has severe ARDS and is placed on the TCAV protocol, the initial Vt are typically <6 mL/kg as Vt is proportional to lung compliance and residual lung volume (i.e. lower compliance, lower Vt). As a result, Vt does not come at the cost of increased driving pressure (DP) since lung Cstat is increased (DP=Vt/Cstat). Thus, it is not only the magnitude of the Vt that is injurious but the residual lung volume and Cstat of the lung to which the Vt is applied. The prolonged CPAP phase enhances time-dependent recruitment of lung tissue and the TLow prevents time-dependent derecruitment, limiting heterogeneous alveolar instability (HAI). Because the slope of the expiratory flow curve reflects the elastance and resistance changes in lung mechanics, the TLow slope and termination functions to provide a dynamic real-time adaptation to evolving lung mechanics. |
Clinical Studies using a Protocol with APRV as the Mode
To date there have been no randomized controlled trials (RCT) using the TCAV protocol in patients with or at high risk of developing acute lung injury. The TCAV protocol is used as a primary mode of ventilation (i.e. criteria for applying the TCAV protocol is intubation) at the R Adam Cowley Shock/Trauma Center in Baltimore, Maryland and thus 1000s of patient have been successfully treated with this ventilation strategy. A meta-analysis comparing patients using the TCAV protocol as the primary ventilation strategy at R Adam Cowley Shock/Trauma Center where compared to patients in 15 other surgical intensive care units (SICU) for incidence of ARDS and mortality. There was a significant decrease in both ARDS incidence and mortality in the patients on the preemptive TCAV protocol (Fig 5).(87) A recent RCT used APRV in a protocol similar, but not identical to, the TCAV protocol.(88) In 138 patients this study compared the ARDSnet protocol with that of protocol similar to TCAV. The studied showed the Protocol using APRV improved oxygenation, respiratory system compliance, and plateau pressure, with a shorter duration of both mechanical ventilation and ICU stay (Fig 6). A recent review of both animal and clinical studies using APRV in the ventilation protocol has been published.(89) Table 2 overviews the basic TCAV protocol APRV settings and weaning strategy.
Fig 5.
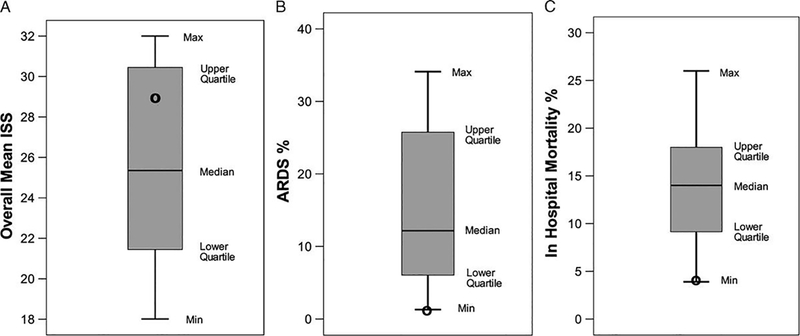
(A) Injury Severity Score (ISS), (B) ARDS incidence (%), (C) in–hospital mortality (%) from 16 SICUs using standard of care ventilation (Bar and Whisker) and from R Adam Cowley Shock/Trauma Center using the preemptive TCAV protocol (Bold Circles)(87)
Fig 6.
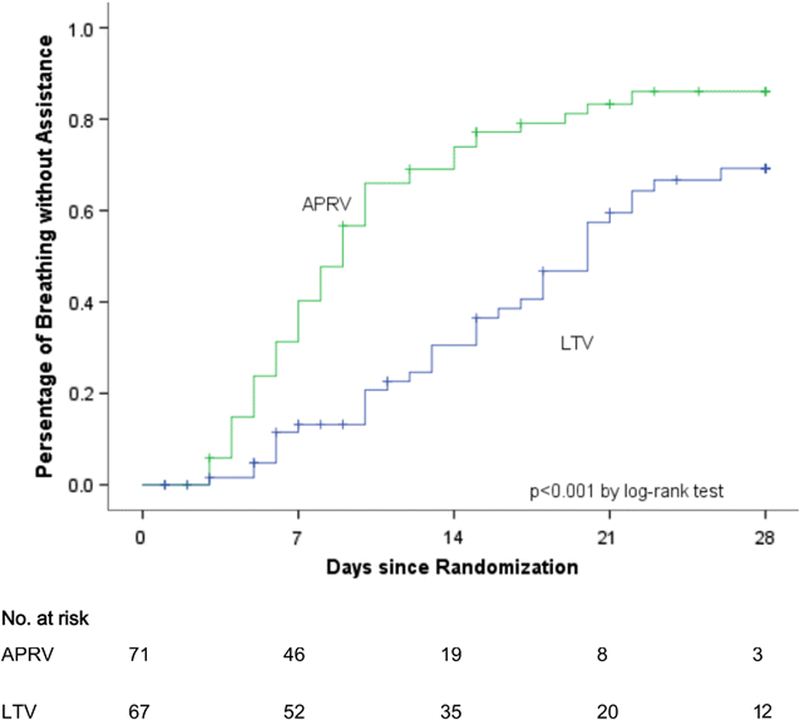
Patients breathing without assistance (%) in the Airway Pressure Release Ventilation (APRV) group and the low tidal volume (LVT) group for 28 days from the time of enrollment.(88)
Table 2.
Time Controlled Adaptive Ventilation (TCAV) Protocol Clinical Guide
|
|
|
Legend: PHigh = high airway pressure, THigh = the time at PHigh, PLow = set low pressure, TLow = the time at PLow, WOB=Work of breathing, PEFR=Peak expiratory flow rate, EEF=end expiratory flow, SB=spontaneous breathing. Table modified from Habashi Crit Care Med 2005
Conclusions
Study of dynamic alveolar physiology during inflation and deflation has revealed alveoli function as a viscoelastic system with a fast and slow phase during both recruitment and collapse in response to the application or removal of the applied force (i.e. tidal volume). This suggests that the mechanical breath component of time during both inspiration and expiration is critical in both maximizing alveolar recruitment and minimizing derecruitment. The TCAV protocol takes advantage of this knowledge by extending the time at inspiration to gradually recruit alveoli with each breath and setting a very short expiratory duration to minimize alveolar collapse by a dual mechanism of time and pressure (TC-PEEP). A recent randomized controlled trial demonstrated that early application of a protocol similar to TCAV improved oxygenation and respiratory system compliance, decreased plateau pressure, and reduced mechanical ventilation and ICU time.
Funding:
Salary Support for GN and JS from NIH R01 HL131143
List of abbreviations:
- ARDS
Acute Respiratory Distress Syndrome
- VILI
Ventilator Induced Lung Injury
- LVt
Low Tidal Volume
- ALI
Acute Lung Injury
- ARF
Acute Renal Failure
- PEEP
Positive End Expiratory Pressure
- Ptp
Transpulmonary Pressure
- RACE
Repetive Alveolar Collapse and Expansion
- MBp
Mechanical Breatth Profile
- A-Vt
Alveolar Tidal Volume
- APRV
Airway Pressure Release Ventilation
- TCAV
Time Controlled Adaptive Ventilation
- TC-PEEP
Time Controlled-PEEP
- RM
Recruitment Maneuver
- OLA
Open Lung Approach
References
- 1.Tremblay LN, Slutsky AS. Ventilator-induced lung injury: from the bench to the bedside. Intensive care medicine. 2006;32(1):24–33. [DOI] [PubMed] [Google Scholar]
- 2.McAslan TC, Cowley RA. The preventive use of PEEP in major trauma. The American surgeon. 1979;45(3):159–67. [PubMed] [Google Scholar]
- 3.Dreyfuss D, Saumon G. Ventilator-induced lung injury: lessons from experimental studies. American journal of respiratory and critical care medicine. 1998;157(1):294–323. [DOI] [PubMed] [Google Scholar]
- 4.Gattinoni L, Pesenti A, Avalli L, Rossi F, Bombino M. Pressure-volume curve of total respiratory system in acute respiratory failure. Computed tomographic scan study. The American review of respiratory disease. 1987;136(3):730–6. [DOI] [PubMed] [Google Scholar]
- 5.Gattinoni L, Mascheroni D, Torresin A, Marcolin R, Fumagalli R, Vesconi S, Rossi GP, Rossi F, Baglioni S, Bassi F, et al. Morphological response to positive end expiratory pressure in acute respiratory failure. Computerized tomography study. Intensive care medicine. 1986;12(3):137–42. [DOI] [PubMed] [Google Scholar]
- 6.Nieman GF, Satalin J, Kollisch-Singule M, Andrews P, Aiash H, Habashi NM, Gatto LA. Physiology in Medicine: Understanding dynamic alveolar physiology to minimize ventilator-induced lung injury. Journal of applied physiology. 2017;122(6):1516–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tobin MJ. Advances in mechanical ventilation. The New England journal of medicine. 2001;344(26):1986–96. [DOI] [PubMed] [Google Scholar]
- 8.Malhotra A Low-tidal-volume ventilation in the acute respiratory distress syndrome. The New England journal of medicine. 2007;357(11):1113–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stewart TE, Meade MO, Cook DJ, Granton JT, Hodder RV, Lapinsky SE, Mazer CD, McLean RF, Rogovein TS, Schouten BD, et al. Evaluation of a ventilation strategy to prevent barotrauma in patients at high risk for acute respiratory distress syndrome. Pressure- and Volume-Limited Ventilation Strategy Group. The New England journal of medicine. 1998;338(6):355–61. [DOI] [PubMed] [Google Scholar]
- 10.Brochard L, Roudot-Thoraval F, Roupie E, Delclaux C, Chastre J, Fernandez-Mondejar E, Clementi E, Mancebo J, Factor P, Matamis D, et al. Tidal volume reduction for prevention of ventilator-induced lung injury in acute respiratory distress syndrome. The Multicenter Trail Group on Tidal Volume reduction in ARDS. American journal of respiratory and critical care medicine. 1998;158(6):1831–8. [DOI] [PubMed] [Google Scholar]
- 11.Brower RG, Shanholtz CB, Fessler HE, Shade DM, White P, Wiener CM Jr., Teeter JG, Dodd-o JM, Almog Y, Piantadosi S. Prospective, randomized, controlled clinical trial comparing traditional versus reduced tidal volume ventilation in acute respiratory distress syndrome patients. Critical care medicine. 1999;27(8):1492–8. [DOI] [PubMed] [Google Scholar]
- 12.Amato MB, Barbas CS, Medeiros DM, Schettino Gde P, Lorenzi Filho G, Kairalla RA, Deheinzelin D, Morais C, Fernandes Ede O, Takagaki TY, et al. Beneficial effects of the “open lung approach” with low distending pressures in acute respiratory distress syndrome. A prospective randomized study on mechanical ventilation. American journal of respiratory and critical care medicine. 1995;152(6 Pt 1):1835–46. [DOI] [PubMed] [Google Scholar]
- 13.Hickling KG, Henderson SJ, Jackson R. Low mortality associated with low volume pressure limited ventilation with permissive hypercapnia in severe adult respiratory distress syndrome. Intensive care medicine. 1990;16(6):372–7. [DOI] [PubMed] [Google Scholar]
- 14.Amato MB, Barbas CS, Medeiros DM, Magaldi RB, Schettino GP, Lorenzi-Filho G, Kairalla RA, Deheinzelin D, Munoz C, Oliveira R, et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. The New England journal of medicine. 1998;338(6):347–54. [DOI] [PubMed] [Google Scholar]
- 15.ARDS-Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. The New England journal of medicine. 2000;342(18):1301–8. [DOI] [PubMed] [Google Scholar]
- 16.Villar J, Sulemanji D, Kacmarek RM. The acute respiratory distress syndrome: incidence and mortality, has it changed? Current opinion in critical care. 2014;20(1):3–9. [DOI] [PubMed] [Google Scholar]
- 17.Maca J, Jor O, Holub M, Sklienka P, Bursa F, Burda M, Janout V, Sevcik P. Past and Present ARDS Mortality Rates: A Systematic Review. Respiratory care. 2017;62(1):113–22. [DOI] [PubMed] [Google Scholar]
- 18.Villar J, Blanco J, Kacmarek RM. Current incidence and outcome of the acute respiratory distress syndrome. Current opinion in critical care. 2016;22(1):1–6. [DOI] [PubMed] [Google Scholar]
- 19.Villar J, Blanco J, Anon JM, Santos-Bouza A, Blanch L, Ambros A, Gandia F, Carriedo D, Mosteiro F, Basaldua S, et al. The ALIEN study: incidence and outcome of acute respiratory distress syndrome in the era of lung protective ventilation. Intensive care medicine. 2011;37(12):1932–41. [DOI] [PubMed] [Google Scholar]
- 20.Phua J, Badia JR, Adhikari NK, Friedrich JO, Fowler RA, Singh JM, Scales DC, Stather DR, Li A, Jones A, et al. Has mortality from acute respiratory distress syndrome decreased over time?: A systematic review. American journal of respiratory and critical care medicine. 2009;179(3):220–7. [DOI] [PubMed] [Google Scholar]
- 21.Bellani G, Laffey JG, Pham T, Fan E, Investigators LS, the ETG. The LUNG SAFE study: a presentation of the prevalence of ARDS according to the Berlin Definition! Critical care. 2016;20:268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kollisch-Singule MC, Jain SV, Andrews PL, Satalin J, Gatto LA, Villar J, De Backer D, Gattinoni L, Nieman GF, Habashi NM. Looking beyond macro-ventilatory parameters and re-thinking ventilator-induced lung injury. Journal of applied physiology. 2017:jap004122017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kollisch-Singule M, Emr B, Smith B, Roy S, Jain S, Satalin J, Snyder K, Andrews P, Habashi N, Bates J, et al. Mechanical Breath Profile of Airway Pressure Release Ventilation: The Effect on Alveolar Recruitment and Microstrain in Acute Lung Injury. JAMA Surg. 2014;149(11):1138–45. [DOI] [PubMed] [Google Scholar]
- 24.Kollisch-Singule M, Jain S, Andrews P, Smith BJ, Hamlington-Smith KL, Roy S, DiStefano D, Nuss E, Satalin J, Meng Q, et al. Effect of Airway Pressure Release Ventilation on Dynamic Alveolar Heterogeneity. JAMA Surg. 2015:1–9. [DOI] [PubMed] [Google Scholar]
- 25.Kollisch-Singule M, Emr B, Smith B, Ruiz C, Roy S, Meng Q, Jain S, Satalin J, Snyder K, Ghosh A, et al. Airway pressure release ventilation reduces conducting airway micro-strain in lung injury. Journal of the American College of Surgeons. 2014;219(5):968–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tabuchi A, Nickles HT, Kim M, Semple JW, Koch E, Brochard L, Slutsky AS, Pries AR, Kuebler WM. Acute Lung Injury Causes Asynchronous Alveolar Ventilation That Can Be Corrected by Individual Sighs. American journal of respiratory and critical care medicine. 2016;193(4):396–406. [DOI] [PubMed] [Google Scholar]
- 27.Bos LD, Schouten LR, van Vught LA, Wiewel MA, Ong DSY, Cremer O, Artigas A, Martin-Loeches I, Hoogendijk AJ, van der Poll T, et al. Identification and validation of distinct biological phenotypes in patients with acute respiratory distress syndrome by cluster analysis. Thorax. 2017;72(10):876–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hasan D, Blankman P, Nieman GF. Purinergic signalling links mechanical breath profile and alveolar mechanics with the pro-inflammatory innate immune response causing ventilation-induced lung injury. Purinergic signalling. 2017;13(3):363–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silva PL, Cruz FF, Samary CDS, Moraes L, de Magalhaes RF, Fernandes MVS, Bose R, Pelegati VB, Carvalho HF, Capelozzi VL, et al. Biological Response to Time-Controlled Adaptive Ventilation Depends on Acute Respiratory Distress Syndrome Etiology. Critical care medicine. 2018. [DOI] [PubMed] [Google Scholar]
- 30.Nieman GF, Gatto LA, Habashi NM. Impact of mechanical ventilation on the pathophysiology of progressive acute lung injury. Journal of applied physiology. 2015;119(11):1245–61. [DOI] [PubMed] [Google Scholar]
- 31.Mead J, Takishima T, Leith D. Stress distribution in lungs: a model of pulmonary elasticity. J Appl Physiol. 1970;28(5):596–608. [DOI] [PubMed] [Google Scholar]
- 32.Nieman GF, Satalin J, Andrews P, Aiash H, Habashi NM, Gatto LA. Personalizing mechanical ventilation according to physiologic parameters to stabilize alveoli and minimize ventilator induced lung injury (VILI). Intensive Care Med Exp 2017;5(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takahashi A, Bartolak-Suki E, Majumdar A, Suki B. Changes in respiratory elastance after deep inspirations reflect surface film functionality in mice with acute lung injury. Journal of applied physiology. 2015;119(3):258–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Faffe DS, Zin WA. Lung parenchymal mechanics in health and disease. Physiological reviews. 2009;89(3):759–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Albert SP, DiRocco J, Allen GB, Bates JH, Lafollette R, Kubiak BD, Fischer J, Maroney S, Nieman GF. The role of time and pressure on alveolar recruitment. J Appl Physiol. 2009;106(3):757–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neumann P, Berglund JE, Fernandez Mondejar E, Magnusson A, Hedenstierna G. Dynamics of lung collapse and recruitment during prolonged breathing in porcine lung injury. Journal of applied physiology. 1998;85(4):1533–43. [DOI] [PubMed] [Google Scholar]
- 37.Deans KJ, Minneci PC, Cui X, Banks SM, Natanson C, Eichacker PQ. Mechanical ventilation in ARDS: One size does not fit all. Critical care medicine. 2005;33(5):1141–3. [DOI] [PubMed] [Google Scholar]
- 38.Eichacker PQ, Gerstenberger EP, Banks SM, Cui X, Natanson C. Meta-analysis of acute lung injury and acute respiratory distress syndrome trials testing low tidal volumes. American journal of respiratory and critical care medicine. 2002;166(11):1510–4. [DOI] [PubMed] [Google Scholar]
- 39.Andrews PL, Sadowitz B, Kollisch-Singule M, Satalin J, Roy S, Snyder K, Gatto LA, Nieman GF, Habashi NM. Alveolar instability (atelectrauma) is not identified by arterial oxygenation predisposing the development of an occult ventilator-induced lung injury. Intensive Care Med Exp 2015;3(1):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baumgardner JE, Markstaller K, Pfeiffer B, Doebrich M, Otto CM. Effects of respiratory rate, plateau pressure, and positive end-expiratory pressure on PaO2 oscillations after saline lavage. American journal of respiratory and critical care medicine. 2002;166(12 Pt 1):1556–62. [DOI] [PubMed] [Google Scholar]
- 41.Formenti F, Chen R, McPeak H, Matejovic M, Farmery AD, Hahn CE. A fibre optic oxygen sensor that detects rapid PO2 changes under simulated conditions of cyclical atelectasis in vitro. Respiratory physiology & neurobiology. 2014;191:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Caltabeloti F, Monsel A, Arbelot C, Brisson H, Lu Q, Gu WJ, Zhou GJ, Auler JO, Rouby JJ. Early fluid loading in acute respiratory distress syndrome with septic shock deteriorates lung aeration without impairing arterial oxygenation: a lung ultrasound observational study. Critical care. 2014;18(3):R91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Amato MB, Meade MO, Slutsky AS, Brochard L, Costa EL, Schoenfeld DA, Stewart TE, Briel M, Talmor D, Mercat A, et al. Driving pressure and survival in the acute respiratory distress syndrome. The New England journal of medicine. 2015;372(8):747–55. [DOI] [PubMed] [Google Scholar]
- 44.Franchineau G, Brechot N, Lebreton G, Hekimian G, Nieszkowska A, Trouillet JL, Leprince P, Chastre J, Luyt CE, Combes A, et al. Bedside Contribution of Electrical Impedance Tomography to Setting Positive End-Expiratory Pressure for Extracorporeal Membrane Oxygenation-treated Patients with Severe Acute Respiratory Distress Syndrome. American journal of respiratory and critical care medicine. 2017;196(4):447–57. [DOI] [PubMed] [Google Scholar]
- 45.Laffey JG, Engelberts D, Kavanagh BP. Injurious effects of hypocapnic alkalosis in the isolated lung. American journal of respiratory and critical care medicine. 2000;162(2 Pt 1):399–405. [DOI] [PubMed] [Google Scholar]
- 46.Laffey JG, Engelberts D, Kavanagh BP. Buffering hypercapnic acidosis worsens acute lung injury. American journal of respiratory and critical care medicine. 2000;161(1):141–6. [DOI] [PubMed] [Google Scholar]
- 47.Kavanagh BP, Laffey JG. Hypercapnia: permissive and therapeutic. Minerva anestesiologica. 2006;72(6):567–76. [PubMed] [Google Scholar]
- 48.Tiruvoipati R, Pilcher D, Buscher H, Botha J, Bailey M. Effects of Hypercapnia and Hypercapnic Acidosis on Hospital Mortality in Mechanically Ventilated Patients. Critical care medicine. 2017;45(7):e649–e56. [DOI] [PubMed] [Google Scholar]
- 49.Jain SV, Kollisch-Singule M, Sadowitz B, Dombert L, Satalin J, Andrews P, Gatto LA, Nieman GF, Habashi NM. The 30-year evolution of airway pressure release ventilation (APRV). Intensive Care Med Exp 2016;4(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Habashi NM. Other approaches to open-lung ventilation: airway pressure release ventilation. Critical care medicine. 2005;33(3 Suppl):S228–40. [DOI] [PubMed] [Google Scholar]
- 51.Meade MO, Cook DJ, Guyatt GH, Slutsky AS, Arabi YM, Cooper DJ, Davies AR, Hand LE, Zhou Q, Thabane L, et al. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA : the journal of the American Medical Association. 2008;299(6):637–45. [DOI] [PubMed] [Google Scholar]
- 52.Brower RG, Lanken PN, MacIntyre N, Matthay MA, Morris A, Ancukiewicz M, Schoenfeld D, Thompson BT, National Heart L, Blood Institute ACTN. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. The New England journal of medicine. 2004;351(4):327–36. [DOI] [PubMed] [Google Scholar]
- 53.Mercat A, Richard JC, Vielle B, Jaber S, Osman D, Diehl JL, Lefrant JY, Prat G, Richecoeur J, Nieszkowska A, et al. Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA : the journal of the American Medical Association. 2008;299(6):646–55. [DOI] [PubMed] [Google Scholar]
- 54.Kacmarek RM, Villar J, Sulemanji D, Montiel R, Ferrando C, Blanco J, Koh Y, Soler JA, Martinez D, Hernandez M, et al. Open Lung Approach for the Acute Respiratory Distress Syndrome: A Pilot, Randomized Controlled Trial. Critical care medicine. 2016;44(1):32–42. [DOI] [PubMed] [Google Scholar]
- 55.Lu J, Wang X, Chen M, Cheng L, Chen Q, Jiang H, Sun Z. An Open Lung Strategy in the Management of Acute Respiratory Distress Syndrome: A systematic review and meta-analysis. Shock. 2017. [DOI] [PubMed] [Google Scholar]
- 56.Cherniack NS, Stanley NN, Tuteur PG, Altose MD, Fishman AP. Effects of lung volume changes on respiratory drive during hypoxia and hypercapnia. J Appl Physiol. 1973;35(5):635–41. [DOI] [PubMed] [Google Scholar]
- 57.Cressoni M, Cadringher P, Chiurazzi C, Amini M, Gallazzi E, Marino A, Brioni M, Carlesso E, Chiumello D, Quintel M, et al. Lung inhomogeneity in patients with acute respiratory distress syndrome. American journal of respiratory and critical care medicine. 2014;189(2):149–58. [DOI] [PubMed] [Google Scholar]
- 58.Makiyama AM, Gibson LJ, Harris RS, Venegas JG. Stress concentration around an atelectatic region: a finite element model. Respiratory physiology & neurobiology. 2014;201:101–10. [DOI] [PubMed] [Google Scholar]
- 59.Perlman CE, Lederer DJ, Bhattacharya J. Micromechanics of alveolar edema. American journal of respiratory cell and molecular biology. 2011;44(1):34–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Retamal J, Bergamini BC, Carvalho AR, Bozza FA, Borzone G, Borges JB, Larsson A, Hedenstierna G, Bugedo G, Bruhn A. Non-lobar atelectasis generates inflammation and structural alveolar injury in the surrounding healthy tissue during mechanical ventilation. Critical care. 2014;18(5):505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Taskar V, John J, Evander E, Robertson B, Jonson B. Surfactant dysfunction makes lungs vulnerable to repetitive collapse and reexpansion. American journal of respiratory and critical care medicine. 1997;155(1):313–20. [DOI] [PubMed] [Google Scholar]
- 62.Writing Group for the Alveolar Recruitment for Acute Respiratory Distress Syndrome Trial I, Cavalcanti AB, Suzumura EA, Laranjeira LN, Paisani DM, Damiani LP, Guimaraes HP, Romano ER, Regenga MM, Taniguchi LNT, et al. Effect of Lung Recruitment and Titrated Positive End-Expiratory Pressure (PEEP) vs Low PEEP on Mortality in Patients With Acute Respiratory Distress Syndrome: A Randomized Clinical Trial. JAMA : the journal of the American Medical Association. 2017;318(14):1335–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sahetya SK, Brower RG. Lung Recruitment and Titrated PEEP in Moderate to Severe ARDS: Is the Door Closing on the Open Lung? JAMA : the journal of the American Medical Association. 2017;318(14):1327–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Serpa Neto A, Cardoso SO, Manetta JA, Pereira VG, Esposito DC, Pasqualucci Mde O, Damasceno MC, Schultz MJ. Association between use of lung-protective ventilation with lower tidal volumes and clinical outcomes among patients without acute respiratory distress syndrome: a meta-analysis. JAMA : the journal of the American Medical Association. 2012;308(16):1651–9. [DOI] [PubMed] [Google Scholar]
- 65.Neto AS, Simonis FD, Barbas CS, Biehl M, Determann RM, Elmer J, Friedman G, Gajic O, Goldstein JN, Linko R, et al. Lung-Protective Ventilation With Low Tidal Volumes and the Occurrence of Pulmonary Complications in Patients Without Acute Respiratory Distress Syndrome: A Systematic Review and Individual Patient Data Analysis. Critical care medicine. 2015;43(10):2155–63. [DOI] [PubMed] [Google Scholar]
- 66.Futier E, Constantin JM, Paugam-Burtz C, Pascal J, Eurin M, Neuschwander A, Marret E, Beaussier M, Gutton C, Lefrant JY, et al. A trial of intraoperative low-tidal-volume ventilation in abdominal surgery. The New England journal of medicine. 2013;369(5):428–37. [DOI] [PubMed] [Google Scholar]
- 67.Determann RM, Royakkers A, Wolthuis EK, Vlaar AP, Choi G, Paulus F, Hofstra JJ, de Graaff MJ, Korevaar JC, Schultz MJ. Ventilation with lower tidal volumes as compared with conventional tidal volumes for patients without acute lung injury: a preventive randomized controlled trial. Critical care. 2010;14(1):R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gong MN, Thompson BT. Acute respiratory distress syndrome: shifting the emphasis from treatment to prevention. Current opinion in critical care. 2016;22(1):21–37. [DOI] [PubMed] [Google Scholar]
- 69.Roy S, Sadowitz B, Andrews P, Gatto LA, Marx W, Ge L, Wang G, Lin X, Dean DA, Kuhn M, et al. Early stabilizing alveolar ventilation prevents acute respiratory distress syndrome: a novel timing-based ventilatory intervention to avert lung injury. The journal of trauma and acute care surgery. 2012;73(2):391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ball L, Costantino F, Orefice G, Chandrapatham K, Pelosi P. Intraoperative mechanical ventilation: state of the art. Minerva anestesiologica. 2017;83(10):1075–88. [DOI] [PubMed] [Google Scholar]
- 71.Mosier JM, Hypes C, Joshi R, Whitmore S, Parthasarathy S, Cairns CB. Ventilator Strategies and Rescue Therapies for Management of Acute Respiratory Failure in the Emergency Department. Annals of emergency medicine. 2015;66(5):529–41. [DOI] [PubMed] [Google Scholar]
- 72.Levin MA, McCormick PJ, Lin HM, Hosseinian L, Fischer GW. Low intraoperative tidal volume ventilation with minimal PEEP is associated with increased mortality. British journal of anaesthesia. 2014;113(1):97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Anaesthesiology PNIftCTNotESo Hemmes SN, Gama de Abreu M, Pelosi P, Schultz MJ. High versus low positive end-expiratory pressure during general anaesthesia for open abdominal surgery (PROVHILO trial): a multicentre randomised controlled trial. Lancet. 2014;384(9942):495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Downs JB, Stock MC. Airway pressure release ventilation: a new concept in ventilatory support. Critical care medicine. 1987;15(5):459–61. [PubMed] [Google Scholar]
- 75.Davis K, Johnson DJ Jr., Branson RD, Campbell RS, Johannigman JA, Porembka D. Airway pressure release ventilation. Archives of surgery. 1993;128(12):1348–52. [DOI] [PubMed] [Google Scholar]
- 76.Gama de Abreu M, Cuevas M, Spieth PM, Carvalho AR, Hietschold V, Stroszczynski C, Wiedemann B, Koch T, Pelosi P, Koch E. Regional lung aeration and ventilation during pressure support and biphasic positive airway pressure ventilation in experimental lung injury. Critical care. 2010;14(2):R34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Roy S, Habashi N, Sadowitz B, Andrews P, Ge L, Wang G, Roy P, Ghosh A, Kuhn M, Satalin J, et al. Early Airway Pressure Release Ventilation Prevents Ards-a Novel Preventive Approach to Lung Injury. Shock. 2013;39(1):28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mireles-Cabodevila E, Kacmarek RM. Should Airway Pressure Release Ventilation Be the Primary Mode in ARDS? Respiratory care. 2016;61(6):761–73. [DOI] [PubMed] [Google Scholar]
- 79.Halter JM, Steinberg JM, Schiller HJ, DaSilva M, Gatto LA, Landas S, Nieman GF. Positive end-expiratory pressure after a recruitment maneuver prevents both alveolar collapse and recruitment/derecruitment. American journal of respiratory and critical care medicine. 2003;167(12):1620–6. [DOI] [PubMed] [Google Scholar]
- 80.Gattinoni L, Caironi P, Cressoni M, Chiumello D, Ranieri VM, Quintel M, Russo S, Patroniti N, Cornejo R, Bugedo G. Lung recruitment in patients with the acute respiratory distress syndrome. The New England journal of medicine. 2006;354(17):1775–86. [DOI] [PubMed] [Google Scholar]
- 81.Allen GB, Leclair T, Cloutier M, Thompson-Figueroa J, Bates JH. The response to recruitment worsens with progression of lung injury and fibrin accumulation in a mouse model of acid aspiration. American journal of physiology Lung cellular and molecular physiology. 2007;292(6):L1580–9. [DOI] [PubMed] [Google Scholar]
- 82.Al-Rawas N, Banner MJ, Euliano NR, Tams CG, Brown J, Martin AD, Gabrielli A. Expiratory time constant for determinations of plateau pressure, respiratory system compliance, and total resistance. Critical care. 2013;17(1):R23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.van Drunen EJ, Chiew YS, Chase JG, Shaw GM, Lambermont B, Janssen N, Damanhuri NS, Desaive T. Expiratory model-based method to monitor ARDS disease state. Biomedical engineering online. 2013;12:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brody AW. Mechanical compliance and resistance of the lung-thorax calculated from the flow recorded during passive expiration. The American journal of physiology. 1954;178(2):189–96. [DOI] [PubMed] [Google Scholar]
- 85.Gattinoni L, Pesenti A. The concept of “baby lung”. Intensive care medicine. 2005;31(6):776–84. [DOI] [PubMed] [Google Scholar]
- 86.Magalhaes PAF, Padilha GA, Moraes L, Santos CL, Maia LA, Braga CL, Duarte M, Andrade LB, Schanaider A, Capellozzi VL, et al. Effects of pressure support ventilation on ventilator-induced lung injury in mild acute respiratory distress syndrome depend on level of positive end-expiratory pressure. European journal of anaesthesiology. 2018. [DOI] [PubMed] [Google Scholar]
- 87.Andrews PL, Shiber JR, Jaruga-Killeen E, Roy S, Sadowitz B, O’Toole RV, Gatto LA, Nieman GF, Scalea T, Habashi NM. Early application of airway pressure release ventilation may reduce mortality in high-risk trauma patients: a systematic review of observational trauma ARDS literature. The journal of trauma and acute care surgery. 2013;75(4):635–41. [DOI] [PubMed] [Google Scholar]
- 88.Zhou Y, Jin X, Lv Y, Wang P, Yang Y, Liang G, Wang B, Kang Y. Early application of airway pressure release ventilation may reduce the duration of mechanical ventilation in acute respiratory distress syndrome. Intensive care medicine. 2017;43(11):1648–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jain SV, Kollisch-Singule M, Satalin J, Searles Q, Dombert L, Abdel-Razek O, Yepuri N, Leonard A, Gruessner A, Andrews P, et al. The role of high airway pressure and dynamic strain on ventilator-induced lung injury in a heterogeneous acute lung injury model. Intensive Care Med Exp 2017;5(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Richard JC, Lyazidi A, Akoumianaki E, Mortaza S, Cordioli RL, Lefebvre JC, Rey N, Piquilloud L, Sferrazza Papa GF, Mercat A, et al. : Potentially harmful effects of inspiratory synchronization during pressure preset ventilation. Intensive care medicine 2013, 39(11):2003–2010. [DOI] [PubMed] [Google Scholar]


